Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The retina is the light-sensitive portion of the eye that contains the following: (1) the cones , which are responsible for color vision; and (2) the rods , which can detect dim light and are mainly responsible for black and white vision and vision in the dark. When either rods or cones are excited, signals are transmitted first through successive layers of neurons in the retina and, finally, into optic nerve fibers and the cerebral cortex. In this chapter, we explain the mechanisms whereby the rods and cones detect light and color and convert the visual image into optic nerve signals.
Figure 51-1 shows the functional components of the retina, which are arranged in layers or boundaries from the outside to the inside, as follows: (1) pigment layer; (2) photoreceptor layer containing rods and cones projecting to the pigment; (3) outer limiting membrane; (4) outer nuclear layer containing the cell bodies of the rods and cones; (5) outer plexiform layer; (6) inner nuclear layer; (7) inner plexiform layer; (8) ganglionic layer; (9) layer of optic nerve fibers; and (10) inner limiting membrane.

After light passes through the lens system of the eye and then through the vitreous humor, it enters the retina from the inside of the eye (see Figure 51-1 ); that is, it passes first through the ganglion cells and then through the plexiform and nuclear layers before it finally reaches the layer of rods and cones located all the way on the outer edge of the retina. This distance is a thickness of several hundred micrometers; visual acuity is decreased by this passage through such nonhomogeneous tissue. However, in the central foveal region of the retina, as discussed subsequently, the inside layers are pulled aside to decrease this loss of acuity.
The fovea is a minute area in the center of the retina, shown in Figure 51-2 ; it occupies a total area a little more than 1 square millimeter. It is especially capable of acute and detailed vision. The central fovea , only 0.3 millimeter in diameter, is composed almost entirely of cones. These cones have a special structure that aids their detection of detail in the visual image—that is, the foveal cones have especially long and slender bodies, in contradistinction to the much fatter cones located more peripherally in the retina. Also, in the foveal region, the blood vessels, ganglion cells, inner nuclear layer of cells, and plexiform layers are all displaced to one side rather than resting directly on top of the cones, which allows light to pass unimpeded to the cones.

Figure 51-3 is a diagrammatic representation of the essential components of a photoreceptor (either a rod or a cone). As shown in Figure 51-4 , the outer segment of the cone is conical in shape. In general, the rods are narrower and longer than the cones, but this is not always the case. In the peripheral portions of the retina, the rods are 2 to 5 micrometers in diameter, whereas the cones are 5 to 8 micrometers in diameter; in the central part of the retina, in the fovea, there are no rods, and the cones are slender and have a diameter of only 1.5 micrometers.
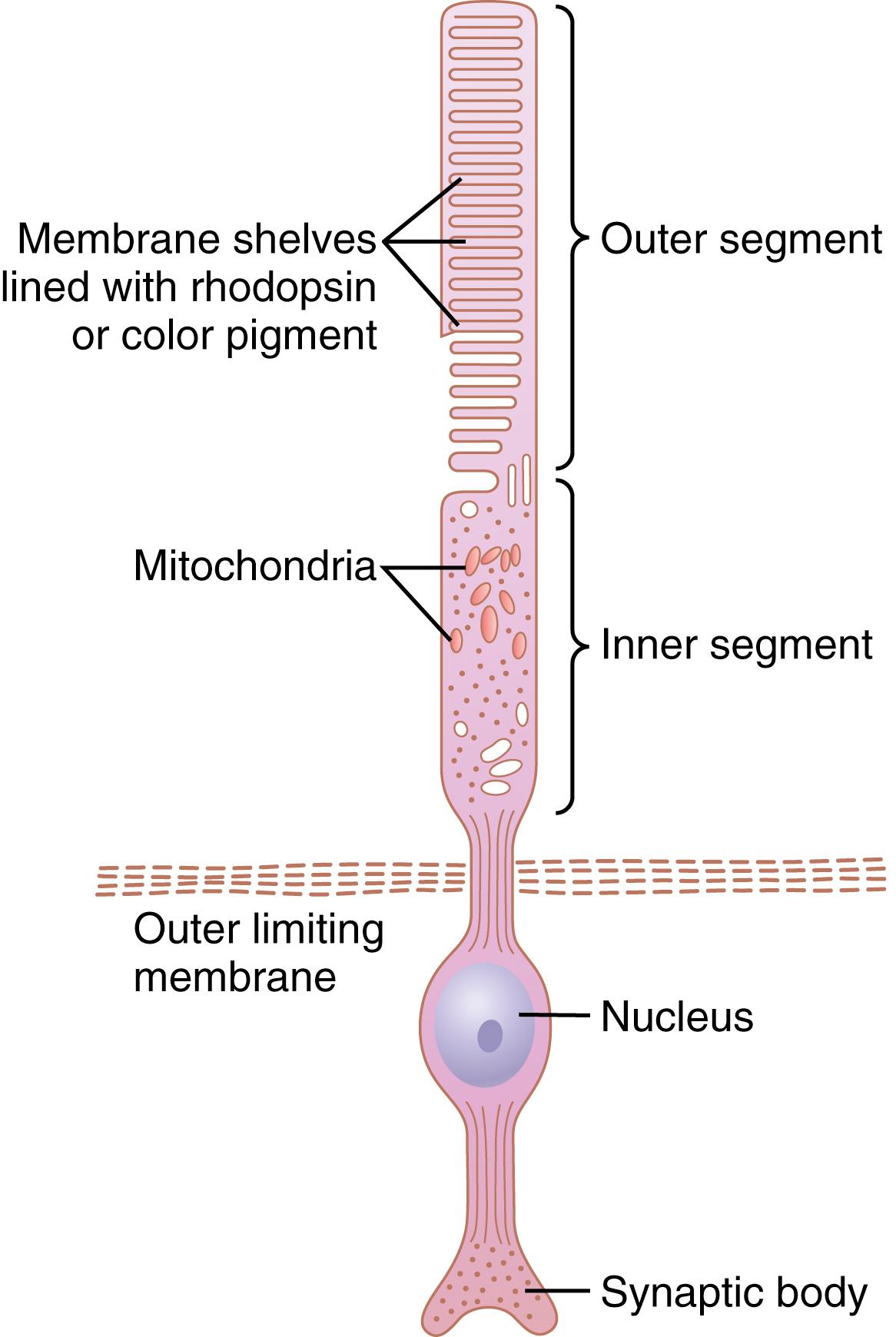

The major functional segments of either a rod or cone are shown in Figure 51-3 : (1) the outer segment; (2) the inner segment; (3) the nucleus; and (4) the synaptic body . The light-sensitive photochemical is found in the outer segment. In the case of the rods, this photochemical is rhodopsin ; in the cones, it is one of three “color” photochemicals, usually called simply color pigments, that function almost exactly the same as rhodopsin except for differences in spectral sensitivity.
In the outer segments of the rods and cones in Figures 51-3 and 51-4, note the large numbers of discs . Each disc is actually an infolded shelf of cell membrane. There are as many as 1000 discs in each rod or cone.
Both rhodopsin and the color pigments are conjugated proteins. They are incorporated into the membranes of the discs in the form of transmembrane proteins. The concentrations of these photosensitive pigments in the discs are so great that the pigments themselves constitute about 40% of the entire mass of the outer segment.
The inner segment of the rod or cone contains the usual cytoplasm, with cytoplasmic organelles. Especially important are the mitochondria, which, as explained later, play the important role of providing energy for function of the photoreceptors.
The synaptic body is the portion of the rod or cone that connects with subsequent neuronal cells, the horizontal and bipolar cells , which represent the next stages in the vision chain.
The black pigment melanin in the pigment layer prevents light reflection throughout the globe of the eyeball, which is extremely important for clear vision. This pigment performs the same function in the eye as the black coloring inside the bellows of a camera. Without it, light rays would be reflected in all directions in the eyeball and would cause diffuse lighting of the retina rather than the normal contrast between dark and light spots required to form precise images.
The importance of melanin in the pigment layer is well illustrated by its absence in people with albinism (congenital absence of melanin pigment in all parts of their bodies). When a person with albinism enters a bright room, light that impinges on the retina is reflected in all directions inside the eyeball by the unpigmented surfaces of the retina and by the underlying sclera, so a single discrete spot of light that would normally excite only a few rods or cones is reflected everywhere and excites many receptors. Therefore, the visual acuity of people with albinism, even with the best optical correction, is seldom better than 20/100 to 20/200 rather than the normal 20/20 values.
The pigment layer also stores large quantities of vitamin A. This vitamin A is exchanged back and forth through the cell membranes of the outer segments of the rods and cones, which are embedded in the pigment. We discuss later that vitamin A is an important precursor of the photosensitive chemicals of the rods and cones.
The nutrient blood supply for the internal layers of the retina is derived from the central retinal artery, which enters the eyeball through the center of the optic nerve and then divides to supply the entire inside retinal surface. Thus, the inner layers of the retina have their own blood supply, independent of the other structures of the eye.
However, the outermost layer of the retina is adherent to the choroid , which is also a highly vascular tissue lying between the retina and the sclera. The outer layers of the retina, especially the outer segments of the rods and cones, depend mainly on diffusion from the choroid blood vessels for their nutrition, especially for their oxygen.
The neural retina occasionally detaches from the pigment epithelium . In some cases, the cause of such detachment is injury to the eyeball that allows fluid or blood to collect between the neural retina and the pigment epithelium. Detachment is occasionally caused by contracture of fine collagenous fibrils in the vitreous humor, which pull areas of the retina toward the interior of the globe.
Partly because of diffusion across the detachment gap, and partly because of the independent blood supply to the neural retina through the retinal artery, the detached retina can resist degeneration for days and can become functional again if it is surgically replaced in its normal relation with the pigment epithelium. If it is not replaced soon, however, the retina will be destroyed and will be unable to function, even after surgical repair.
Both rods and cones contain chemicals that decompose on exposure to light and, in the process, excite the nerve fibers leading from the eye. The light-sensitive chemical in the rods is called rhodopsin ; the light-sensitive chemicals in the cones , called cone pigments or color pigments , have compositions only slightly different from that of rhodopsin.
In this section, we discuss principally the photochemistry of rhodopsin, but the same principles can be applied to the cone pigments.
The outer segment of the rod that projects into the pigment layer of the retina has a concentration of about 40% of the light-sensitive pigment called rhodopsin , or visual purple. This substance is a combination of the protein scotopsin and the carotenoid pigment retinal (also called “retinene”). Furthermore, the retinal is a particular type called 11- cis retinal. This cis form of retinal is important because only this form can bind with scotopsin to synthesize rhodopsin.
When light energy is absorbed by rhodopsin, the rhodopsin begins to decompose within a very small fraction of a second, as shown at the top of Figure 51-5 . The cause of this rapid decomposition is photoactivation of electrons in the retinal portion of the rhodopsin, which leads to instantaneous change of the cis form of retinal into an all- trans form that has the same chemical structure as the cis form but a different physical structure—it is a straight molecule rather than an angulated molecule. Because the three-dimensional orientation of the reactive sites of the all- trans retinal no longer fits with the orientation of the reactive sites on the protein scotopsin , the all- trans retinal begins to pull away from the scotopsin. The immediate product is bathorhodopsin , which is a partially split combination of the all- trans retinal and scotopsin. Bathorhodopsin is extremely unstable and decays in nanoseconds to lumirhodopsin. This product then decays in microseconds to metarhodopsin I , then in about a millisecond to metarhodopsin II , and finally, much more slowly (in seconds), into the completely split products scotopsin and all- trans retinal.

It is the metarhodopsin II, also called activated rhodopsin , that excites electrical changes in the rods, and the rods then transmit the visual image into the central nervous system in the form of optic nerve action potentials, as we discuss later.
The first stage in re-formation of rhodopsin, as shown in Figure 51-5 , is to reconvert the all- trans retinal into 11- cis retinal. This process requires metabolic energy and is catalyzed by the enzyme retinal isomerase. Once the 11- cis retinal is formed, it automatically recombines with the scotopsin to re-form rhodopsin, which then remains stable until its decomposition is again triggered by absorption of light energy.
Note in Figure 51-5 that there is a second chemical route whereby all- trans retinal can be converted into 11- cis retinal. This second route is by conversion of the all- trans retinal first into all- trans retinol, which is one form of vitamin A. Then, the all- trans retinol is converted into 11- cis retinol under the influence of the enzyme isomerase. Finally, the 11- cis retinol is converted into 11- cis retinal, which combines with scotopsin to form new rhodopsin.
Vitamin A is present both in the cytoplasm of the rods and in the pigment layer of the retina. Therefore, vitamin A is normally always available to form new retinal when needed. Conversely, when there is excess retinal in the retina, it is converted back into vitamin A, thus reducing the amount of light-sensitive pigment in the retina. We shall see later that this interconversion between retinal and vitamin A is especially important in long-term adaptation of the retina to different light intensities.
Night blindness occurs in persons with severe vitamin A deficiency because, without vitamin A, the amounts of retinal and rhodopsin that can be formed are severely depressed. This condition is called night blindness because the amount of light available at night is too little to permit adequate vision in vitamin A–deficient persons.
For night blindness to occur, a person usually must remain on a vitamin A–deficient diet for months, because large quantities of vitamin A are normally stored in the liver and can be made available to the eyes. Once night blindness develops, it can sometimes be reversed in less than 1 hour by intravenous injection of vitamin A.
Exposure of the rod to light causes increased negativity of the intrarod membrane potential, which is a state of hyperpolarization. This is exactly opposite to the decreased negativity (the process of “depolarization”) that occurs in almost all other sensory receptors.
How does activation of rhodopsin cause hyperpolarization? The answer is that when rhodopsin decomposes, it decreases the rod membrane conductance for sodium ions in the outer segment of the rod causing hyperpolarization.
Figure 51-6 shows movement of sodium and potassium ions in a complete electrical circuit through the inner and outer segments of the rod. The inner segment continually pumps sodium from inside the rod to the outside, and potassium ions are pumped to the inside of the cell. Potassium ions leak out of the cell through nongated potassium channels that are confined to the inner segment of the rod. As in other cells, this sodium-potassium pump creates a negative potential on the inside of the entire cell. However, the outer segment of the rod, where the photoreceptor discs are located, is entirely different. Here, the rod membrane, in the dark state, is leaky to sodium ions that flow through cyclic guanosine monophosphate (cGMP)–gated channels. In the dark state, cGMP levels are high, permitting positively charged sodium ions to continually leak back to the inside of the rod and thereby neutralize much of the negativity on the inside of the entire cell. Thus, under normal dark conditions, when the rod is not excited, there is reduced electronegativity inside the membrane of the rod, measuring about −40 millivolts rather than the usual −70 to −80 millivolts found in most sensory receptors.

When the rhodopsin in the outer segment of the rod is exposed to light, it is activated and begins to decompose. The cGMP-gated sodium channels are then closed, and the outer segment membrane conductance of sodium to the interior of the rod is reduced by a three-step process ( Figure 51-7 ): (1) light is absorbed by the rhodopsin, causing photoactivation of the electrons in the retinal portion, as previously described; (2) the activated rhodopsin stimulates a G protein called transducin , which then activates cGMP phosphodiesterase, an enzyme that catalyzes the breakdown of cGMP to 5′-GMP; and (3) the reduction in cGMP closes the cGMP-gated sodium channels and reduces the inward sodium current. Sodium ions continue to be pumped outward through the membrane of the inner segment. Thus, more sodium ions now leave the rod than leak back in. Because they are positive ions, their loss from inside the rod creates increased negativity inside the membrane, and the greater the amount of light energy striking the rod, the greater the electronegativity becomes—that is, the greater is the degree of hyperpolarization. At maximum light intensity, the membrane potential approaches −70 to −80 millivolts, which is near the equilibrium potential for potassium ions across the membrane.

Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here