Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The combination of gray-scale, color-flow Doppler, and Doppler spectral analysis is highly accurate in determining plaque characterization and degree of carotid stenosis.
Accurate diagnosis of carotid stenosis is critical for patients who would benefit from surgical and interventional treatment.
Clinicians can accurately follow changes in noncritical carotid stenosis or plaque using ultrasound.
The assessment of the vertebral arteries is an integral component of the carotid ultrasound examination. However, the degree of stenosis of the vertebral arteries cannot be accurately assessed.
Carotid atherosclerotic plaque with resultant stenosis usually involves the internal carotid artery within 2 cm of the carotid bifurcation.
Homogenous plaque, which is stable, has uniform echo pattern with a smooth surface. The amount of sonolucency is less than 50%.
Heterogeneous plaque, which can be unstable, has a more complex echo pattern with sonolucent areas of more than 50%.
Either the consensus table by the Society of Radiologists in Ultrasound or other standard reporting tables and criteria can be used to grade carotid stenosis as long as there is appropriate quality outcome feedback for accuracy.
Stroke secondary to atherosclerotic disease is the third leading cause of death in the United States. Many stroke victims survive the catastrophic event with some degree of neurologic impairment depending on collateral flow. Annually, stroke kills more than 130,000 people in the United States with an incidence of more than 795,000 cases of cerebrovascular accident (CVA). Ischemia from severe, flow-limiting stenosis caused by atherosclerotic disease involving the extracranial carotid arteries is implicated in 20% to 30% of strokes with a decreasing incidence due to improved control of hypertension and hyperlipidemia with medications. An estimated 80% of CVAs are thromboembolic in origin, often with carotid plaque as the embolic source. Cardioembolic stroke carries a higher risk of death, recurrent stroke, hospital readmission, and severe disability than other types of stroke.
Carotid atherosclerotic plaque with resultant stenosis usually involves the internal carotid artery (ICA) within 2 cm of the carotid bifurcation. This location is readily amenable to examination by sonography as well as surgical intervention. Carotid endarterectomy (CEA) initially proved to be more beneficial than medical therapy in symptomatic patients with carotid stenoses of more than 70%, as reported in the North American Symptomatic Carotid Endarterectomy Trial (NASCET) and the European Carotid Surgery Trial (ECST).
Subsequent NASCET results for moderate stenoses have shown a net benefit for surgical intervention with carotid narrowing between 50% and 69% of vessel diameter. A 15.7% reduction in the 5-year ipsilateral stroke rate was seen in patients treated surgically versus 22.2% stroke reduction in those treated medically. These results are not as compelling as those for the higher degree of stenosis seen in the earlier NASCET trial. The benefit from surgery was greatest in men, patients with recent stroke, and those with hemispheric symptoms. In addition, the NASCET trials dealing with moderate carotid stenoses required rigorous surgical expertise, such that the risks for disabling stroke or death should not exceed 2% to achieve the statistical surgical benefit. The Asymptomatic Carotid Atherosclerosis Study (ACAS) trials published in 1995 reported a reduction in ipsilateral stroke in asymptomatic patients with greater than 60% ICA stenoses who undergo CEA. However, these results were less clear-cut than the NASCET trials. According to the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST), carotid artery stenting has been shown to be comparable to CEA with respect to rates of ipsilateral stroke and death. However, there are increased adverse effects in women and elevated stroke rates and deaths in older patients. With implementation of new medical management regimens—including aspirin, clopidogrel, statins, antihypertensive medications, diabetic management, smoking cessation, and lifestyle changes—future trials may change how carotid disease is treated.
Accurate diagnosis of carotid stenosis clearly is critical to identify patients who would benefit from surgical treatment. In addition, ultrasound can assess plaque morphology, such as determining heterogeneous or homogeneous plaque, known to be an independent risk factor for stroke and transient ischemic attack (TIA).
Carotid sonography is the principal screening method for suspected extracranial carotid atherosclerotic disease. Gray-scale examination, color Doppler, power Doppler, and pulsed Doppler imaging techniques are routinely employed in the evaluation of patients with neurologic symptoms and suspected extracranial cerebral disease. Ultrasound is an inexpensive, noninvasive, and highly accurate method of diagnosing carotid stenosis. Magnetic resonance angiography (MRA) and computed tomography angiography (CTA) are additional noninvasive screening tools for the identification of carotid bifurcation disease as well as for clarification of ultrasound findings. Angiography is often now reserved for those patients for whom the ultrasound or MRA was equivocal or inadequate.
Other carotid ultrasound applications include the evaluation of carotid bruits, monitoring the progression of known atherosclerotic disease, assessment during or after CEA or stent placement, screening before major vascular surgery, and evaluation after the detection of retinal cholesterol emboli. Also, nonatherosclerotic carotid diseases can be evaluated, including follow-up of carotid dissection, examination of fibromuscular dysplasia or Takayasu arteritis, assessment of malignant carotid artery invasion, and workup of pulsatile neck masses and carotid body tumors.
Evaluation of patients with hemispheric neurologic symptoms, including stroke, transient ischemic attack, and amaurosis fugax
Evaluation of patients with a carotid bruit
Evaluation of pulsatile neck masses
Evaluation of patients scheduled for major cardiovascular surgical procedures
Evaluation of nonhemispheric or unexplained neurologic symptoms
Follow-up of patients with proven carotid disease
Evaluation of patients after carotid revascularization, including stenting
Intraoperative monitoring of vascular surgery
Evaluation of suspected subclavian steal syndrome
Evaluation of a potential source of retinal emboli
Follow-up of carotid dissection
Follow-up of radiation therapy to the neck in select patients
The first major branch of the aortic arch is the innominate or brachiocephalic artery, which divides into the right subclavian artery and right common carotid artery (CCA). The second major branch is the left CCA, which is generally separate from the third major branch, the left subclavian artery ( Fig. 26.1 ).
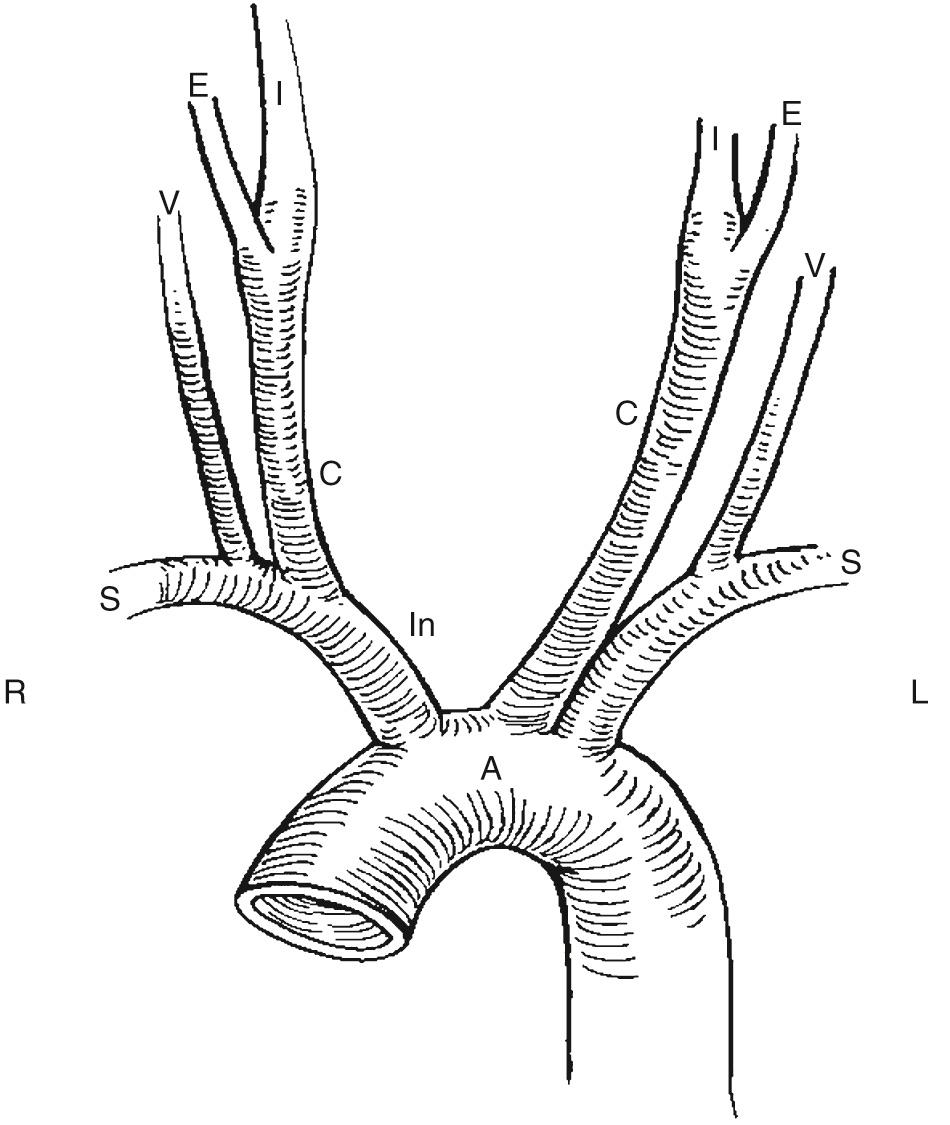
The right and left CCAs ascend into the neck posterolateral to the thyroid gland and lie deep to the jugular vein and sternocleidomastoid muscle. The CCAs have different proximal configurations, with the right originating at the bifurcation of the innominate (brachiocephalic) artery into the common carotid and subclavian arteries. The left CCA usually originates directly from the aortic arch but often arises with the brachiocephalic trunk. This is known as a “bovine arch” configuration. The CCA usually has no branches in its cervical region. Occasionally, however, it may give off the superior thyroid artery, vertebral artery, ascending pharyngeal artery, and occipital or inferior thyroid artery. At the carotid bifurcation, the CCA divides into the external carotid artery (ECA) and the internal carotid artery (ICA). The ICA usually has no branching vessels in the neck. The ECA, which supplies the facial musculature, has multiple branches in the neck. The ICA may demonstrate an ampullary region of mild dilation just beyond its origin.
Carotid artery ultrasound examinations are performed with the patient supine, the neck slightly extended, and the head turned away from the side being examined. Some operators prefer to perform the examination at the patient's side, whereas others prefer to sit at the patient's head. The examination sequence also varies with operator preference. This sequence includes the gray-scale examination, Doppler spectral analysis, and color Doppler blood flow interrogations. Power Doppler sonography may or may not be employed. A 5- to 12-MHz transducer is used for gray-scale imaging and a 3- to 7-MHz transducer for Doppler sonography; the choice depends on the patient's body habitus and technical characteristics of the ultrasound machine. Color Doppler flow imaging and power Doppler imaging may be performed with 5- to 10-MHz transducers. In cases of critical stenosis, the Doppler parameters should be optimized to detect extremely slow flow.
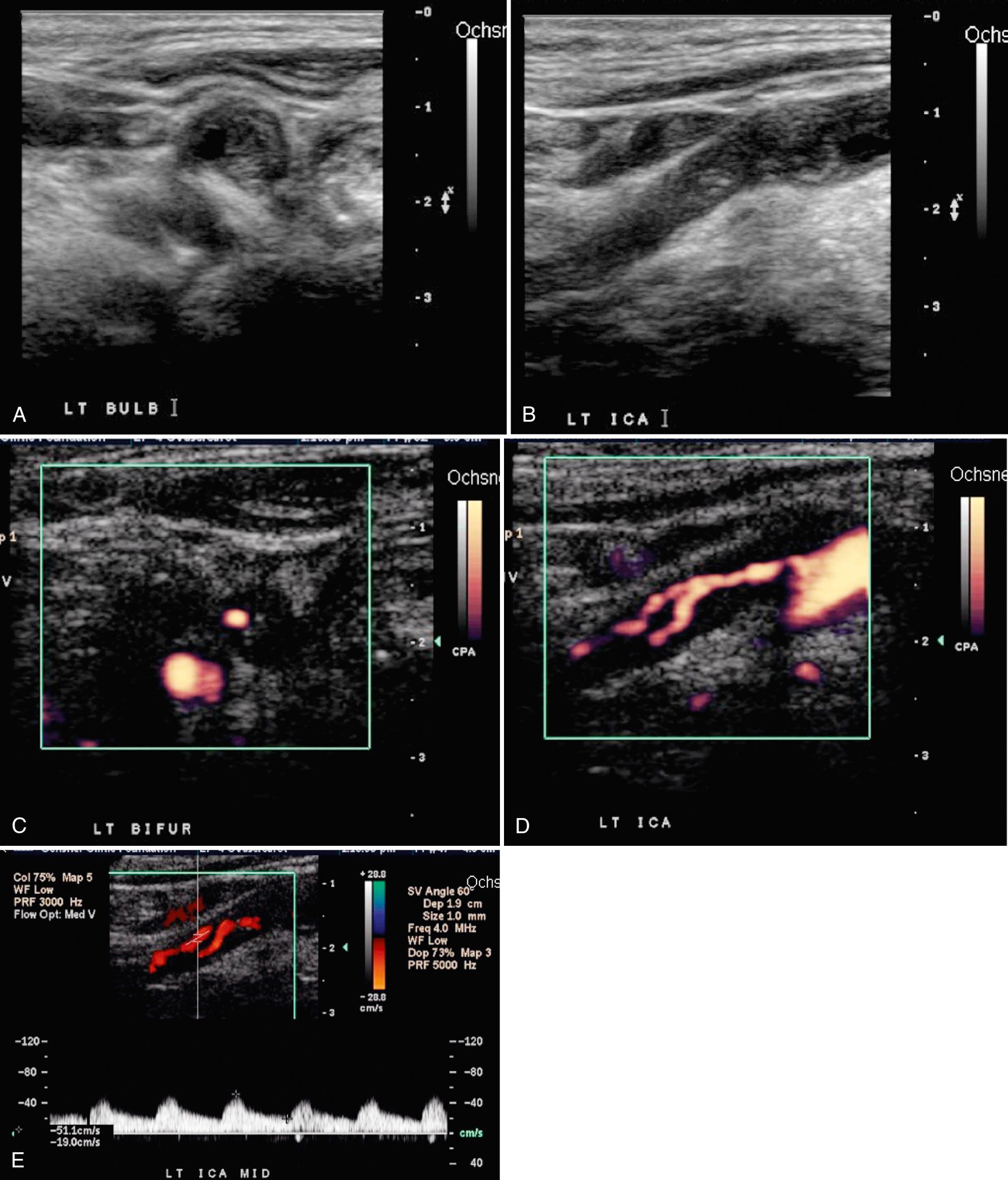
Gray-scale sonographic examination begins in the transverse projection. Scans are obtained along the entire course of the cervical carotid artery, from the supraclavicular notch cephalad to the angle of the mandible ( Fig. 26.2 ). Inferior angulation of the transducer in the supraclavicular area images the CCA origin. The left CCA origin is deeper and more difficult to image consistently than the right. The carotid bulb is identified as a mild widening of the CCA near the bifurcation. Transverse views of the carotid bifurcation establish the orientation of the external and internal carotid arteries and help define the optimal longitudinal plane in which to perform Doppler spectral analysis (Video 26.1). When the transverse ultrasound images demonstrate occlusive atherosclerotic disease, the percentage of “diameter stenosis” or “area stenosis” can be calculated directly using electronic calipers and software analytic algorithms available on most duplex equipment.
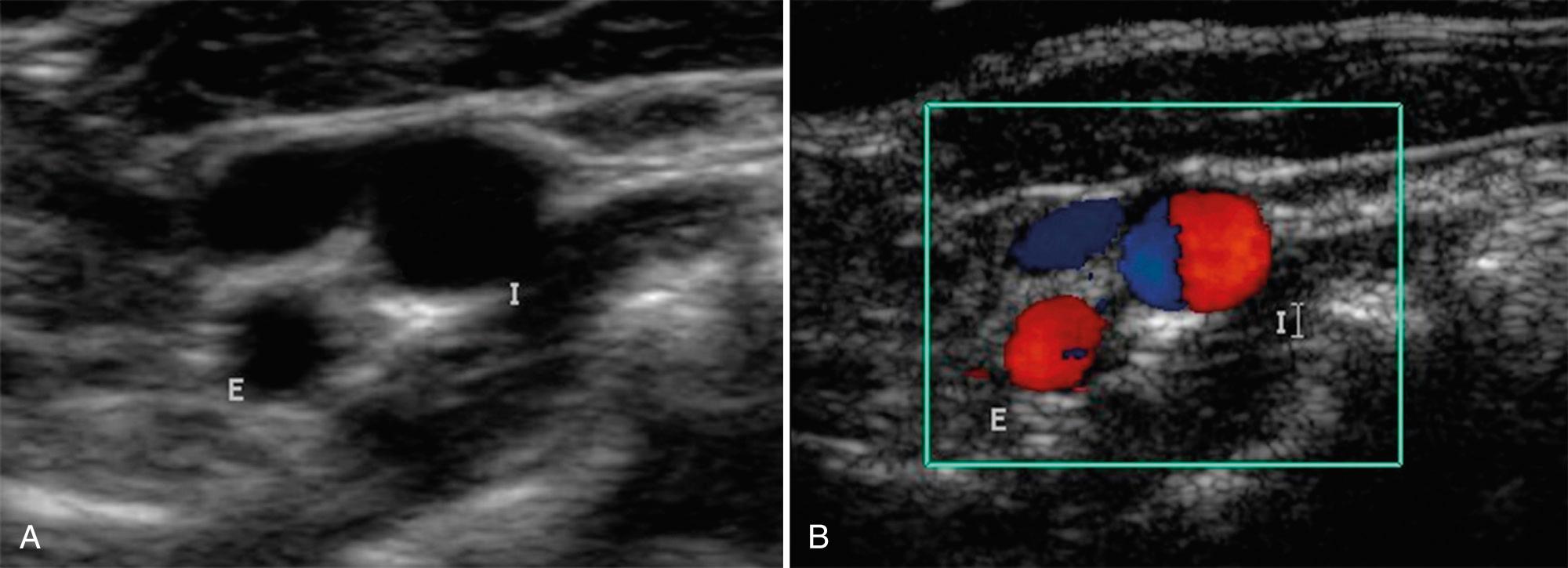
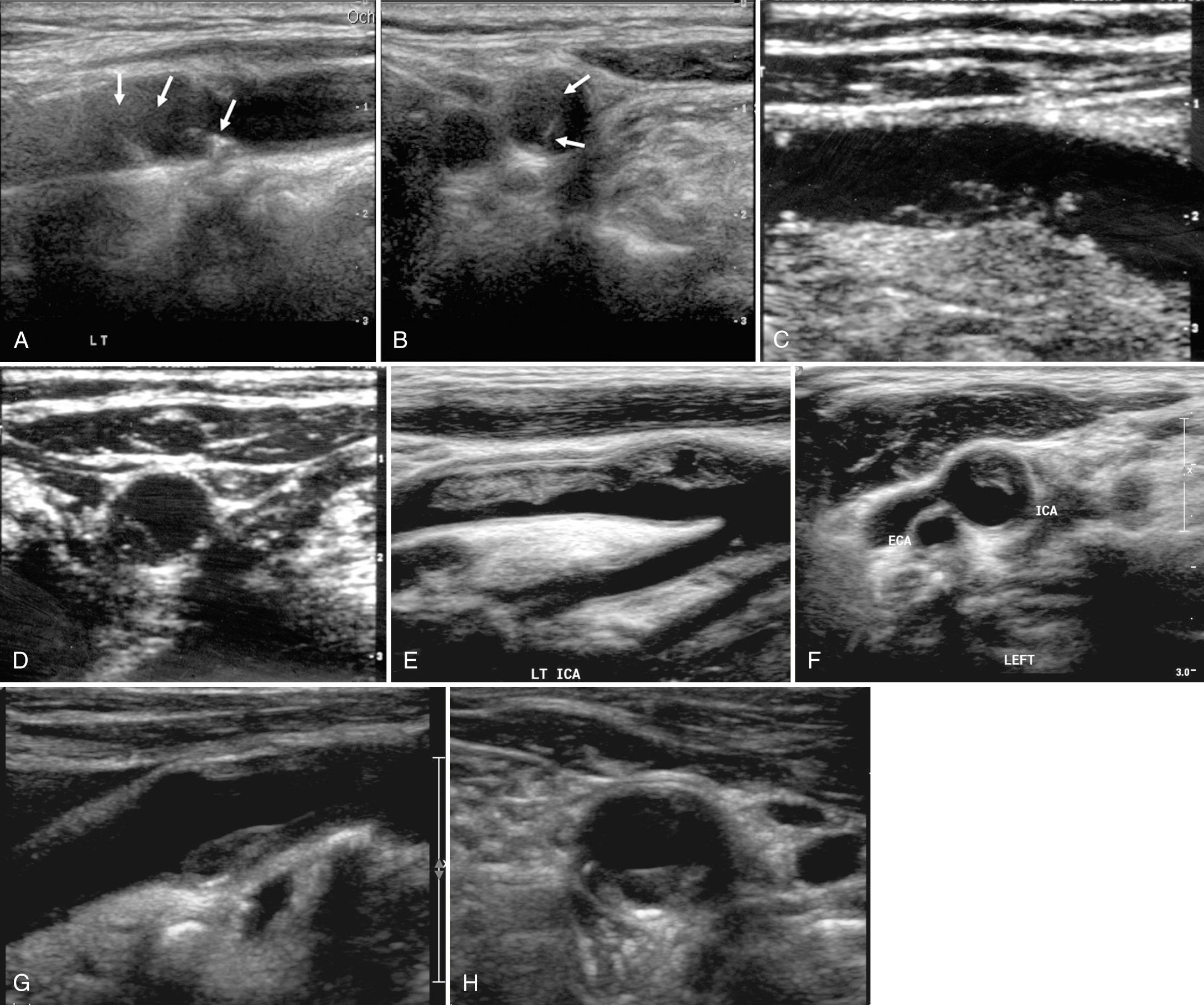
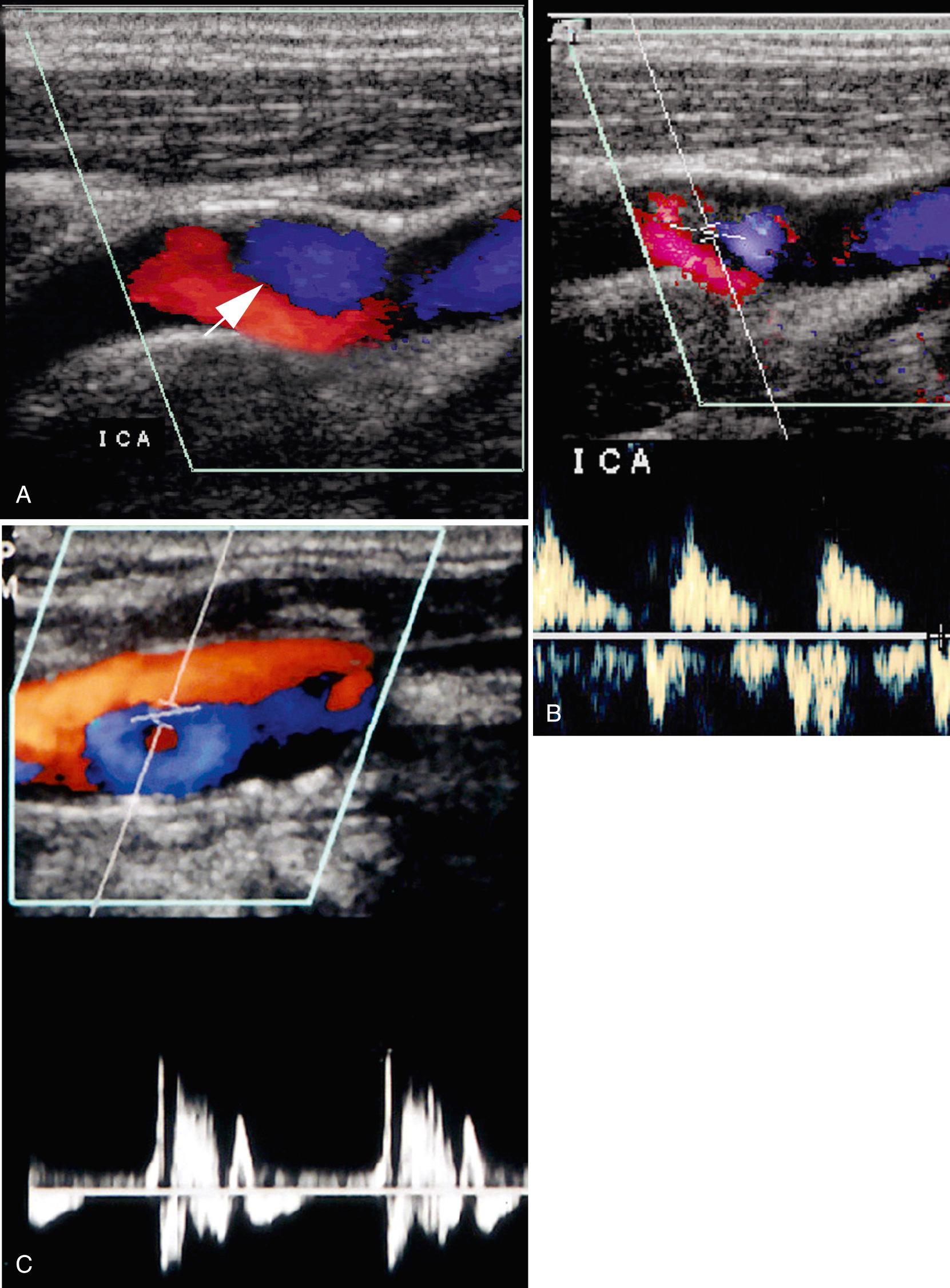
After transverse imaging, longitudinal scans of the carotid artery are obtained. The examination plane necessary for optimal longitudinal scans is determined by the course of the vessels demonstrated on the transverse study. In some patients, the optimal longitudinal orientation will be nearly coronal, whereas in others it will be almost sagittal. In most cases, the optimal longitudinal scan plane will be oblique, between sagittal and coronal. In approximately 60% of patients, both vessels above the carotid bifurcation and the CCA can be imaged in the same plane ( Fig. 26.3 ); in the remainder, only a single vessel will be imaged in the same plane as the CCA. Images are obtained to display the relationship of both branches of the carotid bifurcation to the visualized plaque disease, and the cephalocaudal extent of the plaque is measured. Several anatomic features differentiate the ICA from the ECA. In about 95% of patients, the ICA is posterior and lateral to the ECA. This may vary considerably, and the ICA may be medial to the ECA in 3% to 9% of people. The ICA frequently has an ampullary region of dilation just beyond its origin and is usually larger than the ECA.
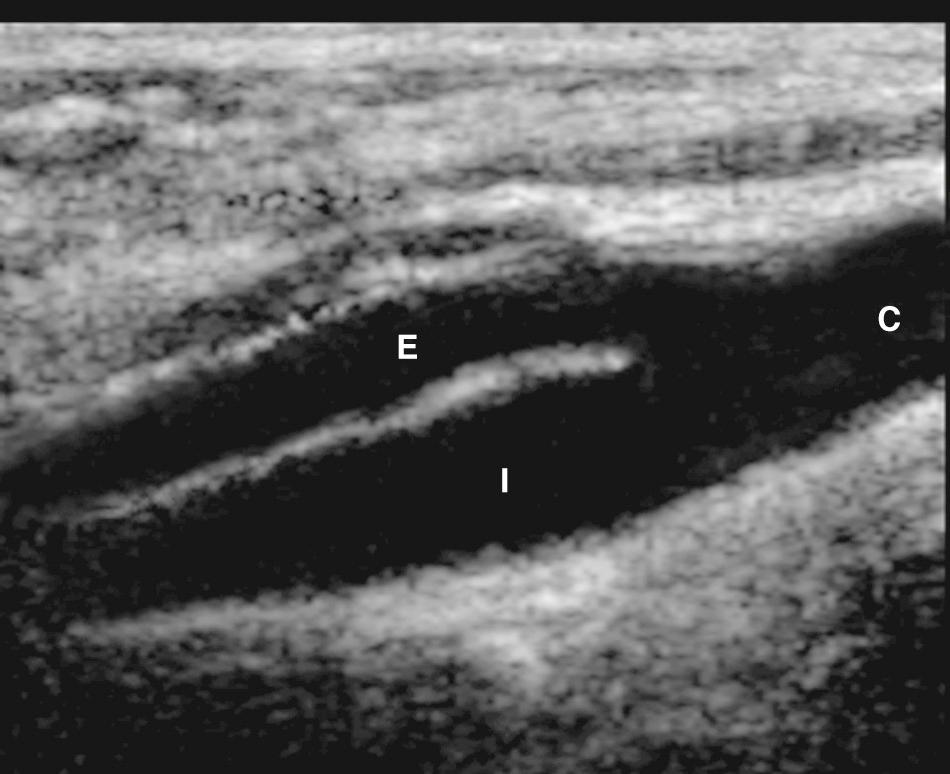
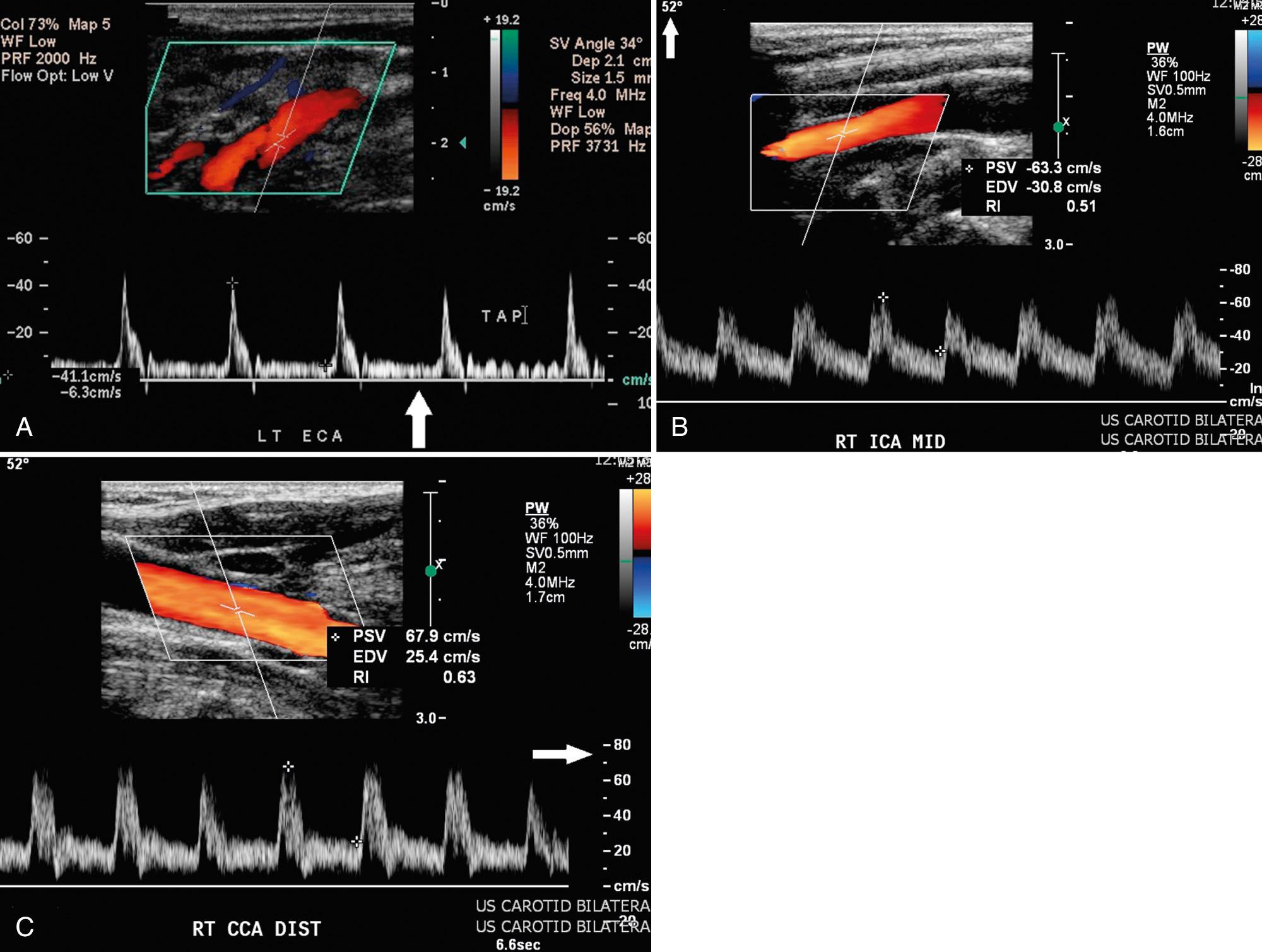
One reliable distinguishing feature of the ECA is that it has branching vessels ( Fig. 26.4A ). A useful method to identify the ECA is the tapping of the ipsilateral superficial temporal artery in the preauricular area, the temporal tap. The pulsations are transmitted back to the ECA where they cause a sawtooth appearance on the spectral waveform ( Fig. 26.4B ). Although the tap helps identify the ECA, this tap deflection may be transmitted into the CCA and even the ICA in certain rare situations.

The superior thyroid artery is often seen as the first branch of the ECA after the bifurcation of the CCA. Occasionally, an aberrant superior thyroid artery branch will arise from the distal CCA. The ICA usually has no branches in the neck, although in rare cases the ICA gives rise to the ascending pharyngeal, occipital, facial, laryngeal, or meningeal arteries. In some patients, a considerable amount of the ICA will be visible, but in others, only the immediate origin of the vessel will be accessible. Very rarely, the bifurcation may not be visible at all. Rarely, the ICA may be hypoplastic or congenitally absent.
Each facet of the carotid sonographic examination is valuable in the final determination of the presence and extent of disease. In most cases, the gray-scale, color Doppler, and power Doppler sonographic images and assessments will agree. However, when there are discrepancies between Doppler ultrasound imaging findings and measured velocities, every attempt should be made to discover the source of the disagreement. The more closely the image and spectral Doppler findings correlate, the higher the degree of confidence in the diagnosis. Generally, gray-scale and color or power Doppler images better demonstrate and quantify low-grade stenoses, whereas high-grade occlusive disease is more accurately defined by Doppler spectral analysis. For plaque characterization, assessment must be made in gray-scale only, without color or power Doppler ultrasound.
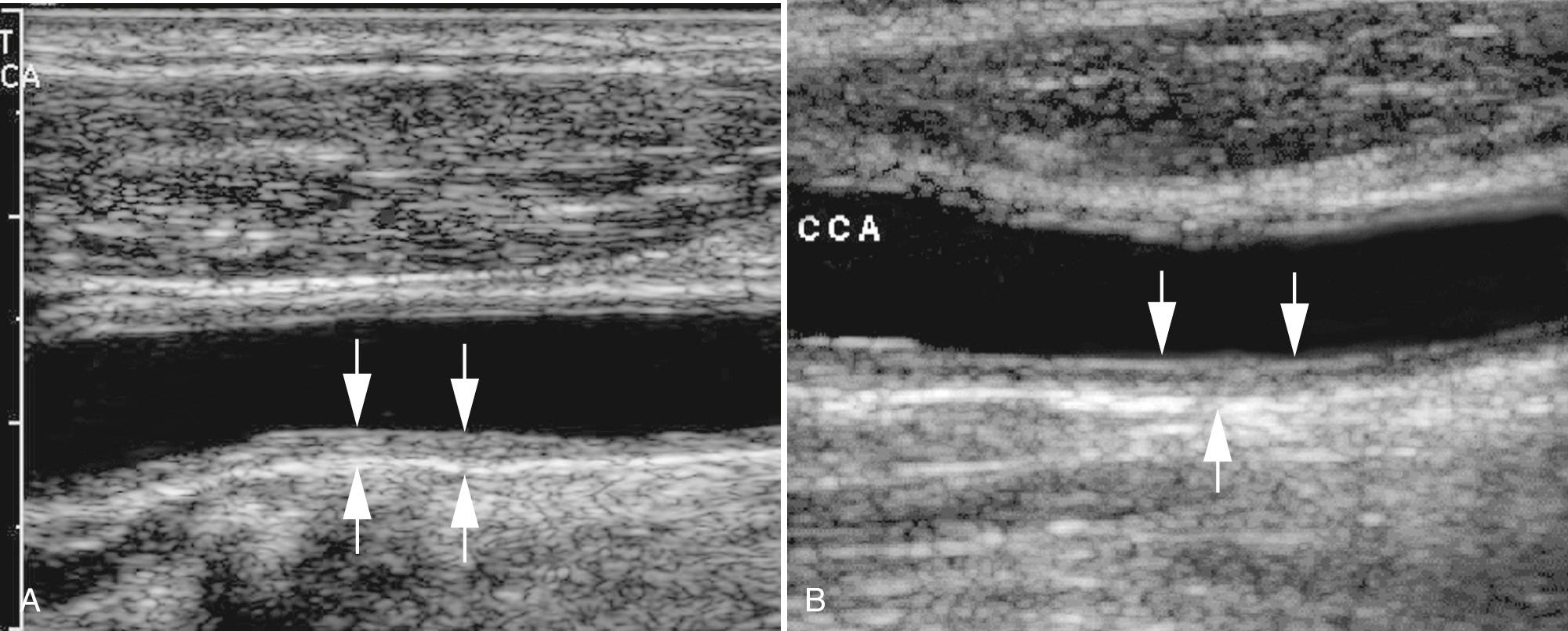
Longitudinal views of the layers of the normal carotid wall demonstrate two nearly parallel echogenic lines, separated by a hypoechoic to anechoic region ( Fig. 26.5 ). The first echo, bordering the vessel lumen, represents the lumen-intima interface; the second echo is caused by the media-adventitia interface. The media is the anechoic/hypoechoic zone between the echogenic lines. The distance between these lines represents the combined thickness of the intima and media (I-M complex). The far wall of the CCA is measured. Many consider measurement of intima-media thickness (IMT) to be a surrogate marker for atherosclerotic disease in the whole arterial system, not only the cerebrovascular system. Some believe that thickening of the I-M complex greater than 0.8 mm is abnormal and may represent the earliest changes of atherosclerotic disease. However, because thickness of the I-M increases with age, absolute measurements of IMT for any given person may not be a reliable indicator of atherosclerotic risk factors ( Fig. 26.6 ).
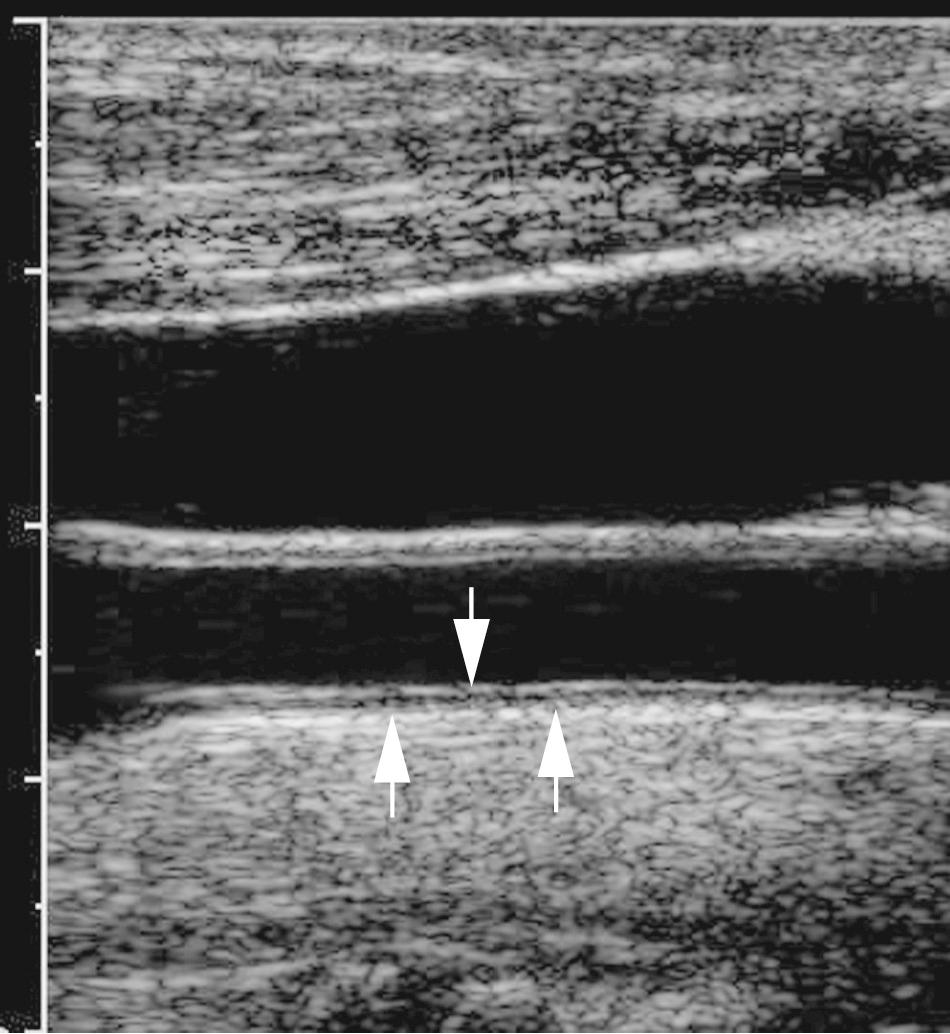
Carotid artery IMT is an independent predictor of new cardiovascular events in persons without a history of cardiovascular disease. Numerous studies support the relationship between IMT and increased risk for myocardial infarction or stroke in asymptomatic patient populations. Assessment of IMT has been advocated as a means of assessing effectiveness of medical interventions to reduce the progression of I-M thickening or even reverse carotid wall thickening. However, because of concerns regarding minimal impact on prediction models and difficulty in standardizing measurement technique, the 2013 American College of Cardiology/American Heart Association (ACC/AHA) Guideline on the Assessment of Cardiovascular Risk does not recommend the carotid IMT test.
Atheromatous carotid plaques should be carefully evaluated to determine plaque extent, location, surface contour, and texture, as well as to assess luminal stenosis. The plaque should be scanned and evaluated in both the sagittal and the transverse projections. The most common cause of TIAs is embolism, not flow-limiting stenosis; less than half of patients with documented TIA have hemodynamically significant stenosis. It is important to identify low-grade atherosclerotic lesions that may contain hemorrhage or ulceration, which can serve as a nidus for emboli that cause both TIAs and stroke. Polak et al. showed that plaque is an independent risk factor for developing a stroke. Of patients with hemispheric stroke symptoms, 50% to 70% demonstrate hemorrhagic or ulcerated plaque. Plaque analysis of CEA specimens has implicated intraplaque hemorrhage as an important factor in the development of neurologic symptoms. However, the relationship between sonographic plaque morphology and the onset of symptoms is controversial.
Currently, substantial gaps in knowledge of the mechanisms involved in atherosclerotic plaque rupture exist and this is a critical barrier to developing methodologies for the prevention of myocardial infarction and stroke. Myocardial infarction and stroke, complications of atherosclerosis, are the most common causes of death in developed countries and are caused by inflammation-driven rupture of atherosclerotic plaques. Stable plaques are characterized by a necrotic core with an overlying fibrous cap composed of vascular smooth muscle cells in a collagen-rich matrix. In vulnerable/ruptured plaques, the fibrous cap has thinned, exhibiting fewer vascular smooth muscle cells, decreased collagen, and increased inflammatory cells.
Heterogeneous plaque has been suggested as unstable and vulnerable, in contrast to homogeneous plaque. Some have suggested that environmental factors and toxins cause abnormal flow patterns, damaging the internal structure of the vessels. This can lead to intimal proliferation and potential wall ischemia and the degradation of the vasa vasorum. Because the vasa vasorum do not have a muscular media, these vessels are subsequently at risk to rupture, perhaps leading, in part, to intraplaque hemorrhage. Others have suggested that a bacterial infection etiology may play a role in inflammatory angiogenesis.
Recently, several groups have demonstrated that microRNAs play a key role in the development and thinning of the fibrous cap. MicroRNAs are a class of noncoding RNAs (ncRNAs) that are approximately 21 nucleotides in length and are potent effectors of gene expression, doing so by binding to messenger RNA and inhibiting protein translation. A role for ncRNA in cardiovascular diseases is emerging, including microRNAs (miRNAs) and their inhibition by circular RNA (circRNAs). MicroRNA-221 and microRNA-222 (miR-221/miR-222) are short ncRNAs that inhibit the expression of the cyclin-dependent kinase inhibitor p27 Kip1 , promoting vascular smooth muscle cell proliferation and intimal thickening. Bazan et al. recently demonstrated an important role for the downregulation of miR-221/222 in the shoulder region of carotid plaques shortly after rupture through increased p27 Kip1 and propose that vascular smooth muscle cell volume is lost, leading to intimal thinning.
Plaque texture is generally classified as homogeneous or heterogeneous. The accurate evaluation of plaque can only be made with gray-scale ultrasound, without the use of color or power Doppler. The plaque must be evaluated in both sagittal and transverse planes. Homogeneous plaque has a generally uniform echo pattern and a smooth surface ( Fig. 26.7 ). Sonolucent areas may be seen, but the amount of sonolucency is less than 50% of the plaque volume. The uniform acoustic texture corresponds pathologically to dense fibrous connective tissue (Videos 26.2 through ). Calcified plaque produces posterior acoustic shadowing and is common in asymptomatic individuals ( Fig. 26.8 , Video 26.5). Heterogeneous plaque has a more complex echo pattern and contains one or more focal sonolucent areas corresponding to more than 50% of the plaque volume ( Fig. 26.9 , Videos 26.6 through ). Heterogeneous plaque is characterized pathologically by containing intraplaque hemorrhage and deposits of lipid, cholesterol, and proteinaceous material. Homogeneous plaque is identified much more often than heterogeneous plaque, occurring in 80% to 85% of patients examined. Sonography accurately determines the presence or absence of intraplaque hemorrhage (sensitivity, 90%-94%; specificity, 75%-88%).
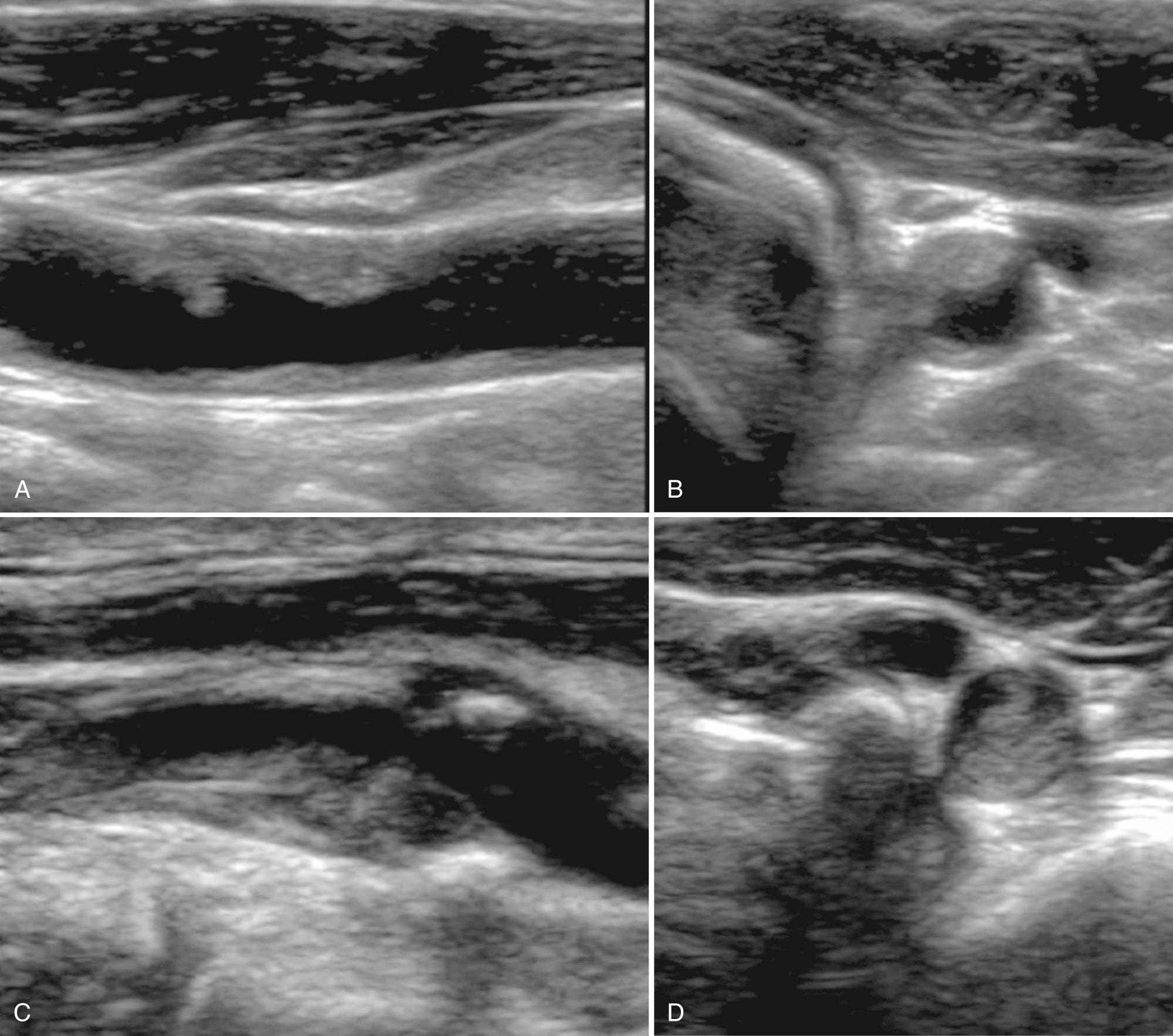
Some sources suggest classifying plaque according to four types. Plaque types 1 and 2, similar to heterogeneous plaque and much more likely to be associated with intraplaque hemorrhage and ulceration, are considered unstable and subject to abrupt increases in plaque size after hemorrhage or embolization. Types 1 and 2 plaque are typically found in symptomatic patients with stenoses greater than 70% of diameter. Plaque types 3 and 4 are generally composed of fibrous tissue and calcification. These plaque types are similar to homogeneous plaque. These are generally more benign, stable plaques typically seen in asymptomatic individuals (see Fig. 26.8 ).
Type 1: Predominantly echolucent, with a thin echogenic cap
Type 2: Substantially echolucent with small areas of echogenicity (>50% sonolucent)
Type 3: Predominantly echogenic with small areas of echolucency (<50% sonolucent)
Type 4: Uniformly echogenic
Other methods are being introduced to characterize plaque in a more automated and reproducible manner. Reiter et al. developed a gray-scale median level for echolucency of plaque after standardizing and adjusting the B-mode images. They obtained standardized gray-scale median levels for asymptomatic patients with greater than 30% stenosis and found that decreasing echolucency of carotid plaques over 6 to 9 months is predictive of major cardiovascular events affecting coronary, peripheral, and cerebrovascular circulation. However, absolute gray-scale median levels were not associated with a specific risk.
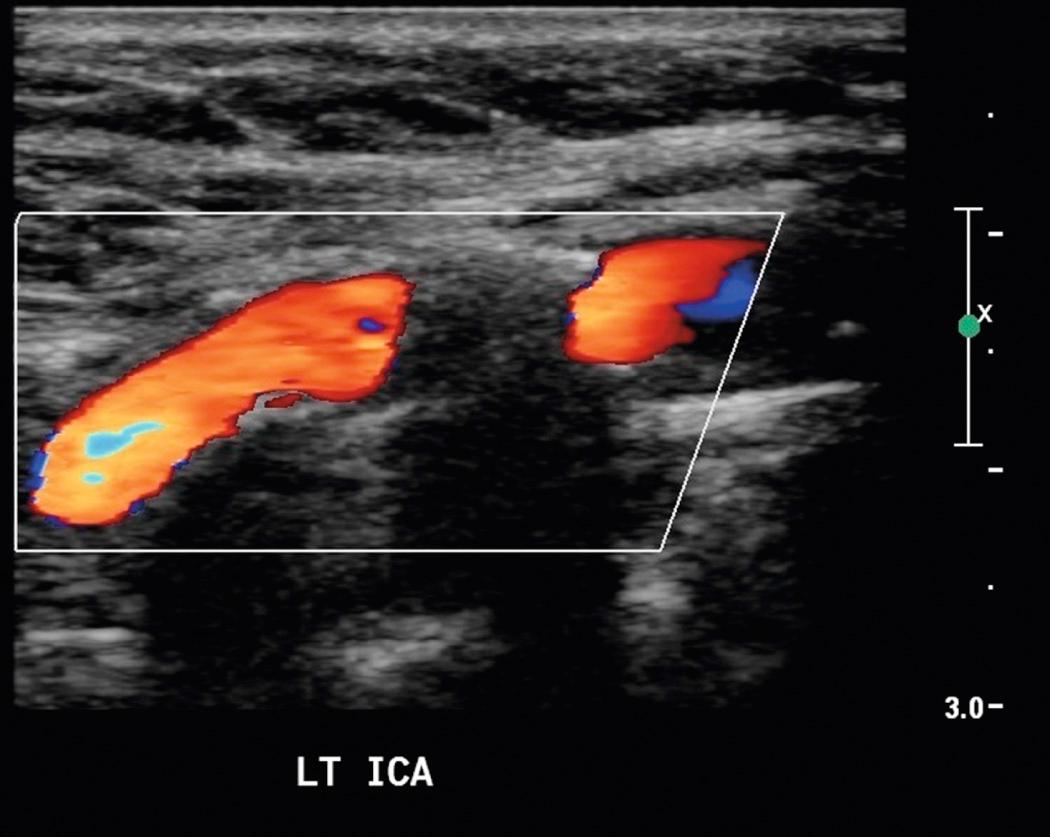
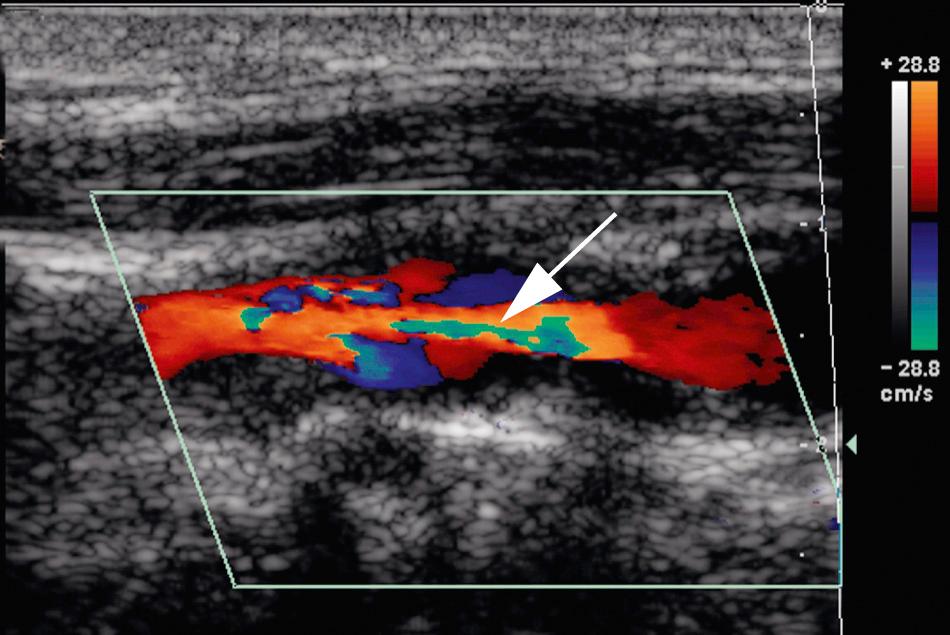
Sonography has a low sensitivity (38%) and high specificity (92%) for plaque ulceration. However, when identified, all ulcerated plaques fit into the heterogeneous plaque pattern. Sonographic findings that suggest plaque ulceration include a focal depression or break in the plaque surface, causing an irregular surface or an anechoic area within the plaque that extends to the plaque surface without an intervening echo between the vessel lumen and the anechoic plaque region. Color and power Doppler ultrasound may improve sonographic identification of plaque ulceration. Color or power Doppler ultrasound or B-flow imaging (a proprietary non-Doppler imaging technique) may demonstrate slow-moving eddies of color within an anechoic region in plaque, which would suggest ulceration ( Fig. 26.10 ). The demonstration of these flow vortices was 94% accurate in predicting ulcerative plaque at surgery in one study. Contrast-enhanced ultrasound represents a promising tool in the characterization of vulnerable carotid plaque with improved detection of intraplaque vascularization and ulceration.
Focal depression or break in plaque surface
Anechoic region within plaque extending to vessel lumen
Eddies of color within plaque
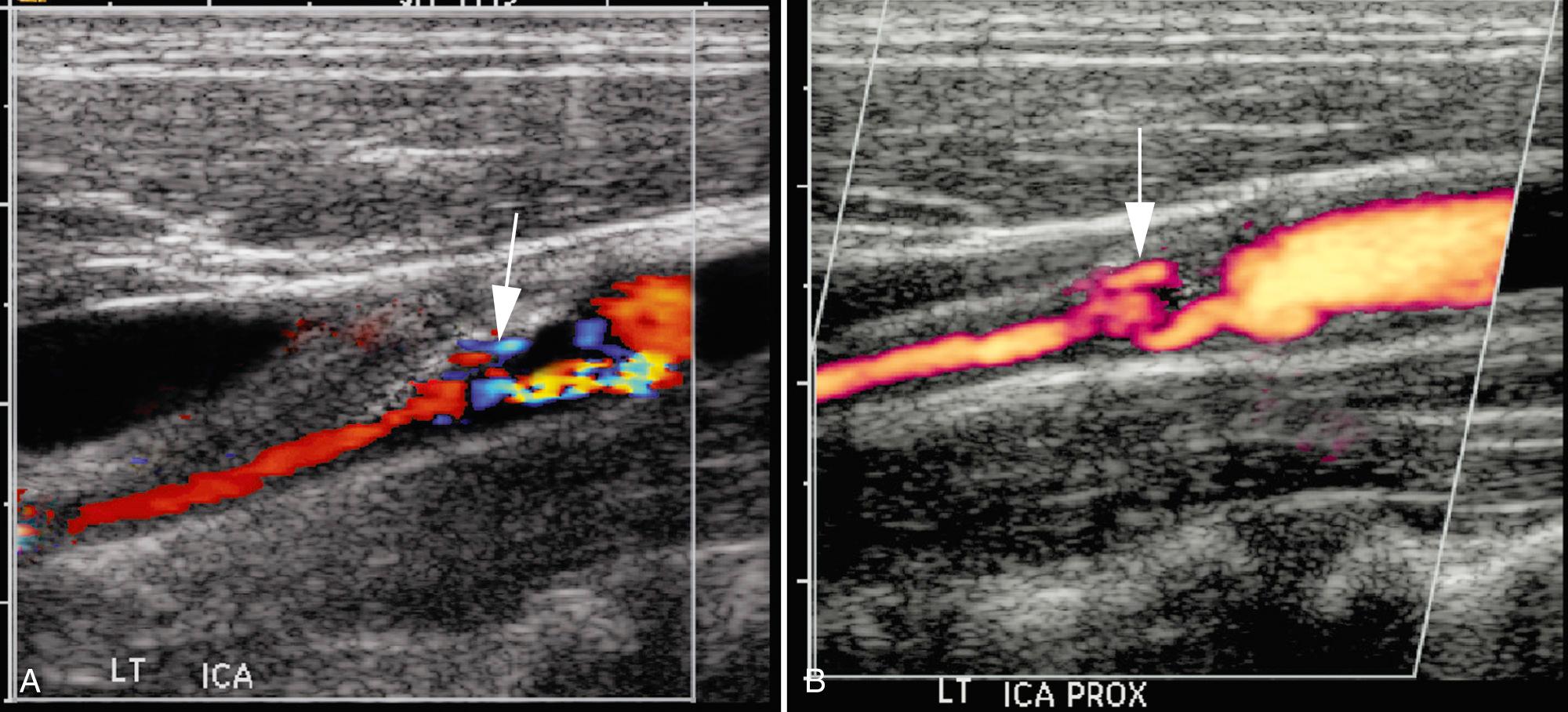
A potential pitfall in the diagnosis of plaque ulceration results from a mirror-image artifact producing pseudoulceration of the carotid artery. Highly reflective plaque can produce a color Doppler ghost artifact simulating ulceration. However, the region of color within the plaque can be recognized as artifactual because the spectral waveform and color shading within the pseudoulceration are of lower amplitude, but otherwise identical to those within the true carotid lumen. Conversely, pulsed Doppler traces from within ulcer craters show low-velocity dampened waveforms ( Fig. 26.11 ).
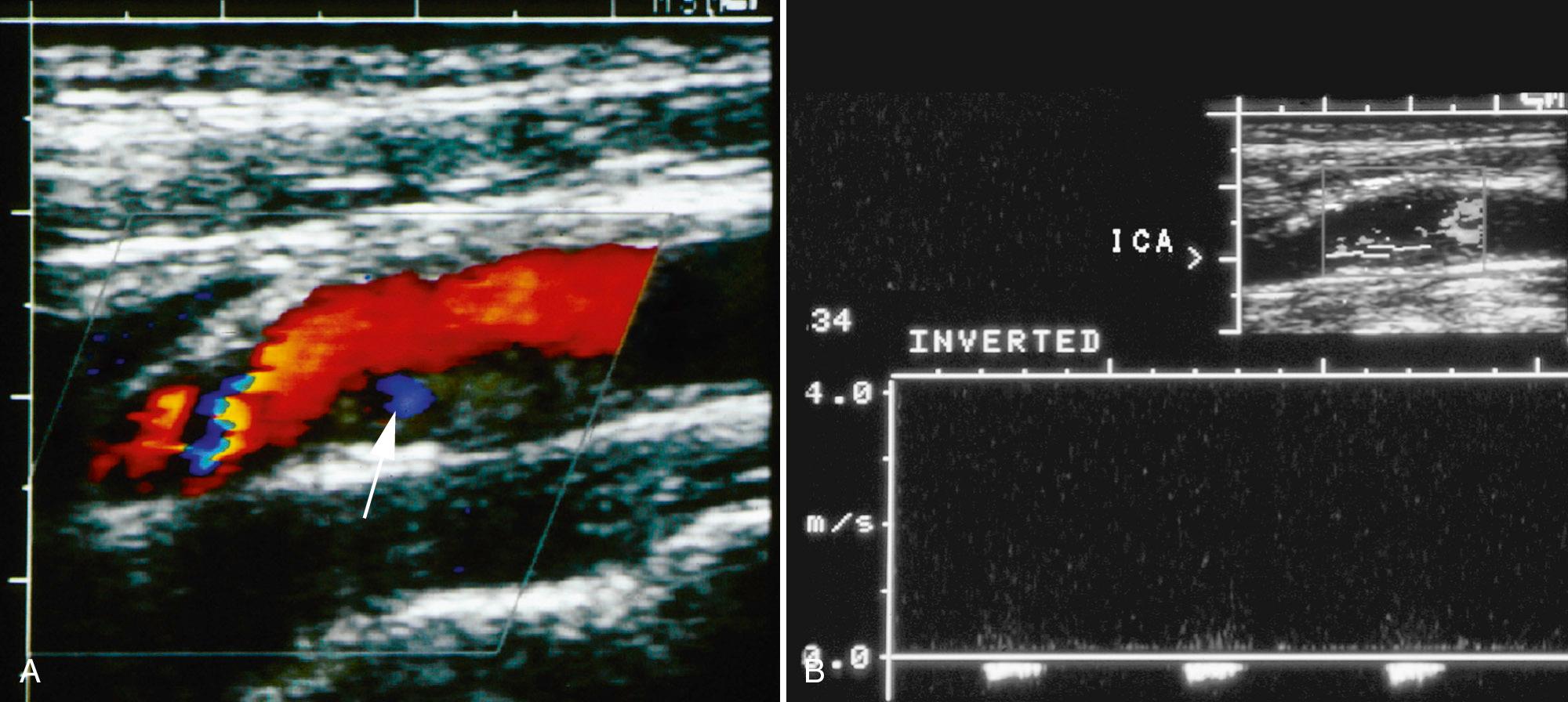
Although the diagnosis of ulceration varies, the ability to predict intraplaque hemorrhage reliably, with its associated clinical implications, underscores the importance of ultrasound plaque characterization. The presence of heterogeneous, irregular plaque should be noted because of the association of heterogeneous plaque, and intraplaque hemorrhage even in a stenosis of less than 50% may warrant medical therapy. Many now consider heterogeneous plaque to be the “vulnerable,” unstable form of plaque that should be treated differently than the more stable homogeneous form of plaque. Plaque characterization should be considered when determining the type of therapy to use in carotid intervention. Angioplasty and subsequent stenting of carotid vessels might be safer if performed in patients with homogeneous plaque than those with heterogeneous plaque.
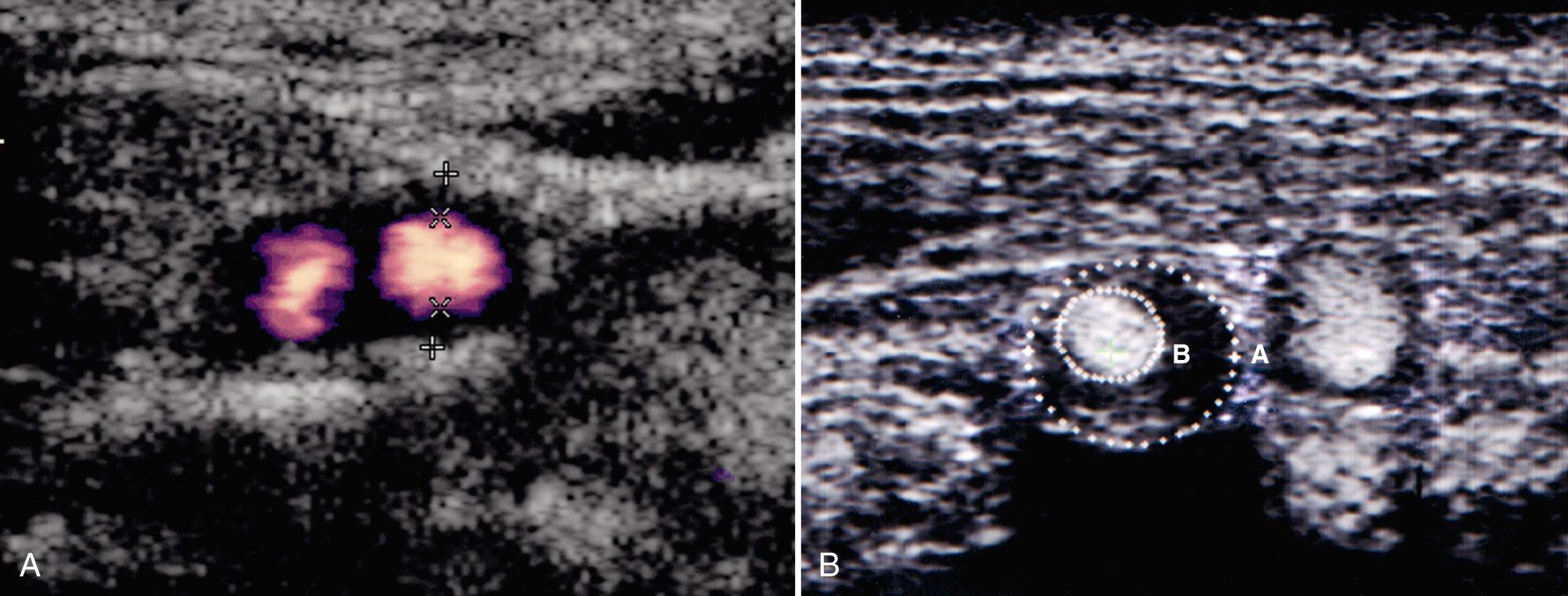
Measurements of carotid diameter and area stenosis should be made in the transverse plane, perpendicular to the long axis of the vessel, using gray-scale, B-flow, or power Doppler sonographic imaging ( Fig. 26.12 , Videos 26.12 and ). Measurements made on longitudinal scans may overestimate or underestimate the severity of stenosis by partial “voluming” through an eccentric plaque. The percentage of diameter stenosis and the percentage of area stenosis are not always linearly related. Clinical records should state the type of stenosis measured. Asymmetrical stenoses are most appropriately assessed with “percentage of area stenosis” measurements, although these are often time consuming and technically difficult. The cephalocaudal extent and length of plaques should be noted, along with the presence of tandem plaques. Recently, three-dimensional ultrasound has been used to measure plaque volume with good intraobserver and interobserver variability as well as plaque characterization.
As the severity of a stenosis increases, the quality of the real-time image deteriorates. Several factors work against successful image assessment of high-grade stenosis. Plaque calcification and irregularity produce shadowing, which obscures the vessel lumen. Heterogeneous plaque often has acoustic properties similar to flowing blood, producing anechoic plaques or thrombi that are difficult to visualize on gray-scale images. In the most extreme cases, vessels can show little visible plaque yet be totally occluded (see Fig. 26.9E and F ). Color Doppler sonography readily identifies such phenomena. For these reasons, real-time gray-scale ultrasound is best suited for the evaluation of nonrate-limiting lesions and not for quantifying high-grade stenoses, which are more accurately determined by spectral analysis. The gray-scale findings and Doppler spectral analysis values must be integrated and correlated for a complete ultrasound assessment of the carotid vessels (Video 26.14).
It is probably unnecessary to make a quantitative assessment of the amount of plaque. Rather, a qualitative assessment of the amount of plaque should be made and compared to the Doppler spectral findings to ensure accuracy in grading stenoses. A mismatch between the qualitative assessment of the amount of plaque and the Doppler findings should alert the examiner to a possible technical error. If these cannot be resolved, further assessment with CTA or MRA should be considered.
The Doppler spectrum is a quantitative graphic display of the velocities and directions of moving red blood cells (RBCs) present in the Doppler sample volume. The Doppler spectral display represents velocities on the y axis and time on the x axis. By convention, flow toward the transducer is displayed above the zero-velocity baseline, and flow away from the transducer is below. For ease of analysis, spectra that project below the baseline are often inverted and placed above the baseline, always keeping in mind the true direction of flow within the vessel. The amplitude of each velocity component (number of RBCs with each velocity component) is used to modulate the brightness of the traces. This is also known as a gray-scale velocity plot. In the normal carotid artery, the frequency spectrum is narrow in systole and somewhat wider in early and late diastole (Videos 26.15 through ). There is usually a black zone between the spectral line and the zero-velocity baseline called the spectral window ( Fig. 26.13 ).
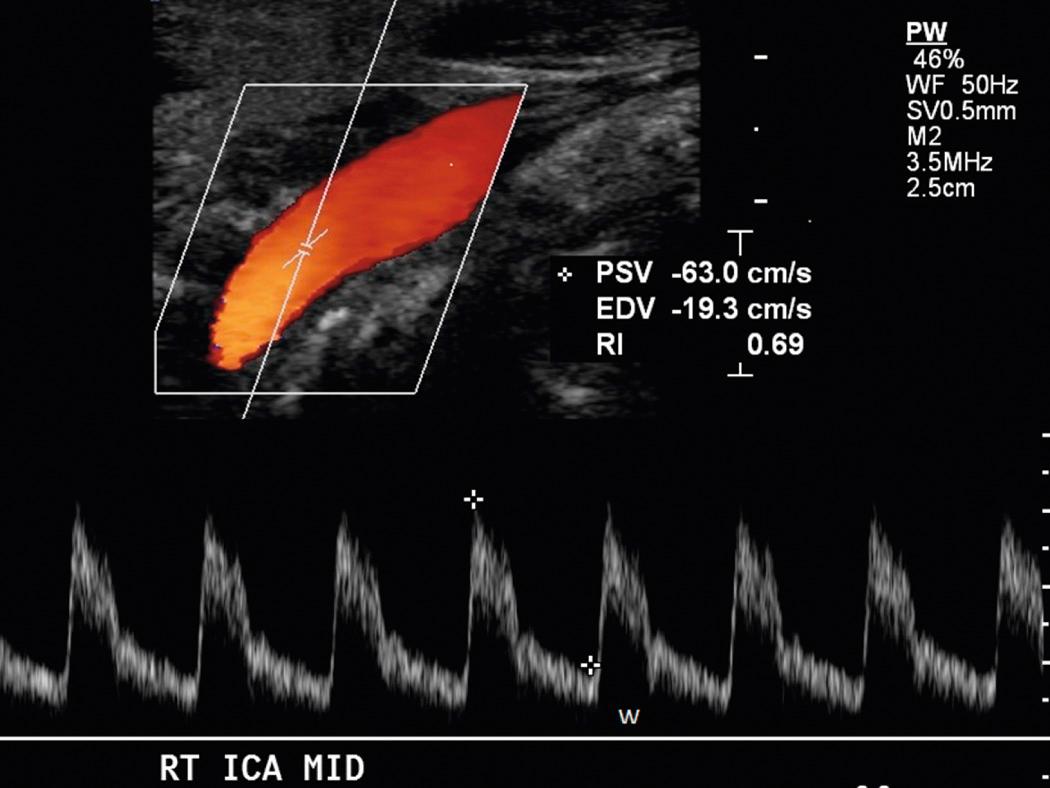
The ICA and ECA branches of the CCA have distinctive spectral waveforms ( Fig. 26.14 ). The external carotid artery supplies the high-resistance vascular bed of the facial musculature, so its flow resembles that of other peripheral arterial vessels. Flow velocity rises sharply during systole and falls rapidly during diastole, approaching zero or transiently reversing direction. The internal carotid artery supplies the low-resistance circulation of the brain and demonstrates flow similar to that in vessels supplying other blood-hungry organs, such as the liver, kidneys, and placenta. The common feature in all low-resistance arterial waveforms is that a large quantity of forward flow continues throughout diastole (see through ). The CCA waveform is a composite of the internal and external waveforms, but most often the CCA flow pattern more closely resembles that of the ICA, and diastolic flow is generally above the baseline (see ). Approximately 80% of the blood flowing from the CCA goes through the ICA into the brain, whereas 20% goes through the ECA into the head musculature. The relative decrease in blood flow through the ECA will cause it to have a generally lower amplitude gray-scale waveform than in either the ICA or the CCA.
Virtually all state-of-the-art ultrasound equipment offers color and power Doppler, as well as gray-scale capabilities and pulsed Doppler, for the carotid examination (Video 26.19). A rapid color Doppler screen allows the detection of abnormal flow patterns, which allows the pulsed Doppler signal volume to be placed in areas that are abnormal, especially those with high-velocity jets (Video 26.20). These high-velocity jets are located in the region of and immediately distal to a high-grade stenosis ( Figs. 26.15 and 26.16 ). In cases where both gray-scale and color and power Doppler images of an entire carotid artery are normal, only representative spectral tracings from the CCA, ICA, and ECA are necessary to complete the examination.
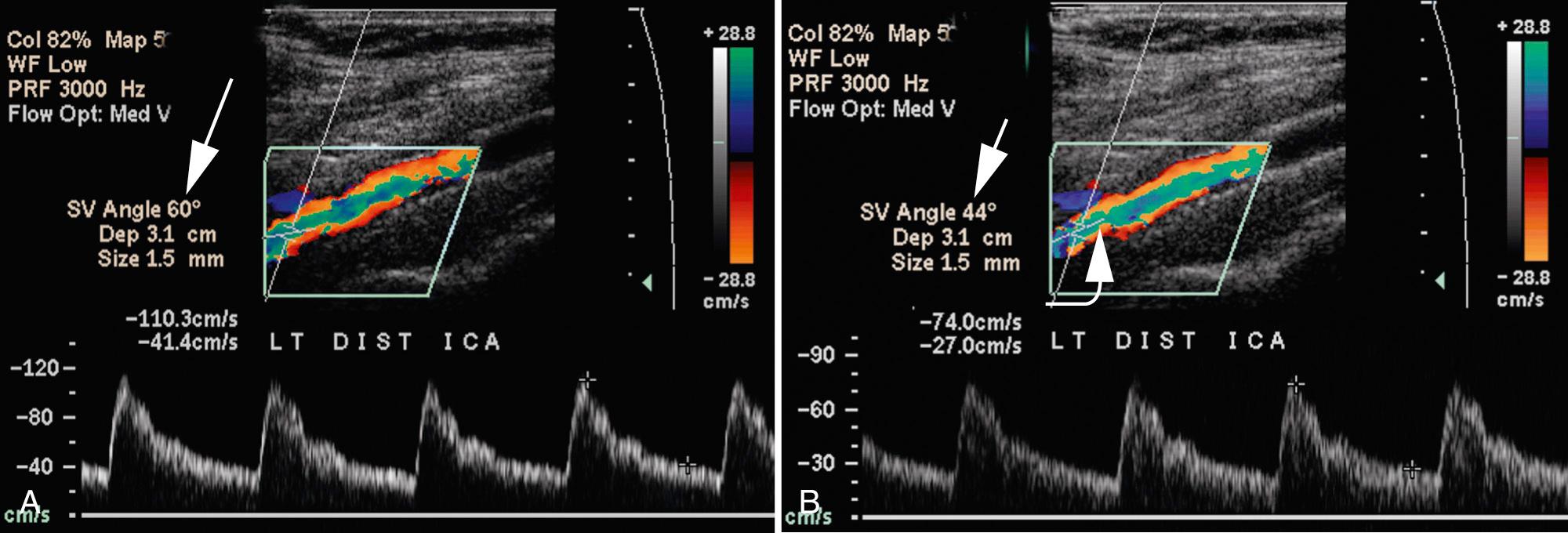
The standard Doppler spectral examination consists of traces obtained from the proximal and distal CCA, carotid bulb, and proximal ECA; samples in the proximal, middle, and distal ICA; and a representative trace from the vertebral artery. Normal velocities are higher in the proximal CCA and lower in the distal vessel; normal ICA velocities tend to increase from proximal to distal. In addition, blood flow velocities are obtained immediately proximal to, at, and just beyond regions of maximal visible stenosis and at 1-cm intervals distal to the visualized plaque as far cephalad as possible. Positioning the Doppler angle cursor parallel to the vessel walls determines angle theta, used to convert frequency information into velocity values (see Fig. 26.14B ). Doppler angle theta is defined as the angle between the Doppler transducer line of sight and the direction of blood flow. The ideal angle theta is 0 degrees, as the cosine of this angle is 1, thus resulting in the greatest possible detectable frequency shift. Because this angle is rarely achievable in the clinical setting, angles from 30 to 60 degrees are considered acceptable for carotid spectral analysis.
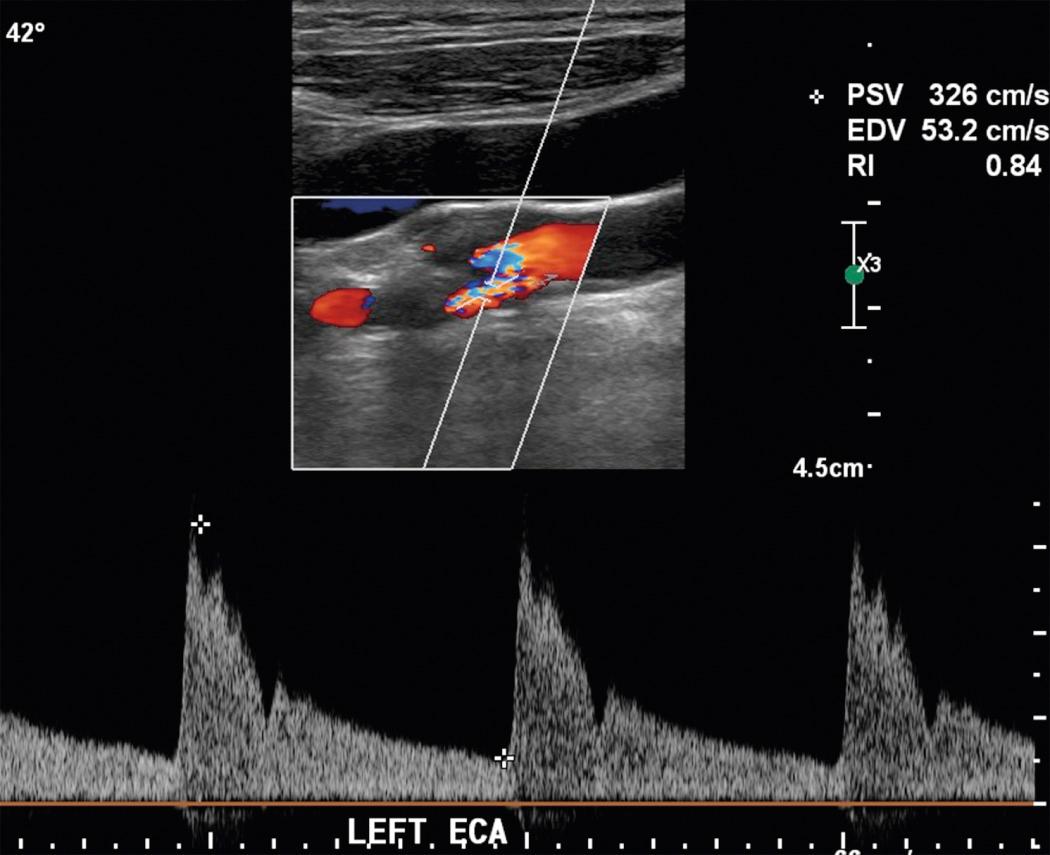
Certain schools of ultrasound use a technique in which the Doppler angle is set at 60% and the transducer is “heel and toed” to parallel the carotid artery for Doppler spectral analysis. In our experience, it is frequently not possible to optimize cursor placement in the midportion of the vessel using this technique in tortuous vessels. Therefore our technique involves selecting the site of spectral analysis and paralleling the wall of the vessel at that point, making certain that the Doppler angle does not exceed 60 degrees. Although either technique can be used, results obtained using these different methodologies can result in different velocities. Thus if the first technique is used, a different set of velocity criteria should be expected than if the second is used. This is one of the factors responsible for the differences in velocity spectral criteria used in different laboratories ( Fig. 26.16 ). When angle theta exceeds 60 to 70 degrees, the accuracy of velocity measurement declines precipitously ( Fig. 26.17 ), to the point that virtually no velocity change is detected at angle theta of 90 degrees. The entire course of the CCA and ICA should be interrogated with a consistent angle theta between the transducer and the vessel maintained throughout the examination, when possible. Generally, only the origin of the ECA is evaluated because occlusive plaque is less common here than in the ICA and is rarely clinically important. A stenosis of the ECA should be noted because it may account for a worrisome cervical bruit when the ICA is normal.
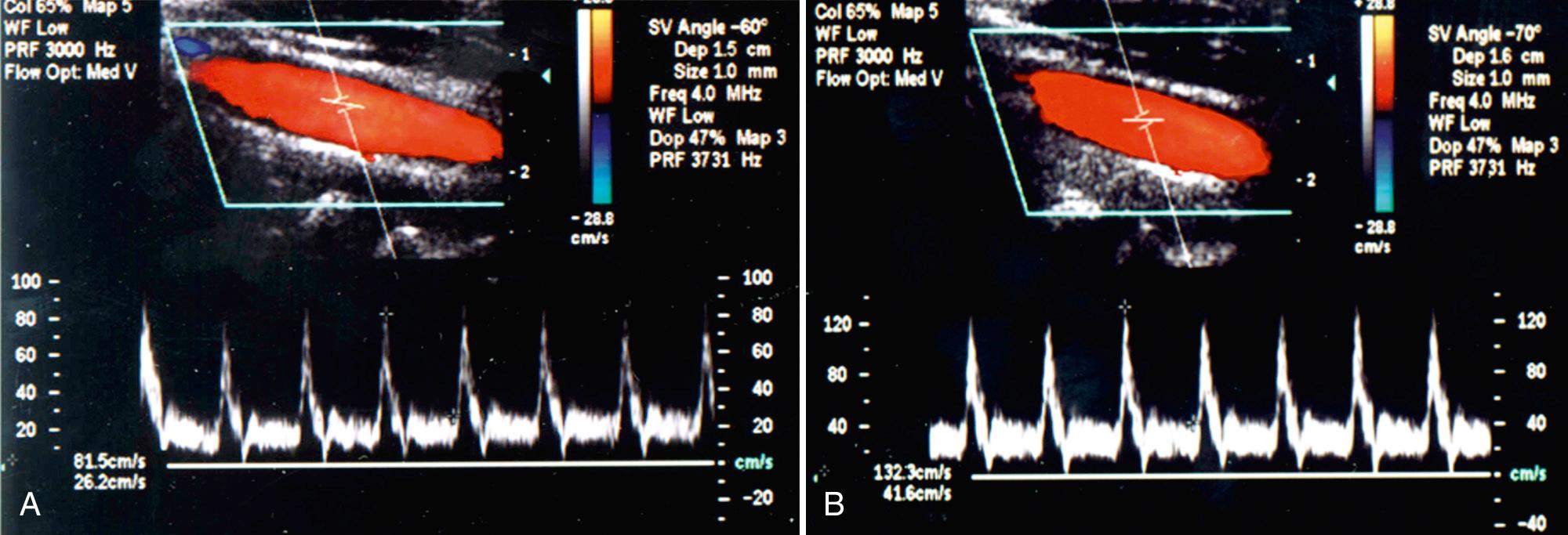
Atheromatous plaque projecting into the arterial lumen disturbs the normal, smooth laminar flow of erythrocytes. The RBCs move with a wider range of velocities, so the spectral line becomes wider, filling in the normally black spectral window. This phenomenon, termed spectral broadening, increases in proportion to the severity of carotid artery stenosis ( Fig. 26.18 , Video 26.21). Some duplex machines allow the operator to measure the spectral spread between the maximal and minimal velocities (bandwidth) and thus quantitate spectral broadening. The validity of these measurements remains unproved, however, and further correlative studies are needed to document the relationship of quantitative spectral broadening parameters to specific degrees of stenosis. Most tables no longer include a measurement for spectral broadening when grading carotid stenosis. Nevertheless, a visible gestalt of the amount of spectral window obliteration, as well as color Doppler heterogeneity, provides a useful, if not quantitative, predictor of the severity of flow disturbance.
Pseudospectral broadening can be caused by technical factors, such as too high a gain setting. In such cases the background around the spectral waveform often contains noise. Whenever spectral broadening is suspected, the gain should be lowered to see if the spectral window clears. Similarly, spectral broadening caused by vessel wall motion can occur when the Doppler sample volume is too large or positioned too near the vessel wall. Decreasing the size of the sample volume and placing it midstream should eliminate this potential pitfall.
Altered flow patterns can normally be found at certain sites in the carotid system. For example, it is normal to find flow separation at the site of branching vessels, such as where the CCA branches into the ECA and ICA. Flow disturbances also occur at sites where there is an abrupt change in the vessel diameter. For example, flow disturbances and bizarre waveforms caused by flow separation may be encountered in a normal carotid bulb where the CCA terminates in a localized area of dilation as it divides into the ECA and ICA ( Fig. 26.19 ).
The tendency for spectral broadening increases in direct proportion to the velocity of blood flow. For example, spectral broadening can be observed in a normal ECA, vertebral arteries, and CCA supplying circulation contralateral to an occluded contralateral ICA. Increased velocity may also account for the disturbed flow that is sometimes observed in the normal extracranial carotid arteries of young athletes with normal cardiac outputs or in patients in pathologically high cardiac output states. It is also seen in arteries supplying arteriovenous fistulas and malformations. Postoperative spectral broadening may persist for months after CEA in the absence of significant residual or recurrent disease, possibly from changes in wall compliance.
Tortuous carotid vessels can demonstrate spectral broadening and asymmetrical high-velocity flow jets in the absence of plaque disease. Other nonatheromatous causes of disturbed blood flow in the extracranial carotid arteries include aneurysms, arterial wall dissections, and fibromuscular dysplasia.
Carotid stenoses usually begin to cause velocity changes when they exceed 50% diameter (70% cross-sectional area) ( Fig. 26.20 ). Velocity elevations generally increase as the severity of the stenosis increases. At critical stenoses (>95%), the velocity measurements may actually decrease, and the waveform becomes dampened.
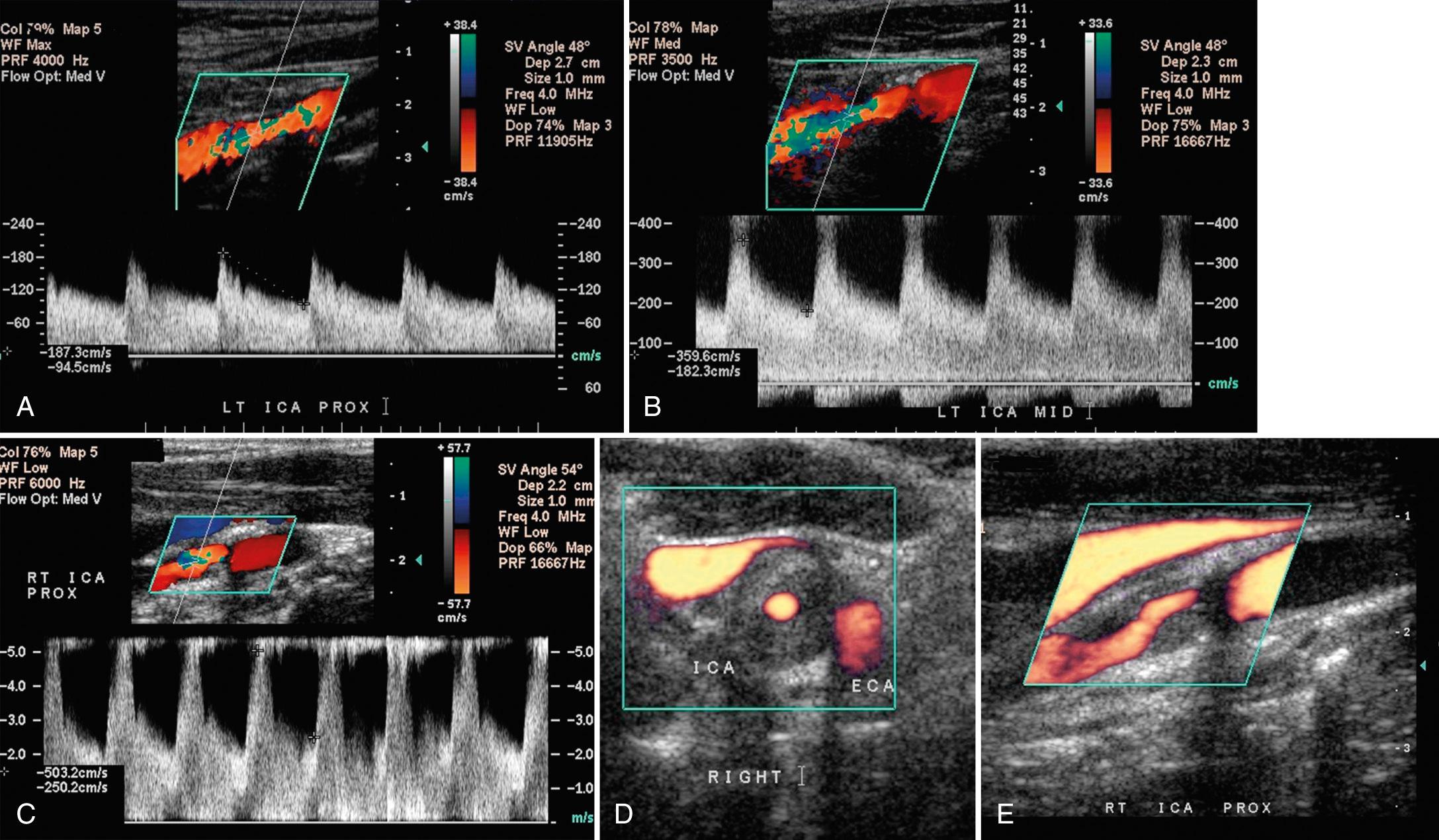
In these cases, correlation with color or power Doppler imaging is essential to diagnose correctly the severity of the stenoses. Velocity increases are focal and most pronounced in and immediately distal to a stenosis, emphasizing the importance of sampling directly in these regions. Moving further distal from a stenosis, flow begins to reconstitute and assume a more normal pattern, provided a tandem lesion does not exist distal to the initial site of stenosis. Spectral broadening results in the jets of high-velocity flow associated with carotid stenosis; however, correlation with gray-scale and color Doppler images can define other causes of spectral broadening. An awareness of normal flow spectra combined with appropriate Doppler techniques can obviate many potential diagnostic pitfalls.
The degree of carotid stenosis that is considered clinically significant in the symptomatic or asymptomatic patient is in evolution. Initially, it was thought that lesions causing 50% diameter stenosis were significant; this perception changed as more information was gathered from two large clinical trials. As noted earlier, NASCET demonstrated that CEA was more beneficial than medical therapy in symptomatic patients with 70% to 99% ICA stenosis. ECST demonstrated a CEA benefit when the degree of stenosis was greater than 60%. Interestingly, the method used to grade stenoses in the ECST study was substantially different than that used in the NASCET trials. The NASCET trials compared the severity of the ICA stenosis on arteriogram with the residual lumen of a presumably more normal distal ICA. The ECST methodology entailed assessment of the severity of stenosis with a “guesstimation” of the lumen of the carotid artery at the level of the stenosis. The ECST assessment is more comparable to ultrasound's visible assessment of the degree of narrowing, whereas velocity tables currently in use have been derived to correspond to the NASCET angiographic determinations for stenosis. The ECST method for grading carotid artery stenosis tends to give a more severe assessment of narrowing than the NASCET technique ( Fig. 26.21 ).
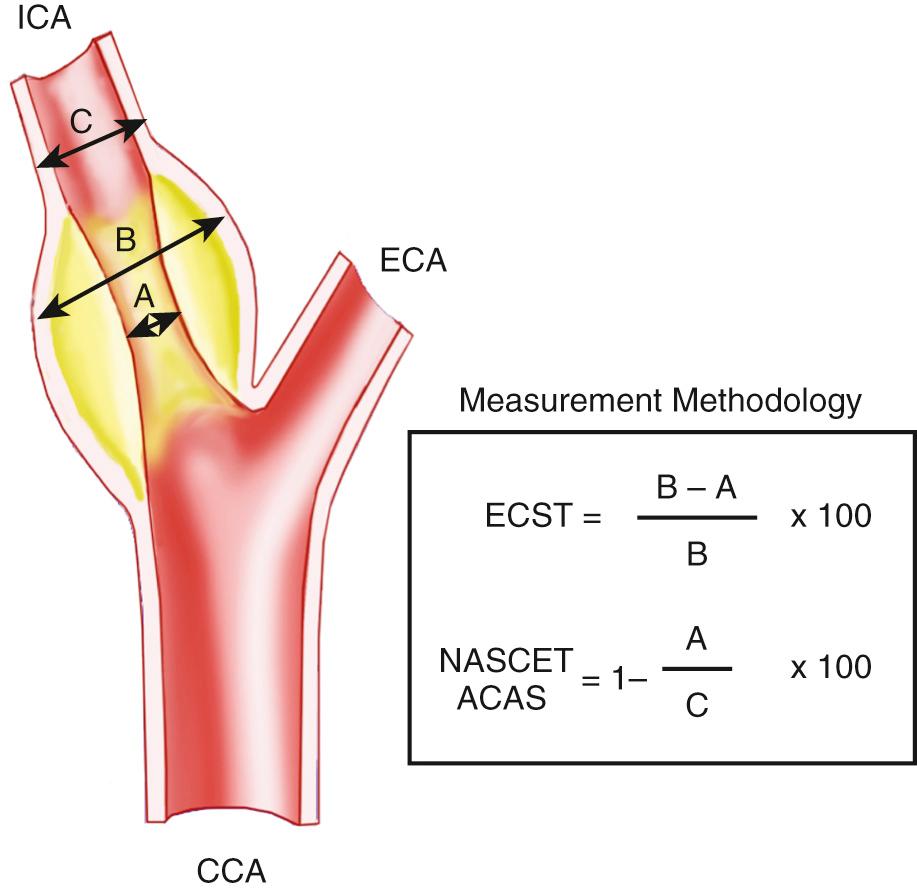
The initial NASCET trials retrospectively compared velocity data obtained on the Doppler examination with angiographic measurements of stenosis. No standardized ultrasound protocol was employed by the numerous centers involved in the trials. Despite the lack of uniformity, moderate sensitivity and specificity ranging from 65% to 77% were obtained for grading ICA stenoses using Doppler velocities. If ultrasound technique is standardized and criteria are validated in a given laboratory, peak systolic velocity (PSV) and peak systolic ratios have proved to be an accurate method for determining carotid stenosis. The ECST group compared three different angiographic measurement techniques: the NASCET, the ECST, and a technique comparing distal CCA measurements with those of ICA stenosis. Researchers concluded that the ECST and NASCET techniques were similar in their prognostic value, whereas the CCA/stenosis measurement was the most reproducible of the three techniques. They also concluded that the CCA method, although reproducible, would be invalidated by the presence of CCA disease. Virtually all investigators advocate using the NASCET angiographic measurement technique.
The results of these trials, as well as the more recent ACAS and moderate NASCET studies, have generated reappraisals of the Doppler velocity criteria that most accurately define 70% or greater stenosis and, more recently, greater than 50% diameter stenoses. Attempts have been made to determine the Doppler parameters or combination of parameters that most reliably identify a certain-diameter stenosis. Most sources agree that the best parameter is the PSV of the ICA in the region of a stenosis. Using multiple parameters can improve diagnostic confidence, particularly when combined with color and power Doppler imaging (see ).
The degree of stenosis is best assessed using the gray-scale and pulsed Doppler parameters, including ICA PSV, ICA end diastolic velocity (EDV), CCA PSV, CCA EDV, peak systolic ICA/CCA ratio, and peak end diastolic ICA/CCA ratio (EDR) (Videos 26.21 and ). PSV has proved accurate for quantifying high-grade stenoses. The relationship of PSV to the degree of luminal narrowing is well defined and easily measured. Although Doppler velocities have proved reliable for defining 70% or greater stenosis, Grant et al. showed less favorable results for substenosis classification between 50% to 69% using PSV and ICA/CCA PSV ratios. In our experience, however, using all four parameters and determining a correct category for the degree of stenosis is the most efficacious way to ensure accuracy. Agreement for all four parameters for a clinical situation is most common. When there is an outlying parameter, further assessment and careful attention to technique and detail are required. EDV and EDR are particularly useful in distinguishing between high grades of stenosis. Additionally, correlating the visual estimation of the degree of stenosis and the velocity numbers will help in correctly grading stenosis, particularly when the degree of stenosis is “near occlusion” ( Figs. 26.22 and 26.23 ; see also Fig. 26.20D and E ). On rare occasions, alternate imaging methodologies (e.g., MRA, CTA) may need to be recommended.

No criteria for grading external carotid artery stenoses have been established. A good general rule is that if the ECA velocities do not exceed 200 cm/sec, no significant stenosis is present. However, we usually rely on a visible assessment of the degree of narrowing associated with velocity changes. Occlusive plaque involving the ECA is less common than in the ICA and is rarely clinically significant.
Similarly, velocity criteria used to grade common carotid artery stenoses have not been well established. However, if one is able to visualize 2 cm proximal and 2 cm distal to a visible CCA stenosis, a PSV ratio obtained 2 cm proximal to the stenosis (vs. in region of greatest visible stenosis) can be used to grade the “percent diameter stenosis” in a manner similar to that used in peripheral artery studies. A doubling of the PSV across a lesion would correspond to at least a 50% diameter stenosis, and a velocity ratio in excess of 3.5 corresponds to a greater than 75% stenosis.
One persistent problem with duplex Doppler with gray-scale ultrasound evaluation of the carotid arteries is that different institutions use PSVs ranging from 130 cm/sec to 325 cm/sec to diagnose greater than 70% ICA stenosis. Factors adding to these discrepancies include technique and equipment. While there is a strong level of correlation between techniques and criteria, the choice of criteria has a significant impact on which patients go to surgery. This wide range of PSVs reinforces the need for individual ultrasound laboratories to determine which Doppler parameters are most reliable in their own institution. Correlation of the velocity ranges obtained by ultrasound with angiographic and surgical results is necessary to achieve accurate, reproducible examinations in a particular ultrasound laboratory.
The Society of Radiologists in Ultrasound, representing multiple medical and surgical specialties, held a consensus conference in 2002 to consider carotid Doppler ultrasound. In addition to guidelines for performing and interpreting carotid ultrasound examinations, panelists devised a set of criteria widely applicable among vascular laboratories ( Table 26.1 ). Although the conference did not recommend all established laboratories with internally validated velocity charts alter their practices, they suggested physicians establishing new laboratories consider using the consensus criteria; those with preexisting charts might consider comparing in-house criteria with those provided by the consensus conference. Velocity criteria corresponding to specific degrees of vascular stenosis are listed in the tables. Our institution uses Table 26.2 , which has a category for 80% to 95% stenoses; our surgeons are more inclined to consider surgery for patients with asymptomatic stenoses greater than 80% than for those with less severe stenoses.
| ICA PSV | Plaque | ICA/CCA PSV Ratio | ICA EDV | |
|---|---|---|---|---|
| Normal | <125 cm/sec | None | <2.0 | <40 cm/sec |
| <50% | <125 cm/sec | <50% diameter reduction | <2.0 | <40 cm/sec |
| 50%-69% | 125-230 cm/sec | ≥50% diameter reduction | 2.0-4.0 | 40-100 cm/sec |
| ≥70% to near occlusion | >230 cm/sec | ≥50% diameter reduction | >4.0 | >100 cm/sec |
| Near occlusion | May be low or undetectable | Visible | Variable | Variable |
| Total occlusion | Undetectable | Visible, no detectable lumen | Not applicable | Not applicable |
| Diameter Stenosis | Peak Systolic Velocity (cm/sec) | Peak Diastolic Velocity (cm/sec) | Systolic Velocity Ratio (VICA/VCCA) | Diastolic Velocity Ratio (VICA/VCCA) |
|---|---|---|---|---|
| 0% (normal) | <110 | <40 | <2.0 | <2.6 |
| 1%-39% (mild) | <110 | <40 | <2.0 | <2.6 |
| 40%-59% (moderate) | <170 | <40 | <2.0 | <2.6 |
| 60%-79% (severe) | >170 | >40 | >2.0 | >2.6 |
| 80%-95% (critical) | >250 | >100 | >3.7 | >5.5 |
| 96%-99% | Velocities demonstrate variability and may be low | |||
| 100% (occlusion) | No flow detected |
The ICA values should be obtained at or just distal to the point of maximum visible stenosis and at the point of greatest color Doppler spectral abnormality. Values from the CCA should be obtained 2 cm proximal to the widening in the region of the carotid bulb. Because velocities normally decrease from proximal to distal in the CCA and increase from proximal to distal in the ICA, it is important that standardized levels be used routinely for obtaining the ICA/CCA velocity ratio.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here