Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Malignant primary tumors of the central nervous system (CNS) occur in about 25,500 individuals and account for an estimated 13,700 deaths in the United States annually, a mortality rate of 6.5 per 100,000. Based on most recent reports, benign tumors of the CNS are about twice as common as malignant brain tumors, but with a much lower mortality rate. Overall, CNS cancer is estimated to represent about 1% of newly occurring malignant tumors. In children and young adults, brain tumors are a major health problem second only to leukemia as a cause of cancer-related deaths, and they are the third leading cause of cancer-related death between ages 15 and 34. The age-adjusted incidence appears to be similar to that observed in other developed countries.
Brain tumors are a diverse group of neoplasms. The particular combination of somatic genetic alterations found in different brain tumors that are histologically distinguishable can vary considerably among individual examples of a particular tumor type. Similarly, extensive molecular and genetic variation is well documented within tumors that are histologically indistinguishable. Recognition of a number of hereditary and nonfamilial syndromes in which brain tumors play a prominent role or occur with increased frequency provide compelling evidence for the importance of several cell regulatory pathways in brain tumor pathogenesis ( Table 40-1 ). Primary CNS tumors have been classified by surgeons and pathologists on the basis of their location and histologic appearance, providing important information that guides treatment with conventional antineoplastic modalities including surgery, irradiation, and chemotherapy as well as contributing to prognostication. More recently, the drive toward more targeted therapy has focused attention on approaches to classification emphasizing tumor type–specific molecular alterations. Glial tumors account for 50% to 60% of all primary brain tumors, and with the exception of some pilocytic astrocytoma, most are malignant. Astrocytomas account for the great majority of the glial tumors, whereas the second most common type is oligodendroglioma. Exposure to ionizing radiation is the only well-documented environmental risk factor for the development of glioma. Benign CNS tumors consist primarily of meningioma and low-grade glial tumors.
| Syndrome | Gene | Chromosomal Location | Associated Central Nervous System Tumors |
|---|---|---|---|
| Neurofibromatosis type 1 | NF1 | 17q | Optic glioma |
| Neurofibromatosis type 2 | NF2 | 22q12 | Acoustic schwannomas, meningioma, ependymoma, glial tumors |
| Retinoblastoma | RB | 13q14 | Retinoblastoma, pinealoblastoma |
| Li-Fraumeni syndrome | P53 | 17p13 | Glioma, medulloblastoma |
| von Hippel-Lindau syndrome | VHL | 3p | Retinal angioma, cerebellar hemangioblastoma |
| Tuberous sclerosis | TSC1 TSC2 |
9q 16p13 |
Giant cell subependymal astrocytoma, astrocytoma, ependymoma |
| Turcot syndrome | APC, hMSH1, hMSH2, hPMS2 | 5q21 | Astrocytoma, medulloblastoma |
| Gorlin syndrome | PTCH1 | 9q22.1-q31 | Medulloblastoma, astrocytoma |
| Ollier disease | IDH1, IDH2 | 2q33.3, 15q26.1 | Endochondroma, rarely glioma |
In addition to rare families in which there is strong evidence for a hereditary basis for the development of familial meningioma or glioma, a number of well-studied cancer predisposition syndromes include among their associated stigmata the development of CNS tumors (see Table 40-1 ). These are important clinical observations and have provided insights into the pathogenesis of several different types of primary brain tumors. Typically these syndromes are associated not only with an increased incidence of brain tumors, but also with the occurrence of tumors at an earlier age than those arising spontaneously and with the finding of multicentric tumors. Inherited syndromes associated with brain tumors are inherited as autosomal dominant disorders and arise as the result of the germline mutation of one allele of a tumor suppressor gene whose other copy is typically inactivated in the tumors that arise. The most commonly occurring brain tumor predisposition syndrome is neurofibromatosis type 1 (NF1). NF1 affects approximately 1 in 3000 live births; approximately 15% of these have radiographic evidence of optic glioma early in childhood, although most cases do not become symptomatic. Although peripheral nervous system tumors are common in NF1 patients, these patients are also at increased risk of developing pilocytic astrocytomas and, more rarely, malignant astrocytomas. The protein product of the NF1 gene, located on the long arm of chromosome 17, is neurofibromin 1 (NF1), which functions to antagonize the proliferative function of p21(ras). Neurofibromatosis type 2 (NF2) is distinct from NF1, affecting approximately one in 50,000 individuals. The NF2 gene, at 22q12.2, encodes a protein known as neurofibromin 2 or merlin (NF2). NF2 patients frequently present before the third decade of life with deafness caused by bilateral schwannomas of the eighth cranial nerve. Less commonly NF2 patients develop neuronal schwannoma including tumors of the spinal and cranial nerves, meningioma, low-grade astrocytoma, and ependymoma. The Li-Fraumeni syndrome is observed in patients with germline TP53 mutations. Although affected family members are predisposed to a number of different tumor types, brain tumors are among the more common and include astrocytomas, medulloblastomas, and choroid plexus tumors. The TP53 protein product, p53, plays an important role in the DNA damage checkpoint of cells and in regulating apoptosis. Its function is commonly inactivated by mutation in a wide variety of human tumors and is thought to play an important role in sporadically occurring astrocytic tumors.
Brain tumors are thought to arise as the result of genetic and epigenetic alterations that activate oncogenes and inactivate tumor suppressor genes ( Figure 40-1 , and see Table 40-1 ). The precise cell of origin in which the most common brain tumors arise is unknown, but emerging evidence suggests that the malignant transformation of different cell types can give rise to high-grade glioma. There is convincing evidence in animal models of glioma that tumors can arise from the malignant transformation of neural stem cells, very early lineage-specific precursor cells, and more differentiated cells that dedifferentiate and take on key characteristics of precursor cells such as proliferative potential, migratory capacity, and multipotentiality. Identifying the cell type in which tumors arise may provide insight into critical pathologic pathways used by tumor cells. Mouse models of glioma provide strong evidence for the possibility of multiple different cells being targets for malignant transformation, resulting in high-grade glioma.
Significant recent progress has also been made in understanding the cell biology of high-grade glial tumors. An emerging body of data indicates that there is a cellular subpopulation in human glioma, typically consisting of very rare cells, that is distinguished from other tumor cells by the ability to grow in vitro in suspension as neurospheres and to recapitulate the tumor of origin when inoculated orthotopically into immunosuppressed mice. This capacity for tumor initiation is typically not shared with the overwhelming majority of tumor cells, and in contrast to other strategies for immortalizing human glioma, tumor cells grown as neurospheres tend to retain a larger number of the genetic alterations present in the tumors from which they are derived. Study of these tumor-initiating cells, sometimes referred to as tumor stem cells, indicates that they exhibit increased resistance to both radiation and chemotherapy, making them a compelling target for the development of future therapeutic interventions.
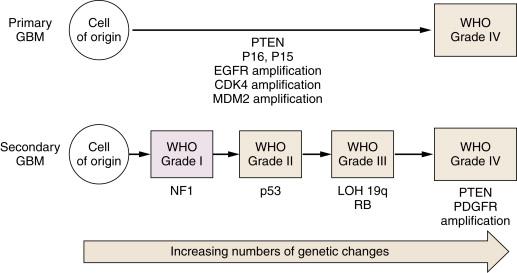
Diffusely infiltrating astrocytomas are so named because they display morphologic and some biochemical evidence of astroglial differentiation. These are the most common tumors of adults and children, occur throughout the CNS, and exhibit a wide range of histopathologic appearances and clinical behaviors. These tumors are organized by the World Health Organization (WHO) according to tumor grade. Histologically, malignancy is manifested as hypercellularity, cellular atypia, endothelial proliferation, necrosis, and invasion of normal adjacent tissue. WHO grade I tumors are variants of astrocytoma that are generally benign, and WHO grade IV, also known as glioblastoma multiforme (GBM), is the most aggressively malignant. Prognosis is closely associated with pathologic grade. WHO grades II and III exhibit intermediate grades of malignancy, but the evidence of increased mitotic activity in grade III tumors is likely to be a key contributor to poor prognosis. Although low-grade tumors can exhibit a circumscribed growth pattern, all pathologic grades of glioma can exhibit invasiveness, and this characteristic compromises the possibility of treating these tumors with surgery alone. The known propensity of some astrocytic tumors that present as low-grade tumors to progress over time to higher grade tumors has provided insight into the pathogenesis of these tumors, suggesting that they are closely related and that progression is associated with the acquisition of sequential genetic alterations (see Figure 40-1 ; Table 40-2 ). High-grade astrocytic tumors, GBMs that arise in this manner, are called secondary GBMs. The finding of selected genetic changes (e.g., TP53 mutation) in lower grade tumors that are also present in higher grade tumors along with additional mutations typically found only in higher grade tumors (e.g., epidermal growth factor receptor [EGFR] amplification) suggests that specific genetic alterations are associated with particular pathologic features characteristic of the corresponding grade. Genetic changes currently thought to be important in this regard are shown in Figure 40-1 and Table 40-2 , an adaptation of the pathogenesis model first proposed for colon cancer.
| Tumor Suppressor Gene | Proto-oncogene | ||
|---|---|---|---|
| Gene or Locus | Chromosomal Location | Gene or Locus | Chromosomal Location |
| VHL | 3p25.3 | EGFR | 7p12 |
| TSC1, TSC2 | 9q34, 16p13.3 | PDGFRA | 5q31-q32 |
| PTCH1 | 9q22.3 | MDM2 | 12q14 |
| REST | Chr. 4 | HRAS, NRAS | 11p15, 1p13 |
| CDKN2A, CDKN2B | 9p21 | CMYC, NMYC | 8q24, 2p23-24 |
| P53 | 17p13.1 | ||
| NF2 | 22q12 | ||
| NF1 | 17q11.2 | ||
| RB1 | 13q14 | ||
| APC | 9q31 | ||
| PTEN | 10q23 | ||
| IDH1, IDH2 | 2q33.3, 15q26 | ||
Mutation of the TP53 gene is likely to be an early event associated with the change of normal cells to low-grade neoplasia. Commonly mutated residues are codons 248 and 273. TP53 has an important role in stabilizing the genome, and the genetic instability resulting from its loss may contribute to the accumulation of multiple mutations in a single cell that are required for the development of highly malignant tumors. Inactivation of TP53 by mutation or epigenetic mechanisms may occur in up to 75% of astrocytomas. MDM2, encoding MDM2, a protein that inhibits the ability of p53 to promote transcription by targeting the protein for degradation, is amplified in approximately 10% of gliomas, and these invariably have a wild-type TP53 gene. A second gene that is also likely to be mutated early in gliomagenesis is IDH1 . Mutations of IDH1 were first identified in studies sequencing glioma cell genomes. Mutations of IDH1 are found both in lower grade glioma and in GBM . IDH1 encodes isocitrate dehydrogenase, a Krebs cycle enzyme, and its role in tumorigenesis is an area of intense investigation.
Loss of heterozygosity (LOH) of Ch9p21 at the site of the CDKN2A and CDKN2B loci leads to homozygous deletion of these adjacent genes in approximately 60% of GBM. This deletion results in the loss of the p15 (INK4B), p16 (INK4A), and p14 (ARF) tumor suppressor proteins. CDKN2A and CDKN2B encode cyclin-dependent kinase inhibitors, p16 (INK4A) and p15 (INK4B), respectively, which bind to the cyclin-dependent protein kinases CDK4 and CDK6 inhibiting the catalytic activity of the cyclin D–CDK complex. p1 (ARF) is expressed from a distinct transcript as the result of alternative splicing of the CDKN2A gene and functions to keep MDM2 in the nucleolus so that it cannot degrade p53. Loss of p14 (ARF) results in the enhanced degradation of p53. Other cytogenetic changes including +7p/q, +19q, and –1p/q are widely recognized in high-grade brain tumors, but the best understood is clearly the deletion of chromosome 10, where PTEN is located. The PTEN protein product, PTEN, is a lipid phosphatase that antagonizes the function of the phosphatidylinositol-3-kinase (PI3K) family of lipid messengers and consequently inhibits downstream signaling through AKT1, a serine/threonine kinase that is a key regulator of critical cell functions including cell proliferation and survival.
Several different receptor tyrosine kinases (RTKs) and their cognate ligands have been implicated in the malignant behavior of astrocytic tumors, and especially GBM. These include PDGF/PDGFR, EGF and TGF-α/EGFR, IGF/IGFR, and others. Although amplification of EGFR is found in approximately 50% of GBMs, this change is rarely found in WHO grade III and never in grade I or II tumors. Also, small deletions and rearrangements of the EGFR gene are commonly found in GBM. As many as 50% of tumors in which amplification of EGFR is detectable also have a rearrangement of EGFR in which exons 2 to 7 are deleted. This results in an in-frame deletion that encodes a constitutively active EGFR ( EGFRvlll ), which, along with EGFR amplification in tumor cells, predicts a poor outcome. Both antibodies and small-molecule therapeutics have been used to target EGFR in glioma, but resistance to such therapies is rapidly manifested when these treatments are used alone. Although members of the PDGF pathway tend to be overexpressed in all grades of astrocytic tumors, amplification and mutation are rare. Activation of RTKs in glioma leads to autophosphorylation of these receptors, which in turn leads to the docking of a series of proteins on phosphorylated sites of the receptors and downstream activation of various effectors. Activation of RAS by the docking protein SOS1 leads to initiation of the RAF/MEK/MAPK (ERK) pathway that leads to the transcriptional activation of genes important for proliferation. Docking of GAB1 to another phosphorylated site on autophosphorylated RTKs leads to activation of PI3K, which in turn initiates a cascade of phosphorylation leading to PDK1 phosphorylation of AKT1. Activated AKT1 in turn phosphorylates both a number of pro-apoptotic proteins, inactivating them, and a series of transcription factors, activating and leading to transcription of other genes important for cellular proliferation. About 80% of GBMs exhibit activation of AKT1, primarily as a result of RTK activation or deletional inactivation of PTEN .
It is now widely recognized that not all GBMs exhibit the same constellation of genetic alterations, and, interestingly, a second clinical presentation of GBM seems to be associated with a distinctive genetic profile (see Figure 40-1 ; Table 40-3 ). Approximately 90% of patients with GBMs present without evidence of a precursor lesion (de novo, primary GBM). Although high levels of EGFR expression are frequently found in primary GBM, these are rarely detectable in secondary GBM, which is associated with lower grade precursor lesions. In primary GBM, amplification of MDM2 is a more common mechanism for inactivation of TP53 than it is in secondary GBM. The CDKN2A and PTEN loci are also frequently inactivated by mutation in primary GBM, whereas IDH1 is more commonly mutated in secondary GBM.
| Glioblastoma Multiforme Subtypes (Frequency of Mutation) | ||||
|---|---|---|---|---|
| Genes Mutated in Glioblastoma | Proneural | Neural | Classical | Mesenchymal |
| TP53 | 54% | 21% | 0% | 12% |
| NF1 | 5% | 16% | 5% | 37% |
| EGFR | 16% | 5% | 0% | 0% |
| EGFRvIII | 3% | 0% | 23% | 3% |
| IDH1 | 30% | 5% | 0% | 0% |
| PDGFRA | 11% | 0% | 0% | 0% |
It has not yet been possible to recognize a characteristic pathology or clinical presentation associated with the genetic heterogeneity that is now well documented to accompany the histologic and cytologic variation characteristic of GBM. Current interpretation of the extensive genomic evaluation of glioma gene structure and gene expression suggests that among histologically indistinguishable tumors, there are definable subtypes of glioma characterized by distinctive molecular alterations (see Table 40-3 ). These classifications may be of prognostic significance, although proven targets for therapeutic intervention have not yet been identified.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here