Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Protocol-based ultrasound carefully performed by a knowledgeable and experienced examiner following established guidelines is very sensitive in evaluating the central nervous system (CNS).
Ventriculomegaly can be due to many underlying conditions, including obstruction, destruction, and dysmorphism, or a combination.
In cases of brain abnormalities in which additional morphologic or functional information is needed beyond that available with ultrasound, magnetic resonance imaging can be helpful.
Best results in assessment of complex CNS abnormalities are obtained when there is a multidisciplinary consultation and collaboration among specialists in imaging, genetics, and prenatal and postnatal care.
Anomalies of the central nervous system (CNS) are the most common cause of referral for prenatal diagnosis and result in great anxiety for parents. CNS anomalies occur with a frequency of about 1.4 to 1.6 per 1000 live births but are seen in about 3% to 6% of stillbirths. The increased use of maternal serum alpha-fetoprotein (MS-AFP) screening and first-trimester nuchal translucency (NT) screening has resulted in increased numbers of pregnancies being referred for evaluation of the CNS and suspected anomalies. Fortunately, protocol-based ultrasound carefully performed by a knowledgeable and experienced examiner following established guidelines is very sensitive in evaluating the CNS. Routine scanning is currently recommended at 18 to 20 weeks of gestation. Although many cerebral anomalies are detectable in the first trimester and early second trimester, others develop or become apparent only later in pregnancy.
Magnetic resonance imaging (MRI) has become an important supplement after initial ultrasound evaluation. Multiparametric MRI using special sequences allows evaluation of areas that are difficult to assess with ultrasound, can be used to evaluate tissue properties beyond morphology, and provides new insights into ischemia, tumor characteristics, bleeding, brain metabolism, and neural tracts, which can allow unprecedented clarification of suspected disorders. Debate continues regarding the role of ultrasound versus MRI in evaluating the fetal CNS. We believe that ultrasound will continue to be the initial screening modality and that MRI will increasingly be used to clarify findings. The important issues for the examiner are familiarity with the strengths and limitations of all imaging modalities, expertise in their use, and collaboration with specialists in other disciplines.
Knowledge of fetal gestational age is particularly important when evaluating anatomy in early pregnancy. In this chapter we use menstrual age and gestational age as typically used clinically and with ultrasound studies to mean “age from last menses” or “postmenstrual weeks.” For consistency will convert “fertilization age” or “conception age” used in publications to menstrual age by adding 2 weeks.
CNS development is controlled by numerous genes with functions not only in the CNS but also in the rest of the body, and the understanding of the molecular basis of development and malformations is occurring at an explosive rate. Morphologic changes start at about the fifth menstrual week when cells destined to form the notochord infiltrate into the embryonic disc and induce the overlying embryonic tissue to thicken, fold over, and fuse as the neural tube. Zipperlike fusion starts in the midtrunk of the embryo and then extends to the cranial and caudal ends ( Table 34.1 ). Final anterior closure, at the rostral neuropore, occurs by about  menstrual weeks, and a few days later at the caudal neuropore. By the sixth week, the cephalic end enlarges and flexes to become the brain. Transvaginal ultrasound and in vitro MRI can demonstrate many of the stages. By 12 to 15 menstrual weeks, almost all structures and processes are in their final form except for the corpus callosum, cerebellar vermis, neuronal migration (from the periventricular germinal matrix), development of the sulci and gyri, and myelination. These latter structures and processes develop from about 15 weeks onward. The corpus callosum starts developing at about 12 weeks. Its development induces the formation of the two septi pellucidi and the intervening space, the cavum septi pellucidi (CSP) and cavum Vergae (after Andrea Verga in 1851).
menstrual weeks, and a few days later at the caudal neuropore. By the sixth week, the cephalic end enlarges and flexes to become the brain. Transvaginal ultrasound and in vitro MRI can demonstrate many of the stages. By 12 to 15 menstrual weeks, almost all structures and processes are in their final form except for the corpus callosum, cerebellar vermis, neuronal migration (from the periventricular germinal matrix), development of the sulci and gyri, and myelination. These latter structures and processes develop from about 15 weeks onward. The corpus callosum starts developing at about 12 weeks. Its development induces the formation of the two septi pellucidi and the intervening space, the cavum septi pellucidi (CSP) and cavum Vergae (after Andrea Verga in 1851).
| Primary Vesicle | Secondary Vesicle | Mature Structure |
|---|---|---|
| Forebrain | Telencephalon | Cerebral hemispheres |
| Basal ganglia | ||
| Olfactory system | ||
| Diencephalon | Thalamus | |
| Hypothalamus | ||
| Midbrain | Mesencephalon | Midbrain |
| Hindbrain | Metencephalon | Pons |
| Cerebellum | ||
| Myelencephalon | Medulla |
The dorsal aspect of the neural tube at the hindbrain (rhombencephalon) is very thin and membranous ( membranous area or area membranosa ). In the first trimester, the enclosed central neural canal, the rhombencephalic cavity, is very large and forms a conspicuous cystic cavity that should not be mistaken for an abnormality. This space eventually becomes the fourth ventricle. The membrane is divided into an anterior rostral area (anterior membranous area or area membranosa rostralis) and posterior caudal area (posterior membranous area or area membranosa caudalis) by a fold, the plica choroidea, which will become the choroid of the fourth ventricle. The cerebellum and vermis develop as lateral and rostral proliferations into the anterior membranous area. The posterior membranous area eventually fenestrates to form the foramina of Magendie and Luschka. The posterior membranous area can bulge into the cisterna magna to a variable extent, forming the Blake pouch. With high-resolution equipment, the Blake pouch can be seen in most fetuses, where it is often mistaken for arachnoid strands. The cerebellum and vermis are essentially formed by 22 weeks. Care must be taken before 22 weeks to avoid mistaking the incompletely developed early vermis for vermian dysplasia or hypoplasia.
Cortex and neuron development starts at about 5 weeks and is complete by 28 weeks. There are three complex overlapping stages: proliferation, migration, and organization. Neurons proliferate and develop from glial stem cells at the surface of the ventricles and ganglionic eminence. Migration occurs via two pathways, projectional and tangential. In projectional migration, neurons follow a radially oriented glial scaffold directly to the cortical surface, with later-forming neurons passing through the earlier layers to the pial surface. From 17 to 34 weeks, this migration can be seen as bands or laminations that are visible on MRI and ultrasound as the ventricular zone, intermediate zone, subplate zone, and cortical plate. This regular pattern is disturbed with migrational disorders, malformations, and infections such as cytomegalovirus (CMV). In tangential migration, GABAergic cells from the ganglionic eminence take a tangential route through the cortex and provide neurons with controlling functions. The ganglionic eminence overlying the thalamus is more evident on MRI than ultrasound. The cortex undergoes folding into gyri to accommodate the extra cells. Finally, neurons in the cortex organize local connections and send axons remotely as large tracts such as the corpus callosum to connect the hemispheres. Development requires normally functioning genes and is easily disrupted by intrinsic and extrinsic insults such as fetal and maternal metabolic disorders, hypoxia, infections, and teratogens. Note that with teratogens, the final morphologic appearance reflects the gestational age and stage at which disruption occurred, rather than the specific agent.
The early embryo is best examined transvaginally. The cephalic end is identifiable by about 8 weeks. By 10 or 11 weeks, bones of the vault show mineralization. At this age, the brain mantle is very thin. The ventricles are large and filled with choroid, which provides nourishment for the developing brain. A large, echo-free space behind the hindbrain represents the rhombencephalic cavity, which decreases in size as the cerebellum forms and is destined to become the fourth ventricle. In the first trimester the normal rhombencephalic cavity appears especially large and prominent and should not be mistaken for an abnormality ( Fig. 34.1A ).

After 13 or 14 weeks, most cerebral structures can be identified ultrasonographically (see Fig. 34.1B ). In the second trimester, three standard transaxial planes or views (thalamic, ventricular, and cerebellar) can lead to the detection of more than 95% of sonographically detectable cerebral anomalies. These three views (thalamic view, ventricular view, and cerebellar view) form the starting point for routine scanning. If abnormality is suspected, then additional views including transvaginal scan and the midline view to assess the corpus callosum and vermis are useful to clarify problems ( Fig. 34.2 , Video 34.1 , , and ).
Measurement of biparietal diameter and head circumference
Head shape
Bone density
Falx and interhemispheric fissure
Ventricle size and appearance
Cavum of the septi pellucidi (CSP)
Thalamus
Cerebellum and vermis
Cisterna magna
Nuchal fold
(Median view for corpus callosum and vermis—not part of routine scanning, but increasingly being done)
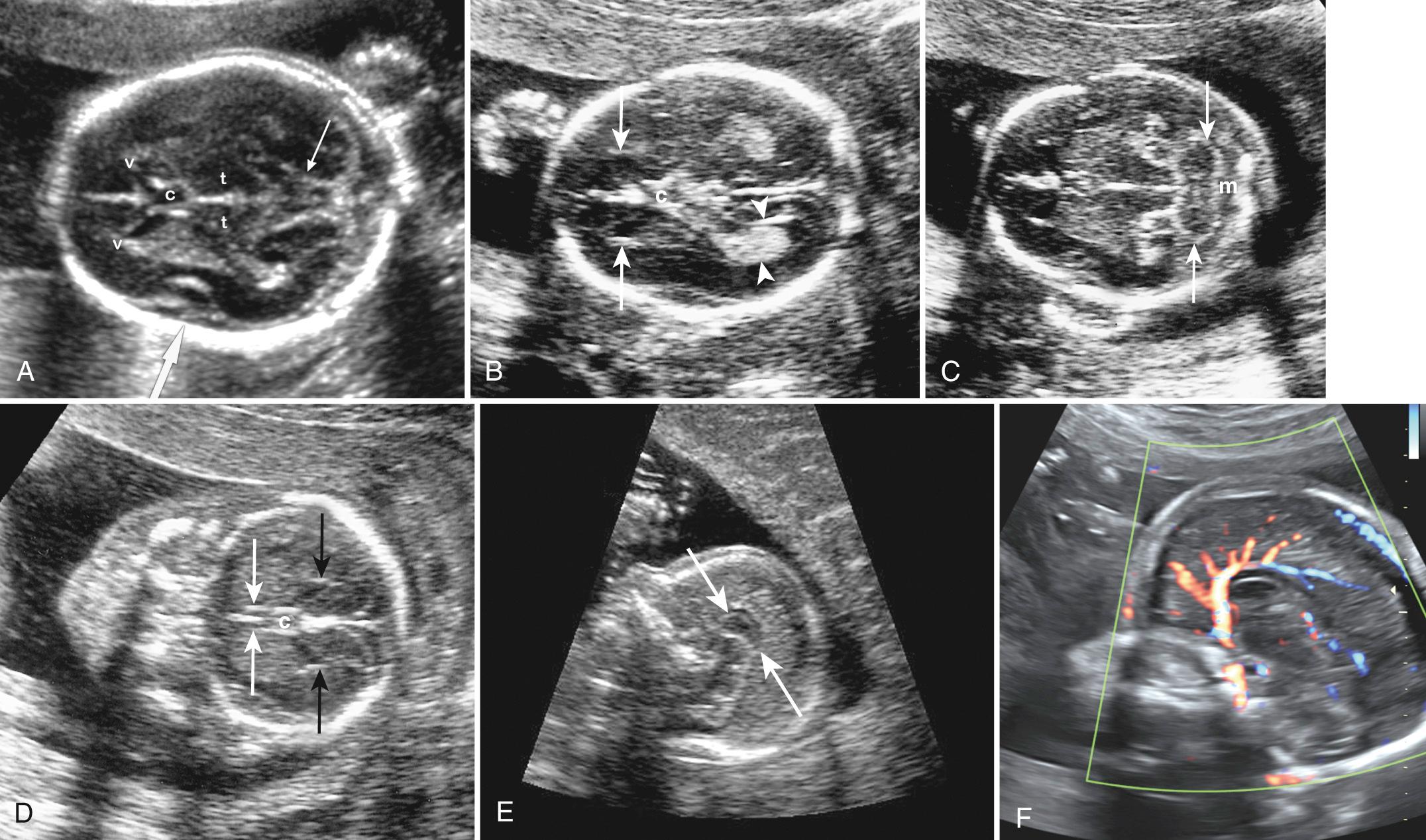
The thalamic view displays the thalamus, third ventricle, fornices, basal ganglia, insula, and ambient cistern and is used to measure the biparietal diameter (BPD), occipitofrontal diameter (OFD), and head circumference (HC) (see Fig. 34.2A ). The smooth straight interhemispheric fissure is visible on this and the ventricular view. If the fissure appears irregular, then abnormalities of the corpus callosum and other cerebral structures should be suspected. The ventricular view is slightly higher than the thalamic view and shows the bodies and, more important, the atria of the lateral ventricles as well as the interhemispheric fissure (see Fig. 34.2B ).
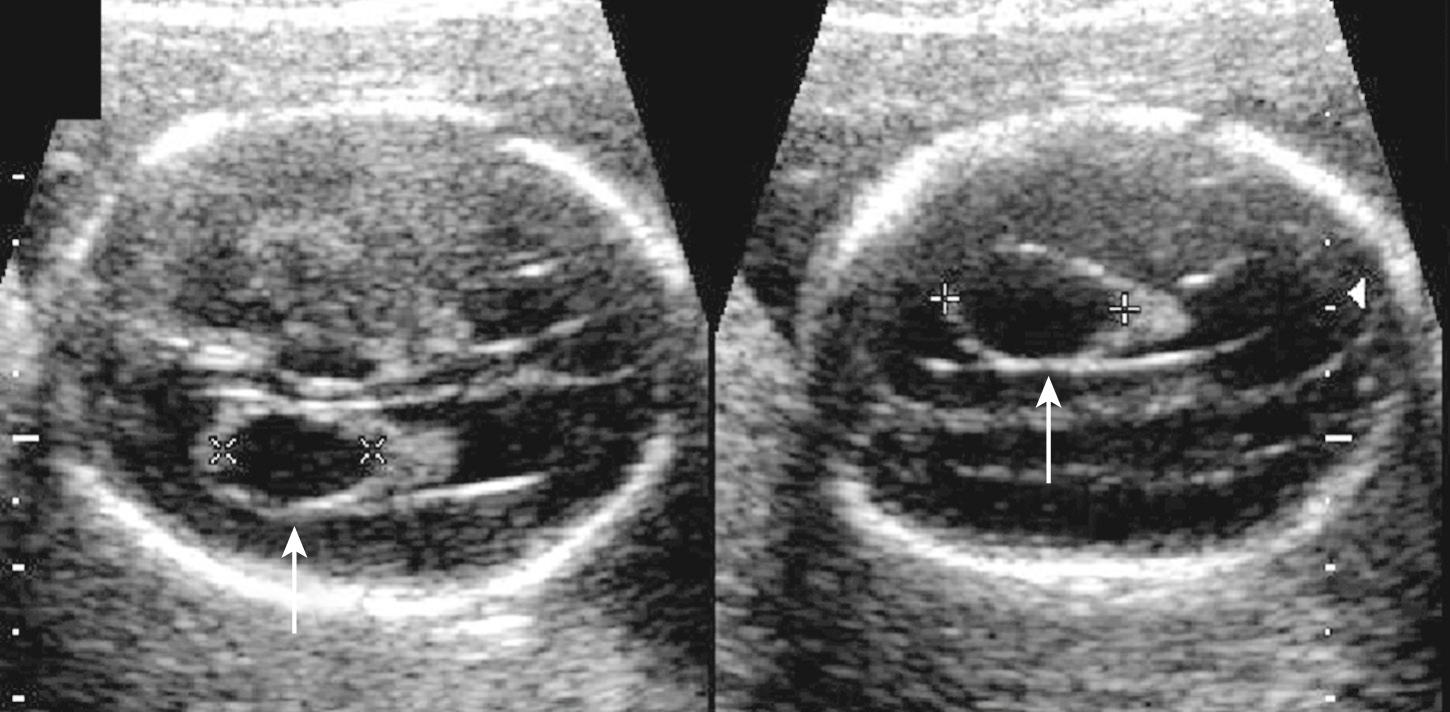
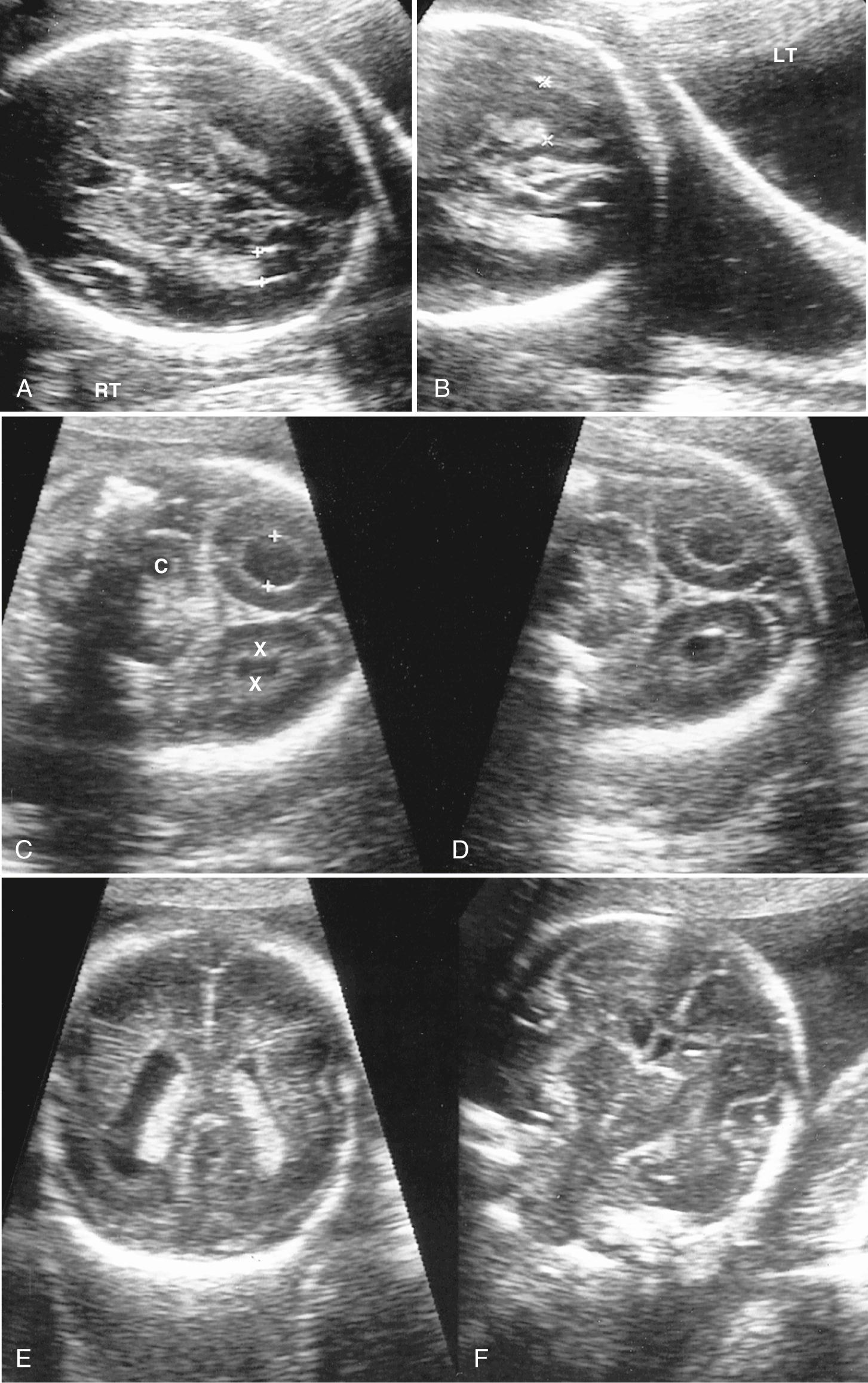
Atrial width (occipital horn width) is the most useful and accepted measurement of ventricular size. The atrium of the lateral ventricles is the site of confluence of the bodies, occipital horns, and temporal horns. Fortuitously, with abnormalities this is the part of the ventricle that undergoes the earliest and most marked enlargement. Ventricular measurement is a required element of routine scans and is easily performed during routine obstetric scanning. Usually only the distal ventricle is measured because the closer ventricle is obscured by artifact; it is assumed that the ventricles are symmetrical, but mild asymmetry is common ( Fig. 34.3 ). Measurements should be performed in a very standardized manner on true axial views. Calipers should be placed touching the inner walls of the ventricles opposite the deepest part of the parieto-occipital fissure; a normal value is taken as below 10 mm. Ventricular abnormality, especially enlargement, is a key finding in the initial detection of CNS abnormalities and is seen in about 88% of fetuses with cerebral abnormalities.
The cerebellar view is obtained by rotating the transducer into a suboccipitobregmatic plane centered on the thalamus to show the cerebellar hemispheres. This view shows the cerebellum, cisterna magna, cavum septi pellucidi (CSP), and frequently the anterior horns of the lateral ventricles. Up to about 24 weeks, the transverse cerebellar diameter corresponds to gestational weeks. The cisterna magna is the cerebrospinal fluid (CSF) space behind the cerebellum and is measured between the cerebellar vermis and inner occipital bone on an axial plane that includes the anterior end of the CSP and the midplane of the cerebellum. It is normally 2 to 10 mm (see Fig. 34.2C ). Cisterna magna obliteration suggests Chiari II malformation and spina bifida. Excessive enlargement may be seen with mega–cisterna magna, Blake pouch cyst, vermian dysplasia, Dandy-Walker syndrome, and arachnoid cysts.
Additional sonographic views and projections using the windows provided by the fontanelles and sutures can help to clarify brain anatomy and development. The median (midsagittal) view through the metopic suture–anterior fontanelle–sagittal suture shows midline structures such as the corpus callosum and occasionally the cerebellar vermis and brainstem (see Fig. 34.2E-F ). The posterolateral mastoid fontanelles can provide access to the cerebellum and occipital lobes and ventricles.
The sulci and gyri undergo predictable development patterns that can be assessed as early as 18 weeks. Special views to evaluate development of sulci and insula can be helpful in detecting abnormal development such as lissencephaly ( Fig. 34.4 ).
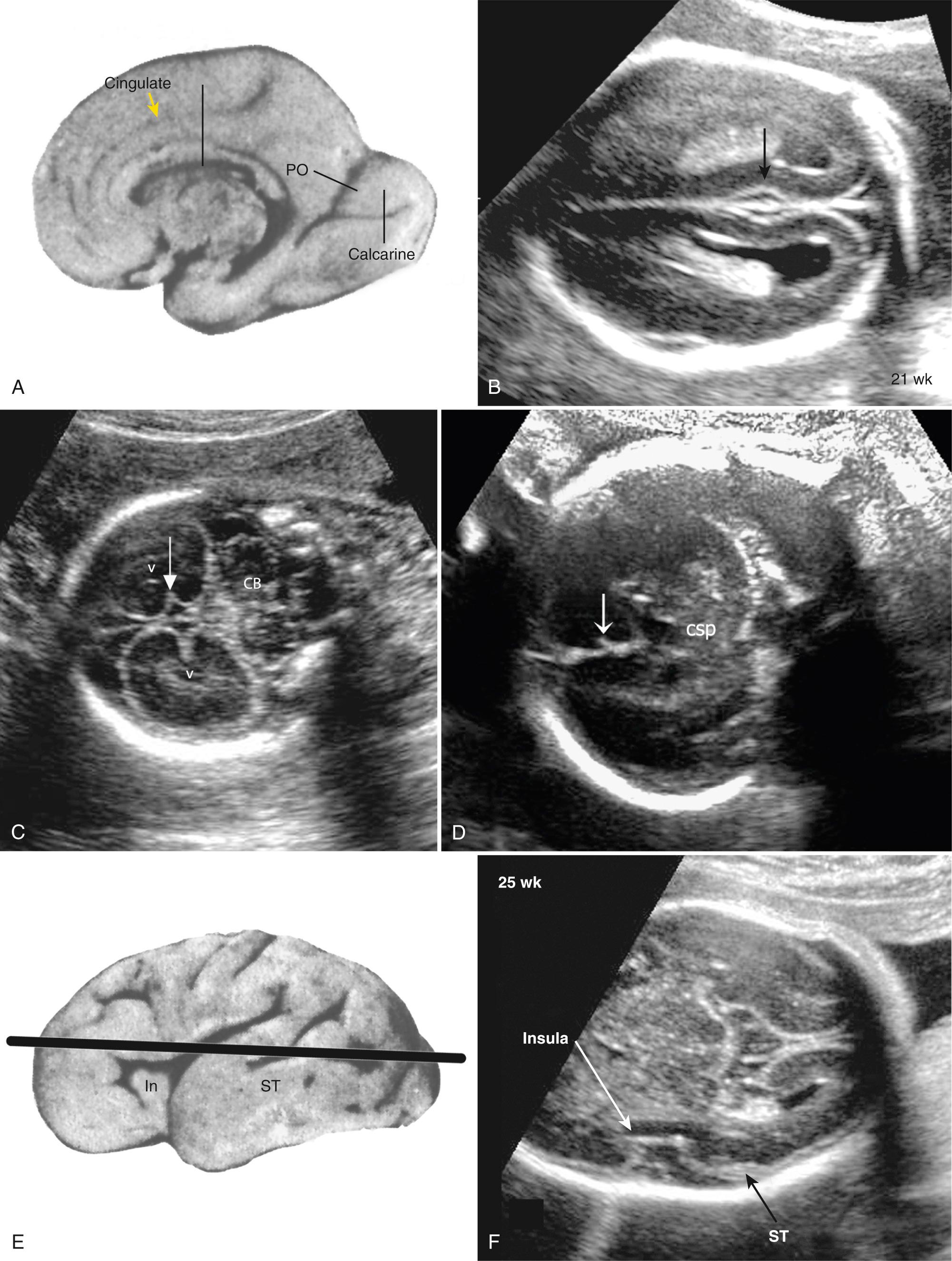
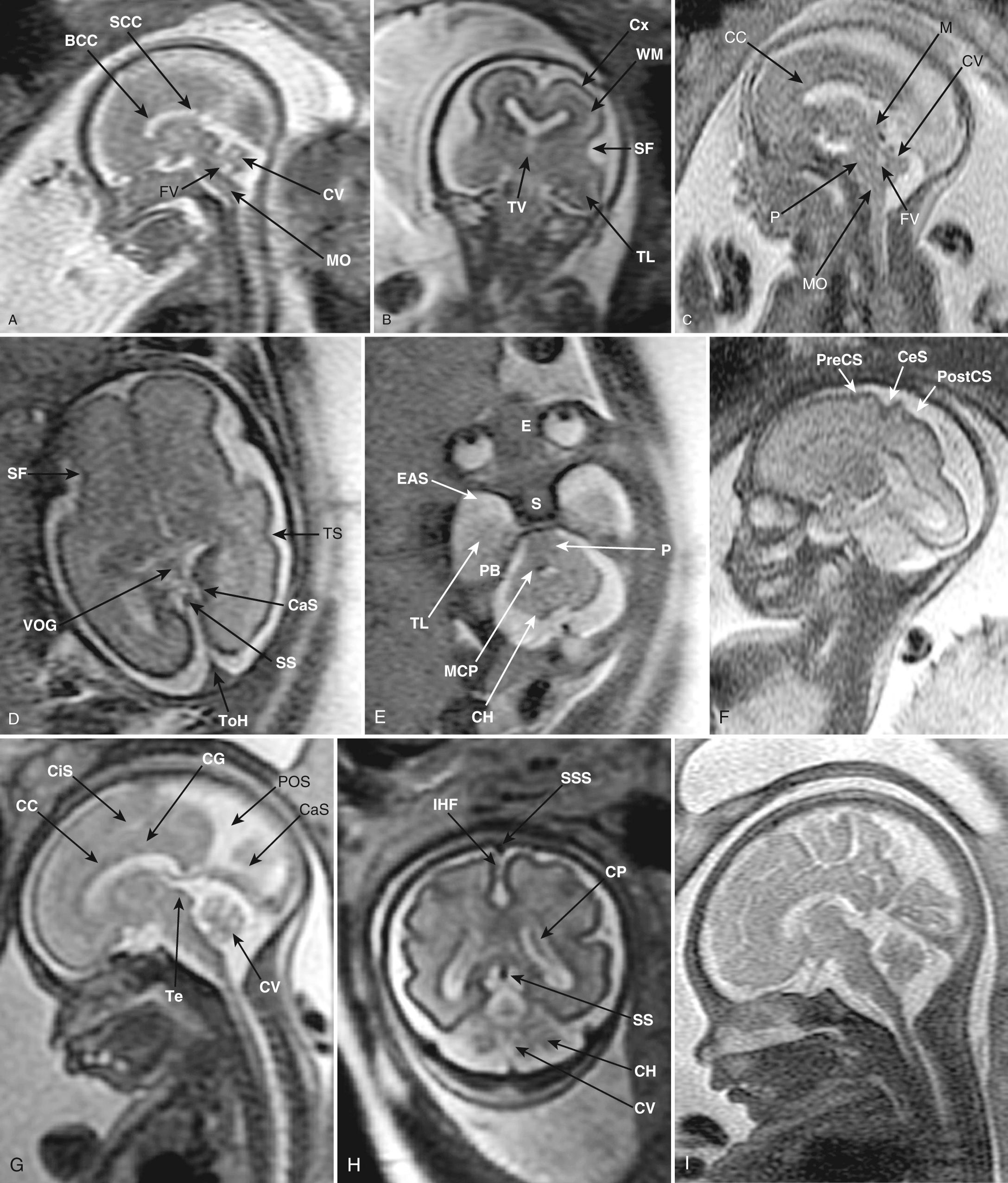
Multiplanar three-dimensional (3-D) imaging can be used to reconstruct axial and median views to assess the brain in any orientation. Midsagittal reconstructions are especially helpful in evaluating abnormalities of the corpus callosum and cerebellum. Head shape and ossification should be noted with all these views (see Chapter 33 ). Although ultrasound is the mainstay of a prenatal examination, MRI has been proven to be valuable to further assess problems found with ultrasound. MRI provides excellent anatomic images after about 22 weeks' gestation and is superior in evaluating the character of brain tissue and the periphery of the brain, where ultrasound visibility is limited ( Fig. 34.5 ). However, MRI has limitations in showing cerebral calcifications and small cysts.
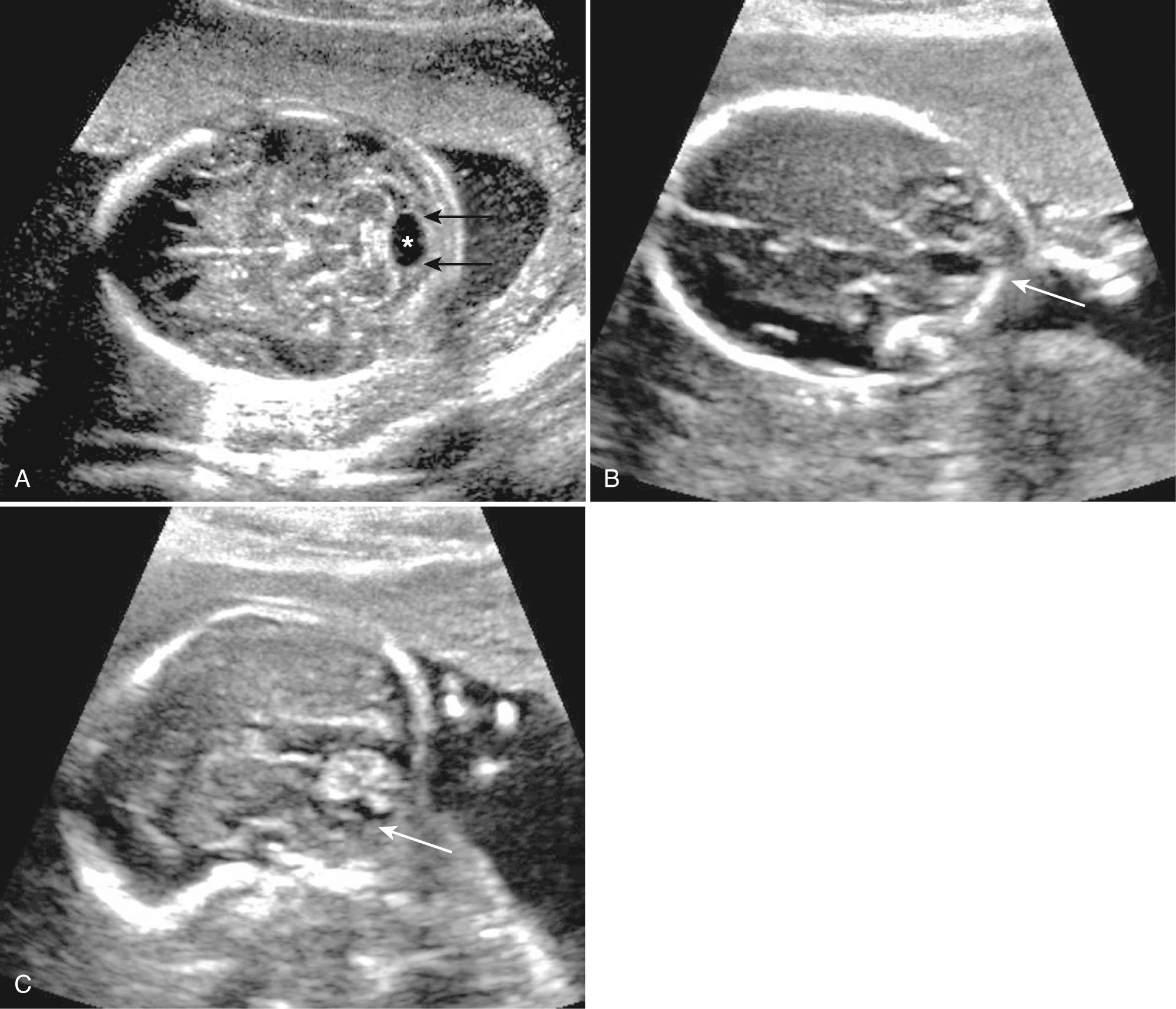
Choroid plexus cysts (CPCs) are cystlike spaces in the choroid plexus and are due to entrapment of CSF within an infolding of neuroepithelium ( Fig. 34.6 , Video 34.4 ). It is suggested that only CPCs over 3 mm should be considered substantial enough to be termed “choroid plexus cyst.” They are common and seen in 1% to 6% of fetuses between 14 and 24 weeks' gestation. Most are small and disappear without consequence by about 28 weeks. Rarely, large cysts may transiently dilate the lateral ventricles. They do not affect neurological development.
The great majority of CPCs are found incidentally in normal fetuses, but they can be associated with trisomy 18. About 50% of trisomy 18 fetuses have CPC, and they are the only obvious initial finding in about 10% of trisomy 18 cases. Likelihood ratios of trisomy 18 with isolated CPC are about 7 (range 4-12) times the mother's background risk. Size and bilaterality of the cysts do not affect incidence of aneuploidy. Fetuses with trisomy 18 almost always have other detectable abnormalities. When CPCs are found, there should be a detailed search for features of trisomy 18, especially the hands, heart, and CNS. Other tests of aneuploidy risk should be reviewed, including maternal age, serum screening, and cell free fetal DNA testing (noninvasive prenatal testing [NIPT]) to ensure low aneuploidy risk. Many feel that patients with isolated CPC and low aneuploidy risk, especially with normal NIPT findings, do not warrant further counseling or testing.
Blake pouch cyst, described by Robert Blake over 100 years ago, is a thin-walled cystic structure commonly seen in the midline of the posterior fossa behind the lower portion of the cerebellar vermis and brainstem. Because it contains CSF, this cyst is echo free, unlike the adjacent subarachnoid fluid, which is slightly echogenic owing to fine strands in the subarachnoid space ( Fig. 34.7 ). Of note, this difference in appearance of the fluid and Blake cyst walls are not visible on MRI. With careful scanning, we have found that a Blake pouch cyst is visible in almost every fetus varying in size from tiny to large and conspicuous. A large Blake pouch can be associated with vermian rotation to 40 to 50 degrees with an intact vermis. Some feel this rotation results from delayed fenestration of the foramina of Magendie and Luschka and secondary cystic dilation of the inferior membranous area, which is the pouch and elevates the vermis. If isolated, the rotation typically decreases in later pregnancy and outcome is good. When isolated, it should not be mistaken for abnormality. Three-dimensional ultrasound with midline reconstruction is especially helpful in this regard and can confirm vermian normality, as can MRI. We agree with those who feel that the mega–cisterna magna (MCM) is simply a large Blake pouch cyst, possibly resulting from delayed fenestration of the foramina; however, a few with isolated MCM may show developmental delay.
Cavum veli interpositi (CVI) is a small cystic collection in the midline, seen below the splenium of the corpus callosum, behind the upper brainstem and above the region of the pineal gland ( Fig. 34.8 ). It represents fluid in the potential space in the telea choroidea above the third ventricle. Most CVIs are seen just below the splenium, but they can extend anteriorly above the third ventricle to the foramina of Monroe. Coronal, midsagittal, and 3-D scans to help to confirm the diagnosis and MRI and color Doppler exclude vascular abnormality such as vein of Galen aneurysm (see Fig. 34.8C ). Although infrequently described in the prenatal ultrasound literature, we have found small CVIs, with maximal diameter less than 8 mm, to be very common on axial scans at 18 to 20 weeks. The pediatric literature reports a CVI incidence of 5% to 34%. Most CVIs are of no clinical consequence, and many recede spontaneously with normal long-term neurologic outcome. Occasionally, however, large cysts can distort the brainstem and adjacent brain, causing obstructive hydrocephalus (HC), and need treatment by unroofing.
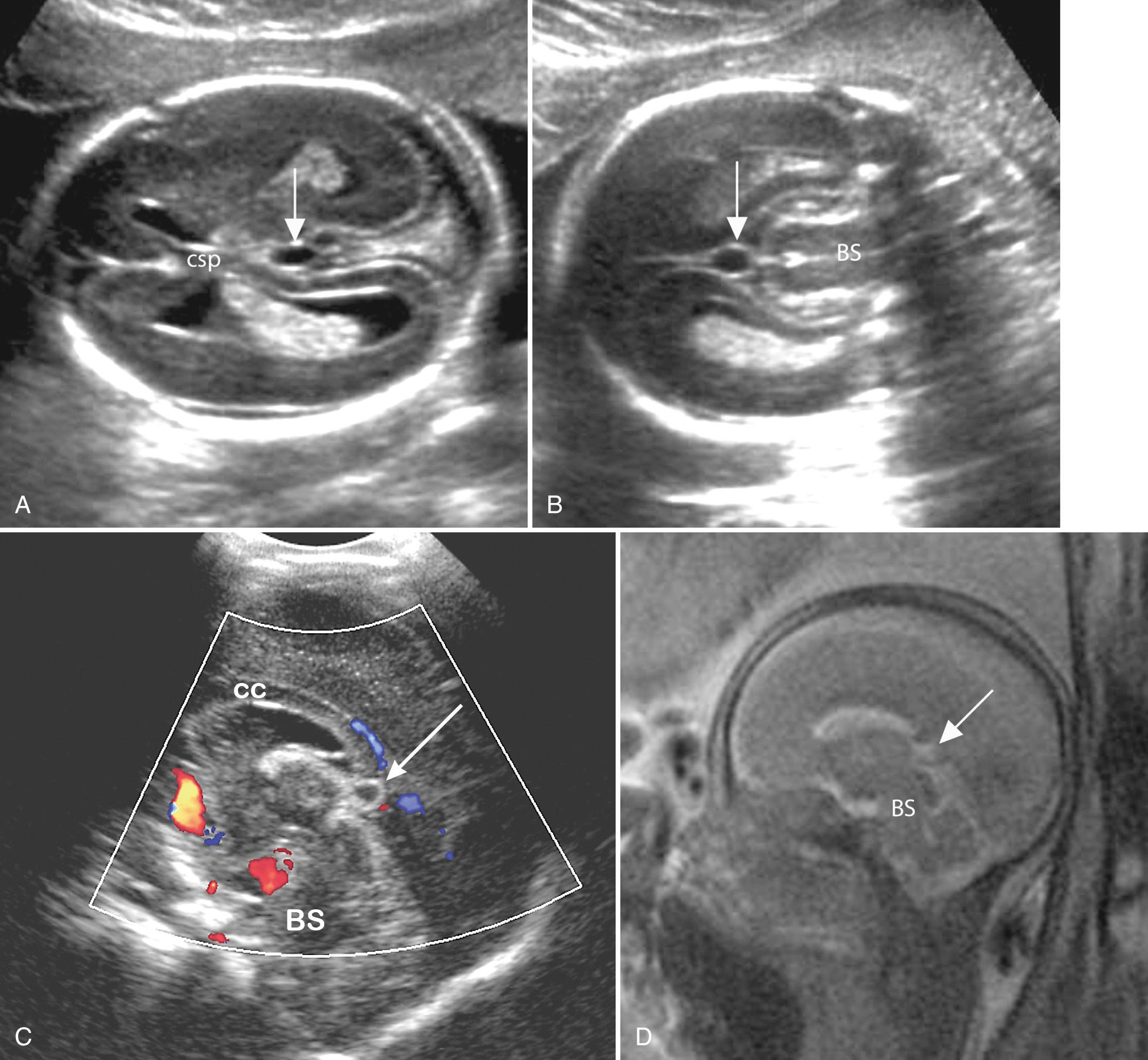
The physiologic CVI cysts tend to be isolated, unilocular, and small (<10 mm) and remain stable or recede over time. There is no known genetic association. The differential diagnosis includes cysts and cystlike conditions that occur in the midline, such as dilated cavum Vergae, glioependymal cysts, arachnoid cysts, cystic tumors (mainly cystic teratomas), vein of Galen aneurysm, pineal cyst, and hemorrhage. Pathologic cystic collections are generally larger, enlarge over time, and have associated abnormalities such as corpus callosum dysgenesis, ventriculomegaly (VM), or solid masses.
Fetuses with suspected CVI cysts should undergo detailed neurosonography and anatomic scan including Doppler to exclude vein of Galen aneurysm. If there are any atypical features or associated findings, then MRI can be helpful in further investigation. We no longer monitor incidentally discovered small (<8 mm) isolated cysts but perform follow-up scans if the cysts are especially conspicuous or if there is parental concern.
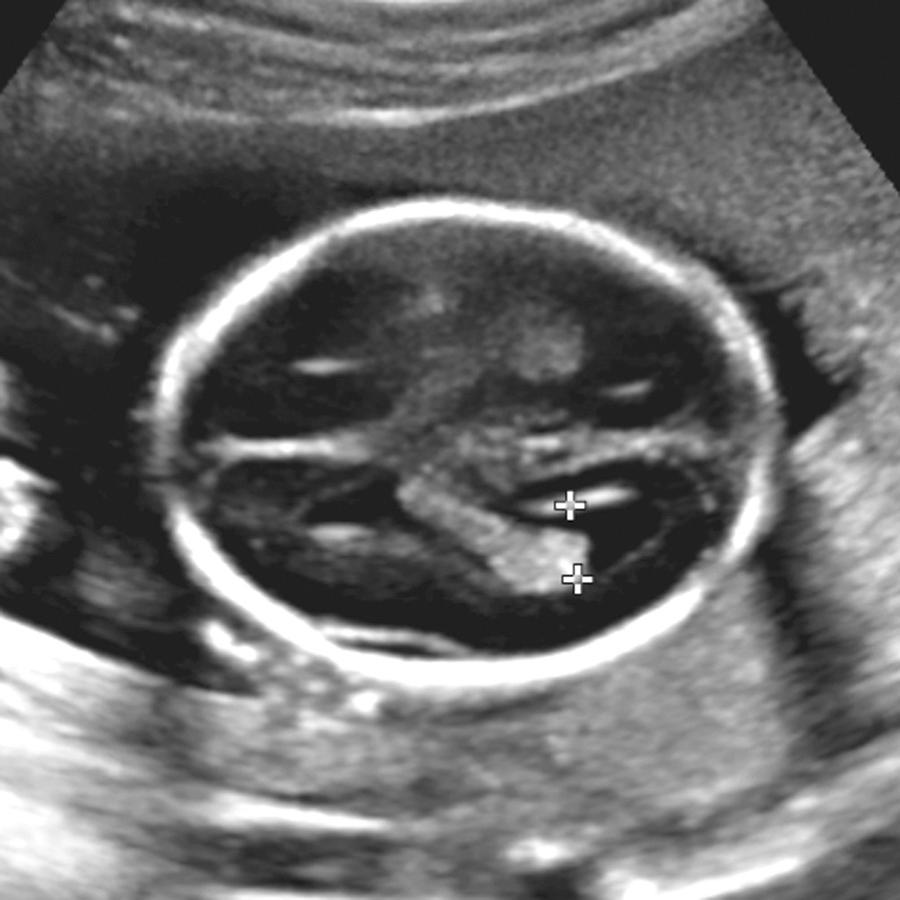
The term ventriculomegaly (VM) describes ventricles 10 mm or larger at the atrium. Care should be taken to measure the ventricles in the appropriate plane; an oblique plane will lead to an apparently enlarged ventricle if the trigone is measured. The head itself may be normal, large, or even smaller than expected for menstrual age. “Hydrocephalus” refers to enlarged ventricles associated with increased intracranial pressure and thus is typically associated with head enlargement. Ventricular abnormality, especially enlargement, is the most commonly encountered cranial abnormality at prenatal ultrasound, with prenatal incidence of 10 to 80 per 10,000 pregnancies. It is often the key finding in the initial detection of CNS abnormalities and is found in about 88% of fetuses with cerebral abnormalities ( Fig. 34.9 ).
Ventricle measurement technique is important and details should be remembered. With the head in a true axial view, measurements are generally taken only of the distal ventricle since the near ventricle is obscured by skull bone artifact and ventricles are presumed to be symmetrical. Calipers are placed at the widest part in the fluid space touching the inner ventricle wall perpendicular to the ventricle axis. For consistency measurements should be taken opposite the parieto-occipital fissure. It is possible, however, to measure the near ventricle directly by waiting for the head to turn or exploiting access provided by the squamosal and lambdoid sutures and the posterolateral (mastoid) fontanelles and on 3-D multiplanar reconstructions and even on coronal views using the “owl's eye” view. But it is important to ensure that all measurements are made in the true axial plane (see Figs. 34.3 and 34.9 ). Visualization of the near ventricle and hemisphere should be performed whenever VM is suspected.
There are pitfalls to ventricular measurement. Errors arise if the plane of view is not axial or if there is an improper choice of ventricle boundary. The insula, the extreme capsule of the basal ganglia, the supraventricular veins, the medial wall of the occipital lobe (see Fig. 34.2A-B and Fig. 34.10 ), and reverberation echoes of the proximal skull all can appear as lines, which should not be mistaken for ventricular walls.
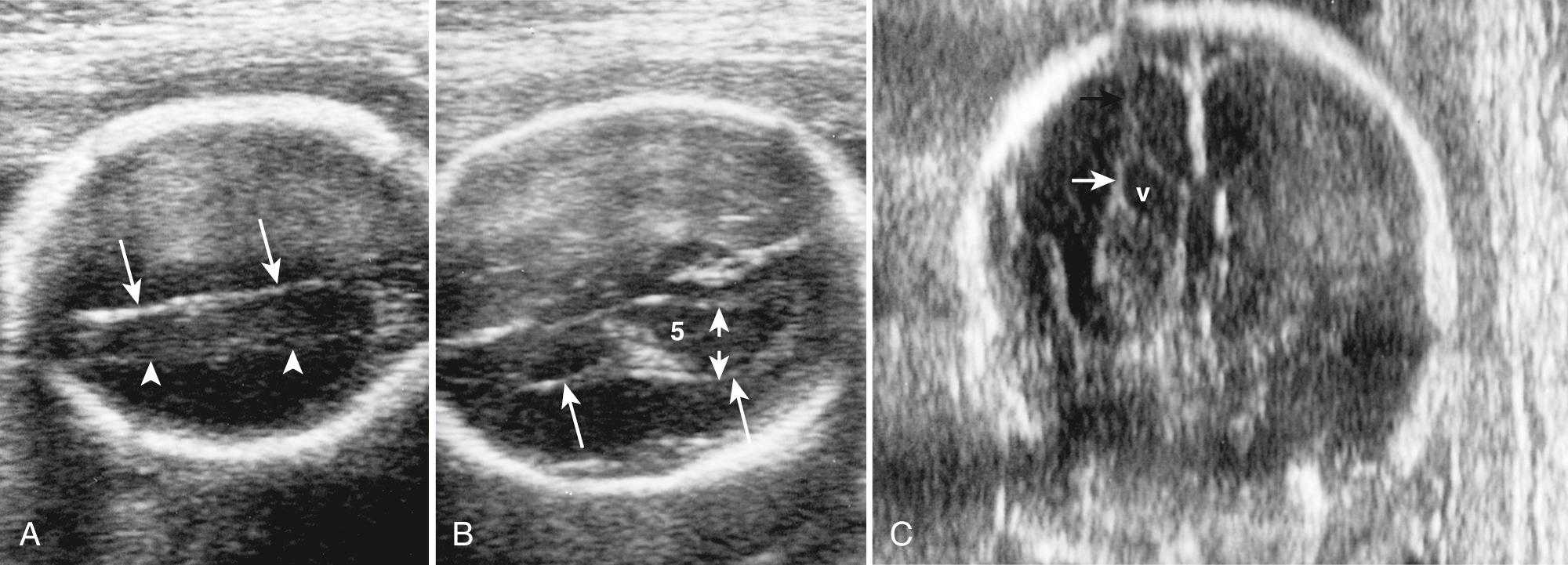
Between 14 and 38 weeks, the transverse atrial measurement is reported constant at 7.5 mm (standard deviation [SD], 0.7 mm). Measurements of 10 mm or larger suggest VM with a low false-positive rate. A finding of 10 mm is not a clear criterion of abnormality, but rather it is generally accepted that at 10 mm or more the ventricle is sufficiently different to warrant additional attention and investigation. Note that the 10-mm limit was initially proposed by Dr. Filly as the upper limit not because it definitively distinguished normal from abnormal, but rather because it was an easy number to remember at all gestational ages and identified most fetuses with findings that were felt to be different enough to warrant counseling and further investigation. It was understood that some fetuses with ventricles larger than 10 mm may be entirely normal.
In general, 10 to 12 mm is termed mild or borderline; 12 to 15 mm is moderate (although some authors consider up to 15 mm in the mild range); and greater than 15 mm is marked VM. Although 10.0 mm has been considered the upper limit of normal, there are reports of normal outcomes with ventricles larger than 10 mm, and some have suggested raising the upper limit of normal to 11 or 12 mm. Ten millimeters is already about 4 SDs above the mean, and we agree with others that 10 mm should remain the criterion above which counseling and investigation should occur ( Fig. 34.11 ).
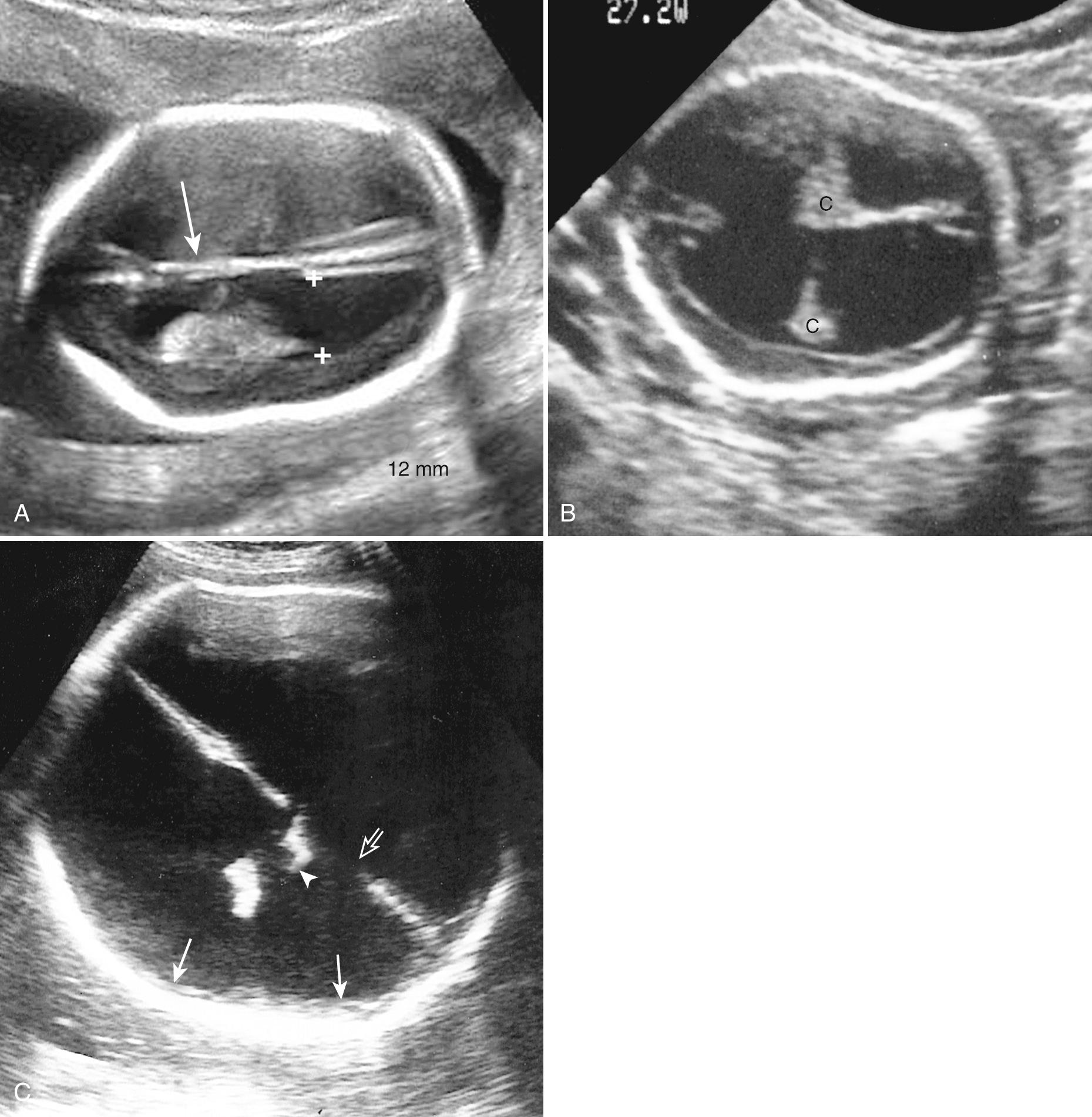
As an alternative approach to detection of mild VM, Mahony and colleagues suggested using choroid separation from the medial ventricle wall and reported that normally this distance is 1 to 2 mm after 15 weeks. Measurements of 3 mm or more were associated with abnormal outcomes when combined with other fetal abnormalities, even if the ventricle measurement was normal. Hertzberg and colleagues found that 20% of such fetuses had abnormal outcomes. However, many believe that this approach is too sensitive and unnecessarily creates anxiety in parents.
Ventricular enlargement is termed isolated ventriculomegaly (IVM) if there are no associated cerebral or somatic findings and the karyotype is normal. At time of diagnosis of VM, about 60% will have additional abnormalities, and 40% will not. Unfortunately, at birth, even after detailed ultrasound and prenatal MRI, about 7% to 16% of infants with apparent IVM are found to have additional abnormalities.
Enlargement of the lateral cerebral ventricles is not the primary problem. Although VM may be an isolated finding, it is often the sonographically conspicuous finding of numerous disorders and syndromes. It is the underlying changes in the brain that are clinically important, not only the size and appearance of the ventricles. Cerebral functional alterations are only variably predicted by ventricular size, cortical thinning, and appearance. A single institution study of 29,000 pregnancies found VM in 0.38% of fetuses (1 in 265). Of these, 57% were not isolated and had other abnormalities. In the 43% with IVM, VM was mild in 40% (10-15 mm) and marked in 60% (>15 mm). Abnormal neurodevelopmental outcome was poorest with nonisolated (91%) and marked IVM cases (68%) but was seen in only 19% of mild VM cases.
The approximate frequencies of cerebral abnormalities seen with VM are aqueduct stenosis, 30% to 40%; Chiari II malformation with spina bifida, 25% to 30%; Dandy-Walker complex, 7% to 10%; and less common conditions including agenesis of the corpus callosum . In general, associated CNS and somatic malformations are common. Chromosomal and genetic abnormalities are more common with nonisolated (25%-36%) than isolated (3%-6%) VM and include the trisomies and X-linked hydrocephalus in males and numerous other conditions.
CSF is secreted by the choroid plexus of the lateral, third, and fourth ventricles, as well as by the cerebral capillaries. CSF flows from the lateral ventricles with assistance of ciliary activity of ependymal cells through the foramina of Monro, third ventricle, aqueduct of Sylvius, and fourth ventricle and out the foramina of Magendie and Luschka into the subarachnoid space of the posterior fossa, from where it courses over the surface of the brain to be absorbed by the pacchionian or arachnoid granulations and lymphatics.
Ventricular enlargement generally occurs in one of four scenarios. First and most commonly, there is obstruction of CSF flow in the brain, usually at the aqueduct (intraventricular obstructive hydrocephalus). Alternatively, the site of blockage may be outside the ventricular system, or there may be failure of absorption ( extraventricular obstructive hydrocephalus or communicating hydrocephalus ). Second and less often, VM results from excess CSF secretion with choroid plexus papillomas. Third, VM can follow cerebral destruction and brain shrinkage as a result of abnormal brain development or diverse insults (hydrocephalus ex vacuo) . Fourth, it may be a feature of generalized cerebral malformation. Apart from the effects of the basic underlying disorder, the degree of brain injury with HC varies with age of onset and magnitude and duration of the dilation, all of which affect periventricular axons, myelin, and microvasculature, resulting in cerebrovascular injury, gliosis, and inflammation and compensation by neuroplasticity.
Obstructive hydrocephalus
Aqueduct stenosis (idiopathic, infections, bleeding, X-linked, obstructing masses)
Spina bifida with Chiari II malformation
Large choroid plexus cyst
Excess cerebrospinal fluid (CSF) production
Choroid plexus papillomas
Destructive processes (encephaloclasis)
Vascular insult
Infection
Ischemia
Schizencephaly
Hydranencephaly
Cerebral malformations
Aneuploidy (trisomies 21, 18, 13)
Agenesis of corpus callosum
Vermian dysgenesis and Dandy-Walker malformation
Holoprosencephaly
Neuronal migration abnormalities (lissencephaly, schizencephaly)
Microcephaly
The detection of VM is generally straightforward, but the determination of its etiology can be difficult. Several approaches to measuring ventricular size have been described: atrial width, choroid separation, ventricle-to-hemisphere ratio, combined anterior horn width, and visual anatomic appearance. Of these, the universally accepted method is the transverse measurement of the atrium of the occipital horn (see Figs. 34.3 , 34.9A , and 34.11A ). Methodological scanning can often determine specific causes. The size of the head is not helpful in detecting VM, and frequently the BPD remains normal even with severe ventricular enlargement. Nomograms of other parts of the ventricles are available but not in common use.
In the first trimester the choroid plexus should fill the prominent-appearing lateral ventricle, except for the conspicuous fluid containing anterior frontal horn of the lateral ventricle, which should not be mistaken for abnormality (see Fig. 34.1B ). VM manifests as a small-appearing choroid plexus with excess surrounding fluid.
Qualitative appearances suggesting ventricular enlargement include convexity (outward bulge) to the lateral wall of the lateral ventricle and asymmetrical position of the left and right choroids, which fall with gravity when unsupported by ventricular walls because the choroids are denser than CSF (“droopy” or “dangling” choroids) ( Fig. 34.11B , Video 34.5 ). With massive ventricular enlargement, the interhemispheric structures, particularly the septum pellucidum, undulate with maternal pulse and movements and can become disrupted (fenestrate), allowing the left and right lateral ventricles to communicate. The upper choroid can fall across the midline through this hole and into the lower ventricle ( Fig. 34.11C ).
Once ventricular enlargement is suspected, attention turns to etiology and a detailed search for associated cerebral and somatic abnormalities that may suggest specific causes or syndromes. Both left and right ventricles should be measured, and both hemispheres evaluated. Associated brain abnormalities may be subtle and not readily appreciated on standard axial scanning. Coronal and sagittal views both transabdominally and transvaginally and use of 3-D multiplanar scans can be especially helpful to detect abnormalities of the cerebellar vermis and corpus callosum. MRI adds additional information in 5% to 50% of cases, especially with respect to parenchymal injury, migrational disorders, ischemia, hemorrhage, and brainstem abnormalities.
Fetuses with suspected VM should be evaluated by a knowledgeable team with experience in the investigation, counseling, management, and follow-up of prenatally discovered abnormalities. Additional testing can be offered including karyotype and molecular analysis, testing for infection (especially CMV, Toxoplasma, and rubella), platelet antibodies, and follow-up examinations to follow ventricle growth and to detect additional late-appearing findings that may relate to prognosis (porencephaly, malformation of cortical development, hemorrhage). Postpartum evaluation is important to evaluate for additional prenatally unsuspected abnormalities and to plan treatments.
Prognosis relates to the degree of VM, progression of VM, and especially the presence of associated abnormalities. In cases of VM associated with chromosomal or other CNS and somatic abnormalities, the prognosis and recurrence risk relate to underlying conditions. When VM is truly isolated, neurodevelopmental outcome relates to the degree of enlargement. Normal functional outcome is reported in about 85% to 96% of mild (10-12 mm), 76% of moderate (12-15 mm), and 28% of severe (>15 mm) cases. Outcome is better if VM is stable or decreases, and worse with enlarging ventricles. Unilateral cases behave similarly.
Counseling of parents remains difficult, and long-term outcome remains guarded. Laskin and colleagues reported that initially 85% of children had a favorable outcome, but this decreased to 79% by 20 months. Remember also that 2% to 3% or more of entirely normal-appearing fetuses and children can also have developmental disability, and it is not clear how this rate compares with those with IVM.
Congenital CNS abnormalities often reflect the time of insult in prenatal life rather than the specific cause. Increasingly, CNS abnormalities are being associated with gene mutations at the molecular level. Genes control cortical development and neuron migration, and the same genes also have roles in somatic development. The understanding of these complex and interrelated molecular mechanisms of disease and maldevelopment is changing rapidly. Increasingly, malformations are starting to be categorized based on their underlying genetic and molecular abnormalities rather than on morphologic appearances. Even if the genes are normal, their functions can be variably disturbed by external influences, such as anoxia, infections, and teratogens, and the final outcomes will typically reflect the timing and intensity of the insults more than their specific nature.
To reach an accurate diagnosis and help with investigation, counseling, and treatment, evaluation should include not only CNS changes, but also additional, subtle cerebral and somatic findings. Additional clues can be found in pregnancy history, family history, course of current pregnancy (including maternal conditions such as diabetes), medications, illnesses, occupations and exposures, and evaluation of parents and close relatives. If there is a known risk of specific conditions, online resources such as Online Mendelian Inheritance in Man (OMIM, http://www.ncbi.nlm.nih.gov/omim ) and consultation with experts in other specialties can be helpful in providing a list of findings to target on ultrasound examination and MRI.
CNS development begins with development of the notochord, which subsequently induces the dorsal development of the neural plate, which closes to form the neural tube, which becomes the spinal cord and part of the brain. Errors of dorsal induction can result from many different genetic and teratogenic factors and affect development of the dorsal neural plate and closure of the neural canal (neurulation).
Anencephaly follows failure of closure of the rostral neuropore leading to failed cranial vault development and unprotected exposed brain tissue (exencephaly), the subsequent destruction of which leaves a flattened, amorphous vascular-neural mass (area cerebrovasculosa) characteristic of anencephaly ( Fig. 34.12 ). Facial structures and orbits are present. Associated spinal and non-CNS abnormalities and polyhydramnios are common. On occasion, the dysraphic abnormality involves the head and entire spine (craniorachischisis). Acrania is sometimes defined as a normal brain with absent skull, and therefore this term should not typically be used in cases of anencephaly or exencephaly, even though the cranial vault is absent.

Prevalence of anencephaly is about 1 per 1000 births. Risk is increased in families with neural tube defects (NTDs), young or old mothers, or diabetes and with antiepileptic drugs. Folate appears protective. Chromosomal abnormality is seen in 2%, and additional abnormalities are common including cleft lip, cardiac defects, club feet, and abdominal wall defects.
Detection of anencephaly before 14 weeks can be difficult because a relatively normal-appearing brain structure can be present, but the diagnosis has been suggested as early as  weeks. The diagnosis can be missed unless the examiner specifically looks for ossified cranial bones. Ultrasonically visible ossification of frontal bones may not be apparent until 10 weeks, and anencephaly should not be diagnosed before this gestational age. Commonly, however, the cerebral mass is deformed, often looking like “Mickey Mouse ears.” An additional clue to diagnosis is the identification of amniotic fluid that is more echogenic than the extraamniotic subchorionic fluid owing to intraamniotic debris shed from the uncovered brain. The introduction of the NT scan and increasing familiarity with first-trimester appearances has shown that most anencephalic fetuses can be detected in the late first trimester.
weeks. The diagnosis can be missed unless the examiner specifically looks for ossified cranial bones. Ultrasonically visible ossification of frontal bones may not be apparent until 10 weeks, and anencephaly should not be diagnosed before this gestational age. Commonly, however, the cerebral mass is deformed, often looking like “Mickey Mouse ears.” An additional clue to diagnosis is the identification of amniotic fluid that is more echogenic than the extraamniotic subchorionic fluid owing to intraamniotic debris shed from the uncovered brain. The introduction of the NT scan and increasing familiarity with first-trimester appearances has shown that most anencephalic fetuses can be detected in the late first trimester.
Differential diagnosis includes other conditions in which the cranial bones are absent or lack mineralization, such as amniotic band sequence, large encephaloceles, osteogenesis imperfecta, and hypophosphatasia. With amniotic band sequence (early amnion rupture sequence) the fetuses typically have an asymmetrical defect accompanied by body wall defects and/or amputation of body parts and occasional oligohydramnios, and membranes may be visible in amniotic fluid, or the fetus may be stuck to the side of the uterus or placenta. Unlike anencephaly and open spina bifida, early amnion rupture sequence is sporadic and without increased recurrence risk. In general, large encephaloceles have more calvarial development than seen with anencephaly, but occasionally the two can appear similar. In either case, the prognosis remains hopeless.
The outcome of anencephaly is invariably fatal, and pregnancy termination is offered at any gestational age.
A cephalocele is a herniation of intracranial structures through a defect in the cranium or skull base. It is termed cranial meningocele when it contains only meninges and CSF and encephalocele when it contains brain tissue. Among live births the incidence is about 1 to 5 per 10,000, but this underestimates the true incidence because many die at or before birth. Most encephaloceles occur in the midline in the occipital (75%), frontal (13%), or parietal (12%) regions ( Fig. 34.13 ). Anteriorly they can extend into the mouth, nasal, and sphenoid areas, where their identification can be difficult. Off-midline lesions are commonly associated with amniotic band syndrome. Cephaloceles can occur as isolated lesions, but most (20%-60%) are associated with other anomalies or syndromes involving the head, spine, face, skeleton, or kidneys. Chromosomal abnormality is seen in 14% to 60%, especially trisomies 18 and 13. Interestingly, in the Western world the occipital location is more common, whereas in Asia frontonasal location predominates.
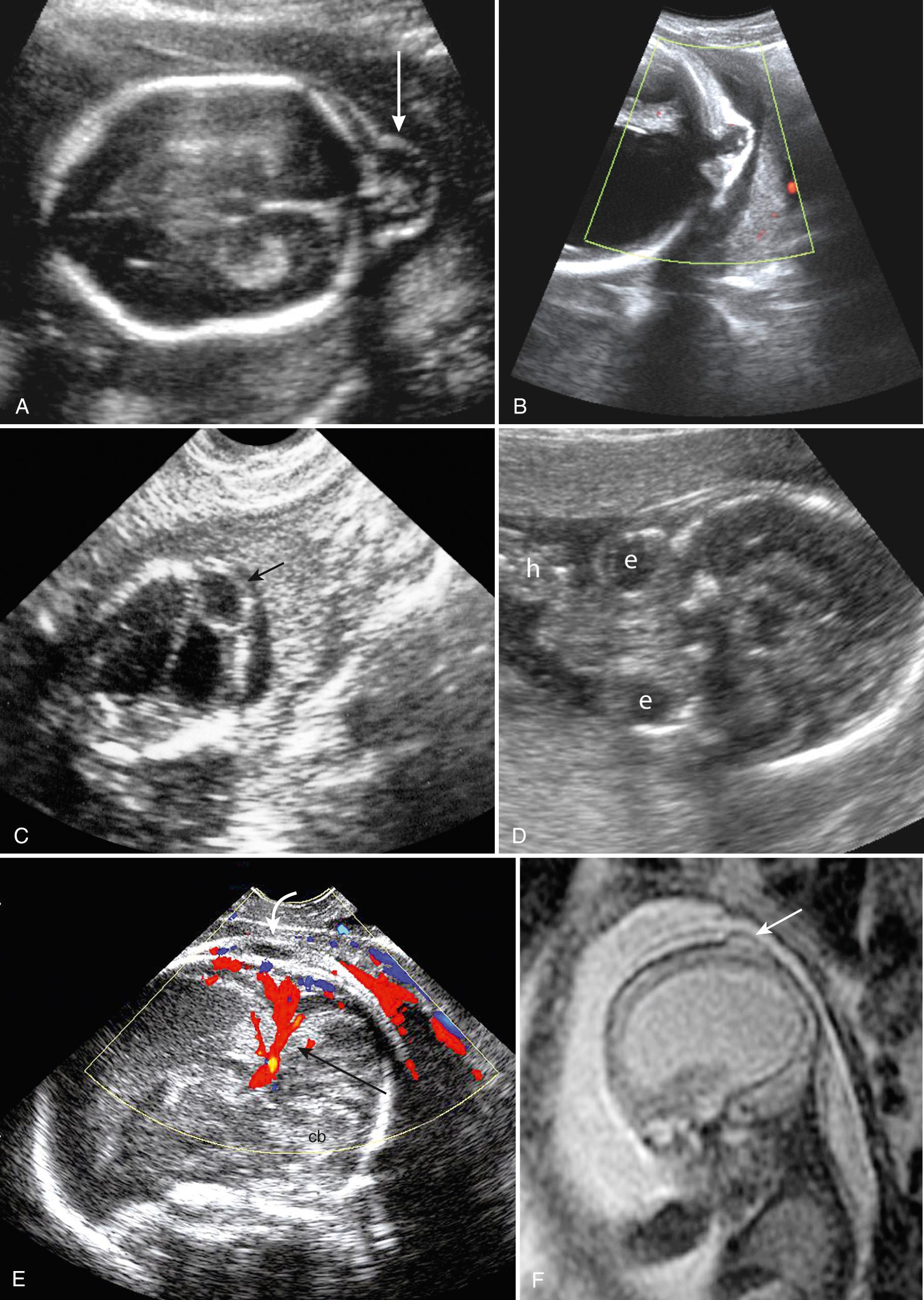
Although at first glance cephaloceles resemble spina bifida, there are some differences. Spina bifida is a failure of primary closure (neurulation), whereas many cephaloceles are skin covered and likely are a secondary abnormality after neurulation, possibly representing abnormal mesenchymal migration or, in the case of frontonasal cephaloceles, a failure of closure of normal diverticulation. Because many are skin covered, MS-AFP is elevated in only 33% compared with spina bifida (62%) and anencephaly (92%). This makes ultrasound the primary diagnostic tool. Cephaloceles are more likely to be associated with additional cerebral and somatic abnormalities and syndromes. Common associated cerebral abnormalities involve the midline structures such as the corpus callosum, cerebellum, and brainstem and include venous abnormalities, holoprosencephaly (HPE), HC, and facial clefting ( Table 34.2 ). Over 30 syndromes include cephaloceles; many are related to gene dysfunctions that affect both CNS and somatic development. These additional abnormalities strongly affect prognosis, so whenever a cephalocele is found, there should be a detailed search for other anomalies, and MRI and genetic evaluation should be considered.
| Meckel-Gruber | Cystic renal dysplasia, polydactyly, other central nervous system abnormalities, microphthalmia, cleft lip and palate, micrognathia, ciliopathy |
| Walker-Warburg | Hydrocephalus, agyria (lissencephaly type 2), retinal dysplasia, vermis hypoplasia, kinked brainstem, muscular dystrophy |
| Joubert and related | Vermian hypoplasia, deep interpeduncular fossa (molar tooth), hydrocephalus, ciliopathy |
| Hydrolethalus | Hydrocephalus, cerebral abnormality, polyhydramnios, micrognathia, polydactyly, heart defects |
| Fraser | Cryptophthalmos, syndactyly, genital abnormality, renal abnormality, laryngeal stenosis |
| Robert | Phocomelia (pseudo-thalidomide), cleft lip and palate, intrauterine growth restriction |
Ultrasonographically, an encephalocele manifests as a cystic mass at the surface of the skull, typically in the midline (see Fig. 34.13 ). Contained brain with a visible bony defect confirms the diagnosis, but it may be difficult to see the contained, often abnormal, brain tissue, and the bony defect may also be small and difficult to detect. Encephaloceles can be seen during first-trimester ultrasound and are often preceded by an enlarged rhombencephalic cavity. MRI is helpful to confirm the diagnosis and, more important, to search for additional features that can affect prognosis and counseling.
Other lesions that should be considered in the differential diagnosis when a scalp lesion is seen include cystic hygroma, hemangioma, scalp edema or cephalohematoma, epidermal scalp cyst, branchial cleft cyst, dermoid cyst, dacryocystocele, epignathus, and cervical teratoma.
The atretic cephalocele is a variant that is infrequently recognized prenatally, manifesting as a small, blisterlike subcutaneous collection in the skin in the midline near the vertex at the surface of a seemingly intact skull (see Fig. 34.13E ). Diagnostic clues are abnormalities of the superior sagittal sinus, which may have multiple channels, and the persistence of the prosencephalic vein of Markowski, which runs in the falx (falcine sinus) from the region of the start of the vein of Galen to the sagittal sinus underlying the encephalocele. As with other cephaloceles, there may be other cerebral abnormalities, especially involving the midline, which can affect outcomes. However, if the cephalocele is isolated, the children in general do well.
The prognosis of cephaloceles depends on the location of the lesion, the amount of herniated brain, the formation of the underlying brain, associated anomalies, and genetic abnormalities. Prenatal and perinatal mortality rates are high, and intellectual impairment is common. If discovered before viability, pregnancy termination is considered. Later in pregnancy, management depends on the size and location of the encephalocele and associated anomalies.
Ciliopathies are a group of disorders resulting from abnormal structure or function of cell surface cilia due to mutations in ciliary genes. Cilia are found on the surface of almost all cells of vertebrates, and their functions are very much more complex than providing simple transport as seen in bronchi and various anatomic ducts. Normally functioning cilia are required for the normal development and function of seemingly unrelated sites including brain, kidneys, eyes, liver, and skeleton. In the brain, cell surface cilia are involved with brain patterning and neuronal migration and help direct CSF flow. Abnormal ciliary function may result in HC and other cerebral malformations including encephaloceles. Over 40 mutations of ciliary genes have been found, resulting in over 1000 polypeptide abnormalities. As result, the expression of ciliary gene mutation is variable. A single gene mutation may cause different phenotypes, and mutations of different genes may result in the same phenotype. Commonly encountered ciliopathies have overlapping features such as encephaloceles and include Meckel-Gruber syndrome, Joubert syndrome, orofaciodigital syndrome, Bardet-Biedl syndrome, autosomal recessive polycystic kidneys, autosomal dominant polycystic kidneys, Jeune asphyxiating thoracic dystrophy, Ellis-van Creveld syndrome, and others.
Joubert syndrome and related disorders (JSRD) have the key feature of molar tooth sign visible on MRI. The molar tooth appearance results from hypoplasia of the cerebellar vermis, horizontal thick elongated cerebral peduncles, and deep interpeduncular fossa at upper pons; on axial MRI of the brainstem, these features look like a molar tooth. This sign is used as the diagnostic test in children. JSRD is clinically characterized by hypotonia, ataxia, psychomotor delay, irregular breathing, and abnormal eye movements and has an incidence of about 1 per 80,000 pregnancies. Different combinations of ciliary gene mutations can result in primary Joubert syndrome, and related disorders have variable abnormalities of the neurons, eye, renal tubules, and bile ducts and polydactyly.
On ultrasound the molar tooth sign findings of vermian hypoplasia, thickened cerebral peduncles, and interpeduncular notch may be visible by 20 weeks and confirmed by MRI if needed. Additional cerebral imaging findings can include abnormalities of the corpus callosum and neuronal migrational abnormalities, Dandy-Walker malformation (DWM), and encephalocele as well as abnormalities in somatic structures. If the mutation is known (about 50%), early diagnosis is possible with chorionic villus sampling (CVS).
Prognosis is generally poor and related to extent of breathing and feeding problems in the short term and renal and hepatic complications in the long term.
Meckel-Gruber syndrome is likely the most common syndromic abnormality of the CNS and is characterized by occipital encephalocele, enlarged dysplastic kidneys, hepatic duct proliferation, polydactyly, posterior fossa abnormalities, and craniofacial and heart defects and has features that overlap with JSRS. Incidence is 1 per 13,000 to 140,000 live births. It is a lethal autosomal recessive disorder associated with mutations in several ciliary genes. The detection of its classic triad of large echogenic kidneys, encephalocele, and postaxial polydactyly can allow diagnosis as early as 14 weeks, but oligohydramnios in later pregnancy may hinder detection of some of these features. Autosomal recessive polycystic disease and trisomy 13 should be considered in the differential diagnosis. Genetic testing may be possible in at-risk families.
Amniotic band sequence/limb–body wall complex describes a variable collection of disruptive abnormalities and is associated with adherence of the fetus to bands in the amnion.
Cases are sporadic, occurring in about 1 to 10 per 10,000 pregnancies, and typically have normal karyotype. Fetuses with these conditions have a very variable range of complex, generally asymmetrical abnormalities that range from minor to massively disruptive. Cranial manifestations may initially mimic anencephaly, encephalocele, and other cerebral abnormalities and are often associated with asymmetrical facial disruptions. The literature on the etiology and pathogenesis of these sporadic conditions is confusing and controversial and includes amniotic rupture, vascular disruption, and abnormal development of embryonic body folds. There is a common theme of combinations of amniotic abnormality and asymmetrical fetal disruptions. Bands may be seen attached to abnormal body parts, or the fetus is partly fixed to the uterine wall. Limb–body wall complex terminology is often used if there is a more severe malformation consisting of encephalocele with facial clefts, thoracoschisis or abdominoschisis, short umbilical cord, and limb defects. Often there are internal abnormalities that cannot be explained by amniotic bands such as congenital heart disease, renal agenesis, and intestinal atresias. Minor variants may have only constrictive amniotic bands resulting in annular constrictions or focal malformations of limb parts.
The findings at ultrasound vary with affected areas. Clues to diagnosis are asymmetrical abnormalities and amniotic bands attaching to the fetus or fetal parts that are stuck to the uterine wall (as with limb–body wall complex). The bands can be hard to see, and technique needs to be optimized and gain increased to demonstrate them. In some cases fetal anatomy is so disrupted that parts become unrecognizable and this massive disruption is the clue to the diagnosis. The condition can mimic anencephaly or encephalocele, and often additional intracranial abnormities are present (see Fig. 34.12C ).
The major disruptions are lethal. Three-dimensional ultrasound and MRI can be helpful in clarifying the extent of the lesions. Prognosis, counseling, and management depend on the nature and degree of disruptions. Fortunately, these anomalies are sporadic, with negligible recurrence risk.
Spina bifida or neural tube defect (NTD) is the second most common birth defect after congenital heart disease. Two general groups are recognized: open (not skin covered) and closed (skin covered). Both result from abnormalities associated with neural tube closure. Open lesions result from failure of closure of the neural tube, leaving a defect that leaks CSF and exposes neural tissue to the damaging effects of amniotic fluid. About 80% to 90% are open, appearing as dorsal cyst (myelocele or meningomyelocele depending on neural content) or rachischisis (uncovered open defect). Open lesions are commonly associated with elevated MS-AFP above 2.50 multiples of the median (MoM). The remaining 10% to 20% are closed (occult) lesions that are skin covered and do not leak AFP and in which MS-AFP levels are normal. Closed defects result from failure of separation of the neural tube from the skin and abnormal adjacent mesenchymal tissue development. These cause variable spinal, vertebral, and cutaneous lesions including lipoma, lipomeningocele, hypertrichosis, tags, dorsal pits, and others.
The incidence is about 0.2 to 10 per 1000 pregnancies and has decreased by about 70% following folate supplementation. The etiology is multifactorial and includes syndromes, genetic abnormalities, and maternal and fetal factors including diabetes and teratogens such as anticonvulsants.
Open spina bifida is virtually always associated with the Chiari II malformation which is a complex deformity involving calvaria, dura, cerebrum, and hindbrain. In Chiari II malformation the striking abnormalities are evident in the small posterior fossa and include herniations of the vermis, pons, and medulla; brainstem kinking; small fourth ventricle; aqueduct stenosis; and tectal beaking. There are, however, additional pancranial changes that involve the skull bones (beaten silver appearance or Lükenschädel ), small posterior fossa, falx (interdigitation of sulci, low-lying tentorium and transverse sinuses), large massa intermedia, cerebral hemispheres (hydrocephalus, dysgenesis of corpus callosum, heterotopias, malformations of cortical development, and distortion of the limbic system). It is believed that leakage of CSF through the spinal defect leads to a cascade of events resulting in a small underdeveloped posterior fossa followed by hindbrain compression and obstruction to CSF flow, which leads to additional cerebral and calvarial abnormalities. Some believe that failure of neural tube closure is not just a single event, but rather is only part of a spectrum of developmental abnormalities not yet fully defined but that result in multiple additional cerebral and somatic abnormalities.
Of newborns with spinal NTDs, it was found that 80% had isolated NTDs. The remaining 20% had additional anomalies including chromosomal abnormalities or sporadic abnormalities involving virtually all systems. An autopsy study found that 68% of cases with spina bifida had non-CNS anomalies, most commonly skeletal (club feet), but also involving kidneys, gastrointestinal tract, abdominal wall, and other structures. A recent multicenter prenatal ultrasound study found chromosome abnormalities in 6.2% of cases (most commonly, trisomy 18 and triploidy) and structural abnormalities in 4.6% (most commonly heart defects).
The actual spinal abnormality of open NTD can be very difficult to see at screening ultrasound, but the defect leaks CSF containing alpha-fetoprotein (AFP), which may become detectable in maternal serum (MS-AFP). This was the basis of earlier serum AFP screening for open defects. Maternal serum levels greater than 2.5 MoM at 16 to 18 weeks detected 88% of anencephalic fetuses and 79% of fetuses with open NTDs but were also found in 3% of normal fetuses. However, all closed, skin-covered NTDs were missed by MS-AFP screening.
In the 1980s it was found that open NTDs are virtually always associated with readily detected ultrasonographic cranial changes of Chiari II malformation in the second trimester ( Fig. 34.14 , Video 34.6 ). This is unlike closed spina bifida, in which the brain and posterior fossa are normal and the spinal abnormality can be detected only with careful direct examination of the spine ( Fig. 34.15 ). Improvements and standardization and use of these cerebral signs now allow second-trimester detection of open spina bifida in 88% to 96% of cases, and even 100% in some expert hands. Many believe that second-trimester ultrasound has replaced MS-AFP screening for open lesions (anencephaly, encephalocele, open spina bifida).
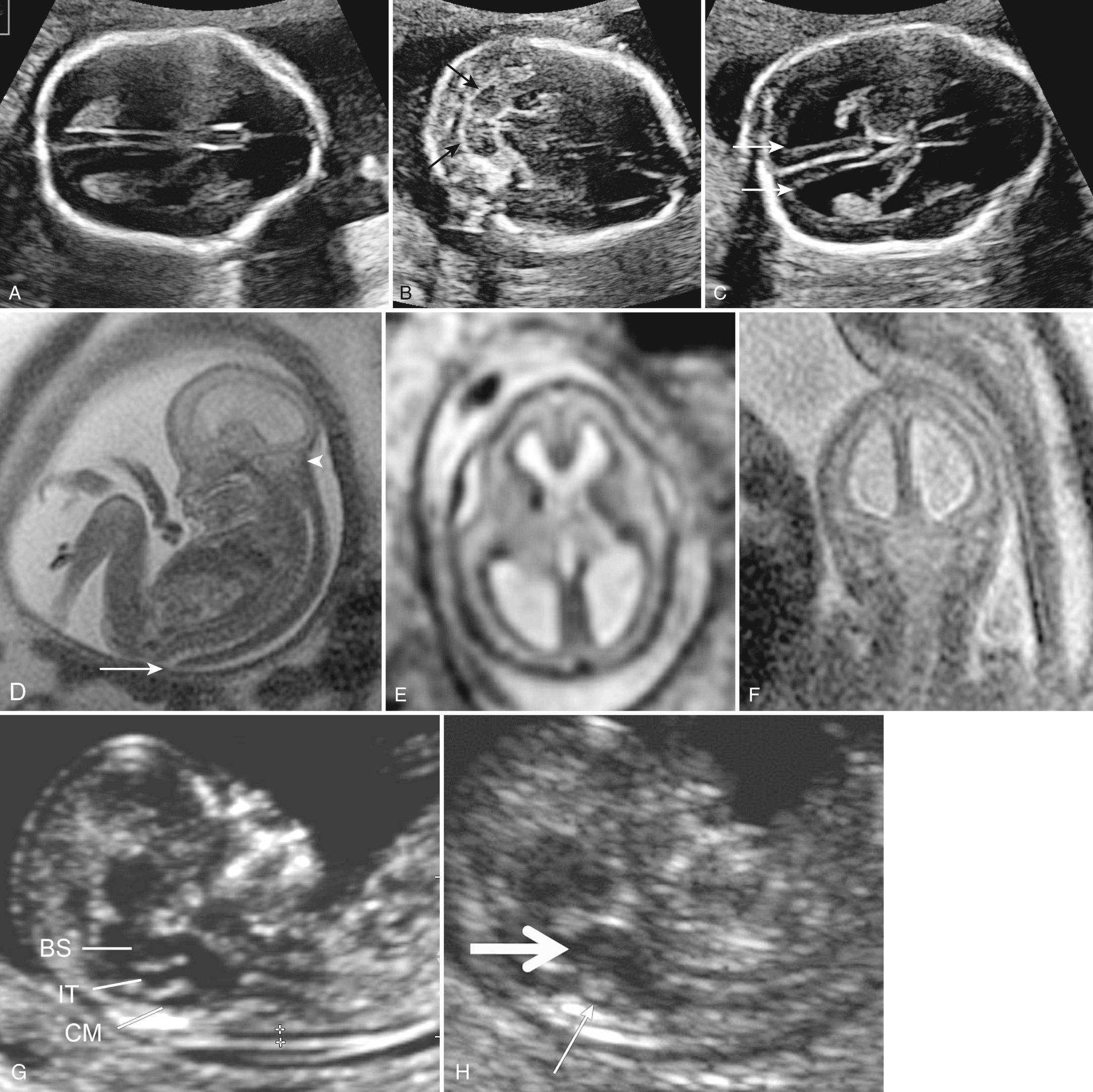
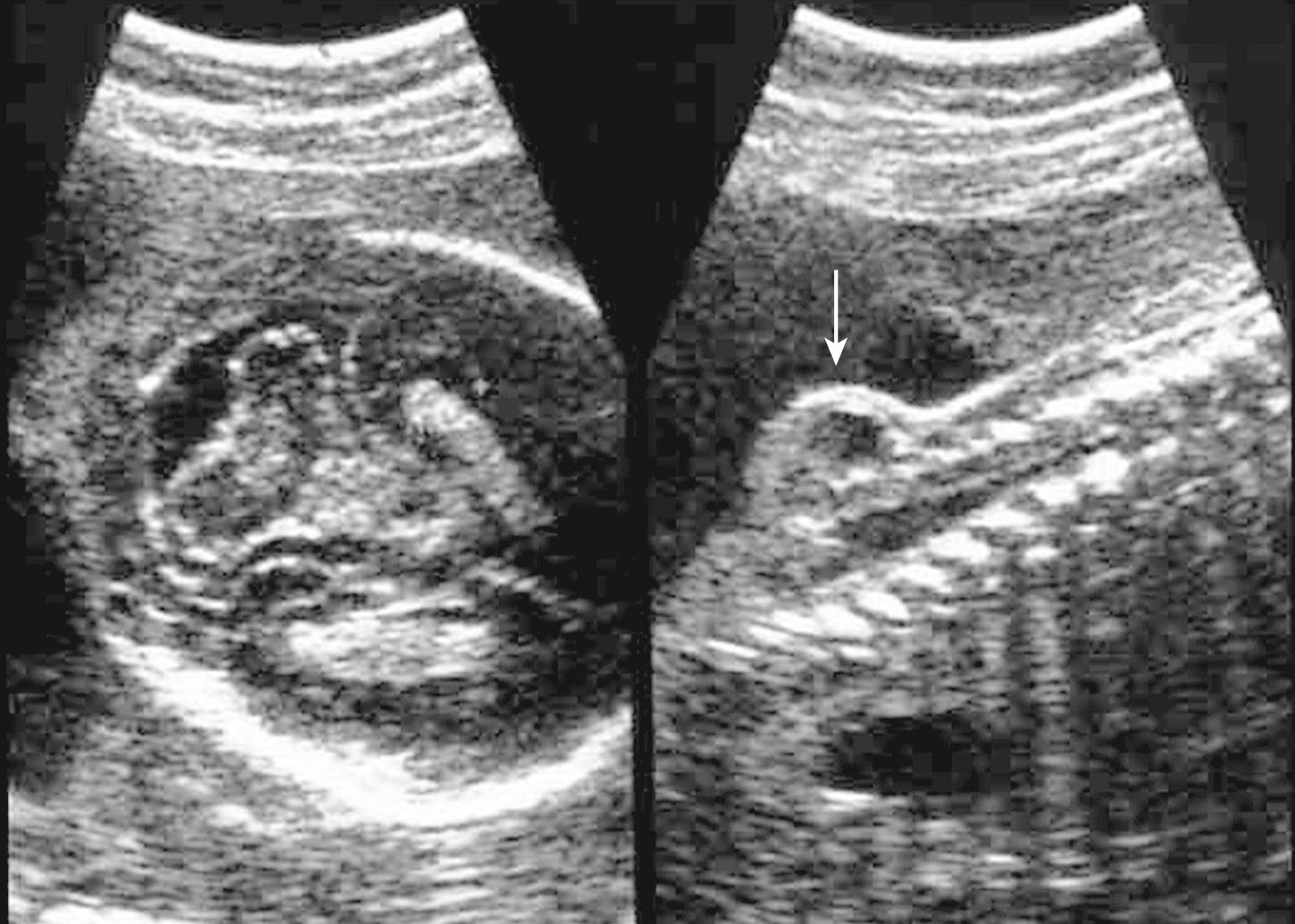
Many second-trimester cranial signs of spina bifida have been suggested ( Table 34.3 ). The most commonly used are obliteration or effacement of the cisterna magna, the “lemon sign” (bifrontal indentation), the “banana sign” (cerebellum wrapped around brainstem) and VM exceeding 10 mm. Less frequently used second-trimester signs include small BPD and head circumference (even if VM is present), funneling of the posterior fossa as measured by a small clivus-supraocciput angle, and pointing of the occipital horn. Some caveats to these signs bear attention. The level or extent of the spina bifida does not relate to the cranial signs. The lemon sign is seen more commonly before 24 weeks (5%-100%) but in only 13% after 24 weeks. It is less pronounced in fetuses with abnormal chromosomes and can also be seen in about 1% of normal fetuses. The banana sign and VM can worsen over the course of gestation.
| Finding | Frequency | Comments |
|---|---|---|
| Effacement of cisterna magna | 95%-100% | From about 14 weeks onward |
| Bifrontal indentation (“lemon sign”) | 53%-100% | 14-24 weeks, disappears later. Also seen in 1% of normal fetuses and in other conditions such as skeletal dysplasia |
| Cerebellar “banana” sign | 62%-97% | Cerebellum wrapped around brainstem |
| Ventriculomegaly > 10 mm | 46%-86% | More common after 24 weeks |
| Small BPD < 5th percentile | 26%-70% | |
| Small head circumference < 5% | 26%-70% | |
| Small cerebellum < 10% | 96% | |
| Funneling of posterior fossa | 96% | Clivus-supraocciput angle < 72 degrees |
| Pointed dilated occipital horns | 70% | Seen on axial view |
There are increasing efforts to screen for spina bifida with ultrasound at time of NT scan. Interestingly, the spinal defect has been detected as a dorsal abnormality as early as 9 weeks before head changes become apparent. After about 12 weeks, cranial changes may begin to be observed (see Fig. 34.14G-H , Table 34.4 ). These include decrease in head size, altered head shape, and alterations in the posterior fossa including posterior displacement of the midbrain and cerebral peduncles, thickening of the brainstem, and loss of fluid spaces in the cisterna magna and fourth ventricle. A recent prospective study of markers in the first trimester by highly trained operators using IT and cisterna magna appearances found positive or suspicious findings in 11 of 11 cases (100%). Management in first-trimester cases differs from management during the second trimester because the indirect cranial findings are only suggestive, and direct confirmation of the spinal defect in the first trimester is difficult. The sensitivity, specificity, and false-positive rate of these signs have not been established. It is prudent to reassess suspected cases at 15 to 16 weeks when spinal abnormalities and more specific cranial findings become more confidently detectable.
| Sign | Sensitivity | Comments |
|---|---|---|
| DIRECT SIGN | ||
| Spinal abnormality (meningocele, kyphoscoliosis) | 50%-60% | 9 weeks onward Dorsal mass or irregularity apparent before cranial changes |
| INDIRECT SIGNS | ||
| BPD plus biomarkers | 70% | BPD small, AFP elevated |
| BPD/abdominal diameter ratio ≤1 | 69% | |
| BPD below 5th percentile | 50%-56% | |
| Frontomaxillary angle below 5% for CRL | 90% | Sloping forehead appearance |
| “Lemon sign” or “acorn sign” | ? | Similar to “lemon sign” in midtrimester |
| Brainstem/occipital bone ratio | 87%-100% | Decreased with spina bifida |
| Brainstem diameter above 95th percentile | 96.7% | Brainstem diameter is increased |
| Four-line view observed | 67 | The normal four lines demarcating the anterior and posterior brainstem, fourth ventricle, and occipital bone are not seen |
| Parallel cerebral peduncles or posterior position of brainstem | ? | Seen on axial view below BPD level |
| Absent IT | 18% | |
| Absent cisterna magna | 64% | Fluid not seen in cisterna magna |
| Prospective studies using IT, four-line view, BPD, IT/CM ratio, brainstem diameter, BSOB | 50%-100% | |
The investigation of fetuses suspected of having spinal abnormalities includes detailed evaluation for associated cranial and somatic abnormalities. Determination of the spinal level of the defect is important for functional prognosis regarding bowel and bladder function and ambulation. MRI may help demonstrate additional brain abnormalities including cerebral hypoplasia and migrational and callosal abnormalities. Karyotype testing should be performed because 7% to 16% have chromosomal abnormality even with apparently isolated NTDs.
Prognosis varies and depends on associated abnormalities. Overall survival is about 66% at 20 years. Prenatal surgery in selected cases is associated with additional maternal and prematurity risks related to the procedure. However, fetuses that undergo surgery have reduced hindbrain herniation, reduced need for ventricular shunting (40% vs. 82%), and improvement in neuromotor functions such as walking (42% vs. 21%), but no improvement in urinary function. Prognosis and quality of life in children with open NTDs relates to manifestations of Chiari II malformation including apnea, swallowing, lower cranial nerve function, and foramen magnum impaction. Other issues include spinal deformities, tethered cord, and progressive orthopedic problems. About 70% have an IQ above 80, half can live independently, and 25% to 38% are capable of active employment.
Errors of ventral induction occur in the rostral end of the embryo and result in brain abnormalities of the prosencephalon, mesencephalon, and rhombencephalon and usually also affect facial development. The prosencephalon is induced to develop through a process of ventral induction consisting of formation, cleavage, and development of midline structures and facial structures. Several developmental abnormalities can occur. Absence of prosencephalic formation is rare and includes aprosencephaly (absence of the prosencephalon) and atelencephaly (absence of the telencephalon). More common is failure of prosencephalic and adjacent facial patterning and cleavage resulting in incomplete separation of prosencephalon into two distinct hemispheres, associated with abnormalities of midline and facial structures manifesting as the different degrees of HPE.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here