Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The pancreas contains two types of glands: (1) exocrine glands, which secrete digestive enzymes (see p. 882 ) and  (see pp. 885–886 ) into the intestinal lumen; and (2) endocrine glands, called the islets of Langerhans. The islets are spread throughout the pancreas and in aggregate comprise only 1% to 2% of its tissue mass.
(see pp. 885–886 ) into the intestinal lumen; and (2) endocrine glands, called the islets of Langerhans. The islets are spread throughout the pancreas and in aggregate comprise only 1% to 2% of its tissue mass.
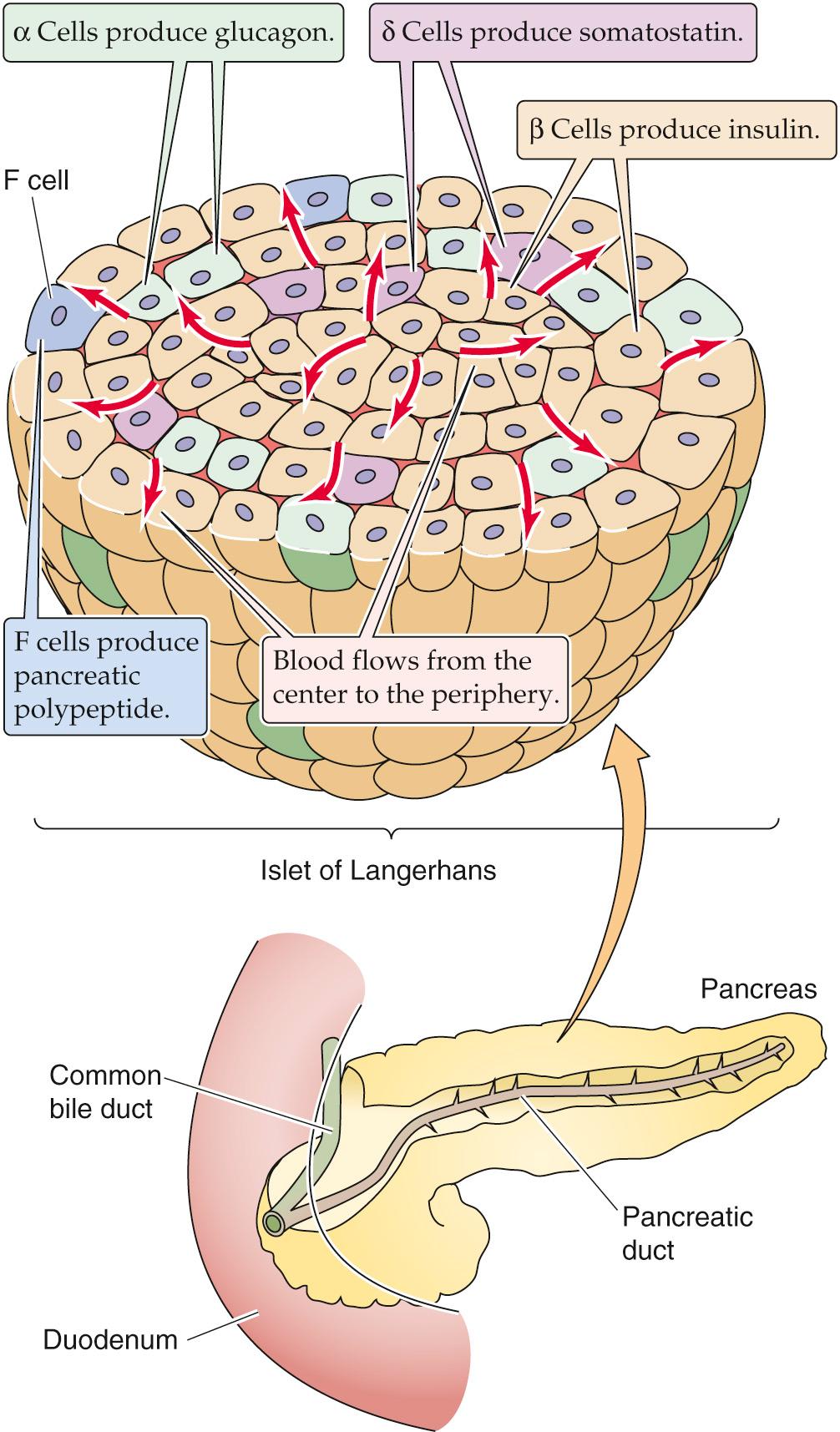
The normal human pancreas contains between 500,000 and several million islets. Islets can be oval or spherical and measure between 50 and 300 µm in diameter. Islets contain at least four types of secretory cells—α cells, β cells, δ cells, and F cells—in addition to various vascular and neural elements ( Fig. 51-1 and Table 51-1 ). β cells secrete insulin, proinsulin, C peptide, and a newly described protein, amylin (or IAPP for islet amyloid polypeptide). β cells are the most numerous type of secretory cell within the islets; they are located throughout the islet but are particularly numerous in the center. α cells principally secrete glucagon, δ cells secrete somatostatin, and F cells (also called pancreatic polypeptide cells) secrete pancreatic polypeptide.
| CELL TYPE | PRODUCT |
|---|---|
| α | Glucagon |
| β | Insulin Proinsulin C peptide Amylin |
| δ | Somatostatin |
| F | Pancreatic polypeptide |
The islets are richly perfused (blood flow per gram of tissue is >5 times that of the myocardium) and receive both sympathetic and parasympathetic innervation. These cells also can communicate with each other and influence each other's secretion. We can group these communication links into three categories:
Humoral communication. The blood supply of the islet courses outward from the center of the islet toward the periphery, carrying glucose and other secretagogues. In the rat—and less strikingly in humans—β cells are more abundant in the center of the islet, whereas α and δ cells are more abundant in the periphery. Cells within a given islet can influence the secretion of other cells as the blood supply courses outward through the islet carrying the secreted hormonal product of each cell type with it. For example, glucagon is a potent insulin secretagogue, insulin modestly inhibits glucagon release, and somatostatin potently inhibits the secretion of both insulin and glucagon (as well as the secretion of growth hormone and other non-islet hormones).
Cell-cell communication. Both gap and tight junctional structures connect islet cells with one another. Cells within an islet communicate via gap junctions, which may be important for the regulation of both insulin and glucagon secretion.
Neural communication. Both the sympathetic and parasympathetic divisions of the autonomic nervous system (ANS) regulate islet secretion. Cholinergic stimulation augments insulin secretion. Adrenergic stimulation can have either a stimulatory or inhibitory effect, depending on whether β-adrenergic (stimulatory) or α-adrenergic (inhibitory) stimulation dominates (see p. 1033 ). ![]() N51-1
N51-1
On page 1033 , we noted that the general rule for α- and β-adrenergic receptors—first noted by Raymond Ahlquist—is that α activation leads to stimulation, whereas β activation leads to inhibition. The pattern in pancreatic islets is just the opposite, as noted in the text.
These three communication mechanisms allow for a tight control over the synthesis and secretion of islet hormones.
The discovery of insulin was among the most exciting and dramatic events in the history of endocrine physiology and therapy. In the United States and Europe, insulin-dependent diabetes mellitus (IDDM), or type 1 diabetes, develops in ~1 in every 600 children. However, the prevalence is only ~1 in 10,000 in eastern Asia. Before 1922, all children with diabetes died within 1 or 2 years of diagnosis. It was an agonizing illness; the children lost weight despite eating well, became progressively weaker and cachectic, were soon plagued by infections, and eventually died of overwhelming acidosis. No effective therapy was available, and few prospects were on the horizon. It was known that the blood sugar level was elevated in this disease, but beyond that, there was little understanding of its pathogenesis.
In 1889, Minkowski and von Mering demonstrated that removing the pancreas from dogs caused hyperglycemia, excess urination, thirst, weight loss, and death—in short, a syndrome closely resembling type 1 diabetes. Following this lead, a group of investigators in the Department of Physiology at the University of Toronto prepared extracts of pancreas and tested the ability of these extracts to lower plasma [glucose] in pancreatectomized dogs. Despite months of failures, these investigators persisted in their belief that such extracts could be beneficial. Finally, by the winter of 1921, Frederick Banting (a surgeon) and Charles Best (at the time, a medical student) were able to demonstrate that an aqueous extract of pancreas could lower blood glucose level and prolong survival in a pancreatectomized dog. ![]() N51-2 Within 2 months, a more purified extract was shown to lower blood glucose level in a young man with diabetes. By the end of 1923, insulin (as the islet hormone was named) was being prepared from beef and pork pancreas on an industrial scale, and patients around the world were receiving effective treatment of their diabetes. For the discovery of insulin, Frederick Banting and John Macleod received the 1923 Nobel Prize in Physiology or Medicine.
N51-2 Within 2 months, a more purified extract was shown to lower blood glucose level in a young man with diabetes. By the end of 1923, insulin (as the islet hormone was named) was being prepared from beef and pork pancreas on an industrial scale, and patients around the world were receiving effective treatment of their diabetes. For the discovery of insulin, Frederick Banting and John Macleod received the 1923 Nobel Prize in Physiology or Medicine. ![]() N51-3
N51-3
In 1923, just 2 years after Frederick Banting (a young faculty member just 5 years out of medical school) and Charles Best (a 22-year-old medical student working in Banting's laboratory) discovered insulin at the University of Toronto, the Nobel Prize in Physiology or Medicine was awarded to Frederick Banting and the head of the research team and chairman of Banting's department, John Macleod. The short delay between the discovery and the award of the prize indicates the enormous significance of the discovery.
It is interesting that Frederick Banting protested the award of the Nobel Prize to John Macleod and gave half of his portion of the monetary award to Charles Best.
For more information about Frederick Banting and John Macleod and the work that led to their Nobel Prize, visit http://nobelprize.org/medicine/laureates/1923/index.html (accessed October 2014).
Since that time, the physiology of the synthesis, secretion, and action of insulin has been studied more extensively than that of any other hormone. Now, nearly a century later, much is known about the metabolic pathways through which insulin regulates carbohydrate, lipid, and protein metabolism in its major targets: the liver, muscle, and adipose tissue. However, the sequence of intracellular signals that triggers insulin secretion by pancreatic β cells, as well as the signal-transduction process triggered when insulin binds to a plasma membrane receptor on target tissues, remain areas of intense study.
What does insulin do? Succinctly put, insulin efficiently integrates body fuel metabolism both during periods of fasting and during feeding ( Table 51-2 ). When an individual is fasting, the β cell secretes less insulin. When insulin levels decrease, lipids are mobilized from adipose tissue and amino acids are mobilized from body protein stores within muscle and other tissues. These lipids and amino acids provide fuel for oxidation and serve as precursors for hepatic ketogenesis and gluconeogenesis, respectively. During feeding, insulin secretion increases promptly, which diminishes the mobilization of endogenous fuel stores and stimulates the uptake of carbohydrates, lipids, and amino acids by insulin-sensitive target tissues. In this manner, insulin directs tissues to replenish the fuel reserves depleted during periods of fasting.
| PARAMETER | AFTER A 24-hr FAST | 2 hr AFTER A MIXED MEAL |
|---|---|---|
| Plasma [glucose], mg/dL | 60–80 | 100–140 |
| mM | 3.3–4.4 | 5.6–7.8 |
| Plasma [insulin], µU/mL | 3–8 | 50–150 |
| Plasma [glucagon], pg/mL | 40–80 | 80–200 |
| Liver | ↑ Glycogenolysis ↑ Gluconeogenesis |
↓ Glycogenolysis ↓ Gluconeogenesis ↑ Glycogen synthesis |
| Adipose tissue | Lipids mobilized for fuel | Lipids synthesized |
| Muscle | Lipids metabolized Protein degraded and amino acids exported |
Glucose oxidized or stored as glycogen Protein preserved |
As a result of its ability to regulate the mobilization and storage of fuels, insulin maintains plasma [glucose] within narrow limits. Such regulation provides the central nervous system (CNS) with a constant supply of glucose needed to fuel cortical function. In higher organisms, if plasma [glucose] (normally ≅ 5 mM) declines to <2 to 3 mM (hypoglycemia; Box 51-1 ) for even a brief period, confusion, seizures, and coma may result. Conversely, persistent elevations of plasma [glucose] are characteristic of the diabetic state. Severe hyper glycemia (plasma glucose levels > 15 mM) produces an osmotic diuresis (see Box 35-1 ) that, when severe, can lead to dehydration, hypotension, and vascular collapse.
Early symptoms are principally autonomic and include palpitations, tachycardia, diaphoresis, anxiety, hyperventilation, shakiness, weakness, and hunger. More severe hypoglycemia manifests principally as neuroglycopenia, with confusion, aberrant behavior, hallucinations, seizures, hypothermia, focal neurological deficits, and coma.
Early manifestations include weakness, polyuria, polydipsia, altered vision, weight loss, and mild dehydration. For prolonged or severe hyperglycemia (accompanied by metabolic acidosis or diabetic ketoacidosis), manifestations include Kussmaul hyperventilation (deep, rapid breathing; see p. 716 ), stupor, coma, hypotension, and cardiac arrhythmias.
Hypoglycemia, which can be viewed most simply as the opposite of diabetes mellitus, has many causes. Perhaps the most frequent setting is a patient with type 1 diabetes who skips a meal or fails to adjust the insulin dose when exercising. Many diabetic patients who seek to maintain tight control over their blood sugar experience frequent hypoglycemic reactions, which they quickly learn to abort with a carbohydrate snack. Patients with type 2 diabetes who take an excessive dose of sulfonylureas are subject to severe hypoglycemia, which may require continuous treatment for several days because the half-life of some of these drugs is quite long.
We saw in Chapter 50 that epinephrine—acting as a β-adrenergic agonist—is a hyperglycemic agent; that is, it promotes glycogenolysis in liver and muscle (see p. 1033 ). Thus, β blockers rarely cause hypoglycemia in healthy individuals because these people can appropriately regulate their insulin secretion. However, because β blockers can mask the early adrenergic response to mild hypoglycemia (sweating, tachycardia, tremulousness), diabetic patients taking both insulin and β blockers commonly progress to severe hypoglycemia without warning. Another drug that can induce hypoglycemia is pentamidine, an agent used to treat Pneumocystis jiroveci pneumonia. Pentamidine is a β-cell toxin that leads to an acute, excessive release of insulin, which can be followed by hypoglycemia.
Alcoholic patients are at great risk of hypoglycemia. Ethanol suppresses gluconeogenesis, and hepatic glycogen stores may already be low because of poor nutrition. Other severe illnesses that can produce persistent hypoglycemia include liver disease, renal failure, and some large tumors that produce a hypoglycemia-inducing peptide, usually IGF-2. Rarely, an insulinoma may develop, which is an islet cell tumor (usually benign) that releases high and unregulated concentrations of insulin into the bloodstream.
Many individuals complain of postprandial hypoglycemia, frequently called reactive hypoglycemia. Despite long years of skepticism, investigators now believe that at least some of these patients do indeed experience true symptoms of hypoglycemia within a few hours of eating. There is no absolute glucose level at which symptoms occur; many people can tolerate extremely low levels of glucose without any problems. However, a rather high rate of decline in the plasma glucose level after a meal may cause symptoms. One cause of postprandial hypoglycemia may be a delay in the timing of insulin release after a meal. Thus, the β cells release too much insulin too late after a meal, so the blood glucose level initially rises markedly and then falls rapidly. In some patients, this defect may herald the development of diabetes mellitus.
Circulating insulin comes only from the β cells of the pancreatic islet. It is encoded by a single gene on the short arm of chromosome 11. Exposing islets to glucose stimulates insulin synthesis and secretion. Though the process is not completely understood, this stimulation requires that the glucose be metabolized.
Transcription of the insulin gene product and subsequent processing produces full-length messenger RNA (mRNA) that encodes preproinsulin. Starting from its 5′ end, this mRNA encodes a leader sequence and then peptide domains B, C, and A. Insulin is a secretory protein (see pp. 34–35 ). As the preprohormone is synthesized, the leader sequence of ~24 amino acids is cleaved from the nascent peptide as it enters the rough endoplasmic reticulum. The result is proinsulin ( Fig. 51-2 ), which consists of domains B, C, and A. As the trans Golgi packages the proinsulin and creates secretory granules, proteases slowly begin to cleave the proinsulin molecule at two spots and thus excise the 31–amino-acid C peptide. The resulting insulin molecule has two peptide chains, designated the A and B chains, that are joined by two disulfide linkages. The mature insulin molecule has a total of 51 amino acids, 21 on the A chain and 30 on the B chain. In the secretory granule, the insulin associates with zinc. The secretory vesicle contains this insulin, as well as proinsulin and C peptide. All three are released into the portal blood when glucose stimulates the β cell.
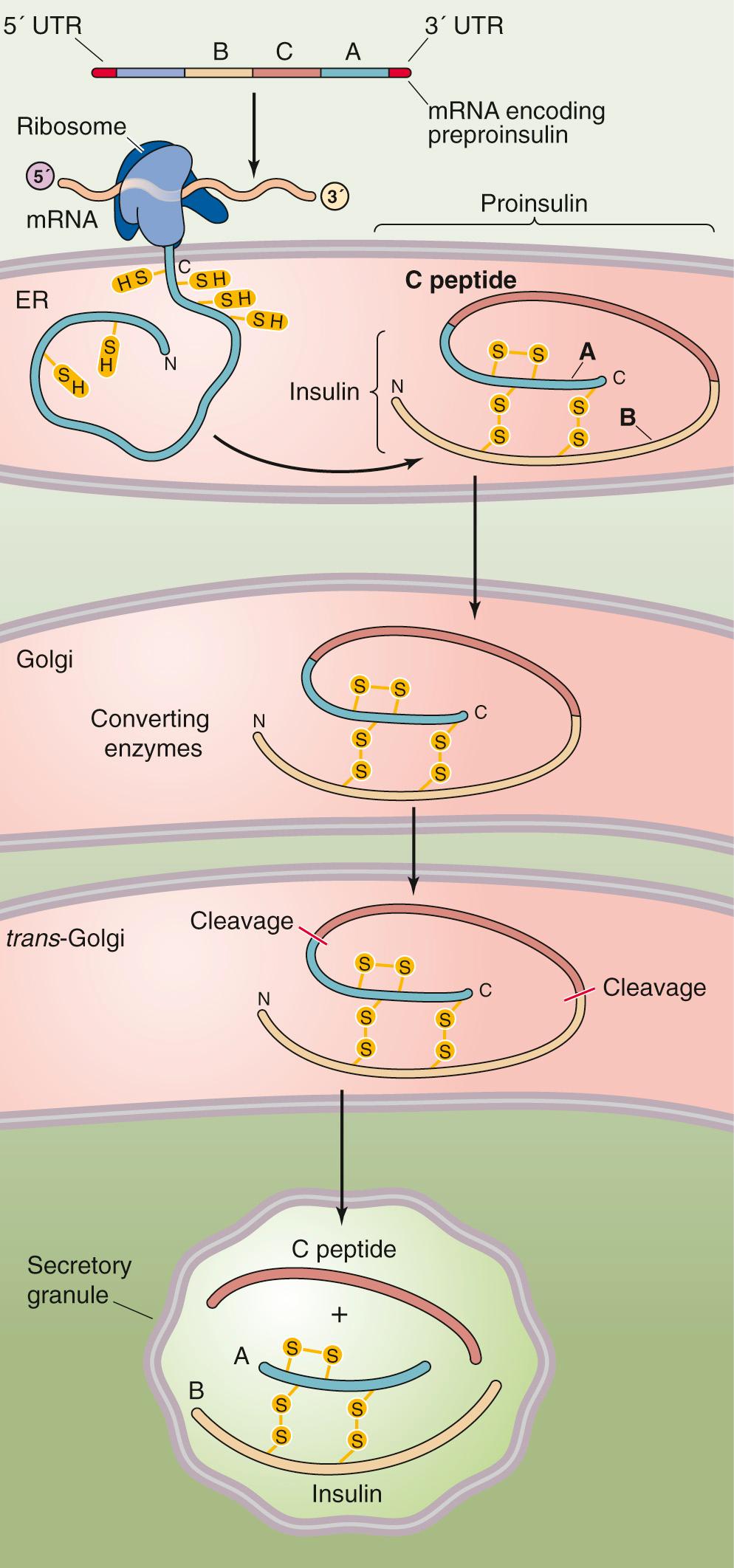
C peptide has no established biological action. Yet because it is secreted in a 1 : 1 molar ratio with insulin, it is a useful marker for insulin secretion. Proinsulin does have modest insulin-like activities; it is ~  th as potent as insulin on a molar basis. However, the β cell secretes only ~5% as much proinsulin as insulin. As a result, proinsulin does not play a major role in the regulation of blood glucose.
th as potent as insulin on a molar basis. However, the β cell secretes only ~5% as much proinsulin as insulin. As a result, proinsulin does not play a major role in the regulation of blood glucose.
Most of the insulin (~60%) that is secreted into the portal blood is removed in a first pass through the liver. In contrast, C peptide is not extracted by the liver at all. As a result, whereas measurements of the insulin concentration in systemic blood do not quantitatively mimic the secretion of insulin, measurements of C peptide do. C peptide is eventually excreted in the urine, and the quantity of C peptide excreted in a 24-hour period is a rough measure of the amount of insulin released during that time.
In healthy individuals, the plasma glucose concentration remains within a remarkably narrow range. After an overnight fast, it typically averages between 4 and 5 mM; the plasma [glucose] rises after a meal, but even with a very large meal it does not exceed 10 mM. Modest increases in plasma [glucose] provoke marked increases in the secretion of insulin and C peptide and hence raise plasma [insulin], as illustrated by the results of an oral glucose tolerance test (OGTT) as shown in Figure 51-3 A . Conversely, a decline in plasma [glucose] of only 20% markedly lowers plasma [insulin]. The change in the concentration of plasma glucose that occurs in response to feeding or fasting is the main determinant of insulin secretion. In a patient with type 1 diabetes mellitus caused by destruction of pancreatic islets, an oral glucose challenge evokes either no response or a much smaller insulin response, but a much larger increment in plasma [glucose] that lasts for a much longer time (see Fig. 51-3 B ).
![Figure 51-3, Glucose tolerance test results. A, When a person ingests a glucose meal (75 g), plasma [glucose] (green curve) rises slowly, reflecting intestinal uptake of glucose. As a result, plasma [insulin] ( solid red curve) rises sharply. When a lower glucose dose is given intravenously (IV) over time—in a manner that reproduces the green curve—plasma [insulin] rises only modestly (dashed red curve). The difference between the insulin responses indicated by the solid and dashed red lines is due to the “incretin effect” of oral glucose ingestion. B, In a patient with type 1 diabetes, the same oral glucose load as that in A causes plasma [glucose] to rise to a higher level and to remain high for a longer time. The diagnosis of diabetes is made if the plasma glucose level is above 200 mg/dL at the second hour. C, If a large IV glucose challenge (0.5 g glucose/kg body weight given as a 25% glucose solution) is administered as a bolus, plasma [glucose] rises much more rapidly than it does with an oral glucose load. Sensing a rapid rise in [glucose], the β cells first secrete some of their stores of presynthesized insulin. Following this “acute phase,” the cells secrete both presynthesized and newly manufactured insulin in the “chronic phase.” Figure 51-3, Glucose tolerance test results. A, When a person ingests a glucose meal (75 g), plasma [glucose] (green curve) rises slowly, reflecting intestinal uptake of glucose. As a result, plasma [insulin] ( solid red curve) rises sharply. When a lower glucose dose is given intravenously (IV) over time—in a manner that reproduces the green curve—plasma [insulin] rises only modestly (dashed red curve). The difference between the insulin responses indicated by the solid and dashed red lines is due to the “incretin effect” of oral glucose ingestion. B, In a patient with type 1 diabetes, the same oral glucose load as that in A causes plasma [glucose] to rise to a higher level and to remain high for a longer time. The diagnosis of diabetes is made if the plasma glucose level is above 200 mg/dL at the second hour. C, If a large IV glucose challenge (0.5 g glucose/kg body weight given as a 25% glucose solution) is administered as a bolus, plasma [glucose] rises much more rapidly than it does with an oral glucose load. Sensing a rapid rise in [glucose], the β cells first secrete some of their stores of presynthesized insulin. Following this “acute phase,” the cells secrete both presynthesized and newly manufactured insulin in the “chronic phase.”](https://storage.googleapis.com/dl.dentistrykey.com/clinical/TheEndocrinePancreas/2_3s20B9781455743773000513.jpg)
A glucose challenge of 0.5 g/kg body weight given as an intravenous bolus raises the plasma glucose concentration more rapidly than glucose given orally. Such a rapid rise in plasma glucose concentration leads to two distinct phases of insulin secretion (see Fig. 51-3 C ). The acute-phase or first-phase insulin response lasts only 2 to 5 minutes, whereas the second-phase insulin response persists as long as the blood glucose level remains elevated. The insulin released during the acute-phase insulin response to intravenous glucose arises from preformed insulin that had been packaged in secretory vesicles docked at, or residing near, the β-cell plasma membrane. The second-phase insulin response also comes from preformed insulin within the vesicles with some contribution from newly synthesized insulin. One of the earliest detectable metabolic defects that occurs in both type 1 and type 2 diabetes is loss of the first phase of insulin secretion, as determined by an intravenous glucose tolerance test. If a subject consumes glucose or a mixed meal, plasma [glucose] rises much more slowly—as in Figure 51-3 A —because the appearance of glucose in plasma depends on gastric emptying and intestinal absorption. Given that plasma [glucose] rises so slowly, the acute-phase insulin response can no longer be distinguished from the chronic response, and only a single phase of insulin secretion is apparent. However, the total insulin response to an oral glucose challenge exceeds the response observed when comparable changes in plasma [glucose] are produced by intravenously administered glucose (see Fig. 51-3 A ). This difference is referred to as the incretin effect ( Box 51-2 ).
Cloning of the insulin gene has led to an important therapeutic advance, namely, the use of recombinant human insulin for the treatment of diabetes. Human insulin was the first recombinant protein available for routine clinical use. Before the availability of human insulin, either pork or beef insulin was used to treat diabetes. Pork and beef insulin differ from human insulin by one and three amino acids, respectively. The difference, although small, is sufficient to be recognized by the immune system, and antibodies to the injected insulin develop in most patients treated with beef or pork insulin; occasionally, the reaction is severe enough to cause a frank allergy to the insulin. This problem is largely avoided by using human insulin.
Sequencing of the insulin gene has not led to a major understanding of the genesis of the common forms of human diabetes. However, rare patients with diabetes make a mutant insulin molecule with a single amino-acid substitution in either the A or B chain. In each case that has been described, these changes lead to a less-active insulin molecule (typically only ~1% as potent as insulin on a molar basis). These patients have either glucose intolerance or frank diabetes, but very high concentrations of immunoreactive insulin in their plasma. In these individuals, the immunoreactivity of insulin is not affected to the same extent as the bioactivity.
In addition to revealing these mutant types of insulin, sequencing of the insulin gene has allowed identification of a flanking polymorphic site upstream of the insulin gene that contains one of several common alleles. In some populations, certain polymorphisms are associated with an increased risk of development of type 1 diabetes mellitus.
The pancreatic β cells take up and metabolize glucose, galactose, and mannose, and each can provoke insulin secretion by the islet. Other hexoses that are transported into the β cell but that cannot be metabolized (e.g., 3- O -methylglucose or 2-deoxyglucose) do not stimulate insulin secretion. Although glucose itself is the best secretagogue, some amino acids (especially arginine and leucine) and small keto acids (e.g., α-ketoisocaproate, α-ketoglutarate), as well as ketohexoses (fructose), can also weakly stimulate insulin secretion. The amino acids and keto acids do not share any metabolic pathway with hexoses other than oxidation via the citric acid cycle (see p. 1185 ). These observations have led to the suggestion that the ATP generated from the metabolism of these varied substances may be involved in insulin secretion. In the laboratory, depolarizing the islet cell membrane by raising extracellular [K + ] provokes insulin secretion.
From these data has emerged a relatively unified picture of how various secretagogues trigger insulin secretion. Key to this picture is the presence in the islet of an ATP-sensitive K + channel and a voltage-gated Ca 2+ channel in the plasma membrane ( Fig. 51-4 ). The K + channel ( K ATP ; see p. 198 ) is an octamer of four Kir6.2 channels (see p. 196 ) and four sulfonylurea receptors ( SURs; see p. 199 ; Box 51-3 ), Glucose triggers insulin release in a seven-step process:
Step 1: Glucose enters the β cell via the GLUT2 glucose transporter by facilitated diffusion (see p. 114 ). Amino acids enter through a different set of transporters.
Step 2: In the presence of glucokinase (the rate-limiting enzyme in glycolysis), the entering glucose undergoes glycolysis as well as oxidation via the citric acid cycle (see p. 1185 ), phosphorylating ADP and raising [ATP] i . Some amino acids also enter the citric acid cycle. In both cases, the following ratios increase: [ATP] i /[ADP] i , [NADH] i /[NAD + ] i , and [NADPH] i /[NADP + ] i (NADH and NAD + are the reduced and oxidized forms of nicotinamide adenine dinucleotide [NAD], and NADPH and NADP + are the reduced and oxidized forms of NAD phosphate) ![]() N51-5
N51-5
Step 3: The increase in the ratio [ATP] i /[ADP] i , or [NADH] i /[NAD + ] i , or [NADPH] i /[NADP + ] i causes K ATP channels (see p. 198 ) to close.
Step 4: Reducing the K + conductance of the cell membrane causes the β cell to depolarize (i.e., the membrane potential is less negative).
Step 5: This depolarization activates voltage-gated Ca 2+ channels (see pp. 190–191 ).
Step 6: The increased Ca 2+ permeability leads to increased Ca 2+ influx and increased intracellular free Ca 2+ . This rise in [Ca 2+ ] i additionally triggers Ca 2+ -induced Ca 2+ release (see pp. 242–243 ).
Step 7: The increased [Ca 2+ ] i , perhaps by activation of a Ca 2+ -calmodulin phosphorylation cascade, ultimately leads to insulin release.
![Figure 51-4, Mechanism of insulin secretion by the pancreatic β cell. Increased levels of extracellular glucose trigger the β cell to secrete insulin in the seven steps outlined in this figure. Metabolizable sugars (e.g., galactose and mannose) and certain amino acids (e.g., arginine and leucine) can also stimulate the fusion of vesicles that contain previously synthesized insulin. In addition to these fuel sources, certain hormones (e.g., glucagon, somatostatin, cholecystokinin [CCK]) can also modulate insulin secretion. ER, endoplasmic reticulum; IP 3 , inositol 1,4,5-trisphosphate; PLC, phospholipase C. Figure 51-4, Mechanism of insulin secretion by the pancreatic β cell. Increased levels of extracellular glucose trigger the β cell to secrete insulin in the seven steps outlined in this figure. Metabolizable sugars (e.g., galactose and mannose) and certain amino acids (e.g., arginine and leucine) can also stimulate the fusion of vesicles that contain previously synthesized insulin. In addition to these fuel sources, certain hormones (e.g., glucagon, somatostatin, cholecystokinin [CCK]) can also modulate insulin secretion. ER, endoplasmic reticulum; IP 3 , inositol 1,4,5-trisphosphate; PLC, phospholipase C.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/TheEndocrinePancreas/3_3s20B9781455743773000513.jpg)
An entire class of drugs—the sulfonylurea agents—is used in the treatment of patients with type 2 diabetes, or non–insulin-dependent diabetes mellitus (NIDDM). Type 2 diabetes arises from two defects: (1) β cells are still capable of making insulin but do not respond adequately to increased blood [glucose], and (2) insulin target tissues are less sensitive or “resistant” to insulin.
The sulfonylurea agents were discovered accidentally. During the development of sulfonamide antibiotics after the Second World War, investigators noticed that the chemically related sulfonylurea agents produced hypoglycemia in laboratory animals. These drugs turned out to have no value as antibiotics, but they did prove effective in treating the hyperglycemia of type 2 diabetes. The sulfonylureas enhance insulin secretion by binding to the SUR subunits (see p. 199 ) of K ATP channels, thereby decreasing the likelihood that these channels will be open. This action enhances glucose-stimulated insulin secretion (see Fig. 51-4 ). By increasing insulin secretion, sulfonylureas overcome insulin resistance and decrease blood glucose in these patients.
Unlike insulin, which must be injected, sulfonylureas can be taken orally and are therefore preferred by many patients. However, they have a therapeutic role only in type 2 diabetes; the β cells in patients with type 1 diabetes are nearly all destroyed, and these patients must be treated with insulin replacement therapy.
Figure 58-1 mentions that glucose-6-phosphate can have three major fates. The anabolic series of reactions summarized in this figure convert glucose-6-phosphate to glycogen. The glycolytic pathway summarized in Figure 58-6 A is a catabolic pathway that converts glucose-6-phosphate to pyruvate. The third fate— the pentose phosphate pathway —is another catabolic series of reactions that converts glucose-6-phosphate to ribose-5-phosphate.
The pentose phosphate pathway has two major products, NADPH and ribose-5-phosphate. The cell can use the reducing equivalents in NADPH (i.e., energy “currency”) to reduce double bonds in the energy-consuming synthesis of fatty acids and steroids. These reactions are particularly important in such tissues as liver, adipose tissue, mammary gland, and adrenal cortex. Note that the cell cannot use NADH to create NADPH. Thus, the pentose phosphate pathway is critical. The second product of the pathway, ribose-5-phosphate, is important for the synthesis of ribonucleotides, which is particularly important in growing and regenerating tissues. The pentose phosphate pathway involves four reactions, the first and third of which involve the conversion of NADP + to NADPH and H + .
If the cell does not use the ribose-5-phosphate to generate ribonucleotides, the cell can use a complex series of reactions to convert the ribose-5-phosphate to fructose-6-phosphate. This sequence of reactions (i.e., from glucose-6-phosphate to ribose-5-phosphate to fructose-6-phosphate) bypasses or “shunts” the conversion of glucose-6-phosphate to fructose-6-phosphate, which would otherwise be catalyzed by phosphoglucose isomerase (see Fig. 58-6 A ). For this reason, the pentose phosphate pathway is also called the hexose monophosphate shunt. *
* Note that the term shunt is a bit of a misnomer, inasmuch as the “shunt” is not a shortcut from glucose-6-phosphate to fructose-6-phosphate (normally catalyzed in one step by phosphoglucose isomerase), but rather a lengthy detour!
However, the reader should be reassured that the shunt does not permit the cell to generate two NADPH molecules for free. Three glucose-6-phosphate molecules (3 × 6 = 18 carbons) must traverse the hexose monophosphate shunt to generate six NADPH molecules (3 × 2 = 6) plus two fructose-6-phosphate (2 × 6 = 12 carbons) molecules, a single glyceraldehyde-3-phosphate (1 × 3 = 3 carbons), and three CO 2 molecules that arise from a decarboxylation reaction in the pentose phosphate pathway (3 × 1 = 3 carbons). If those three glucose molecules had gone through the classical glycolytic pathway, they would have generated 3 × 2 = 6 net ATPs and 6 NADHs (see Table 58-3 ). However, if those same three glucose molecules all go through the pentose phosphate pathway, the net result is only five ATPs, only five NADHs, but six NADPHs. Thus, the cell gives up only one ATP and one NADH for the sake of generating six NADPHs—not a bad deal for the cell!
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here