Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Biogerontology, the field of biologic aging research, is the final biomedical research frontier. The sequencing of the human genome and advancements in molecular technology have provided enormous potential for regenerative medicine. The list of readily replaceable body parts (e.g., eye lenses) and organs (e.g., hip joints, arterial transplants) will continue to grow. Even 30 years ago, little could be done to treat cataracts, but now lens replacements are routine surgical procedures. Advances in many disciplines have resulted in considerable insight into the diseases and disorders of aging. As we will discuss in this chapter, we propose that many more of the current common causes of morbidity and mortality can be eliminated in the upcoming decades. The final puzzle to be solved is the basic underlying cause of how we age. The exceptional life span of humans among primates may uncover aging changes that shorter lived species do not live long enough to incur. Human life span, already longer than any primate in premodern times, has more than doubled, whereas for those at age 70, the remaining life span has also more than doubled. Will this remarkable increase in longevity continue?
We have different backgrounds and have different expectations. CEF, as a molecular biologist, is more reserved about the pace of discovery on aging processes and demographic predictions for further increases than Edward Schneider LS who, as a physician-scientist, is more optimistic about the future benefits of biogerontology research. However, we agree on the challenges ahead from the current epidemic of obesity, as well as from antibiotic resistance and global environmental deterioration. Whatever the future of aging may be, we believe that a deeper understanding of aging will provide the gateway to extended life spans that are increasingly free of disease and disability. We have been debating these issues for several decades and hope that this chapter engages a broad audience of readers to explore the complexities of human aging along with us.
First, let us look at historical changes in the life span. Before 1800, life spans were very short, with life expectancies at birth of 30 to 40 years. However, since 1800, human life expectancy has expanded in developing countries and has more than doubled, whether measured at birth or at age 70 years ( Figure 3-1 ).
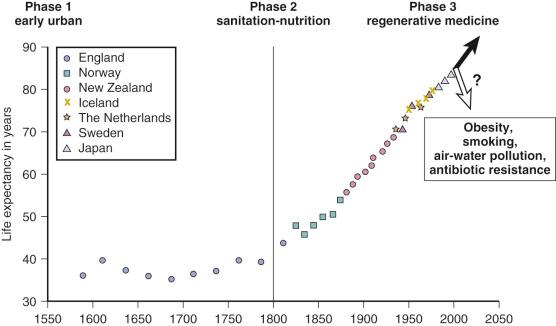
About half of those born before 1800 did not reach the age of parenthood, and a mere 10%, at best, reached age 70. Then, during the industrial revolution, country after country developed better living conditions, with increased food distribution and improved hygiene, even before the understanding of infectious disease; after that came pasteurization and vaccination and finally, after WWII, antibiotics. Infectious disease dwindled from the major cause of death before 1900 to less than 5% of total deaths. Now, most of us survive to older ages, where we accumulate the chronic diseases of aging, from atherosclerosis to cancer, and, if we live long enough, a rapidly increasing risk of Alzheimer disease (AD).
These survival data can be further understood when plotted as mortality rates at each age of life ( Figure 3-2 ). These are known as Gompertz curves, first described in 1825 by the Scottish actuary Benjamin Gompertz. After age 40, mortality rates accelerate, with a doubling every 7 to 8 years. Sweden has the most comprehensive data obtained from nationwide household surveys that were initiated in the mid-eighteenth century (see Figure 3-2 ). The mortality rates in 1800 were high in the early years, starting with 10% to 30% infant mortality. Even young adults in the eighteenth century had a 1% annual mortality rate. After about the age of 40, Sweden, like all other countries, shows accelerating (exponentially increasing) mortality rates, which are the basic manifestation of aging.
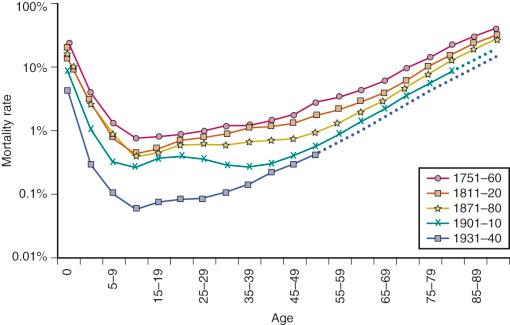
Note how the slope of the more recent population increases steadily with improving conditions, corresponding exactly to the increase in life expectancy shown in Figure 3-1 . In fact, the curves get progressively steeper; paradoxically, as life spans have increased, the rates of mortality acceleration have also increased. Note also that as infections were progressively minimized as a cause of early-age mortality, mortality at ages 10 to 40 years approached a minimum, below 0.1%/year. Those born most recently may now have an even lower mortality of 0.02%/year (2/10,000). This historically unprecedented low baseline mortality represents deaths from conditions in which significant further reductions are unlikely (e.g., accidents, congenital defects, rare familial diseases). Across all ages, women have slightly lower mortality rates. Nonetheless, both genders incur mortality accelerations by the age of 40 years.
From these data on mortality rates, it can be calculated that the maximum human life spans are 120 for women and 113 for men, which are very close to the reported records. Because world mortality data clearly show an approaching lower limit to baseline mortality without delay of the Gompertz mortality acceleration, we must consider that the continued expansion of human life span will soon reach a limit for the most populations. Since Jean Calment's record life span of 122 years in 1997, no one has exceeded 119 years, despite the avalanche of centenarians who are currently comprise the fastest growing age group. CF is thus reserved about predictions for 100-year life spans, based on forecasts from the trends shown in Figure 3-1 .
With increasing life spans came a new profile of disease. Instead of dying from infection, which was the norm before 1900, cancer, heart disease, and other chronic diseases of aging became increasingly prominent. Fries hypothesized 3 decades ago that life expectancy had hit a barrier at age 85 years; as survival curves became more rectangular, the old age time of morbidity was hypothesized to become shorter before death. This compression of morbidity has the important implication that the shorter duration of morbidity would not increase health care costs for seniors, despite their longer life spans. The late Jacob Brody and ELS challenged this hypothesis. Moreover, the recent analysis by Crimmins and Beltran-Sanchez shows that increases in life expectancy have brought increased, not decreased, morbidity, with consequent skyrocketing increases in health care costs for seniors. Nonetheless, the faster acceleration of mortality has continued to rectangularize the survival curve, with little to no change in the maximum life span since 1980.
What about future morbidity? To examine the future burden of disease, we must consider the major causes of death and disability at old ages. First, however, let's look at the potential impact of aging research, personalized medicine, artificial joints, and stem cells.
Almost all the diseases that we will discuss are diseases of older ages. The incidence of these conditions increases exponentially with aging, foreshadowing and anticipating the accelerating mortality rates of the Gompertz curve. Some diseases have been accelerating in regard to incidence even faster than the Gompertz curve. For example, AD incidence doubles every 5 years after age 60, and total mortality doubles every 7 to 8 years. Longevity futurists are confronted with the depressing fact that most centenarians have clinical grade dementia. Therefore, before considering expanding life expectancy, we must develop effective interventions to reduce or delay the incidence of AD and slow its course. For example, delaying the onset of AD by 5 years could cut its prevalence in half. Biologists think this is possible because mice that are calorically restricted not only have increased life spans, but also have delayed onset of AD-like brain changes.
Laboratory models have amply documented that every aspect of aging can be manipulated, from DNA damage to cross-linking of connective tissue collagen and elastin to ovarian egg cell loss to arterial lipids to brain amyloid levels. In addition to food intake and exercise, aging processes can be manipulated by regulating gene activity without changing DNA sequence . We believe that it is within reach of the current younger generation of aging researchers to discover the molecular basis for aging fully. However, it is unlikely that aging is controlled by a single gene or single biochemical or cellular mechanism. Thus, we anticipate that multiple interventions will be developed for different aging pathways to slow or possibly reverse aging processes. Aging can be treated, but interventions need to be initiated long before old age.
It is likely that antiaging interventions by specialized drugs and regenerative medicine for damaged organs is likely to be expensive. Already, even in nations with fully socialized medicine, older adults are given lower priority for major organ replacement. Drugs to slow AD and other dementias will be very expensive because of the huge costs in drug development However, those in poverty already age 10 years faster than the general U.S. population. Thus, the so-called health elite, with ample private funds for medical treatment and potential rejuvenating therapy, may further deepen social disparities in health at later ages.
In the very near future, we anticipate that all initial health visits will include entire genome sequencing. You and your physician will discuss potential genetic risk factors for various conditions and specific preventive measures. For example, carriers of genetic risk factors for type 2 diabetes would be counseled to avoid gaining substantial weight and exercise sufficiently. For cancer risk factors, frequent focused screening would be advised. Genome sequencing is already used to optimize cancer chemotherapy. In the future, there will be customized treatments for many other diseases and disorders that accompany aging, such as arthritis, hypertension, cardiovascular disease, and diabetes. DNA data will also decrease the incidence of adverse drug responses. For individuals with hypertension, the choice among antihypertensive treatments would be based on sequences that are most responsive to specific drugs.
Detrimental genes may also be removed, neutralized, or inactivated through targeted genetic therapies. Thus, the defective gene causing Huntington disease could theoretically be replaced after birth with the normal Huntington gene, even postnatally. Inherited disease–precipitating genes for hypercholesterolemia, hypertension, diabetes, and obesity could similarly be replaced by normal genes. Although personalized aging may permit the detection of probable causes of morbidity and mortality and lead to successful prevention, there will still be a need to repair damaged tissues and organs.
Osteoarthritis remains one of the leading causes of disability with aging. In the upcoming decades, we anticipate improvements in joint replacement and repair that will minimize the impact of this condition. Over the last few decades, knee and hip replacements have become commonplace, allowing relief from pain and increased function for those with severe arthritis of these joints. We anticipate additional experience with shoulder, ankle, elbow, and wrist replacement surgeries that will make these procedures a viable approach to reduce pain and loss of functioning in these joints. Finally, vertebroplasty to restore compressed vertebrae to their original size can now effectively repair the vertebral compression fractures that occur commonly with aging. We are optimistic about diminishing future disability from arthritis with the new technology for joint repair and replacement.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here