Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Tissue damage after surgery triggers a complex inflammatory response termed sterile inflammation characterized by the release of intracellular molecules into the extracellular environment leading to receptor-mediated immune signaling cascades. A key feature of this inflammatory response involves removing necrotic cells and launching a program of tissue repair. Patients may not only respond differently to a similar sterile insult because of genetic differences, but also as a consequence of preexisting disease that can substantially modify the inflammatory phenotype. The presence of comorbid diseases frequently fuels an established dysregulated inflammatory response, which contributes to organ injury and immunosuppression. Postoperative complications, including (subclinical) organ dysfunction and infection, increase morbidity and mortality following surgery, even after discharge from hospital. Immune alterations are not only the result of direct tissue injury but are also influenced by perioperative medications and interventions. This chapter will discuss the broad current understanding of the immune response to tissue damage, the interaction of this response with comorbid diseases, and clinical factors that influence perioperative inflammation.
Although the acute systemic inflammatory response is widely referred to, defining it objectively in the modern perioperative era is rather elusive. Consensus definitions are lacking, in contrast to sepsis. First described by Sir David Cuthbertson, the classic textbook response entails a biphasic immune, inflammatory, and metabolic response to injury. The first ebb phase, as coined by Cuthbertson, involves peripheral vasoconstriction, concomitant hypothermia, and shunting of blood and substrates to vital organs. A transition then occurs (termed flow by Cuthbertson), with a hypermetabolic phase ensuing. In the modern perioperative setting, these classic characteristics are rather less distinct for several reasons. First, many of the cardiometabolic features following trauma/surgery have been derived from critical illness, chiefly because these measurements are more attainable in this patient population. Despite significant morbidity in noncritically ill surgical patients, it is not clear as to whether this classic temporal pattern occurs in other patients—particularly in the modern era of multimorbidity, anesthesia, and surgery. Second, various clinical measures are nonspecific (e.g., heart rate, temperature), and are frequently unhelpful in convincingly differentiating between the systemic inflammatory response syndrome and alternative pathophysiologic/nonpathologic etiologies. Third, the interaction between common (treated) chronic comorbidity and acute systemic inflammation is poorly defined. Fourth, the nonspecificity of various commonly used inflammatory biomarkers cannot distinguish easily between sterile inflammation and alternative triggers of the inflammatory response. Table 2.1 summarizes common clinically used physiologic and biochemical markers for systemic inflammation after surgery.
| Type | Marker | Description | Associations | References |
|---|---|---|---|---|
| Clinical | Systemic inflammatory response syndrome (SIRS) | SIRS diagnosed if 2 + criteria are met without evidence of an infectious source:
|
Length of duration of SIRS associated with increased mortality and complication rate after gastrointestinal and vascular surgery and trauma. | Haga et al. Shepherd et al. |
| Sequential Organ Failure Assessment (SOFA) | Combined total of scores (0-4) in each of six organ systems (nervous, respiratory, cardiovascular, hepatic, renal, and coagulation). | Increasing score associated with increased mortality after elective gastrointestinal and cardiac surgery. | Bota et al. | |
| Biochemical | Neutrophil-lymphocyte ratio (NLR) | The ratio of neutrophils to lymphocytes in blood. | Increased ratio associated with adverse events in cardiac, vascular, gastrointestinal, and orthopedic surgery. | Malietzis et al. |
| C-reactive protein | Acute phase protein synthesized in the liver and produced largely in response to interleukin-6. | Increased levels correlated with the degree of tissue trauma caused by orthopedic surgery and with anastomotic leak rate after upper gastrointestinal resection. | Rettig et al. | |
| Albumin | Family of abundant globular proteins representing the majority of protein present in plasma. | Hypoalbuminemia associated with increased rates of wound infection, need for further surgery, and respiratory complications. | Neumayer et al. |
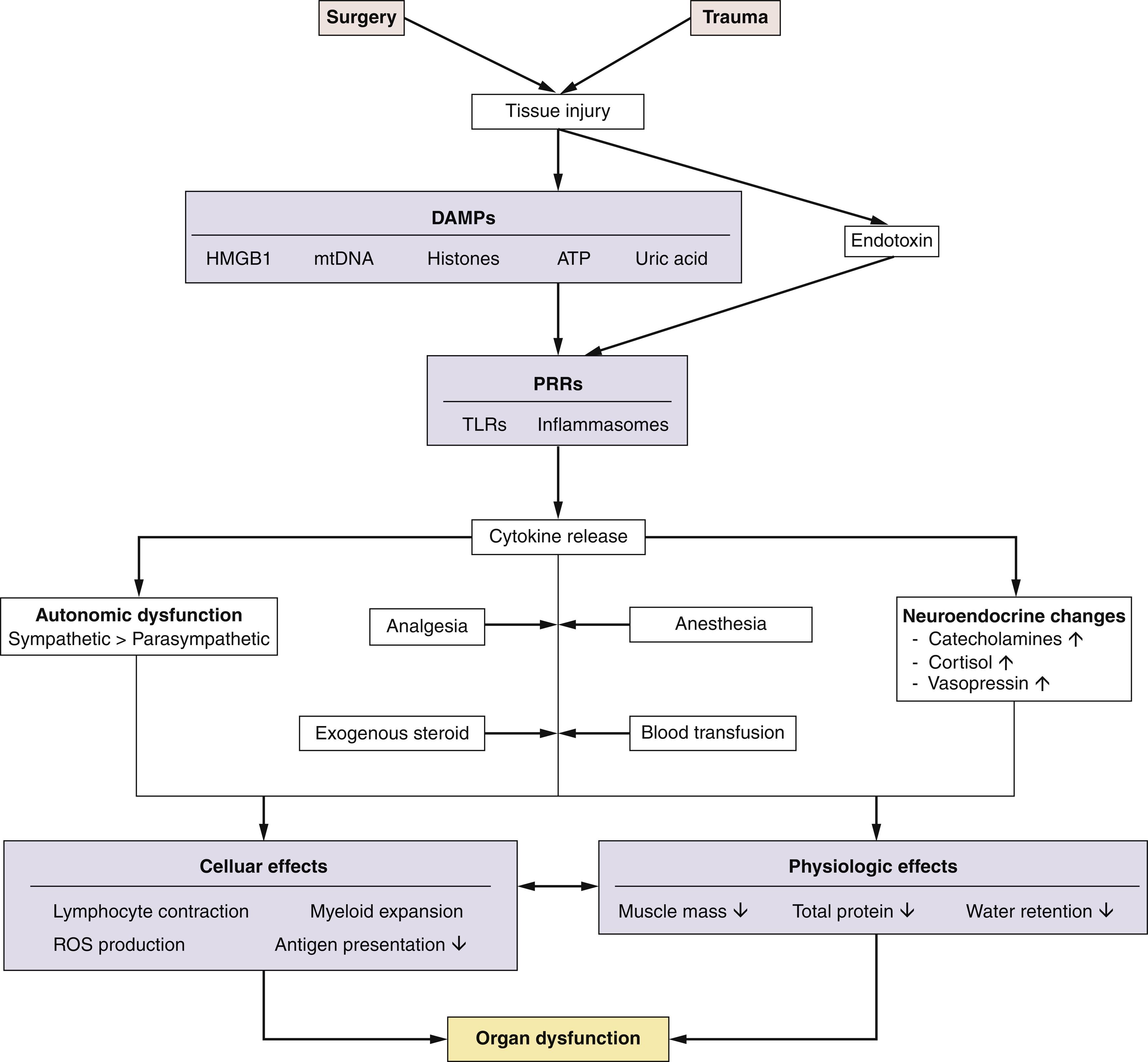
The activation of the host response to sterile inflammation is, unsurprisingly, triggered directly by cellular injury as a result of surgery. Surgical tissue injury shares many similarities with the immune response to infectious pathogens. Upon tissue injury, loss of plasma membrane integrity and cell death (necrosis) leads to the release of danger-associated molecular patterns (DAMPs) into the extracellular environment ( Fig. 2.1 ). Pattern recognition receptors (PRRs), including highly conserved Toll-like receptors (TLRs), are expressed on a wide range of immune and nonimmune (epithelial, endothelial and fibroblasts) cells. PRRs recognize both pathogen-associated molecular patterns (PAMPs) and DAMPs (also known as alarmins) following tissue injury. Thus host innate immune surveillance monitors endogenous (self) markers of tissue damage as well as nonself antigens, such as bacterial infection—a process known as the “danger hypothesis.” Receptors and downstream signaling pathways common to both sterile and pathogenic stimuli, such as nuclear factor-ĸβ and mitogen-activated protein kinase, lead to similar patterns of cytokine release and cellular activation. A diverse array of stimuli synonymous with surgical tissue trauma activate the ubiquitous NLRP3 inflammasome, including multiple microbial products, endogenous molecules, and particulate matter. The biochemical function of inflammasomes is to activate caspase-1, which leads to the maturation and release of interleukin 1β (IL-1β) and IL-18, and induction of pyroptosis (a form of cell death) fueling the release of other inflammatory mediators. Human monocytes (unlike tissue resident macrophages) can release mature IL-1β in response to TLR ligands alone, but most microbial stimuli appear to prime the NLRP3 inflammasome for activation. Clinically, sterile and pathogenic inflammatory responses are frequently indistinguishable. DAMPs implicated in generating inflammation and organ dysfunction after surgery ( Table 2.2 ) include mitochondrial DNA, which activate innate inflammation via TRLs.
| Molecule | Physiologic function | Location | Receptor | Reference |
|---|---|---|---|---|
| HMGB1 | Chromatin regulation | Nucleus of immune cells | TLR4 | Andersson et al. |
| Histones | Nucleosome structure | Nucleus | TLR2/4/9 and NLRP3 | Silk et al. |
| Mitochondrial DNA | Mitochondrial genome | Mitochondria | TLR9 / NLRP3 | Zhang et al. |
| ATP | Cellular energy source | Mitochondria | P2RX7 / NLRP3 | Gombault et al. |
| Uric acid | Byproduct of purine metabolism | Cytoplasm | NLRP3 | Hughes and O’Neill |
TLRs were first characterized for their recognition of PAMPs derived from microbial organisms. Gut microbes may also contribute to systemic inflammation following surgery, particularly when hypoperfusion of the gastrointestinal tract occurs as a result of hypovolemia. Breakdown of the epithelial mucosa may promote the release of enterically derived bacterial endotoxin—termed bacterial translocation. Gram-negative bacteria also activate the NLRP3 inflammasome through the transport of lipopolysaccharide into the cell, which activates caspase-11 and release of IL-1β. In patients, endogenous antibodies to endotoxin appear to be associated with protection from endotoxin-mediated toxicity. Bacterial infection is also an early risk, as features of immunosuppression appear rapidly within hours of surgery. The cellular features of the immunosuppressive response include a rapid decrease in monocyte human leucocyte antigen-DR (HLA-DR) expression and an expansion of myeloid-derived suppressor-like cells that impair adaptive immune responses. Both of these features persist for several days and are associated with postoperative infection and delayed recovery. The underlying mechanism is likely to be multifactorial but may be partially attributed to high levels of circulating catecholamines.
The onset of inflammation heralds a chain of events that in many patients results in organ dysfunction, much of which is subclinical. Typically, different morbidities cluster, as reflected by various clinical measures of subclinical organ dysfunction. For example, many clinicians would overlook a decline in serum albumin that occurs after the vast majority of surgery types, yet there are compelling adverse clinical outcomes linked to this phenomenon that are driven by pivotal inflammatory mechanisms, including endothelial damage. The release of DAMPs triggers a stereotypical response characterized by a dramatic change in circulating blood leukocyte subsets, following the peripheral redistribution of white blood cells to sites of injury and/or lymph nodes. For example, a striking feature of this early phase is a typical doubling of the number of neutrophils in the blood. This process is driven, in part, by autonomic and neuroendocrine activation. Given the changing landscape of modern surgery, both in terms of surgical techniques and increasingly physiologically challenging patients with multiple comorbidities, the classic stress/inflammatory response, as discussed before, is likely to be a gross oversimplification of the broad (rather classic) descriptions that follow.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here