Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The extreme hypermetabolic and hypercatabolic stress responses induced by a severe burn injury are characterized by increased proteolysis, lipolysis, and production of endogenous glucose via glycogenolysis and gluconeogenesis. The critical organ controlling these processes is the liver. With major roles in metabolism, inflammation, immunity, and the acute-phase response, the liver orchestrates the basic functions that modulate survival and recovery in severely burned patients. The function of the liver following a severe burn injury has been elucidated, demonstrating that the preservation of liver function is associated with survival. The strong correlation between postburn survival and the expression of cytokines and acute-phase proteins (APPs) produced by the liver further supports this contention.
Worldwide, the World Health Organization (WHO) attributes approximately 265,000 deaths each year to burn injuries and their sequelae, with the vast majority of these injuries occurring in low- and middle-income countries. In the United States alone, burns result in approximately 4000 deaths, 24,500 hospitalizations, and more than 745,000 nonhospitalized injuries per year, with an incidence rate of 280 burns (per 100,000 people). The effects induced by burns are not limited to the injured area alone. A severe burn injury has devastating effects on the injured patient by affecting almost every organ system, resulting in greater morbidity and mortality. Amplified glucose availability leads to increased protein catabolism and lipolysis, initiating the postburn hypermetabolic stress response. Systemic inflammation, including pathophysiologic regulation of cytokines, hormones, and APPs, drives the hypermetabolic response. Prolongation or amplification of the hypermetabolic or inflammatory responses may result in dysregulation of counterregulatory stress hormones (catecholamines, cortisol, glucagon), thereby exacerbating postburn hypercatabolism, multiorgan failure, and death.
For more than 20 years, reductions in morbidity and mortality have resulted from research efforts focused on improving postburn resuscitation, hypermetabolism, infection control, ventilation, and wound healing. Greater advances in clinical care, however, are needed to reduce morbidity and mortality even further. With a series of studies, we have concluded that the liver plays a fundamental role in the systemic response to burn. Through the modulation of immune, inflammatory, metabolic, and acute-phase response signal transduction pathways, the liver contributes greatly to survival and recovery following a severe burn injury. This chapter discusses liver function under normal conditions and following a severe insult such as a burn injury.
In an average-sized adult, the liver weighs approximately 1500 g, making up almost 2% of the total body weight. Following a severe burn injury, the liver size can increase significantly to meet additional demands. The Couinaud's segmental system, the preferred anatomy classification system, divides the liver into eight independent functional units (termed segments) rather than relying on the traditional morphological description based on the external appearance of the liver. Roman numerals (I–VIII) delineate the segments based on the dual vascular inflow, biliary drainage, and lymphatic drainage within each segment.
A broad spectrum of biological functions is orchestrated by the liver. The interrelated physiologic-anatomic units of the liver direct the following processes:
Energy homeostasis and nutrient metabolism : the synthesis, degradation, and coupled interconversion of amino acids, carbohydrates, and lipids are closely linked to hepatic energy metabolism.
Protein synthesis and amino acid metabolism : The liver uses amino acids directly for protein synthesis and as a source of organic nitrogen for nonessential amino acid synthesis. The overall balance of amino acid synthesis, degradation, dietary supply, and body distribution is reflected by plasma amino acid levels.
Carbohydrate metabolism : The liver plays an important role in maintaining carbohydrate homeostasis, principally through glucose catabolism, production, and storage. The ability to use, store, synthesize, and release glucose gives the liver a central role in maintaining stable serum glucose levels. Compromise of this function can result in hypoglycemia or hyperglycemia.
Lipid metabolism : Hepatic metabolic energy requirements are met principally through β-oxidation of free fatty acids (FFA). Fatty acids synthesized in the liver or derived from peripheral fat depots combine with glycerol in the liver to form triglycerides (TG). Through the production of very-low-density lipoproteins (VLDL), the liver provides peripheral tissues with indirect access to the convergent metabolic processes in the hepatocyte that lead to triglyceride synthesis. Following synthesis by the liver, high-density lipoproteins (HDL) circulate in the plasma, where they scavenge free cholesterol released from aging cell membranes. The liver also produces apoprotein CII, which is required for the peripheral activation of lipoprotein lipase, and cholesterol.
Biotransformation : Many environmental compounds (including drugs) and endogenous metabolic products are lipid soluble and nonvolatile, precluding their efficient excretion in urine or feces. Through biotransformation reactions, the liver transforms these substances into more water-soluble analogs and enhances their excretion via urine or bile.
Excretion : The biliary tract is the principal excretory route for numerous exogenous and endogenous substances. Six hundred to eight hundred milliliters of bile are secreted daily, using a canalicular surface area of approximately 10 m 2 . Inorganic ions account for most of the osmotic activity in bile, keeping it approximately isotonic with plasma. Organic solutes present in bile include conjugated bile acids, phospholipids (lecithin), cholesterol, bile pigments, hormones, and small amounts of protein.
Immunologic functions : A major portion of the mononuclear phagocyte system is centered in hepatic sinusoids. Kupffer's cells use phagocytosis and pinocytosis to clear bacteria, particulate matter, and old erythrocytes from sinusoidal blood. Kupffer's cells are also the major site of lipopolysaccharide (endotoxin) detoxification. The liver also contributes to the humoral arm of body defense through uptake and secretion of IgA.
Vitamin metabolism : Vitamin uptake, storage, and mobilization are additional important functions of the liver. The absorption of fat-soluble vitamins (A, D, E, and K) is dependent on bile salts. Because vitamin A is stored exclusively in the liver, excess ingestion may be associated with significant hepatic injury. Hepatic stellate cells play a role in vitamin A storage as well. The initial vitamin D activation step, conversion of vitamin D 3 to 25-hydroxycholecalciferol, occurs in the liver. Coagulation factors II, VII, IX, and X are dependent on vitamin K, which is essential for the γ-carboxylation and activation of these factors. Vitamin E has recently garnered much attention due to its potent antioxidative properties. Following a severe thermal or traumatic injury, vitamin E might reduce oxidative stress and subsequent damage.
Hormonal system : The liver is an important site of hormonal synthesis, secretion, or interaction. Growth factors that are important for growth and development such as insulin-like growth factor-I (IGF-I) and the IGF binding proteins (IGFBPs) are made and secreted by the liver. Production and secretion into the bloodstream of angiotensinogen occurs within the liver. Synthesis of hepatocyte growth factor (HGF), a major hepatic regenerative growth factor, occurs in the liver.
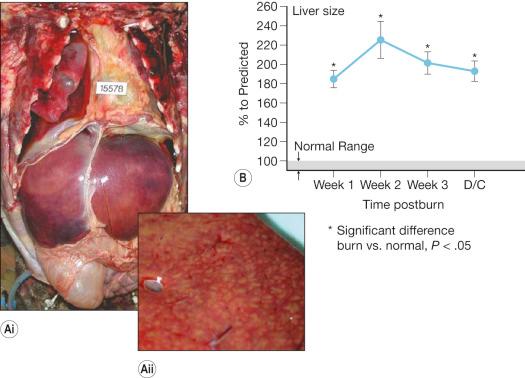
Burn-induced liver injury is variable and is typically proportional to the severity of the burn injury. Hepatomegaly, or a fatty liver, is a common postburn finding ( Fig. 24.1 ). These changes can be reversed; however the significance of these alterations is related to the extent of fat deposition and its etiology. Autopsies of deceased pediatric burn victims revealed that fatty infiltration of the liver is associated with hepatic failure and endotoxemia. Increased hepatic edema, typically observed within 12 hours after burn injury, is associated with damage to the liver. Both liver and body weight significantly increase at 2–7 days postburn, as compared to nonburned liver/body weight. In burned rats, total hepatic protein concentrations are reduced significantly, suggesting that the increase in liver weight is due to edema and not to increased protein levels or hepatocyte numbers. Hepatic edema may induce release of hepatic enzymes into the circulation as a result of cellular damage or by altering membrane permeability. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are typically detected only at low levels in plasma. Therefore, detection of elevated levels in the circulation indicates possible hepatocyte injury. Severe hepatic damage can also be detected by elevations in serum glutamate dehydrogenase or alkaline phosphatase (ALKP). Elevations in ALKP provide insight into the function of the extrahepatic biliary tract and are frequently elevated in hepatobiliary disease. The liver damage induced by thermal injury is secondary to edema formation, hypoperfusion, and inflammation. Following a severe burn, elevations between 50% and 200% of AST, ALT, and ALKP are observed ( Fig. 24.2 ). These serum markers peak early during the first 24 hours after the burn injury, indicating that burn-induced liver damage is a rapid phenomenon.
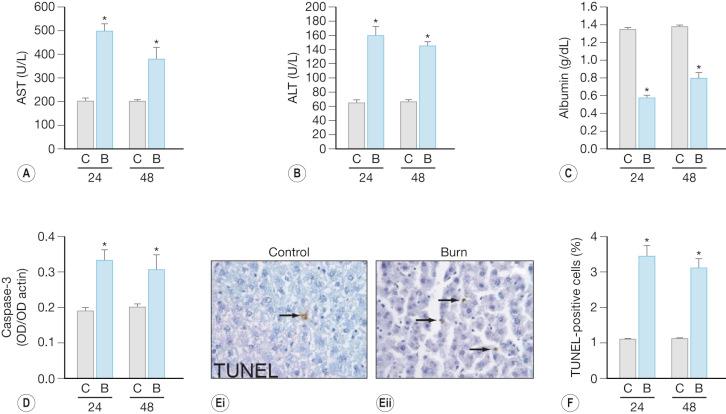
Increased hepatocyte death, both by apoptosis and necrosis, is associated with liver damage. Two distinctly different pathways result in cell death: programmed cell death (apoptosis) and necrosis. Cell shrinkage, uniform fragmentation of DNA, and membrane blebbing characterize apoptotic cells. Necrosis, on the other hand, is characterized by cellular swelling, fragmentation of the DNA in a random manner, activation of lysosomes, and complete breakdown of the cellular membrane enabling cellular contents to be extruded into the interstitium. These final steps induce an inflammatory response by attracting inflammatory cells, causing the release of free radicals and proinflammatory cytokines, leading to additional tissue breakdown. The morphological hallmarks unique to each process are used to differentiate between apoptotic and necrotic cells. At the time of autopsy, 10–15% of severely burned decedents had signs of liver necrosis, as determined by pathological examination.
Apoptosis also occurs in the liver following a cutaneous thermal injury ( Fig. 24.2 ). The liver tries to maintain homeostasis when hepatocyte apoptosis increases by a compensatory increase in hepatocyte proliferation. Despite the attempt to maintain homeostasis in overall hepatocyte number, the liver is unable to immediately regain mass or maintain protein concentration. The molecular mechanisms that initiate and propagate hepatocyte apoptosis following a cutaneous burn are not known. Blood flow to the bowel is decreased by approximately 60% for up to 4 hours following a thermal injury. Hepatic blood flow is likely decreased as well, and this may be one of the early events inducing programmed cell death. Apoptotic signals, including interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α), increase systemically during this same time frame. Additional studies have revealed that the elevations of proinflammatory cytokines are not limited to the serum. Local elevations also occur after a thermal injury, as seen with increased concentrations of hepatic IL-1α, IL-1β, IL-6, and TNF-α. Taken together, these events are probably early events in the induction of hepatocyte apoptotic signaling.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here