Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
For as long as the practice of medicine has existed, physicians have attempted to develop a tool to accurately measure pain. We now realize that it is not possible to measure pain because pain is not a quantifiable or measurable entity but, rather, a subjective and highly variable perception. The one thing that all skeletal trauma, and all trauma for that matter, has in common is acute pain. Acute pain interferes with bodily functions and must, and can, be managed appropriately. This is only possible if physicians understand the physiology and pathophysiology of pain: the timing with which it progresses from a symptom to a disease (and possibly how to prevent this from happening; see Chapter 15 ); how to interpret it; and finally, how to treat it. Because we deal with people who have different genetic, cultural, ethnic, personality, and physical backgrounds with vastly differing perceptions and experiences of pain, which are not straightforward concepts, we must often rely on our judgment and interpretation, as physicians, of the pain the patient is suffering.
Furthermore, older and obsolete concepts regarding pain have now reached the stage where we must abandon them and replace them with more modern ideas and strategies based on lessons learned from experience and research. These outdated concepts come from both sides of the spectrum and vary from “no pain no gain,” “pain is weakness leaving the body,” “pain is protective,” “pain is necessary to warn the body/limb from overuse or further injury,” “pain sets the boundaries for activity,” “pain is necessary to inform us of acute compartment syndrome developing,” to “having no pain is a basic human right,” “it is the right of all patients to have no pain,” and “pain is the fifth vital sign.”
If we adopt the first set of values, we tend to undertreat pain, whereas ascribing to the second set of values would invariably lead to overtreatment. Both of these extremes potentially harm our patients, and the purpose of this chapter is to provide some insight into and a reference for the physiology and pathophysiology of pain: how to interpret pain so that we can treat it appropriately and how to describe the different pharmacologic and interventional measures for treating acute pain. Throughout, we also focus on function; we emphasize that having no pain after trauma or surgery is not realistic for either the patient or the treating physician. We should not strive for this but rather focus on “managed” pain and thus on function. If a patient can function properly and appropriately for his or her condition (e.g., sleep, eat, drink, communicate, ambulate, have satisfactory bowel and bladder function, etc.) and be comfortable, and the pain does not interfere with these functions, the pain is generally acceptable and has been treated appropriately. If the pain (or its overtreatment) interferes with normal and acceptable bodily functions, the pain management is not appropriate, and physicians need to intervene further.
The consequence of oversubscribing to the ideas of “pain being the fifth vital sign” and that the absence of pain is a “basic human or patient right” is the fact that today in the United States and elsewhere in the world, we have the worst opioid addiction epidemic and opioid-related death crises in history. We provide a comprehensive outline of the pharmacology of the opioids, which is emphasized from a more chronic pain angle in Chapter 15 , to help with this enormous problem. We are also of the opinion that continuous peripheral nerve blocks and regional anesthesia (RA) provide a valuable and safe alternative to opioids and other potent analgesics if used correctly. For that reason, we also highlight new discoveries, emerging developments, and various philosophies regarding continuous peripheral nerve blocks and RA without going into the details of performing any particular block (which falls outside the scope of this chapter). Finally, we address a few controversial issues, such as liposomal bupivacaine and the use of RA in patients at risk of acute compartment syndrome.
The United States has only 5% of the world's population but uses 80% of the world's opioids.
Opioid addiction and opioid-related deaths are now more common than death by suicide and those due to motor vehicle accidents in the United States.
Hydrocodone was the most frequently prescribed medication between 2007 and 2011.
Between 1999 and 2007, opioid-related deaths increased by 124%.
Orthopaedic surgeons prescribe 7.7% of all opioids in the United States.
Only family physicians and pain specialists prescribe more opioids than orthopaedic surgeons.
Opioid use before trauma or surgery is the single best predictor of prolonged opioid use.
The American Academy of Orthopaedic Surgeons has released an information statement on opioids specific to orthopaedic practice.
There have been widespread and dramatic changes related to pain, pain management, and ultimately, opioid management over the past two decades. In 1998 an initiative making pain the fifth vital sign was introduced. The Joint Commission established standards for pain assessment and treatment in 2001, and the US Congress declared 2000 to 2010 as the decade of Pain Control and Research. Opioid consumption more than doubled between 1999 and 2010. Currently, the United States has less than 5% of the world's population but consumes 80% of the world's opioid supply. Hydrocodone was the number one prescription medication between 2007 and 2011. Between 1999 and 2007, unintentional opioid-related deaths increased by 124% in the United States and exceeded death rates related to suicide and motor vehicle accidents.
Orthopaedic surgery in general, as well as orthopaedic trauma in particular, has been involved and impacted extensively in terms of practices and outcomes related to opioid usage. Orthopaedic surgeons are the third-highest prescribers of opioids, prescribing 7.7% of all opioids in the United States. A comparison of prescribing practices between the United States and the Netherlands revealed that patients with fractures in the United States receive significantly more narcotic prescriptions. For hip fractures, 85% of patients in the United States received opioids during admission and 77% after discharge. This was compared with 58% and 0%, respectively, for Dutch patients. For ankle fractures, 98% of patients in the United States received opioids during admission and 82% after discharge. This was compared with 64% and 6%, respectively, in Dutch patients.
Patients who suffer trauma have a higher rate of preinjury opioid use (15.5%) than the general population (9.2%). Opioid use for acute trauma or after trauma surgery can become a chronic issue. For patients who did not use opioids preinjury, 12.2% continued to use opioids beyond 3 months after surgery (compared with 62.1% in patients who used opioids preinjury). Elderly patients are not exempt from opioid dependence after acute injury. In a sample of 38,963 elderly trauma patients (average age of 79.3 years) over a 10-year period, it was found that 6.8% of opioid-naive patients were still using opioids 1 year after injury.
Opioid use before trauma or trauma surgery is the single-best predictor of prolonged opioid use. In addition, the category of surgery predicts prolonged opioid use, with acetabular/pelvic injuries having higher use than lower extremity surgery, with upper extremity surgery being lowest. Psychological distress, catastrophic thinking, poor coping ability, greater symptoms, and disability are more predictive of prolonged opioid use than injury or procedure type (see Video 14.1).
The Department of Health and Human Services has acknowledged the opioid crisis and created a website with statistics, guidelines, and resources related to it ( https://www.hhs.gov/opioids/about-the-epidemic/index.html ). The Centers for Disease Control and Prevention similarly has acknowledged the opioid crisis and created guidelines for primary care physicians for prescribing opioids ( https://www.cdc.gov/drugoverdose/prescribing/guideline.html ). These guidelines include a factsheet with 12 recommendations that essentially discourage opioids as first-line management of pain and the use of prolonged opioid therapy. They also encourage the decreased use of long-acting opioids, titration of the dose/agent, and most importantly, patient education and engagement regarding the risks of opioid use ( https://www.cdc.gov/drugoverdose/pdf/Guidelines_Factsheet-a.pdf ). Although this is not specific to surgery or surgeons, several of the guidelines apply and can be adopted for orthopaedic trauma. It has been shown that patients are often overprescribed narcotics, and it is prudent to titrate dosage and opioid agents based on the anatomic location of surgery and the procedure type. Additionally, preoperative counseling and adjustment of patient expectations related to postoperative pain and opioid use have been effective in reducing opioid consumption. Having prescription guidelines for surgeons and establishing opioid prescription protocols is effective in reducing opioid prescriptions initially as well as with refills.
The American Academy of Orthopaedic Surgeons has released an information statement on opioids specific to orthopaedic practice ( https://www.aaos.org/uploadedFiles/PreProduction/About/Opinion_Statements/advistmt/1045%20Opioid%20Use,%20Misuse,%20and%20Abuse%20in%20Practice.pdf ). This statement has relevant information about the opioid crisis as well as important strategies. The need for an “opioid culture change” is outlined, and the conclusion is of importance to our entire specialty: “In the United States, the current cultural expectation of opioid use as the primary treatment for acute and chronic pain has created an opioid epidemic. Only a culture change led by physicians dedicated to limiting inappropriate opioid use will solve this epidemic. Physicians, patients, and caregivers in the United States need to learn how to treat pain with less dependency on opioid medications.”
The complex and elusive continuum of surgery, trauma, rehabilitation, acute pain, and chronic pain ideally should be handled through evidence- and outcomes-based partnerships.
Pain is a unique personal experience and is different from nociception.
The relationship between nociception and pain is complex and variable; it is heavily influenced/modulated by higher centers and emotions.
Pain control should begin long before surgical intervention.
Patients should be educated to have realistic expectations regarding pain associated with trauma and surgery.
Surgery and trauma hurt.
The focus should be on function and not on zero pain. Zero pain does not exist after trauma and surgery.
The emphasis should be on managed pain and not on the elimination of pain.
Patients should be forewarned that surgery hurts; they should not discover this after surgery.
Before we can begin any serious discussion of the physiology of pain, we first need to clarify the terms. Pain includes both the physiology of nociception, the system of peripheral transduction of tissue injury and the processing of those signals in the central nervous system, and also the uniquely individual experience of pain. As such, pain control should begin long before any surgical intervention. When the decision to operate is made, patients should be educated on pain expectations. By beginning with a realistic understanding that surgery hurts and by evaluating an individual patient's coping skills and propensity to catastrophize, plans can begin to be made to best manage, not eliminate, pain and focus on function. When, on the other hand, patients first learn that they will have pain after surgery only when they begin to experience it, they will undoubtedly find their pain control, and perhaps their care as a whole, to be unsatisfactory.
Nociception is the body's ability to detect tissue injury.
Nociception comprises four basic processes:
Transduction
Conversion of signals of actual or impending tissue injury in the environment where the injury is taking place.
Believed to be primary sensory apparatus consisting of a network of free nerve endings that are capable of responding to a variety of signals, including mechanical (pressure), thermal, and chemical stimulation.
Transmission
Nociceptive signals are carried to the central nervous system (CNS) by two types of afferent neurons:
Large and lightly myelinated Aδ-fibers that rapidly carry information about sharp, localized pain—fast fibers.
Small unmyelinated C-fibers fibers that slowly carry information about dull, achy, throbbing diffuse pain—slow fibers.
Both originate in the dorsal root ganglion.
The short process of these neurons (that have no dendrites) enters the spinal cord and synapses with secondary afferent neurons in the dorsal horn.
Although pain coming from the skin can be located, pain originating from viscera, muscles, and bone is often poorly localized and can be referred.
Visceral pain is transmitted to CNS via sympathetic nerves.
It is becoming increasingly evident that bone pain and ischemic pain are visceral pain.
Central processing, which consists of:
Modulation
Modulation decreases the transmission of nociceptive signals up the spinal cord to the brain.
The endogenous opioid system can be activated by a variety of stressors, including pain. This system is activated by surgery, but short-acting nerve blocks (single-shot blocks) may inhibit this, leaving the patient vulnerable to severe pain when the block wears off.
Central processing can also increase pain through spinal cord wind-up (see Chapter 15 ) or central sensitization (enhances responses to stimulation of noninflamed tissue near the area of inflammation).
Perception
Nociceptive information is processed and combined with emotional and contextual information (among other information).
The hippocampus is involved in the development of avoidance behavior in response to pain- and anxiety-induced hyperalgesia.
Connections between the secondary somatosensory cortex and the limbic system are also important for the emotional, motivational, and memory aspects of pain.
Sensory and affective aspects of pain
Pain is an experience and not simply a sensory event.
The emotional experience of pain must be understood alongside the physiology of pain.
The ability to tolerate pain is a complex interplay between the sensory and affective aspects of pain and is influenced by factors as diverse as the particular circumstance(s) under which the pain is experienced, personality traits, attitudes, previous experience, gender, and socioeconomic factors.
Nociception is the term used to describe the body's ability to detect tissue injury, or impending tissue injury, and comprises four basic processes: transduction, transmission, modulation, and perception. Transduction is the conversion of signals from the external or internal environment to a format the nervous system can interpret. Transmission is the conveyance of that information to brain regions responsible for processing and interpreting those signals. Modulation is a relatively recently discovered phenomenon that describes how the signal is modified during transmission. Perception is subjective awareness and is the result of the integration of a multitude of signals. Perception is heavily influenced by attention, expectation, and interpretation in light of psychological factors and past experiences.
The conversion of signals of actual or impending tissue injury begins in the environment where the injury is taking place. It is believed that the primary sensory apparatus for the transduction of nociceptive signals is a network of free nerve endings that are capable of responding to a variety of signals, including mechanical (pressure), thermal, and chemical stimulation. These free nerve endings are believed to exist in most tissues throughout the body (the one notable exception being the brain itself) but are predominantly found in the skin. How various signals activate these nerve endings is poorly understood because studying these free nerve endings directly is very challenging. They are so small that they are difficult to locate and identify in vivo, let alone study. Histologically, it has been demonstrated that they are connected to Aδ- and C-type primary afferent fibers. Although it is not understood how various signals activate nociceptive nerve endings, it has been demonstrated that several biologically relevant chemicals either directly activate these nerve endings or sensitize them, prompting easier activation by other stimuli when they are present. Some of these chemicals, such as potassium, histamine, or serotonin, would be expected to be present in injured tissue, either released from the cytoplasm of damaged cells or from the bloodstream when nearby blood vessels are damaged. Others, such as bradykinins, prostaglandins, and leukotrienes, are synthesized by enzymes activated by tissue injury. Much like the skin, the nociceptive primary afferent fibers in muscle are now understood to respond to mechanical stimuli and chemical signals, but interestingly, muscle contraction itself can also generate signals in the nociceptors in muscle. One of the most potent stimuli that activate muscle nociceptors is muscle contraction under conditions of ischemia, believed to be transmitted by sympathetic nerves.
Nociceptive signals are carried from the tissue in which they originate to the central nervous system by two types of primary afferent neurons ( Table 14.1 ): Aδ- and C-type fibers. Aδ fibers are large, lightly myelinated fibers that rapidly carry information about sharp, localized pain. C fibers are small, unmyelinated fibers that slowly carry information about dull, achy, throbbing, diffuse pain. Both of these fiber types originate from cells whose bodies are in the dorsal root ganglia. Anatomically, they are referred to as pseudounipolar cells. This type of neuron has an axon that divides but has no dendrites. The long, peripheral process generates the fibers discussed previously, and a shorter, central process enters the spinal cord and synapses with secondary afferent neurons in the dorsal horn of the spinal cord. Nociceptive signals are encoded in the firing pattern of these neurons, both in terms of frequency and pattern. Studies conducted in monkeys and humans have demonstrated that reported increases in pain intensity correlate with increased firing frequency in relevant sensory nerves, but this relationship is not linear or direct. It is possible to have pain in the absence of activity in nociceptive fibers, and it is possible to have activity in nociceptive fibers in the absence of pain. Situations like these most commonly occur after injuries to the nervous system, centrally, peripherally, or both, but they can also occur in normal individuals as a product of modulation, which is discussed later. Suffice it to say that the relationship between nociceptive input and pain is complex and variable.
| Generic Name |
MME (Milligrams Unless Otherwise Indicated) |
|---|---|
| Alfentanil b | 0.03 |
| Buprenorphine | |
| Buccal/sublingual | 37.5 (mg) |
| Patch c | 12.6 (mcg/hr) |
| Film c | 10 (mcg/hr) |
| IV, IM | 75 (mg/day) |
| Butorphanol c | 7 |
| Carfentanil | Not listed |
| Codeine c | 0.15 |
| Dextropropoxyphene | 0.1 |
| Diamorphine (heroin) | Not listed |
| Fentanyl | |
| Buccal/sublingual | 0.13 (mcg/day) |
| Film/Oral spray | 0.18 (mcg/day) |
| Nasal spray | 0.16 (mcg/day) |
| Patch | 7.2 (mcg/day) |
| IV, IM, SC | 0.2 (mcg/day) |
| Hydrocodone | 1 |
| Hydromorphone c | 4 |
| Meperidine (Pethidine) |
0.1 |
| Methadone d | 4.7 |
| Methadone c | 3 |
| Morphine | 1 |
| Nalbuphine c | 1 |
| Oxycodone | |
| IV, SC | 3 |
| Rectal | 1.5 |
| Oxymorphone | 3 |
| Pentazocine | 0.37 |
| Remifentanil | 0.2 (mcg/day) |
| Sufentanil | |
| Buccal/sublingual | 0.5 (mcg/day) |
| IV, SC | 2 (mcg/day) |
| Tapentadol | 0.4 |
| Tramadol | 0.2 |
| Tramadol c | 0.1 |
| (Opium) | 1 |
a Conversion factors are based on clinical guidelines for chronic opioid dosing.
b Rowans Hospice. Opioid conversions. https://www.palliativedrugs.com/download/090714_opioid_conversions.pdf .
c Centers for Medicare and Medicaid Services. Opioid morphine equivalent conversion. https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-March-2015.pdf .
d Many guidelines publish conservative rates due to concerns about incomplete cross-tolerance or opioid toxicity with long-acting opioids. This conversion is based on published transfers in the direction of methadone to morphine.
Unlike pain arising from the skin, pain arising from deeper structures such as the viscera, and to a lesser extent, muscle and bone, is often dull, aching, and poorly localized, and it can often generate what is known as referred pain—that is, pain that is misperceived as originating from a site distant from the site of tissue injury. Referred pain can be a major source of confusion when examining a patient whose primary complaint is one of pain. Although it is widely recognized that visceral pain is often referred to somatic structures—for example, cardiac pain is often referred to the chest, back, left arm, and/or upper abdomen—it is far less widely understood that myofascial pain is also often referred and can generate the perception of pain in areas quite distant from the actual site of injury. This was demonstrated as early as the late 1930s by Kellgren. There are now clinically recognizable patterns of pain referred from specific muscles that have been well described, for example, by Travell and colleagues, both in the 1950s and in the 1980s.
There are four major physiologic mechanisms proposed to explain the phenomenon of referred pain: (1) activity in the sympathetic nervous system, (2) peripheral branching of primary afferent nociceptors, (3) convergence projection, and (4) convergence facilitation. Of these, the first two are mechanisms active in the peripheral nervous system, whereas the last two are central mechanisms.
A further explanation of these four major physiologic mechanisms is as follows:
Activity in the sympathetic nervous system may cause referred pain by producing and releasing substances in the area of referred pain that sensitize nociceptors or perhaps by altering blood supply to the sensory nerve fibers themselves.
Peripheral branching of nerve fibers to separate parts of the body may result in the brain misinterpreting signals originating in nerve endings in one area of the body as arising from nerve endings in the other area of the body.
The convergence-projection hypothesis states that because a single nerve cell in the spinal cord receives input from multiple primary nociceptive fibers that supply both visceral and somatic structures, the brain has difficulty distinguishing visceral input from somatic input. In such situations, the brain defaults to interpreting the input as coming from the somatic structures because they are much more commonly the source of such input.
The convergence-facilitation hypothesis states that the background activity of second-order pain-projection neurons in the spinal cord that receive input from one somatic region is amplified (facilitated) in the spinal cord by signals arising from nociceptors originating in another region of the body. Nociceptors producing the background activity originate in the region of perceived pain and tenderness; the nerve activity producing the facilitation originates elsewhere, for example, at a myofascial trigger point. This convergence-facilitation mechanism is of clinical interest because one would expect that blocking sensory input in the area of referred pain with cold or a local anesthetic (LA) should provide temporary pain relief. One would not expect such relief according to the convergence-projection theory. Both of these responses (the presence and the absence of temporary relief offered by blocking sensory input in the area of referred pain) have been demonstrated in clinical experiments.
Referred pain can pose serious problems for both patients and physicians when it goes unrecognized. Because the source of pain actually lies at a location distant from the perceived painful area, physical examination and diagnostic testing and imaging regularly fail to demonstrate any issue at the area perceived to be the source of the pain. This often leads physicians to believe that there is no physical reason for the patient to be experiencing pain and prompts both the physician and patient to suspect strong psychological components to the pain. This results in frustration on the part of the physician and ever-increasing anxiety on the part of the patient. Combined, these can lead to “doctor shopping,” no treatment, or inappropriate treatment.
As discussed previously, nociception does not invariably result in pain, nor is it a prerequisite for pain. This tells us, in no uncertain terms, that just as nociception describes the peripheral physiologic process, pain describes the central processes involved in managing nociceptive signals, both in the spinal cord and in various brain regions.
Modulation is a broad term used to describe the activity of a multitude of circuits and pathways throughout the central nervous system that ultimately terminate in the dorsal horn of the spinal cord. The earliest discovered of these systems functioned to decrease the transmission of nociceptive signals up the spinal cord to the brain. The concept of “spontaneous analgesia” was pioneered when Basbaum and Fields were exploring how electrical stimulation of specific brain regions could block typical responses to noxious stimuli in animal models. This phenomenon was termed “stimulation-produced analgesia” and quickly became a topic of serious interest when it was noted that stimulation of homologous regions of the human brain could provide relief for patients suffering from chronic pain. Electrical stimulation is not the only way to activate this pathway; opioids have been known to activate this same neuronal circuitry. Activation of this system by opioids is responsible for at least a portion of their analgesic effects.
One of the most important discoveries in the understanding of pain was the revelation that there are substances in the brain that mimic the pharmacologic activity of plant-derived opioids. These “endogenous opioids,” known as endorphins, enkephalins, and dynorphins, are found within neurons throughout the central and peripheral nervous systems. Particularly important is that they are concentrated in the brainstem regions previously known to be involved in spontaneous analgesia. The endogenous opioid system can be activated by a variety of stressors, including pain. This system has been shown to be activated after surgery and can have a significant analgesic effect. There are thus systems built into humans whose purpose is to provide analgesia, and these systems, like all biologic systems, show a large degree of interindividual variability. The variability in the activity of the endogenous opioid system and personality differences well explain why different patients undergoing the same operation can have such differences in their postsurgical pain.
Activity taking place in the spinal cord for the modulation of noxious signals does not always serve to suppress pain. There are also important processes that, when triggered, can serve to increase the transmission of signals from afferent nociceptors. A phenomenon referred to as spinal cord wind-up is, in the context of orthopaedic practice, one of the more important of these processes (more about this in Chapter 15 ). Wind-up refers to the observation that activation of peripheral nociceptive fibers, in particular, C fibers, is stimulated at a frequency of approximately 1 Hz. In this situation, second-order neurons in the dorsal horn of the spinal cord become increasingly responsive to the repetitive stimulation incoming from C fibers. Physiologically, this represents the temporal summation of excitatory postsynaptic potentials in the second-order spinal neuron that is driven by near-constant activation of the slow-conducting C fibers. This can not only serve to increase perceived pain in the presence of unchanging stimulation but can also widen the receptive field of the involved spinal cord neurons, which will produce pain that appears to be spreading in the face of unchanging stimulation. Spinal cord modulation can also result in increased perception of pain through a process referred to as central sensitization, which is effectively an increase in the sensitivity of neurons in the dorsal horn of the spinal cord to input from nociceptive fibers originating in inflamed tissue. This central sensitization can be quite pronounced, likely evolved as a protective mechanism to limit further injury to an already injured body part, and may contribute to the evolution of chronic pain states. Typical changes observed in neurons undergoing central sensitization include increased responses to noxious stimulation of inflamed tissue, lowering of the activation threshold of spinal cord neurons with an initially high threshold, increased responsiveness to stimulation applied to noninflamed tissue surrounding the area of inflammation, and expansion of the receptive field of the involved spinal neurons. In particular, the enhanced responses to stimulation of noninflamed tissue near the area of inflammation indicates that the sensitivity of the spinal cord neurons is enhanced so that previously subthreshold input is sufficient to activate the neuron under inflammatory conditions. The sensitization of individual spinal cord neurons will lead to an increased percentage of neurons in a segment that respond to stimulation of an inflamed tissue. Thus the population of responsive neurons increases. Central sensitization can persist for weeks. It is important to keep in mind that tissue inflammation will not only lead to increased responsiveness of the chemosensitive nociceptive fibers in that tissue but will also lead to increased responsiveness of the spinal cord neurons with which the peripheral nociceptors synapse.
It appears that nociceptive information is further processed within the brain and integrated with other information, ultimately culminating in awareness and the subjective experience we call pain. In the amygdala, nociceptive information is processed and combined with, among other things, emotional and contextual information. It is from this interaction that we develop a sense of fear and/or anxiety linked to pain and that reciprocal relationships can develop between pain and affective disorders. Likewise, the hippocampus is involved in the development of avoidance behaviors in response to pain and anxiety-induced hyperalgesia. Connections with higher brain centers and the amygdala facilitate the development of “pain memory,” allowing associations to be made between painful experiences and environmental cues. The nucleus accumbens (NAcc) in the striatum is also a key player in the central processing of pain. The NAcc functions in probability assessment, reward, and reinforcement processing. It is critically involved in risk–benefit analysis and reward processing such that it is a key structure involved in the development of behaviors such as drug abuse. Opioids and stimulants induce changes in the activity of neurons in the striatum that are consistent with other rewarding behaviors, such as food and sex. Not surprisingly, there are important connections between the NAcc, the amygdala, and the cortex. In light of these connections, the NAcc functions like a gatekeeper, either suppressing or enhancing the perception of pain based in part on the assessment of a reward in any particular situation. In the cortex, it appears that the secondary somatosensory cortex is the dominant area for the summation of all the various input and processing areas for pain. It is likely here that perception truly occurs, in that this is the area where noxious stimuli are recognized as painful and intensity is coded, along with other discriminatory aspects of painful stimuli. Connections between the secondary somatosensory cortex and the limbic system are also vitally important for the emotional, motivational, and memory aspects of pain.
As stated throughout this chapter, pain is an experience, not simply a sensory event, and the emotional experience of pain must be discussed alongside the physiology of pain transduction, transmission, modulation, and perception. Pain sensation, or nociception, exists to allow us to detect, localize, assess, and identify injurious or potential injurious internal and external stimuli. The affective experience of pain, on the other hand, describes the unpleasantness of a painful experience. It is tied to the emotional urge to terminate the experience and is usually accompanied by mood changes such as anxiety or depression. This affective experience is also a driving force in the generation of pain memory and a forward-looking desire to avoid similar stimuli in the future.
This disparity can be illustrated in the differences between pain threshold and pain tolerance. When we examine temperature, for example, we find that people report increasing temperature to become painful between 43°C and 46°C. The temperature at which 50% of the population reports pain is the pain, or sensory, threshold. In contrast to this highly reproducible sensory threshold lies the phenomenon of pain tolerance, which varies widely among individuals. When asked to immerse their arm in ice water and leave it there as long as they can tolerate, people would reveal their innate differences in tolerance to pain. Some will remove their arm in under 90 seconds, whereas others will tolerate it for well over 5 minutes. Perhaps more interesting is the observation that this high degree of variability exists even within the same individual. If the test conditions or the emotional context is changed, the duration for which one individual can tolerate the ice-water bath will change. The ability to tolerate pain is a complex interplay between the sensory and affective aspects of pain and is influenced by factors as diverse as the particular circumstance(s) under which the pain is experienced, personality traits, attitudes, previous experience, gender, and even socioeconomic factors. If we want to apply the “threshold” verbiage, then pain tolerance is not so much about sensory detection but, rather, is a response threshold. At what pain intensity and duration will a given individual in a given situation alter his or her behavior—choose to take medicine, stay home from work, or seek medical attention—rather than simply endure the pain because it, although unpleasant, is bearable? That is to say, there is a point at which the suffering, anxiety, or anguish brought on by pain is great enough that the given individual in the given context is no longer able to continue without some change taking place.
Pain is not a measurable entity because of its complexity.
Attempts to quantify:
Verbal rating scale (consist of adjectives “no pain” and “extremely intense pain”)
Visual analog scale (10- or 100-mm line; patient indicates where pain is on this line from 0 to 10 mm or 100 mm)
Numerical rating scale (0 represents no pain and 10 the worse pain imaginable; of value in “big data” and research)
Picture or face scale (used in pediatrics)
Behavioral measurement (grimacing, splinting behavior, rigid body postures, limping, frowning, crying, vocalizing, etc.)
As discussed elsewhere in this chapter, pain is not simply a conscious understanding of nociceptive input but is a much more complex experience that includes not only nociception but also affective factors such as mood, memory, emotional state, motivational state, and desires to avoid or, in some cases, seek out such unpleasant experiences, along with the potential perception of reward or secondary gain. In this light, the assessment of pain is not a simple task. There needs to be an exploration of not only the nociceptive stimuli the patient is responding to but also his or her unique affective context, which will be a great help in explaining how two patients can undergo the same procedure and report wildly different pain.
There are many tools available for the assessment of pain. In fact, a brief Wikipedia article on “Pain Scale” lists over 30 different instruments for the assessment of pain and is quite direct that this list is incomplete ( https://en.wikipedia.org/wiki/Pain_scale ). An ideal pain assessment tool would be sensitive and free from bias; provide immediate information about accuracy and reliability; distinguish between pain, unpleasantness, and emotion; be useful in assessing both experimental and clinical pain; be absolute rather than relative in its scale(s); and estimate the confidence of its predictions. Even with the vast array of instruments available, none meets all of these goals.
To simplify the understanding of these myriad pain assessment tools, we can group them into five categories: verbal rating scales, visual analog and graphic rating scales, numerical rating scales, picture or face scales, and behavioral measurements. Each of these types of measures has its own set of advantages and disadvantages.
Verbal rating scales consist of a list of adjectives to describe pain intensity. Those that are well constructed consist of adjectives that reflect the extremes of pain, from “no pain” to “extremely intense pain,” and sufficient additional adjectives to capture gradations of pain intensity that may be experienced between these two extremes. These scales are easy to administer, understand, and score, and as such, compliance with their use is high. They have been validated against other measures of pain intensity and are consistently sensitive to interventions known to affect pain intensity. Criticisms raised against verbal rating scales include the fact that these scales are based on the assumption that there are equal intervals between the adjectives, even though it is extremely unlikely that those intervals are perceived to be equal. That is, the interval between no pain and mild pain may be much smaller than that between moderate pain and severe pain, yet the interval is scored as if the difference were equivalent. Similarly, patients must be familiar enough with the terms used in the scale to be able to understand them, they may not find a term that appropriately describes their pain, and these scales are likely inappropriate for use in illiterate patients.
Visual analog scales consist of a line, typically 10 cm in length, whose ends are labeled as extremes of pain: no pain and worst possible pain. Some visual analog scales have points along the line labeled with indicators of pain intensity—either adjectives or numbers. Patients are asked to place a mark on the line that best represents their pain. The distance is measured from the “no pain” end of the scale to the mark made by the patient and is used as the pain intensity score. These scales have been validated against other methods of assessing pain, including self-reported pain and behavioral observations, and are sensitive to interventions that reduce pain. Visual analog scales are arguably more sensitive than other pain assessment tools because, conceptually, there are 101 response levels (zero plus 100 mm of the length of the scale). Unfortunately, these scales are more time consuming to use, require the patient to have the ability to place an accurate mark on the scale, and can be difficult for patients to understand, especially the very young and those with cognitive impairments or receiving high-dose opioids.
Numerical rating scales consist simply of asking patients to rate their pain on a scale of 0 to 10 or 0 to 100, with 0 representing no pain and 10 or 100 representing the worst pain possible. At their simplest, numerical rating scales require nothing more than a verbal response from patients, which is then recorded, although several and more complex written numerical rating scales exist. Numerical rating scales are very popular, in large part due to their simplicity. The vast majority of patients can easily understand the scale and respond appropriately. These scales are easy to administer and interpret, and they can even be administered remotely, such as over the telephone. Unfortunately, these scales are designed only to evaluate pain intensity, and they do a very poor job of evaluating affective components that may be contributing to pain.
Picture or face scales employ photos or drawings depicting facial expressions of people experiencing a range of pain severity. Patients are instructed to indicate which of the illustrations best represents their pain. The illustrations are often overlaid on a visual analog scale with numbers indicating pain severity. These scales have the distinct advantage of not requiring the patient to be literate and are particularly useful in pediatric populations, where they have been validated against other measures of pain. They also respond appropriately to the administration of interventions that would be expected to reduce pain. These, too, are imperfect tools. They require explanation, which requires a degree of comprehension that may not be present in very young children. They can also introduce an element of confusion because it may be difficult to separate how aspects of the images represent pain versus misery, discomfort, emotional distress, depression, or anxiety.
Behavioral measurements of pain rely on the idea that when people experience pain, especially acute pain, there will be both physiologic and behavioral indicators of their discomfort. Examples of behavioral indicators of pain include grimacing, splinting behavior, rigid body postures, limping, frowning, crying, vocalizing, and so forth. Changes in vital signs would also be expected, such as tachycardia, tachypnea, elevated blood pressure, and so forth. Again, due to the complex interplay between nociception and affective state, as well as coping skills in individual patients, the absence of changes in vital signs or the absence of common behavioral indicators of pain cannot be used to definitively indicate the absence of pain. Similarly, medications (e.g., beta blockers) or physiologic conditions (e.g., hypothyroidism) could mask physiologic indicators of pain. Guidelines from the American Pain Society indicate that observations of vital signs and behavioral indicators should not be used alone, in place of patients self-reporting regarding their pain, and that it must be understood that physiologic measures are neither sensitive nor specific indicators of pain. That being said, it takes little effort for most clinicians to draw upon memories of patients who self-report “10 out of 10” pain while they carry on conversations with loved ones and play with their electronic devices.
Selection of the most appropriate tool, or combination of tools, to evaluate an individual patient's pain can be complex. If you believe you are having difficulty appropriately assessing pain in a patient, please consult with a pain physician for specific recommendations. In the current age of opioid overuse, the idea of self-reported pain being treated as a fifth vital sign is quickly slipping away, and with it the idea that pain should be eliminated. These ideas are being replaced with a framework that pain comes hand-in-hand with injury and surgery and that pain should be managed, not eliminated. Pain should be treated such that pain itself does not interfere with other vital functions; patients should be able to breathe, cough, eat, sleep, urinate and defecate, and participate in physical and occupational therapy without their pain preventing them from doing these activities. That certainly does not mean that they should be completely pain-free .
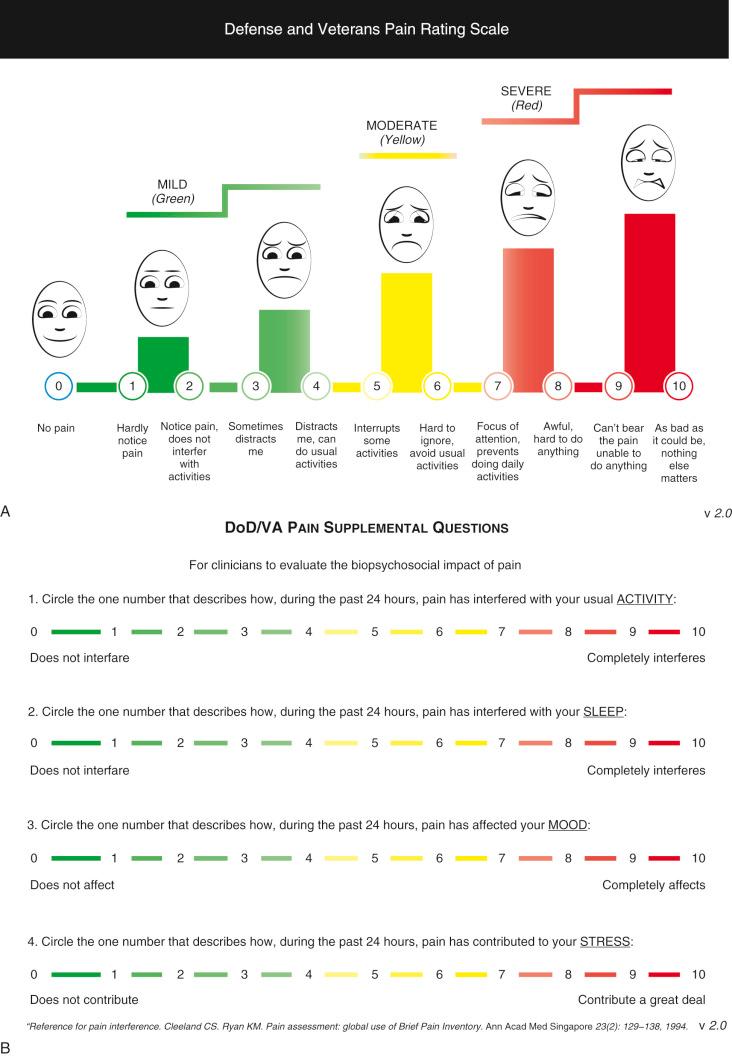
The Defense and Veterans Pain Rating Scale (DVPRS; Fig. 14.1 ) attempts to put all the measurement scales together.
Opioids influence the pain signal at multiple locations in the pain pathway.
They work in the brain, the spinal cord, and the periphery.
In the brain, opioids have mood-altering effects, cause sedation, and can also decrease the emotional response to pain.
Opioids block the signal from the primary nociceptor to the primary afferent neurons, then from the primary afferent neurons in the dorsal root ganglion to the secondary excitatory neurons.
Opioids also work on neurons that descend from the brainstem to the spinal cord that modulate pain signals.
Those descending pathways have fibers that either amplify or inhibit pain signals being transmitted to the brain.
Opioids depress the fibers that amplify the signal and enhance the fibers that inhibit the pain signal.
There is evidence that opioids can work peripherally to increase activation of primary neurons and inhibit immune and inflammatory responses to noxious stimuli.
There are three types of opioid receptors:
µ (mu) receptors (now called MOP) are associated with analgesia.
κ (kappa) receptors (now called KOP) are associated with hallucinations, dysphoria, and an overwhelming sense of dissatisfaction, anxiety, or restlessness.
δ (delta) and µ-receptor agonism can cause respirat ory depression because opioid stimulation of the midbrain suppresses the body's ability to appropriately detect carbon dioxide levels.
Other side effects:
Sedation and somnolence
Urinary retention
Constipation
Nausea and vomiting
Dizziness, itching
Tolerance (which means higher and higher doses are required to achieve the same level of pain relief)
Four naturally occurring compounds (morphine, codeine, thebaine, and papaverine)
Four semisynthetic compounds (diamorphine [heroin], dihydromorphine, buprenorphine, and oxycodone)
At least seven synthetic compounds (meperidine [pethidine], fentanyl, methadone, alfentanil, remifentanil, carfentanil, and tapentadol)
Lack of high-quality evidence that supports opioid use in chronic pain, osteoarthritis, and low back pain
Effect on cardiac function:
Little direct effect on contractility
Can affect cardiac function if given in combination with other drugs (e.g., benzodiazepines)
Cognitive effects and sedation (CES):
CES in populations receiving long-term opioid therapy for chronic nonmalignant pain syndromes ranges from 20% to 62%.
Memory deficits: 73% to 81%
Sleep disturbance: 35% to 57%
Fatigue: 10%
Opioid-induced constipation (OIC):
Opioid bowel dysfunction is a common side effect of opioid therapy as a result of the action exerted via µ-opioid receptors throughout the gastrointestinal tract
Continues throughout treatment period
Newer therapies target the underlying mechanism of OIC by displacing opioids from the receptors in the gastrointestinal tract without affecting the centrally activated mechanism that gives rise to analgesia.
Subcutaneous methylnaltrexone (MNTX; Relistor)
Oral naloxegol (Movantik)
Opioid-related endocrinopathy (opioid-induced androgen deficiency)
Side effect of µ-opioid analgesics
Impaired sexual function
Decreased libido
Infertility
Osteoporosis and osteopenia
No endocrine effect on women found
Opioid-induced hyperalgesia
True incidence not known
Prevalent in:
Former opioid addicts
Patients administered opioids during the preoperative period
Healthy human volunteers receiving remifentanil
The central and peripheral nervous systems are composed of neurons, which themselves are composed of cell bodies, which are responsible for neuronal activity. Each neuron has a number of dendrites, which are short fibers that receive signals from other neurons and transmit them to the cell body. Also attached to the neuron is an axon, a long fiber that transmits messages from the cell body to other dendrons and from there to other neurons. Axons are also present in other tissues such as muscles and transmit electrical signals to them as needed. Nerves communicate with one another by neurotransmission, which occurs by releasing chemical messengers that transmit the message across the spaces or synapses between cells. These chemicals are released by the proximal cells of the synapses and bind to the receptors in the distal cells in the synapses of the dendrites (Video 14.2).
There are different receptors that send messages to the brain when they are exposed to stimuli, including temperature, mechanical, pH, and so forth. Nociceptors detect stimuli that can cause tissue damage and are found in most locations, but they proliferate in the skin, the walls of organs, and deep within other tissues such as muscles, joint capsules, and joints. The electrical impulse transmitted to the brain by nociceptors is pain. The signal is first sent via a primary afferent neuron to the dorsal root ganglion of the spinal roots. There the electrical current caused by the impulse initiates a release of neurotransmitters that passes the pain signal from the primary afferent neuron to a secondary excitatory neuron in the spinal cord. There are several neurotransmitters involved in pain signaling, the major ones being glutamate and substance P. The message is then sent to various locations in the brain, where it is interpreted as pain.
The thalamus receives the signal and provides context to the message. The thalamus releases the pain message to the hypothalamus and limbic system, which in turn help us to learn from the pain and avoid the cause of the pain in the future. This can modify our emotions and behaviors regarding pain. Catastrophizing, which is outside the scope of this chapter but more thoroughly covered in Chapter 15 , is perhaps a good example of such a behavioral/emotional modification to pain.
Opioids influence the pain signal at multiple locations in this pathway. They work in the brain, the spinal cord, and in the periphery. In the brain, opioids have mood-altering effects, cause sedation, and can also decrease the emotional response to pain. Opioids block the signal from the primary nociceptor to the primary afferent neurons, then from the primary afferent neurons in the dorsal root ganglion to the secondary excitatory neurons.
Opioids also work on neurons that descend from the brainstem to the spinal cord that modulate pain signals. Those descending pathways have fibers that either amplify or inhibit pain signals being transmitted to the brain. Opioids depress the fibers that amplify the signal and enhance the fibers that inhibit the pain signal. There is evidence that opioids can work peripherally to increase activation of primary neurons and inhibit immune and inflammatory responses to noxious stimuli.
When the signal is passed from the primary neuron to the secondary neuron at the synaptic cleft, an influx of calcium ions causes the release of neurotransmitters into the synapse. These neurotransmitters flow across the synapse and then bind with receptors on the postsynaptic neuron. This initiates the chain of events within the secondary neuron that further propagates the pain signal to the brain. There are specific opioid receptors on both pre- and postsynaptic neurons. When an opioid compound binds to the opioid receptor on the presynaptic neuron, it decreases the amount of calcium ions that can enter the cell, ultimately decreasing the amount of excitatory neurotransmitters that are released into the synapse. Opioids binding to the postsynaptic opioid receptors, in turn, decrease the response to any of the neurotransmitters released from the presynaptic neuron, which results in fewer pain stimuli transmitted to the brain.
It has been known for hundreds of years that opium possesses analgesic effects. During the 1970s, however, it became apparent that humans and other animals have an analgesic mechanism through endogenous opioids that modulate pain signals. These endogenous opioids, collectively known as endorphins, include beta-endorphins, enkephalins, and dynorphins. The endorphins interact with the opioid receptors to varying degrees, but they all have the tetra-peptide sequence tyrosine-glycine-glycine-phenylalanine (tyr-gly gly phe) in common. Exogenous opioid drugs and medications bind to opioid receptors in the same way but with more powerful and varying consequences.
There are three types of opioid receptors: µ (mu) receptors, κ (kappa) receptors, and δ (delta) receptors. When mu receptors are activated by an opioid agonist like morphine, hydrocodone, or heroin, for example, they will all produce analgesia, but they all will come with side effects as well. Kappa receptor stimulation is associated with hallucinations and dysphoria and an overwhelming sense of dissatisfaction, anxiety, or restlessness. Delta- and mu-receptor agonism can cause respiratory depression because opioid stimulation of the midbrain suppresses the body's ability to appropriately detect carbon dioxide levels. Other side effects include sedation and somnolence, urinary retention, constipation, nausea and vomiting, dizziness, and itching, among others. One of the worst side effects of opioids is tolerance, which means higher and higher doses are required to achieve the same level of pain relief. The exact cellular mechanism for this is not known, although there are many plausible theories. This is the basic cause of opioid dependence and addiction.
Opioids can be classified according to their effect at opioid receptors. In this manner, opioids can be considered agonists, partial agonists, and antagonists. Agonists interact with a receptor to produce a maximal response from that receptor (analgesia after morphine administration is an example). Conversely, antagonists bind to receptors but produce no functional response while at the same time prevent an agonist from binding to that receptor (naloxone). Partial agonists bind to receptors but elicit only a partial functional response no matter the amount of drug administered (buprenorphine). In addition, opioids can be categorized according to the type of opioid receptor at which they produce their effects. Classically, there are considered to be three opioid receptors. These receptors are all G-protein-coupled receptors and were originally named mu (after morphine, its most commonly recognized exogenous ligand), delta (after the vas deferens, the tissue within which it was first isolated), and kappa (after the first ligand to act at this receptor, ketocyclazocine). In 1996 the International Union of Pharmacology renamed the receptors OP1 (the delta receptor), OP2 (the kappa receptor), and OP3 (the mu receptor). In 2000 this nomenclature was changed again to DOP, KOP, and MOP. Now, however, owing to the extensive literature previously using the Greek nomenclature for opioid receptors (δ, κ, and µ), the International Union of Pharmacology recommends using this classification and the DOP, KOP, and MOP classification of 2000 (δ = DOP; κ = KOP; and µ = MOP). There are currently four naturally occurring compounds (morphine, codeine, thebaine, and papaverine), four semisynthetic compounds (diamorphine [heroin], dihydromorphine, buprenorphine, and oxycodone), and at least seven synthetic compounds (meperidine [pethidine], fentanyl, methadone, alfentanil, remifentanil, carfentanil, and tapentadol; see Table 14.1 and Box 14.1 for trade and street names of opioids).
Abstral (fentanyl)
Actiq (fentanyl)
Avinza (morphine sulfate extended-release capsules)
Butrans (buprenorphine transdermal system)
Demerol (meperidine [also known as isonipecaine or pethidine])
Dilaudid (hydromorphone [also known as dihydromorphinone])
Dolophine (methadone hydrochloride tablets)
Duragesic (fentanyl transdermal system)
Fentora (fentanyl)
Hysingla (hydrocodone)
Methadose (methadone)
Morphabond (morphine)
Nucynta ER (tapentadol extended-release oral tablets)
Onsolis (fentanyl)
Oramorph (morphine)
Oxaydo (oxycodone)
Roxanol-T (morphine)
Sublimaze (fentanyl)
Xtampza ER (oxycodone)
Zohydro ER (hydrocodone)
Anexsia (hydrocodone containing acetaminophen)
Co-Gesic (hydrocodone containing acetaminophen)
Embeda (morphine sulfate and naltrexone extended-release capsules)
Exalgo (hydromorphone hydrochloride extended-release tablets)
Hycet (hydrocodone containing acetaminophen)
Hycodan (hydrocodone containing homatropine)
Hydromet (hydrocodone containing homatropine)
Ibudone (hydrocodone containing ibuprofen)
Kadian (morphine sulfate extended-release tablets)
Liquicet (hydrocodone containing acetaminophen)
Lorcet (hydrocodone containing acetaminophen)
Lorcet Plus (hydrocodone containing acetaminophen)
Lortab (hydrocodone containing acetaminophen)
Maxidone (hydrocodone containing acetaminophen)
MS Contin (morphine sulfate controlled-release tablets)
Norco (hydrocodone containing acetaminophen)
Opana ER (oxymorphone hydrochloride extended-release tablets)
OxyContin (oxycodone hydrochloride controlled-release tablets)
Oxycet (oxycodone containing acetaminophen)
Palladone (hydromorphone hydrochloride extended-release capsules)
Percocet (oxycodone containing acetaminophen)
Percodan (oxycodone containing aspirin)
Reprexain (hydrocodone containing ibuprofen)
Rezira (hydrocodone containing pseudoephedrine)
Roxicet (oxycodone containing acetaminophen)
Targiniq ER (oxycodone containing naloxone)
TussiCaps (hydrocodone containing chlorpheniramine)
Tussionex (hydrocodone containing chlorpheniramine)
Tuzistra XR (codeine containing chlorpheniramine)
Tylenol #3 and #4 (codeine containing acetaminophen)
Vicodin (hydrocodone containing acetaminophen)
Vicodin ES (hydrocodone containing acetaminophen)
Vicodin HP (hydrocodone containing acetaminophen)
Vicoprofen (hydrocodone containing ibuprofen)
Vituz (hydrocodone containing chlorpheniramine)
Xartemis XR (oxycodone containing acetaminophen)
Xodol (hydrocodone containing acetaminophen)
Zolvit (hydrocodone containing acetaminophen)
Zutripro (hydrocodone containing chlorpheniramine and pseudoephedrine)
Zydone (hydrocodone containing acetaminophen)
Fentanyl (fentanyl extended-release transdermal system)
Methadone hydrochloride (methadone hydrochloride tablets, methadone hydrochloride oral solution)
Morphine sulfate (morphine sulfate extended-release capsules, morphine sulfate extended-release tablets)
Oxymorphone hydrochloride (oxymorphone hydrochloride extended-release tablets)
OxyContin — oxy, hillbilly heroin, kickers, oxycottons, killers, OC (Jammed is a term that means someone is “under the influence of Oxycontin.”)
Percocet/Percodan — percs, percodoms
Vicodin or Lorcet/Lortab — vikes, Watson-387
Codeine with Robitussin or Tylenol — Captan Cody or Cody, schoolboy
Codeine with glutethimide — doors & fours, pancakes and syrup, loads
Fentanyl — Apache, Duragesic, Sublimaze, dance fever, Actiq, TNT, China white, China girl, Tango and Cash, jackpot, friend, goodfella
Morphine — Roxanol, Miss Emma, M, white stuff, monkey, Duramorph
Methadone — Amidone, fizzies, chocolate chip cookies
Numorphan/Numorphone — blues, Mrs. O, O bomb, octagons/stop signs, biscuits, blue heaven
Dilaudid — D, smack, juice, footballs
Demerol — pain killer, demmies
Heroin — smack, junk, dope, H, white horse, horse, China white, skunk, skag, brown sugar, hell dust, thunder, chiva, big H, c heese
Opium — black stuff, gum, block, hop, big O, ah-pen-yen, zero, hop/hops, Chinese molasses, Chinese tobacco, black pill, mira, O, pox, skee, Dover's powder, dopium, gee, God's medicine, toys, toxy, guma, joy plant, easing powder, dream stick/gun
Although there is good evidence from clinical trials to suggest that opioids are effective at treating pain in the short and medium term, there is a lack of high-quality evidence supporting their use for chronic pain states, with a series of recent Cochrane reviews questioning the use of opioids in this setting. These reviews report weak evidence to support using opioids to manage long-term noncancer pain and show only a small to moderate benefit with opioid administration in osteoarthritis coupled with an increased frequency of adverse events. Similarly, the use of opioids in the management of low back pain is not supported by high-quality evidence, whereas opioids in neuropathic pain conditions is supported only by evidence for their use in the intermediate term.
Most opioids have little direct negative effect on cardiac contractility. However, opioid administration can be associated with decreased cardiac function when administered in combination with other medications, such as benzodiazepines. Opioids can lead to bradycardia and vasodilation, and rarely, they can lead to edema, hypotension, orthostatic hypotension, and syncope when used at analgesic doses. Although most opioids have no effect on cardiac conductivity, methadone and buprenorphine can prolong the QT interval, the interval between the start of the Q-wave and the end of the T-wave (QTc), on electrocardiogram, especially when used in patients at increased risk for it. Electrocardiogram monitoring of QTc at baseline and after dose increases is appropriate in patients receiving these medications.
There are limited data to suggest that chronic opioid administration may be associated with an increased risk for cardiac-related adverse effects. However, this observation has not yet been confirmed.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here