Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The chemical composition of food is complex and little of it is water soluble; therefore, it cannot enter the body fluids unaltered. A series of digestive processes enables food to be broken down and absorbed. These processes take place in the alimentary canal, which consists of the mouth, oesophagus and gastrointestinal tract, and associated exocrine glands producing secretions that act on food.
The functions of the alimentary canal are concerned with storage, digestion and absorption of food together with the excretion of undigested food and waste products. Digestion is the process of breaking down complex food molecules, by mechanical and chemical methods, into simple ones that can be absorbed. The digestion products, together with salts and water, are absorbed into the blood and lymphatic systems. In addition, the alimentary canal serves to host microorganisms (microbiota) that contribute to health and disease and also protects the body from bacterial toxins and swallowed noxious chemicals.
Four activities of the alimentary canal can be identified. These are
Motility
Secretion
Digestion
Absorption.
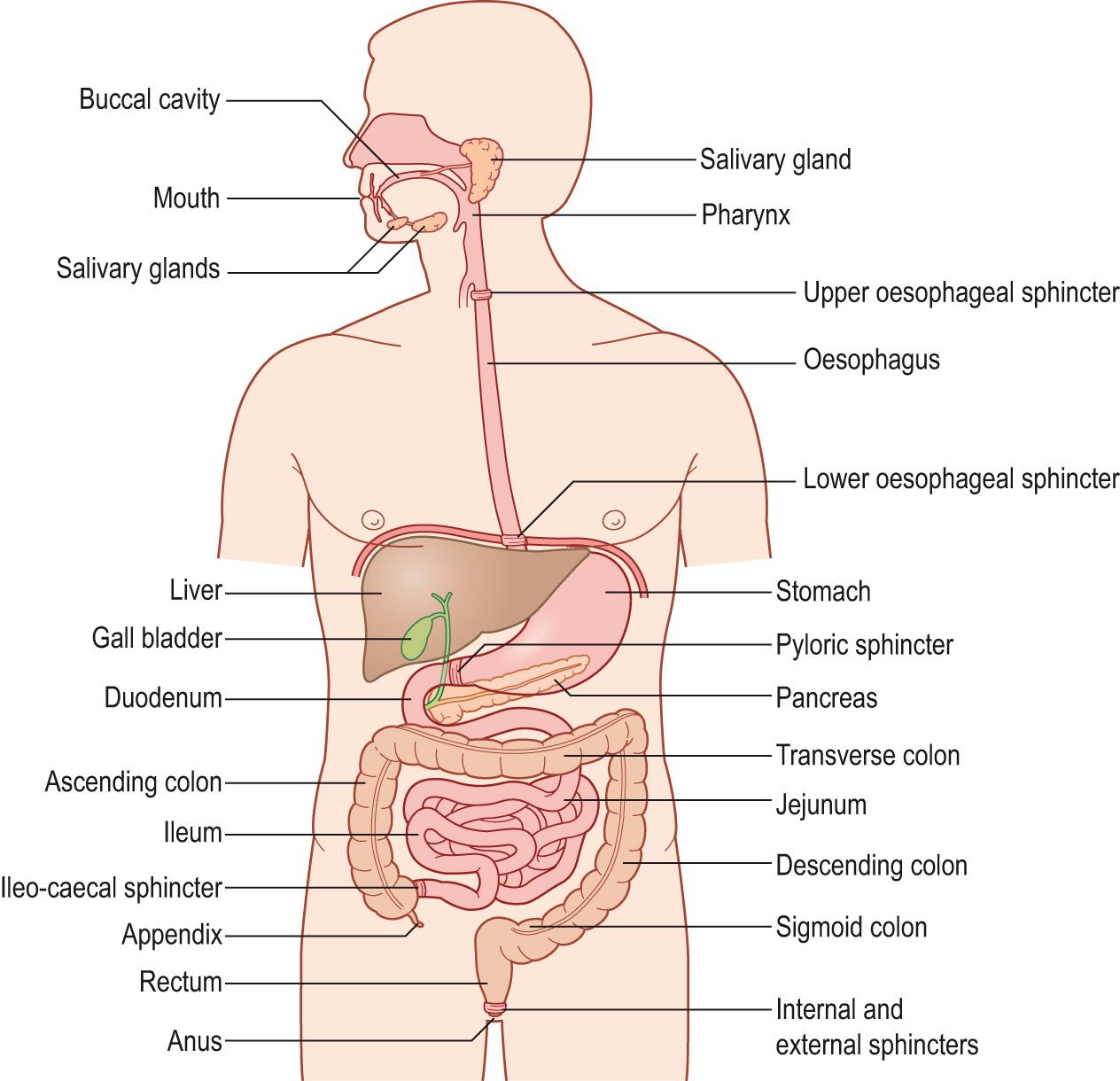
Motility is the term used to describe movements of the alimentary canal that are responsible for propelling partly digested food along the canal and for mixing the food with the digestive secretions so that digestion and absorption can take place in a regulated manner. These activities are inter-related. In health there is a balance, and we pay little attention to alimentary function. Disruption of one activity in disease leads to an imbalance, and we become conscious of gastrointestinal function, e.g. pain and peptic ulceration, diarrhoea, constipation, etc. Coordination of alimentary function depends on the combined action of the nervous, endocrine (circulating hormones) and paracrine (local hormones) systems. The anatomy of the alimentary system and associated glands is shown in Fig. 15.1 .
The mouth or oral cavity consists of the lips, tongue, gums, teeth, hard and soft palate and the pharynx, together with the salivary glands. Food is ingested, mixed with saliva, chewed and swallowed.
The oesophagus is a muscular tube lying in the thorax and abdomen that connects the pharynx to the stomach. It is separated from the pharynx by the upper oesophageal sphincter. Food and drink are propelled to the stomach by the action of the oesophageal muscles.
The stomach lies in the abdomen below the diaphragm. It is separated from the oesophagus and small intestine by the lower oesophageal (cardiac) and pyloric sphincters, respectively.
The duodenum forms the first part of the small intestine. It receives the pancreatic and biliary ducts from the pancreas and liver, respectively.
The jejunum and ileum are a continuation of the small intestine. The ileum terminates at the ileo-caecal junction .
The large intestine consists of the caecum with the appendix , the colon (divided into three sections: ascending, transverse and descending) and the rectum .
The anus is the opening at the end of the large intestine. An internal and an external anal sphincter control the opening.
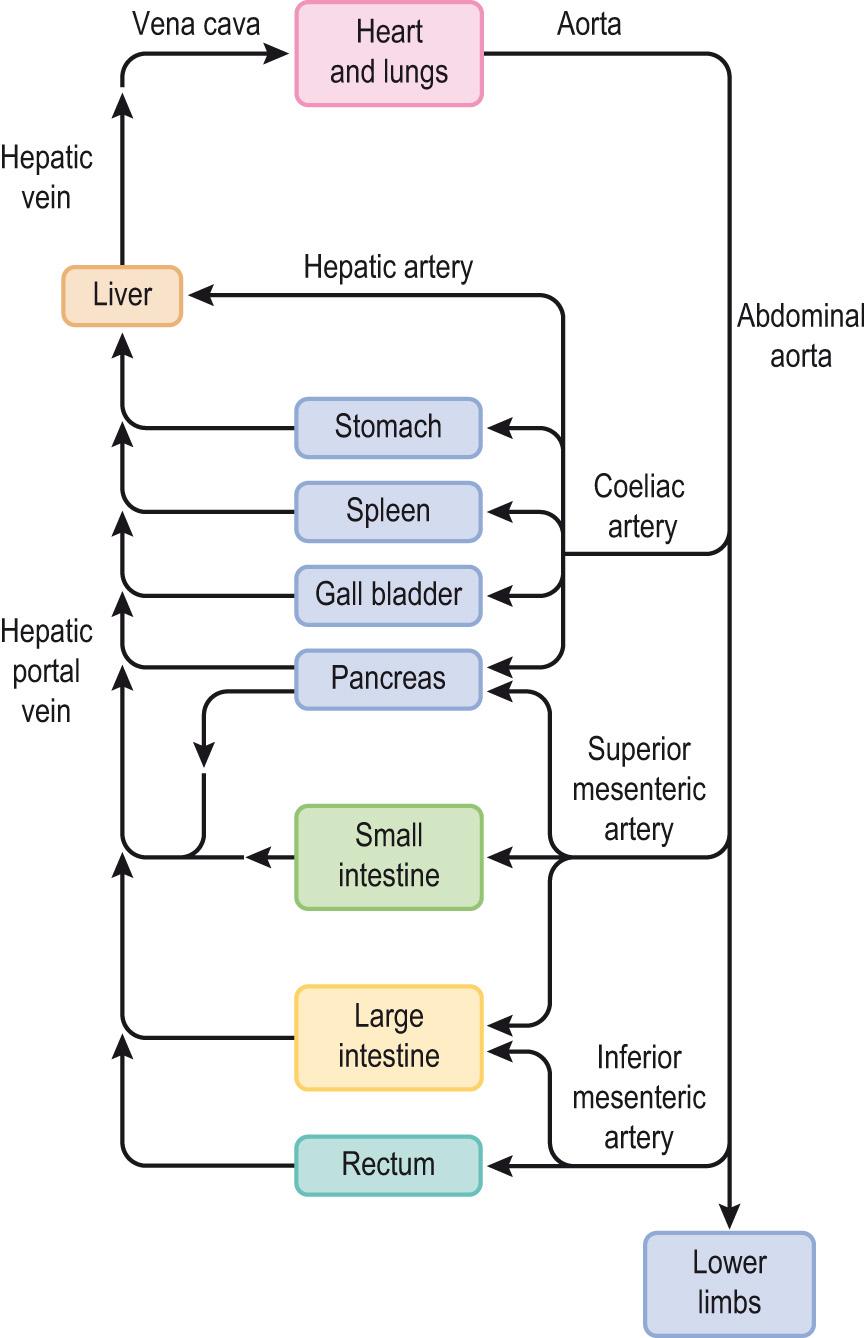
The gastrointestinal tract, liver, gall bladder, pancreas and spleen are supplied with blood from the splanchnic circulation in a number of parallel circuits ( Fig. 15.2 ) . Blood is delivered via three major arteries:
Coeliac artery to the liver, gall bladder, pancreas, stomach and spleen
Superior mesenteric artery to the pancreas, small intestine and most of the large intestine
Inferior mesenteric artery to the terminal portions of the large intestine and rectum.
The arterial supply divides into a capillary network within the digestive organs that subsequently drains into the hepatic portal vein, which enters the liver. The blood from the hepatic portal vein flows through liver sinusoids before being returned to the heart by the inferior vena cava. The liver also receives approximately 20%–25% of its blood supply from the hepatic branch of the coeliac artery. Blood flow through the splanchnic circulation is enhanced during feeding.
The control of blood flow to the different mucosal and muscle layers of the gastrointestinal tract is regulated independently. The neural, endocrine and paracrine mechanisms controlling gastrointestinal blood flow are shown in Table 15.1 .
| Neural mechanisms | Vasoconstrictor action (neurotransmitters) | Vasodilator action (neurotransmitters) |
| Parasympathetic nerves | ACh, VIP | |
| Sympathetic nerves | NE via α adrenoceptors | NE via β 2 adrenoceptors |
| Sensory nerves | CGRP, SP, NKA | |
| Enteric nerves | ACh, VIP, NO | |
| Hormonal mechanisms | Catecholamines (mainly epinephrine (adrenaline)) | Gastrin |
| Angiotensin II | Cholecystokinin | |
| Vasopressin | Secretin | |
| Local mechanisms | Somatostatin Endothelin 1 |
Adenosine Low p O 2 |
| Histamine | ||
| Prostacyclin (PGI 2 ) | ||
| Prostaglandin E 2 |
Mucosal blood flow is required to
Maintain the viability of the mucosa
Provide the precursors for secretory products
Deliver hormones to their target cells
Remove absorbed digestion products, toxins and drugs from the mucosa.
Before nutrients can be absorbed, ingested carbohydrates, protein and fat have to be digested. Disruption of the normal mechanisms for either digestion or absorption (malabsorption), or both, leads to disease. Two mechanisms for the digestion of ingested food can be identified. These are
Physical digestion
Chemical digestion.
Physical digestion is produced by the mechanical activity of the alimentary canal, breaking down pieces of food into smaller particles. The food retains its complex chemical structure, but its surface area is increased to expose more sites to enzymic action. Physical digestion takes place in the
Mouth
Antrum and pylorus of the stomach
Small intestine.
It is dependent upon muscular contractions squeezing and grinding the food, and mixing it with secretions. In addition, bile salts and lecithin act as detergents to emulsify fat globules. These actions are produced by the physical properties of bile salts and lecithin, having fat- and water-soluble components in the molecules ( Clinical box 15.1 ).
Beginning in the mouth, pain from diseased mucous membranes or dental problems interferes with chewing (mastication), the first step in the process of breaking down large food particles. A number of systemic diseases are associated with oral manifestations. Common conditions include the following:
Oral candidiasis (thrush) may be associated with either diabetes mellitus, debilitating illness such as human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS), cancer and blood dyscrasias, or chemotherapy.
Mouth ulcers may be due to infections, e.g. herpes simplex, erythema multiforme (Steven–Johnson syndrome), etc.; other ulcerations of the gastrointestinal tract, e.g. coeliac disease, regional ileitis (Crohn disease), ulcerative colitis, disseminated (or systemic) lupus erythematosus, Behçet disease, etc.; squamous cell carcinoma and other less common tumours; and trauma including ill-fitting dentures.
Absence of teeth or neuromuscular defects in old age (e.g. after a stroke) can interfere with mastication.
Physical digestion in the stomach, i.e. storage of ingested food, mixing the contents to promote fat emulsification, enzyme and acid action, may be impaired through infiltration of gastric musculature by tumour (‘leather bottle stomach’), affecting motility. Pyloric stenosis from peptic ulceration or tumour delays gastric emptying. Surgical resection (partial gastrectomy) increases the rate of gastric emptying (see Clinical box 15.10 ).
Conditions that affect small intestine motility are usually manifested by increased motility and may lead to diarrhoea, abdominal pain and discomfort. Irritable bowel syndrome (IBS) is an example. Other causes include infection, food sensitivities, endocrine disease and radiation enteritis.
Enzymes are responsible for chemical digestion. This involves the hydrolysis of the complex food molecules, i.e. being broken down into their simpler constituents, which are capable of being absorbed. Chemical digestion may occur by means of enzyme activity present in the lumen of the alimentary canal or on the luminal-facing membrane of the epithelial cells (enterocytes) in the small intestine.
The predominant carbohydrates in the diet are
Starch and glycogen (polysaccharides)
Sucrose and lactose (disaccharides)
Fructose (monosaccharide).
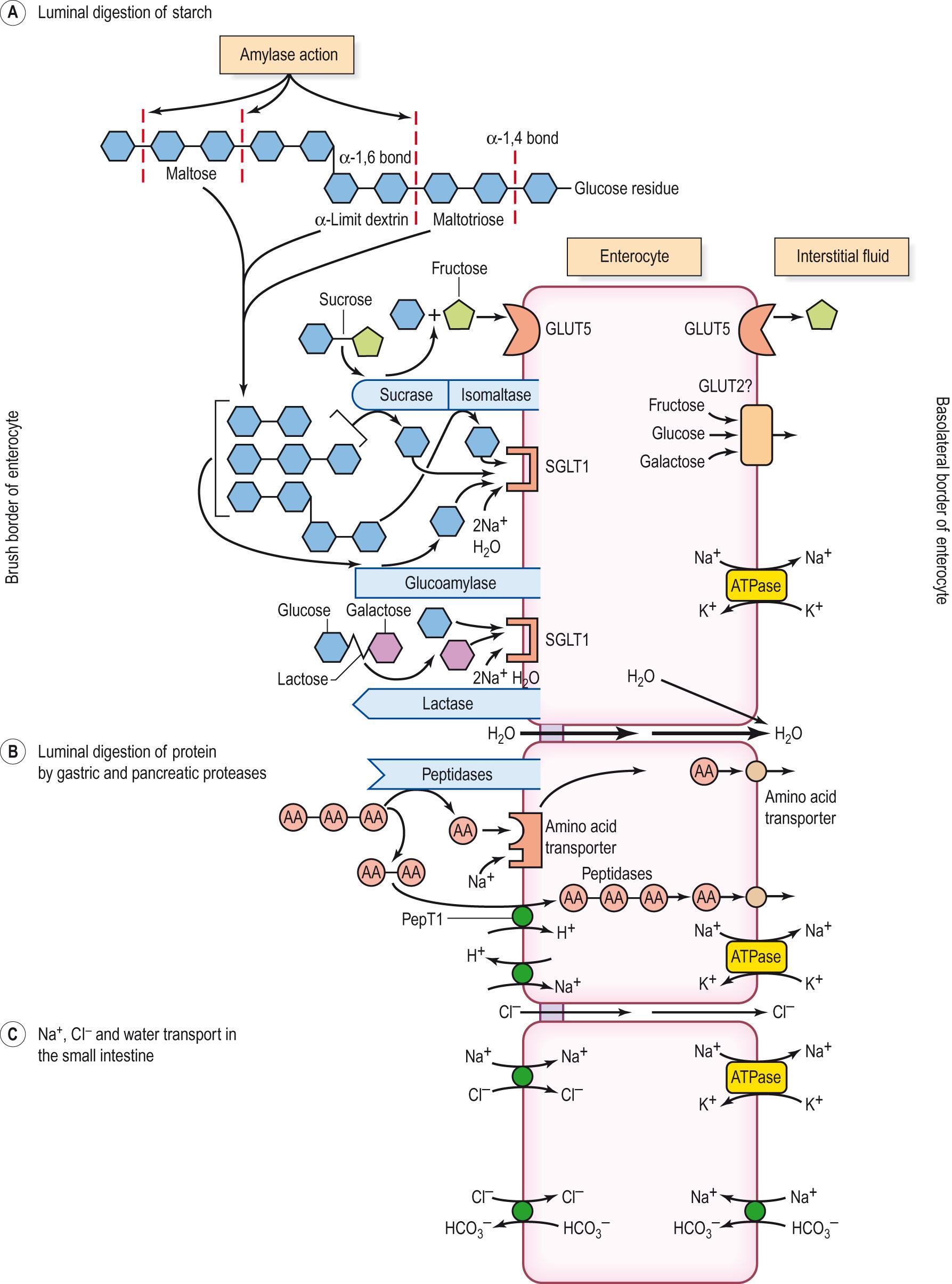
Starch and glycogen are polymers comprising chains of glucose molecules joined together by α-1,4 glycosidic bonds and, at branch points, by α-1,6 glycosidic bonds. Salivary and pancreatic amylases catalyse the hydrolysis of the interior α-1,4 bonds but do not split either the terminal α-1,4 glycosidic bonds or the α-1,6 glycosidic bonds at the branches of starch and glycogen. The products of amylase action are maltose (a disaccharide), maltotriose (a trisaccharide) and α-limit dextrins (branched oligosaccharides) ( Fig. 15.3 ) . However, there are no transport systems for the absorption of these carbohydrates in the intestine. The oligosaccharides derived from starch, together with maltose, sucrose and lactose, are further digested by enzymes present on the brush border of enterocytes to their monosaccharides – glucose, galactose and fructose ( Table 15.2 and Clinical box 15.2 ).
| Enzyme | Substrate | Products |
|---|---|---|
| Glucoamylase (maltase) | Maltose Maltotriose |
Glucose Glucose |
| α-Limit dextrins | Glucose | |
| Isomaltase (α-dextrinase) | α-Limit dextrins | Glucose |
| Sucrase | Sucrose | Glucose and fructose |
| Maltose | Glucose | |
| Maltotriose | Glucose | |
| Lactase | Lactose | Glucose and galactose |
Deficiencies in enzymes concerned with carbohydrate digestion prevent the breakdown of oligo- and di-saccharides to monosaccharides that can be absorbed; therefore, these are excreted undigested instead. This gives rise to diarrhoea after ingesting foods rich in these particular carbohydrates. A common example is milk intolerance due to lactase deficiency, a condition more common in some Asian and Mediterranean countries. It is not a major clinical problem, as most people with lactase deficiency simply avoid milk. A lactose tolerance test is available, but rarely used for adults.
The various sources of protein for digestion are from food and also desquamated gastrointestinal cells and digestive secretions. Proteins and polypeptides consist of amino acids linked together by a peptide bond formed between the amino terminal of one amino acid and the carboxy terminal of another (see Ch. 2 ). Protein digestion is accomplished by enzymes (protease or peptidase) hydrolysing peptide bonds either within a polypeptide chain or protein (endopeptidase) or at the free ends (exopeptidase). Thus, a protein may be broken down to a mixture of small polypeptides and amino acids.
Peptic cells in the stomach secrete pepsinogens, the precursor of a family of enzymes known as pepsins. The acid contents of the stomach activate pepsinogens to pepsins and denature the structure of proteins. Pepsin is an endopeptidase and specifically hydrolyses peptide bonds, containing an aromatic L-amino acid such as phenylalanine or tyrosine. Pepsin is inactivated by the alkaline pH found in the duodenum ( Clinical box 15.3 ).
Deficiency in pancreatic enzyme secretion, notably of trypsinogen and chymotrypsinogen, prevents the conversion of dietary protein into polypeptides for absorption. This can occur in chronic pancreatitis, mainly related to sustained alcohol overuse. Cystic fibrosis sufferers have the same enzyme deficiency. The inability of children with cystic fibrosis to digest protein and fat because of the lack of pancreatic enzymes, trypsin and lipase, leads to nutritional deficiencies in essential amino acids, fatty acids and fat-soluble vitamins. High-dose pancreatic enzyme supplements to treat the resulting steatorrhoea, as well as nutritional supplementation, will be necessary.
Protein digestion in the small intestine occurs owing to the presence of pancreatic proteases ( Table 15.3 ). The most important are trypsin, chymotrypsin and carboxypeptidase. These are secreted as precursors into the duodenum and activated by enteropeptidase (enterokinase) secreted by the duodenal and jejunal mucosa. Enteropeptidase activates trypsinogen to trypsin. Once some trypsin is formed, it acts autocatalytically to convert more trypsinogen to trypsin and also activates chymotrypsinogen to chymotrypsin. Trypsin, chymotrypsin and elastase are endopeptidases and convert protein into polypeptides. Carboxypeptidases release single amino acids from the carboxyl end of polypeptides. The pancreatic proteases active in the lumen of the intestine produce small peptides and some amino acids before being inactivated by autodigestion. The next stage of protein digestion occurs at the brush border of the enterocytes by the action of peptidases present in the luminal membranes to produce small peptides (di-, tri- and tetrapeptides) and single amino acids. Finally, small peptides (usually di- and tri-) are hydrolysed to single amino acids after they have been absorbed into the enterocyte (see Fig. 15.3 ).
| Precursor | Activator | Protease |
|---|---|---|
| Pepsinogens | Acid | Pepsins |
| Trypsinogen | Enteropeptidase | Trypsin |
| Chymotrypsinogen | Trypsin | Chymotrypsin |
| Procarboxypeptidase | Trypsin | Carboxypeptidase |
| Proelastase | Trypsin | Elastase |
The majority of the fats in the diet are triacylglycerols (triglycerides), consisting of glycerol to which three fatty acids are attached (see Ch. 2 ). Fats separate from the water phase of the partly digested food in the stomach and empty slowly. The contractile action of the stomach breaks the fat into small droplets and mixes them with the water phase. The nature of fats and their insolubility in the water create difficulties for their digestion by water-soluble lipases. There are three lipases:
Lingual
Gastric
Pancreatic.
The lingual and gastric lipases have acidic pH optima and function in the stomach, producing fatty acids and diacylglycerols (diglycerides) by attacking the outer ester linkages between glycerol and the fatty acids.
The major site of fat digestion is in the small intestine ( Information box 15.1 ). Fat enters the duodenum relatively slowly so that there is time for the process of emulsification and fat hydrolysis. Fat is emulsified into droplets approximately 500–1000 nm in diameter by the action of bile salts, with their hydrophobic side (fat soluble) dissolving in the fat and their hydrophilic side (water soluble) facing outwards in the water of the intestinal fluid. This process is aided by lecithin and cholesterol, which are also found in bile. The emulsifying agents reduce the surface tension of the fat droplets and keep them apart. These actions increase the surface area of the ingested fat. An additional pancreatic enzyme, colipase, anchors pancreatic lipase to the fat/water interface and activates it. This allows an interaction between the water-soluble pancreatic lipase and the ingested fat. Pancreatic lipase (optimum pH 8.0) preferentially hydrolyses the bonds between glycerol and the fatty acid residues at positions 1 and 3 to produce 2-monoacylglycerols (monoglycerides) and free fatty acids. A small quantity of the 2-monoacylglycerols is hydrolysed to glycerol and a free fatty acid. The presence of fat digestion products, bile salts, cholesterol and phospholipids causes the emulsion to break up into smaller particles, known as mixed micelles, with diameters of up to 5 nm. The bile salts form an outer coat, with the fatty digestion products in the centre. The micelles are small enough to diffuse between the microvilli of the enterocytes.
Failure of secretion of pancreatic lipase and deficiency in bile salts affect fat digestion in the small intestine. Pancreatic lipase deficiency occurs in many conditions, including chronic pancreatitis, carcinoma of the pancreas and cystic fibrosis. Deficiency in bile salts is usually due to obstruction, which may be intra-hepatic, as in cirrhosis of the liver, or extra-hepatic due to gall bladder disease, stones in the biliary tree or tumours. The resulting failure to digest fat leads to the excretion of undigested fat globules in the form of pale, bulky, offensive and sometimes frothy stools that float (steatorrhoea). Steatorrhoea may be confirmed by measuring the fat content of stools. The absorption of fat-soluble vitamins may also be impaired (see later).
Absorption is the term used to describe the transfer of nutrients or their digestion products from the lumen of the alimentary canal to either the blood or the lymph. The small intestine possesses efficient mechanisms for the absorption of nutrients and prevents their passage to the large intestine. The presence of nutrients in the large intestine produces diarrhoea resulting from water being drawn into the lumen by osmosis or by bacterial overgrowth. The absorbed molecules have to overcome a barrier ( Fig. 15.4 ) made up of the
Unstirred layer of fluid covering the microvilli
Glycocalyx covering the microvilli
Luminal plasma membrane
Cytoplasm
Basal or lateral border of the cell
Intercellular space
Basement membrane (basal and reticular lamina)
Plasma membrane of the capillary or lymph vessel.
Most carbohydrate, protein and lipid absorption occurs in the small intestine, the duodenum, the jejunum and the early sections of the ileum. These sites, together with the stomach, are also where orally administered drugs are absorbed. Disease of the membranes through which absorption takes place, and defects in the normal mechanisms for absorption, leads to nutritional deficiencies and also adverse or toxic drug effects. Absorption of solutes may occur by either passive or active processes ( Clinical box 15.4 ).
Disease of the gastrointestinal tract prevents efficient absorption by compromising the transfer of nutrients across the intestinal epithelium. This can be caused by inflammatory processes through infection (gastroenteritis, dysentery and parasitic infestations), immune responses (Crohn disease, ulcerative colitis, coeliac disease, etc.), antibiotic use, hereditary digestive enzyme deficiency, the presence of defective Na + /glucose transporter (SGLT1) or a reduction in surface area after surgical resection.
Clinically, diarrhoea occurs when there is an increase in daily stool weight to more than 300 g, increased fluid content and stool volume, and there is usually an associated increase in frequency of bowel action. Steatorrhoea occurs when there is impaired fat absorption and the stools have a high fat content. Diarrhoea may be
Osmotic – when the presence of undigested hypertonic substances draws fluid into the large bowel by osmosis
Secretory – usually after intestinal resection, in the presence of toxins or laxative use, when fluid and electrolyte absorption is decreased at the same time as an increase in secretion occurs
Inflammatory – due to damage of the intestinal mucosa through infection or inflammation.
There is increased fluid and electrolyte loss as well as reduced absorption. The fluid and electrolyte loss can lead to severe dehydration and metabolic imbalance. Diarrhoea is one of the leading causes of death among children in developing countries.
There are two types of passive diffusion. These are simple diffusion, where small molecules, such as O 2 and CO 2 , and fat-soluble molecules diffuse through the lipid bilayer of the cell membrane, and facilitated diffusion, where the transported molecule moves through the membrane by means of a protein carrier. In a passive process, the absorbed solute will move from a high to a low concentration. Passive processes do not require the use of energy from cellular metabolism.
Water and some solutes pass through membranes at a faster rate than would be expected from a knowledge of the lipid solubility. This suggests that there are routes through the membrane for water and small hydrophilic solutes. These may be in the spaces between the membrane phospholipids and through specific membrane proteins called aquaporins. Ions can diffuse through specific protein ion channels that span the membrane.
In contrast, an active process involves cellular energy to drive an absorbed solute against either a concentration gradient or an electrical gradient, i.e. an ‘uphill’ movement from a low to a high concentration or for a charged solute moving to a region of the same charge, e.g. Na + ions being transported out of cells. The two types of active transport use adenosine triphosphate (ATP) either directly, as in the Na + /K + -ATPase pump, or indirectly as a source of energy to power secondary active transport.
For the absorption of many solutes in the alimentary canal, there are carrier-mediated mechanisms that use the concentration gradient built up as a result of the primary active transport of Na + . This process is known as secondary active transport. The carriers have binding sites for the organic molecules and for Na + . The movement of Na + down its concentration gradient can lead to the simultaneous transfer of the organic molecule into the cell against its concentration gradient. Metabolic inhibitors that block the primary active transport system will lead to inhibition of the secondary active transport system once the potential energy stored in the Na + concentration gradient across the cell membrane has run down.
The carbohydrate digestion products are glucose, galactose and fructose. A small quantity of these sugars is absorbed by passive diffusion through aqueous channels between enterocytes and in the cell membranes. The main transport mechanisms involve carrier proteins in the brush and basolateral borders of enterocytes. All the sugars enter the hepatic portal vein for delivery to the liver.
Glucose and galactose are absorbed by secondary active transport involving a sodium-dependent glucose and galactose transporter (sodium/glucose-linked transporter, SGLT1) in the brush border membrane of the enterocyte (see Fig. 15.3 ). The glucose and galactose compete for the sugar site on the transporter, which also binds two Na + ions at a different site. Once loaded, the transporter moves the Na + down its electrochemical gradient into the cell together with either the glucose or the galactose. The Na + diffuses into the intracellular fluid and is transported out of the cell by the Na + /K + -ATPase (sodium pump) on the basolateral membrane. The glucose and galactose accumulate in the cell until they are removed by simple diffusion and facilitated diffusion involving GLUT2, a transporter found in the basolateral borders of the enterocyte. The importance of GLUT2 has been questioned because patients with the rare Fanconi–Bickel syndrome in which GLUT2 transporters are defective absorb glucose from the intestine but not from the kidney tubules, which also have GLUT2 transporters located on the basolateral borders of the tubular cells.
Fructose enters the enterocyte by facilitated diffusion using the specific fructose transporter GLUT5, which does not require the co-transport of Na + to function (see Fig. 15.3 ). Young children may have difficulty in absorbing fructose in fruit juices owing to a less developed transport system compared with adults, leading to the production of gas and diarrhoea. As with glucose and galactose, GLUT2 is believed to transport fructose across the basolateral border of the enterocyte to the tissue fluid; however, patients with Fanconi–Bickel syndrome are able to absorb fructose. GLUT5 has been identified in the basolateral border of human enterocytes and will provide a route for fructose transport to the tissue fluid.
The proteases in the gastrointestinal tract produce a variety of peptides and free amino acids for absorption. The enterocytes have a relatively high cytosolic concentration of free amino acids for protein synthesis that, in turn, can make amino acid absorption more difficult. This difficulty is overcome by the absorption of di- and tripeptides that are subsequently hydrolysed by peptidases to release free amino acids within the cell. This hydrolysis maintains a concentration gradient for peptides across the luminal cell membrane. After passing across the basolateral membrane, the absorbed amino acids enter the hepatic portal vein for delivery to the liver.
Di- and tripeptides are absorbed into the enterocyte by means of a brush border carrier, PepT1. The transport of di- and tripeptides is a secondary active transport process. The movement of Na + down its concentration gradient, to enter an enterocyte in exchange for a proton moving into the lumen, provides the electrochemical energy for absorbing the peptides (see Fig. 15.3 ). The PepT1 transporter co-transports H + from the lumen and peptides into the cell down an electrochemical gradient for H + . It is interesting to note that the absorption of β-lactam antibiotics (penicillins) and angiotensin-converting enzyme inhibitors (captopril) is by means of the PepT1 transporter.
There are a number of transport systems ( Table 15.4 ) for L-amino acids in the brush border of enterocytes that require the co-transport of Na + to allow the transfer of amino acids against a concentration gradient by means of secondary active transport. Facilitated diffusion of some amino acids also occurs. The presence of carrier systems working in parallel for single amino acids and small peptides in the intestine enhances the overall absorption of amino acids by allowing the uptake of the same amino acid, e.g. glycine in peptide form, as well as of free amino acid. Five carrier mechanisms are present in the basolateral borders of enterocytes. Three amino acid carriers not requiring Na + to function transport the intracellular amino acids to the extracellular fluid for diffusion into the blood. The Na + -requiring carriers provide amino acids from the circulation for protein synthesis in crypt cells.
| Site and system | Amino acid transporter | Co-transported ion |
|---|---|---|
| Apical membrane | ||
| B | Neutral amino acids, e.g. alanine | Na + |
| B o | Neutral and cationic (basic) amino acids, e.g. lysine and also cystine | Na + |
| b ot | Neutral and cationic amino acids | None |
 |
Anionic (acidic) amino acids, e.g. glutamate, aspartate | 2 Na + , 1 H + , inward; 1 K + , outward |
| y + | Cationic (basic) amino acids, e.g. arginine, lysine | None, but will also co-transport small neutral amino acids with Na + |
| Imino | Proline and hydroxyproline | Na + and Cl − |
| β | β amino acids: β-alanine and taurine | Na + and Cl − |
| Basolateral membrane | ||
| A | Most neutral and imino acids | Na + |
| ASC | Neutral amino acids with 3–4 carbons | Na + |
| b ot | Neutral and cationic amino acids | None |
| L | Neutral amino acids with hydrophobic side chain | None |
| y + | Cationic (basic) amino acids, e.g. arginine, lysine | None but will also co-transport small neutral amino acids with Na + |
The process of fat absorption (see Fig. 15.4 ) differs from that of carbohydrate and proteins. Short- and medium-chain fatty acids (C8–14) and glycerol are water soluble and diffuse directly through the luminal and basolateral membranes of the enterocyte to enter the capillaries. The micelles, containing 2-monoacylglycerols and larger free fatty acids (chain length greater than 14 carbon atoms) in their cores, being water soluble, diffuse through the unstirred layer of water at the surface of the enterocyte at a faster rate than can be achieved by fat digestion products alone. The hydrophobic 2-monoglycerides and free fatty acids are delivered by this mechanism to the enterocytes of the jejunum, where they are absorbed by diffusion through the lipid portions of the surface of the cell membrane.
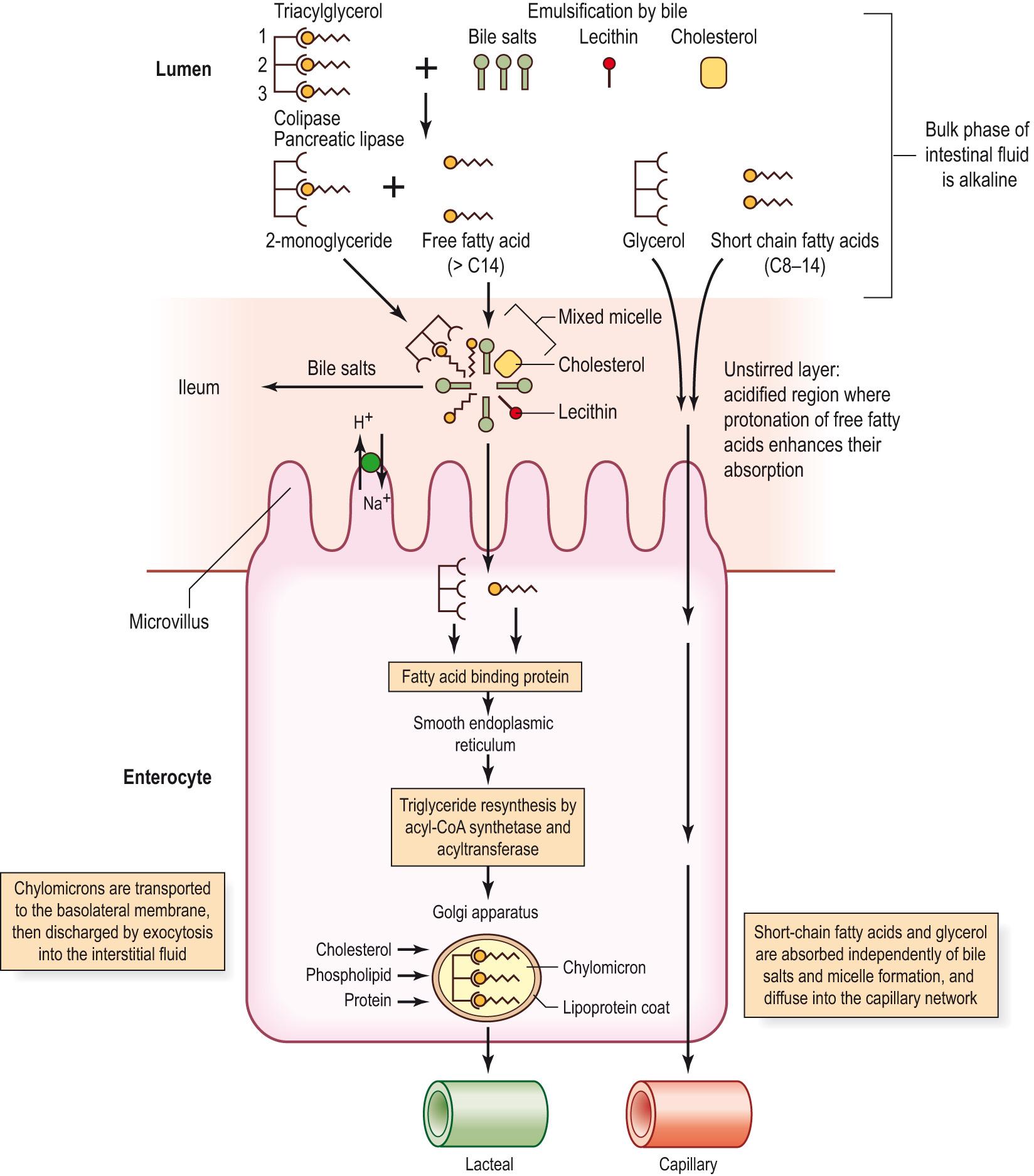
The absorbed long-chain fatty acids and 2-monoglycerides bind to a fatty acid-binding protein within the enterocyte, thus maintaining the concentration gradient across the cell membrane for these products. The fatty acid-binding protein transfers the fatty acids and 2-monoglycerides to the smooth endoplasmic reticulum for the resynthesis of triglyceride by acyl-CoA synthetase and acyltransferase. The triglyceride is coated with lipoprotein, derived from cholesterol, phospholipid and apoprotein B in the rough endoplasmic reticulum, to form a chylomicron that is transferred to the Golgi apparatus where the protein coat is glycosylated prior to exocytosis across the basolateral membrane. Chylomicrons (diameter 75–600 nm) are too large to enter the capillaries, but can diffuse through spaces in the walls of lacteals (lymphatic vessels) in the villi. The lacteals drain into larger lymphatic vessels, leading to the thoracic lymphatic duct, which, in turn, distributes the lymph to the venous system at the junction of the left subclavian vein and left jugular vein. In contrast to other nutrients, the absorbed lipids are available for either energy production or storage in all organs of the body before reaching the liver.
The bile salts are ionised at intestinal pH and require a carrier mechanism for their absorption. The sodium-dependent carrier is present in the enterocytes of the terminal ileum. Absorption is by means of secondary active transport similar to that described for glucose or amino acid transport. The absorbed bile salts enter the venous system draining the ileum and are transported to the liver in the enterohepatic circulation. The liver extracts the bile salts and secretes them into the bile. The bile salt pool may recirculate two or three times during a large meal.
Vitamins (A, B, C, D, E and K) are organic compounds that the body is unable to synthesise; therefore, they must be absorbed from the small intestine (see Ch. 16 ).
The water-soluble vitamins (B group and C) are absorbed by passive diffusion and, in some cases, by secondary active transport. Specialised sodium-dependent transport systems exist for thiamine (B 1 ), niacin, folate and vitamin C in the apical membrane of enterocytes. A facilitated transport system is responsible for the exit of niacin, folate and thiamine across the basolateral border.
The absorption of vitamin B 12 (cobalamin) involves a complex series of events. Vitamin B 12 in food is bound to proteins. The action of acid and pepsins in the stomach releases the vitamin B 12 . The free vitamin B 12 binds to glycoproteins, known as R proteins, that are present in the stomach contents derived from saliva and gastric juice. Another glycoprotein capable of binding vitamin B 12 is intrinsic factor secreted by parietal cells. The affinity of intrinsic factor for ingested B 12 is less than that of R proteins; therefore, most of the vitamin B 12 in the chyme leaving the stomach and entering the intestine is bound to R protein. Once in the intestine, the pancreatic proteases digest the R protein and vitamin B 12 is free once again. It binds to intrinsic factor, which is resistant to digestion by pancreatic proteases. The vitamin B 12 –intrinsic factor complex passes along the small intestine to the terminal ileum where there are receptors in the brush borders of the enterocytes for the vitamin B 12 –intrinsic factor complex. Binding of the vitamin B 12 –intrinsic factor complex to the membrane receptor triggers endocytosis (receptor-mediated endocytosis) of the complex into the cell. Vitamin B 12 is released from the complex within the cell and exported across the basolateral border and diffuses into the blood where it binds to transcobalamin II, a transport protein ( Information box 15.2 ).
Vitamin B 12 deficiency is caused by the malabsorption of vitamin B 12 . The commonest cause is pernicious anaemia . There is atrophic gastritis leading to a failure in the production of intrinsic factor . This is an autoimmune condition and is associated with other autoimmune diseases, such as thyroid disease and vitiligo. It is sometimes also associated with gastric carcinoma. Intrinsic factor antibodies are present, inhibiting the binding of intrinsic factor to B 12 in the stomach and also blocking the vitamin B 12 –intrinsic factor complex coupling to receptors in the terminal ileum, where B 12 would normally be absorbed. A 3–6-year store of vitamin B 12 is present in the liver, and, therefore, patients with pernicious anaemia may take years to develop symptoms of anaemia. Treatment is by intramuscular injections of vitamin B 12 . Other causes of vitamin B 12 malabsorption include coeliac disease, surgical resection of the stomach or ileum, and the long-term use of drugs such as proton pump inhibitors.
The fat-soluble vitamins A, D, E and K entering the small intestine are solubilised by diffusion into micelles containing bile salts and fat digestion products (see Ch. 16 ). Absorption of these vitamins occurs by diffusion across the brush border of the enterocyte together with fatty acids and monoglycerides. The fat-soluble vitamins are exported to the lymphatic system in chylomicrons ( Information box 15.3 ).
Chronic steatorrhoea could lead to malabsorption of fat-soluble vitamins. Although this could be the cause of vitamin D deficiency ( rickets , osteomalacia ), other factors are anticonvulsant therapy and renal failure, which interfere with vitamin D metabolism.
Each day, approximately 9 L of fluid enters the alimentary canal. This is derived from the diet (2 L) and the digestive secretions (7 L) ( Fig. 15.5 ) . In health, approximately 99% of the water and electrolytes are absorbed into the blood as the fluid passes along the small and large intestine. The major site of fluid absorption is the jejunum and ileum (8.5 L), with a relatively small quantity being absorbed from the colon (0.4 L). The faeces contain approximately 0.1 L of water. The absorption of water is secondary to the uptake of electrolytes, in particular Na + and Cl − , sugars and amino acids.
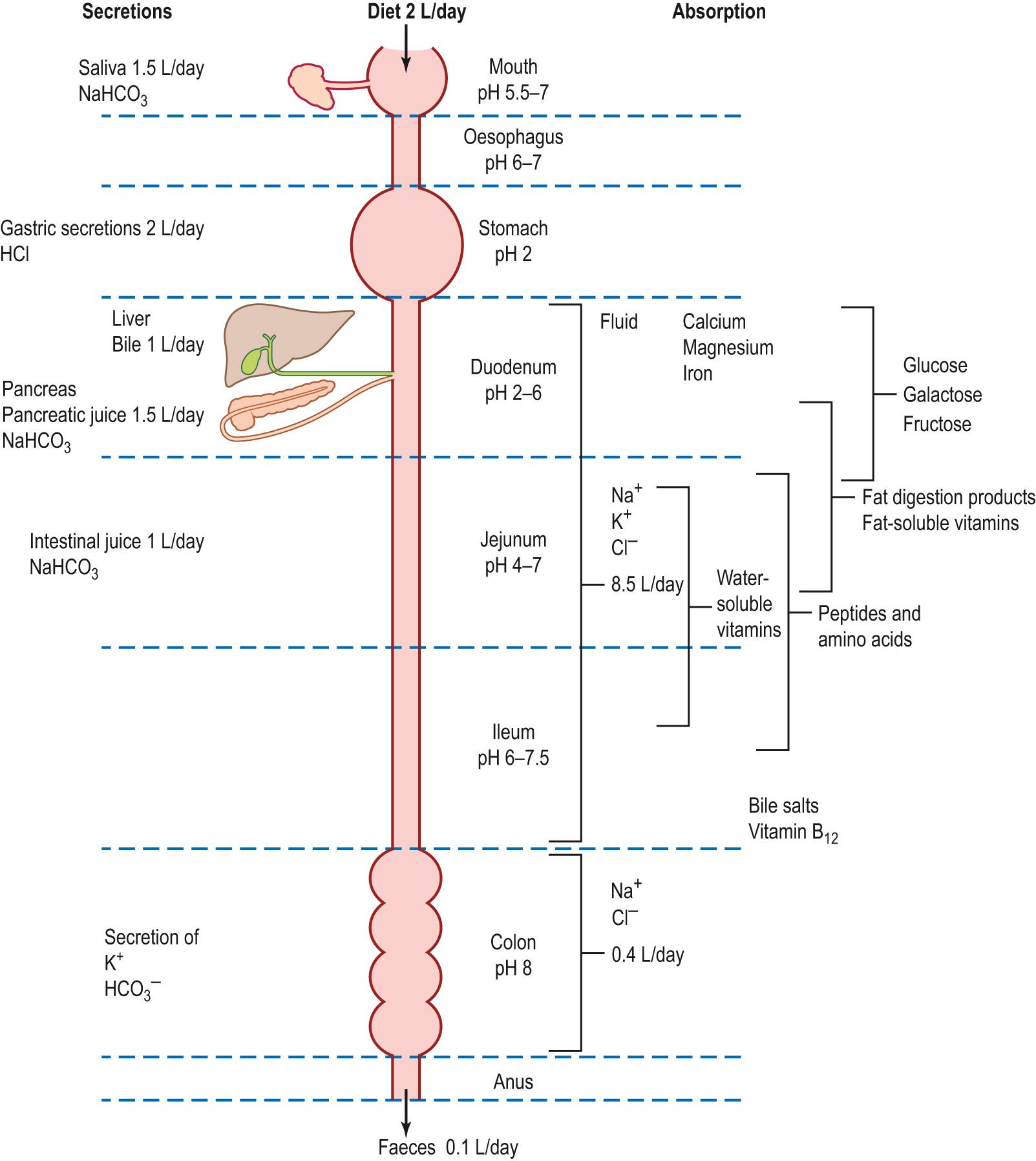
The electrolytes and water may be absorbed by passing between the enterocytes (paracellular route) via aqueous channels, through the tight junctions linking the cells together. The alternative route is by passing through the cells (transcellular route). This may involve both carrier-mediated mechanisms and passage through water-permeable channels ( Table 15.5 ).
| Intestinal region | Na + | K + | Cl − |  |
|---|---|---|---|---|
| Duodenum and jejunum | Actively absorbed
|
Passively absorbed by diffusion through paracellular pathways as the luminal concentration rises after water absorption | Passively absorbed
|
Absorbed as CO 2 following neutralisation of secreted H + |
| Ileum | Actively absorbed as above but reduced importance of co-transport with organic solutes | Passively absorbed as above | Passively absorbed
|
Passively absorbed
|
| Colon | Actively absorbed
|
Secretion Passive leakage from enterocytes through K + channels in their apical membranes when luminal concentration is <25 mmol/L Actively reabsorbed by luminal H + /K + ATPase during K + deprivation or depletion |
Passively absorbed
|
Secretion Counter transport exchange with Cl − |
| Stimulated by aldosterone | Stimulated by aldosterone |
Water movement from the lumen of the intestine may occur via membrane proteins, including the SGLT1, sodium/glucose co-transporter and channels named aquaporins that allow the passage of water. The unstimulated SGLT1 acts as a water-permeable protein channel. When stimulated by the presence of Na + and glucose in the intestinal lumen, it co-transports water together with Na + and glucose into the intestinal mucosal cell. In addition, the absorption of water is secondary to organic and ionic solute movement. The transfer of solutes across the intestinal epithelium creates an osmotic gradient between the lumen and the interstitial fluid and blood. Water is absorbed by osmosis via paracellular and transcellular routes (see Fig. 15.3 ). The principle of oral rehydration therapy, used to treat diarrhoea, is based on promoting water absorption, either by means of co-transport or by osmosis, following an isotonic drink containing glucose and electrolytes, including sodium chloride.
Dietary calcium is found in a variety of foods including dairy products. Calcium may exist bound to oxalates, phosphates and phytates or in the ionised form (Ca 2+ ) in the intestine. Ionised calcium is available for absorption by enterocytes in the upper small intestine. The free Ca 2+ concentration in the cell is low, giving rise to a steep concentration gradient between the lumen and the cytoplasm. Ca 2+ ions bind to a protein in the brush border and are transported down their concentration gradient into the cell. The free Ca 2+ concentration in the cytoplasm is kept low by Ca 2+ binding to calcium-binding proteins that are sequestered in intracellular organelles such as the endoplasmic reticulum. Ca 2+ ions are exported across the basolateral border of the cell against an electrochemical gradient by active transport. There are two mechanisms:
Ca 2+ -ATPase, which uses energy derived from the hydrolysis of ATP to transfer Ca 2+ out of the cell
Na + /Ca 2+ exchanger, in which Na + moving down its electrochemical gradient into the cell drives Ca 2+ extrusion.
The Ca 2+ -ATPase mechanism is the more important one.
Calcium absorption is regulated by 1,25-dihydroxycholecalciferol, the active form of vitamin D, which stimulates the synthesis of both calcium-binding proteins and Ca 2+ -ATPase (see Ch. 10 ). Note: a high dietary phytate content, as in chapatti flour, may inhibit calcium absorption.
Dietary iron is present in two forms:
The haem portion of haemoglobin, myoglobin and cytochromes
An insoluble, non-absorbable state complexed with phytate, tannins and plant fibres. Insoluble iron salts may also form with hydroxide, phosphate and bicarbonate found in digestive secretions.
The acidic conditions of the stomach mobilise the iron compounds by converting the ions from the ferric (Fe 3+ ) to the ferrous (Fe 2+ ) state. Similarly, vitamin C reduces iron to the ferrous state and also forms soluble complexes with it that enhance absorption. Ferric ions are reduced to ferrous ions by duodenal cytochrome b ferric reductase in the brush border of the duodenal enterocyte.
The enterocytes of the duodenum absorb haem and ferrous ions by two separate mechanisms:
Haem is absorbed by endocytosis and digested in the enterocyte by haem oxidase to release ferric ions, carbon monoxide and biliverdin. The ferric ions are reduced to ferrous ions that bind to ferroportin 1 in the basolateral membrane for export from the cell.
Ferrous ions and a proton bind to a divalent metal transporter to cross the brush border. The movement of a proton down its concentration gradient into the enterocyte is the driving force for ferrous ion absorption. In the cytosol, ferrous ions either bind to a storage protein, apoferritin, to form ferritin or are transferred to ferroportin 1 in the basolateral borders of the cell for export to the tissue fluid.
An additional protein, hephaestin, is considered to have a role in the export of iron from the enterocytes to the plasma. It may be involved in the release of iron from its storage sites and transfer to ferroportin 1 for export to the tissue fluid. Ferrous ions diffuse into the blood and are transported around the body in association with transferrin, a plasma protein.
The absorption of iron needs to be regulated because
There are no active excretory mechanisms
This metal plays an essential biological role in oxygen transport and redox reactions in cellular respiration.
In addition, iron is involved in the formation of reactive oxygen species that may be useful in killing bacteria. However, reactive oxygen species are also toxic to cells when large quantities are produced following excessive iron accumulation (see Ch. 16 and Information box 15.4 ).
The ferrous state is more readily absorbed than ferric iron, and oral iron supplement formulations are usually in the ferrous state. Iron absorption increases during pregnancy and iron-deficiency anaemia. High dietary content of phosphates and phytate (in chapatti flour) can inhibit iron absorption by forming insoluble complexes.
Iron transport is stimulated either through erythropoiesis following haemorrhage or by moving to a high altitude and is decreased when iron stores are high. The development of the iron-absorptive mechanisms in the immature enterocytes of the crypts is controlled by the uptake of transferrin-bound iron from the plasma. In states of iron deficiency, the immature enterocyte becomes programmed to increase iron absorption by the upregulation of the divalent metal ion transporter and ferroportin 1 synthesis. An increase in iron accumulation in the body leads to a decrease in the synthesis of the transport systems involved in iron uptake and to the lysosomal degradation of ferroportin 1 initiated by the action of peptide hepcidin following its increased secretion from the liver. The accumulation of iron in the enterocyte stimulates apoferritin synthesis and ferritin formation to protect the body from iron overload. The ferritin is released into the intestinal lumen when the enterocytes are shed from the villus tip.
The upper abdomen is bound by the lower rib (costal) margins formed by the seventh to the tenth costal cartilages and joined in the middle at the xiphisternum ( Fig. 15.6 ) . The lower abdomen extends to the inguinal ligaments and the pubis. The inguinal ligaments appear convex in thin people. To locate the surface positions of abdominal organs, we could roughly divide the anterior surface of the abdomen into nine regions or four quadrants. The nine regions are demarcated by two vertical and two horizontal imaginary planes that transect the abdominal cavity.
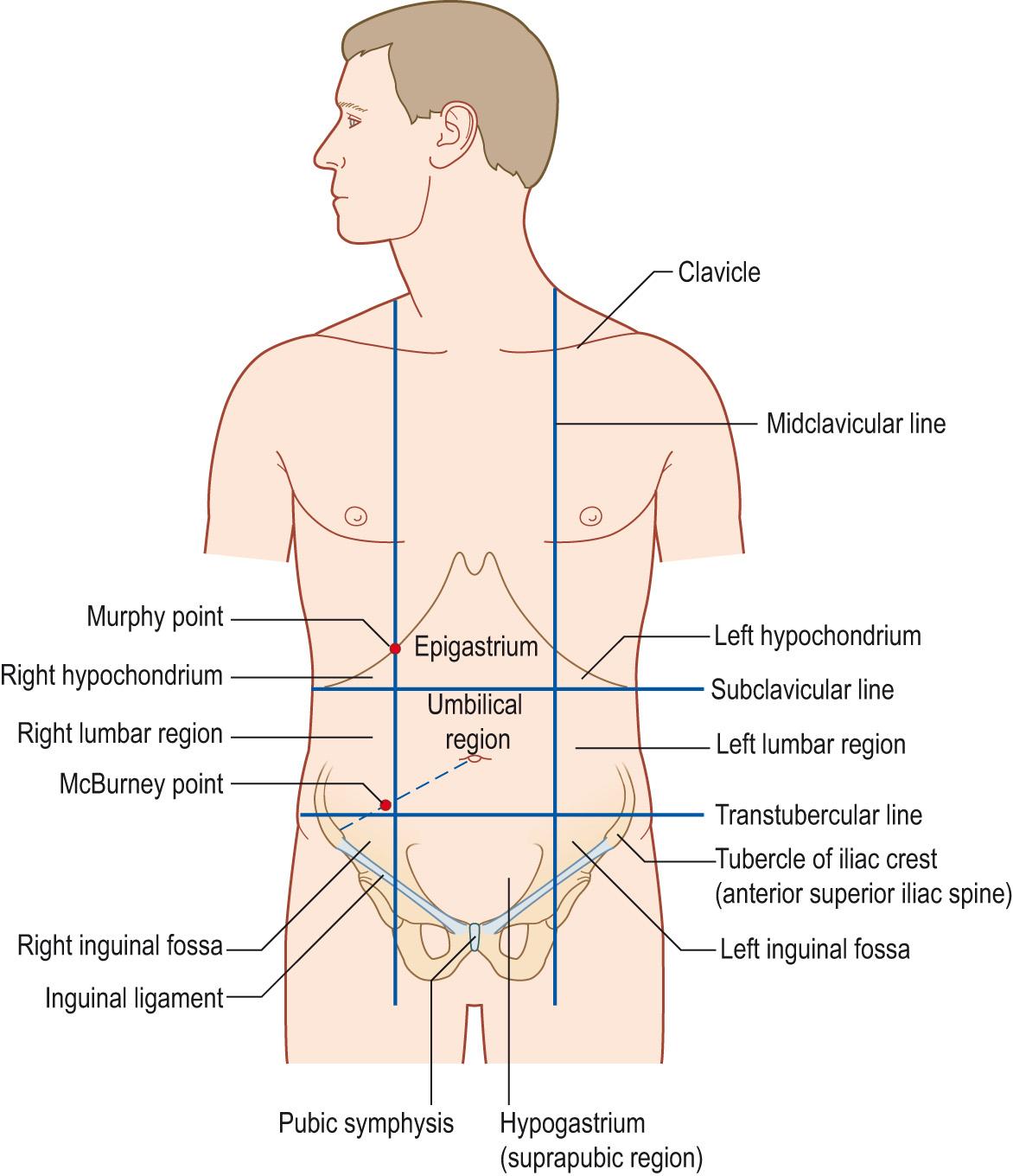
Abdominal regions are demarcated by four imaginary planes into nine regions. The two vertical planes pass through the midclavicular lines. The upper horizontal plane is marked by the lowest edge of the rib cage, the subcostal line, and the lower horizontal plane passes through the line drawn between the tubercles of the iliac crest: the transtubercular line. In practice, the anterior superior iliac spines (ASIS) are easier to feel than the tubercles, which are palpable a little way behind the ASIS. The plane passing through the ASIS is sometimes substituted for the transtubercular plane. From top to bottom in the midline, the regions are epigastric, umbilical and hypogastric (also known as suprapubic). The lateral regions are left and right hypochondrial, lumbar and inguinal (or iliac fossa).
Two important landmarks are the McBurney point, approximately one-third of the way on a line drawn from the right ASIS to the umbilicus, marking the base of the appendix where the point of maximal tenderness is felt in classical acute appendicitis. The Murphy point is roughly below the plane where the midclavicular line crosses the lower margin of the rib cage (costal margin) at approximately the level of the ninth costal cartilage and is classically where the fundus of the gall bladder may be found. It is also the point of maximum tenderness in acute cholecystitis.
Typically, pain and tenderness in the epigastrium are referred from the upper abdominal organs, such as the stomach and duodenum. Palpable masses usually arise from the stomach, and the pulsations of the abdominal aorta can also just be felt in thin people.
Pain from intestinal obstruction, e.g. in acute appendicitis, could be referred to the area around the umbilicus. Pain and tenderness from disease of the transverse colon are also located in the umbilical region. The transverse colon is extremely variable in position, sometimes reaching down to the suprapubic region. Tumours of underlying structures and pulsations from the abdominal aorta are palpable here.
Pain and tenderness from the lower bowel, e.g. descending colon and anal canal, bladder and uterus, and, sometimes, the fallopian tubes, are referred to this region. Enlargement or tumours of the pelvic organs, e.g. bladder and uterus, are palpable here.
The spleen is situated behind the left lower ribs. Splenic tenderness and enlargement are felt in the left hypochondrium, although the spleen has to be enlarged to more than three times its normal size to be palpable. The liver and gall bladder are behind the rib cage on the right. An enlarged or tender liver and gall bladder may be palpated in the right hypochondrium.
The lumbar regions refer to the ‘flanks’. Normally, the kidneys lie deep against the posterior abdominal wall and are not palpable. The lower poles of enlarged kidneys may be felt in the lumbar regions. The right kidney is usually lower than the left one. Posteriorly, the angles formed by the ribs and the spine are the renal angles where maximal renal tenderness may be felt when the kidneys are dilated or inflamed. The ascending colon may be palpable in the right lumbar region and the descending colon in the left.
Pain and tenderness in the left iliac fossa could be related to problems with the descending or sigmoid colon, whereas signs in the right iliac fossa signify disease of the appendix, caecum or ascending colon. Deep iliac fossa pain may relate to fallopian tube disease. Masses in either iliac fossa are suspicious of underlying tumours, but sometimes a loaded colon or impacted faeces may be palpable.
The pulsations of the femoral artery are easily felt at the midpoint between the anterior superior iliac spine and the pubic symphysis along the inguinal ligament. A weakness in the inguinal canal can cause some abdominal contents to be forced through this potential weakness in the inguinal region (hernia) when the intra-abdominal pressure is significantly raised, as in coughing and lifting heavy weights. This manifests as palpable ‘bulges’ medial to the femoral artery when a patient is asked to cough, particularly when standing.
For the purposes of description, the surface of the abdomen is also commonly divided into quadrants – the right and left upper and lower quadrants (the gall bladder and liver being palpable in the right upper quadrant, and enlarged spleen in the left upper quadrant). The right lower quadrant is where the signs of disease in the appendix and caecum become evident, and the left lower quadrant is where signs of disease of the sigmoid colon become evident.
The gastrointestinal tract can be considered as a muscular tube 7–10 m in length, consisting of four major layers ( Fig. 15.7 ) . From the lumen, these are
Mucosa
Submucosa
Muscle
Serosa.
There are variations in the general structure related to the functions of the different regions of the gastrointestinal tract. The histological structure of the mucosal layers shows the greatest variation ( Fig. 15.8 ) . The mucosa consists of
A lining layer of epithelial cells that have secretory and absorptive functions
The lamina propria , a connective tissue support for the mucosa, together with blood vessels and the glandular ducts in some regions
The muscularis mucosae , a thin layer of smooth muscle cells that moves the epithelium, producing mixing activity, and leads to folding of the mucosal layer.
The submucosa contains connective tissues, blood vessels and lymphatics together with nerves innervating the structures within the mucosa.
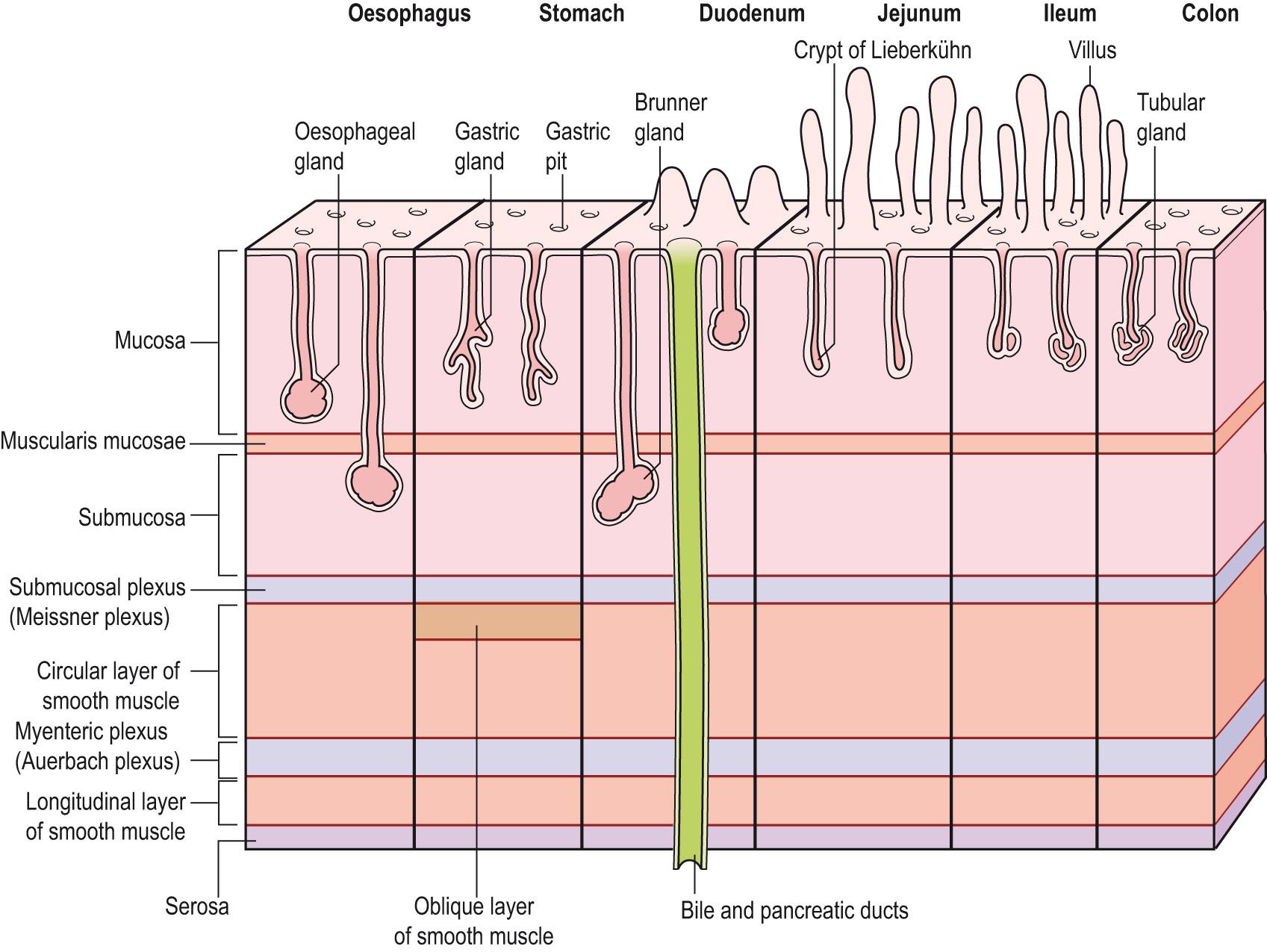
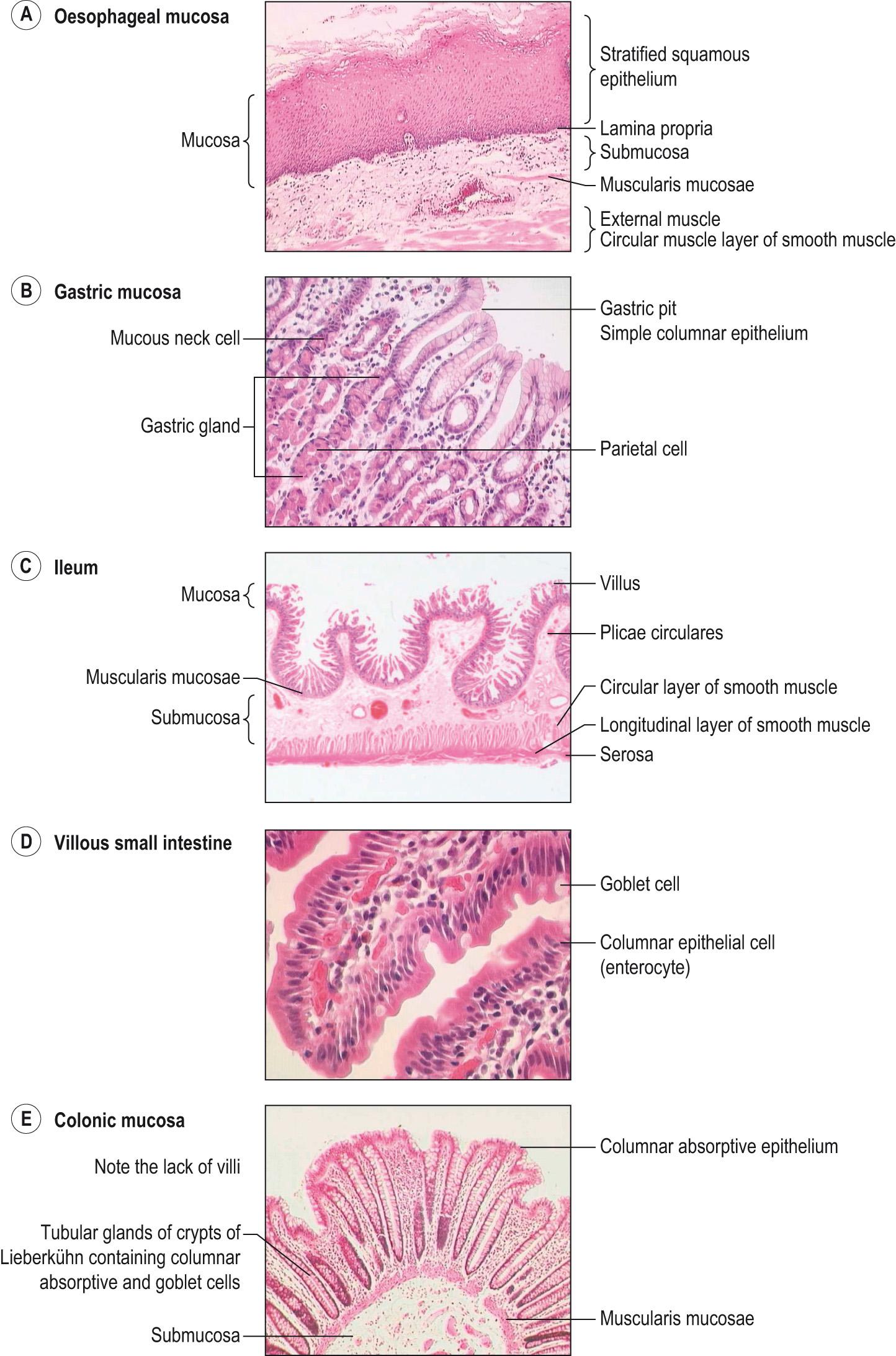
In most regions of the alimentary canal, the external muscle consists of two layers of smooth muscle: the inner layer in which the long axis of the muscle fibres is arranged in a circular manner around the canal ( circular muscle ) and an outer layer with the muscle fibres lying along the length of the canal ( longitudinal layer ). Contraction of the circular and longitudinal muscle layers narrows the lumen and shortens the canal, respectively. These muscles are responsible for mixing the food with the digestive secretions and for propelling the contents along the digestive tract. The first third of the oesophagus and the anal sphincter contain skeletal muscle.
The serosa is the outermost layer of the alimentary canal and is continuous with the parietal peritoneum lining the inner surface of the body wall. It consists of connective tissues covered with a squamous epithelium.
Sensory and motor neurons are found within the wall of the alimentary canal. Major aggregations of neurons are found in
The submucosal plexus or Meissner plexus on the outer edge of the submucosa adjacent to the circular muscle layer
The myenteric plexus or Auerbach plexus lying between the circular and the longitudinal muscle layers.
The plexi are innervated by sensory neurons from the mucosa and muscle layers, and by preganglionic parasympathetic nerves and postganglionic sympathetic nerves from the central nervous system. The nerves, with their cell bodies within the alimentary canal, form the enteric nervous system.
The function of nerves within the alimentary canal is to coordinate muscular, secretory and absorptive activity so that the digestive tract functions as a coordinated unit. Many functions of the alimentary canal continue to be coordinated following the cutting of the parasympathetic and sympathetic nerves innervating the canal. For this reason, it may be said that the nerves within the alimentary canal constitute ‘a little brain’. The central nervous system (or ‘large brain’) modifies the action of the enteric nervous system via the parasympathetic and sympathetic branches of the autonomic nervous system.
Table 15.6 shows the hormones and neuropeptides secreted by the alimentary system. Some gastrointestinal hormones and neuropeptides also act centrally on the hypothalamus to control appetite, hunger and satiety (see also Chs 8 and 16 ).
| Hormone/neuropeptide | Source | Target organ | Action |
|---|---|---|---|
| Cholecystokinin | I cells in the duodenum and jejunum and neurons in the ileum and colon | Pancreas Gall bladder |
↑ Enzyme secretion ↑ Contraction |
| Calcitonin gene-related peptide | Enteric neurons Splanchnic afferent nerves |
Blood vessels Neurons |
↑ Vasodilatation Sensory neurotransmitter |
| Enkephalins | Enteric neurons | Gastrointestinal smooth muscle Intestinal mucosa |
↑ Contraction ↓ Fluid secretion |
| Enteroglucagons Glucagon-like peptide 1 Glucagon-like peptide 2 |
L cells in the ileum, colon and rectum | Pancreas Stomach Gastrointestinal mucosa |
↑ Insulin release ↓ Acid secretion ↓ Gastric emptying ↑ Mucosal growth, integrity, function |
| Epidermal growth factors | Salivary and Brunner glands | Gastrointestinal mucosa | ↑ Growth and mucosal protection |
| Gastric-inhibitory peptide or glucose-dependent insulinotropic peptide | K cells in the duodenum and jejunum | Pancreas Stomach |
↑ Insulin release ↓ Acid secretion |
| Gastrin | G cells, antrum of stomach | Parietal cells in the body of the stomach | ↑ Acid secretion |
| Enterochromaffin-like cells | ↑ Histamine secretion | ||
| Gastrointestinal mucosa | ↑ Growth | ||
| Gastrin-releasing peptide | Enteric neurons | G cells in the antrum of the stomach | Gastrin release |
| Ghrelin | Gr or X/A cells in the stomach adjacent to parietal cells | Anterior pituitary Hypothalamus Stomach |
↑ Growth hormone release ↑ Feeding ↑ Acid secretion ↑ Motility and gastric emptying |
| Guanylin | Endocrine cells in the ileum and colon | Small and large intestine | ↑ Fluid secretion |
| Motilin | Endocrine cells in the upper gastrointestinal tract | Oesophageal sphincter Stomach Duodenum |
↑ Smooth muscle contraction migrating myoelectric complex |
| Neurotensin | N cells, throughout the gastrointestinal tract | Gastrointestinal smooth muscle | Relaxation of LOS ↓ Antral motility |
| Gastrointestinal mucosa | ↑ Growth | ||
| Stomach | ↓ Acid secretion | ||
| Pancreatic polypeptide | Endocrine cells in the ileum | Pancreas | ↓ Enzyme and fluid secretion |
| Peptide YY | Endocrine cells in the ileum and colon | Stomach Pancreas |
↓ Vagally mediated acid secretion ↓ Enzyme and fluid secretion |
| Pituitary adenylate cyclase-activating peptide (PACAP) | Enteric neurons | Intestinal smooth muscle | Relaxation |
| Secretin | S cells in the small intestine | Pancreas | ↑  and fluid secretion by pancreatic ducts and fluid secretion by pancreatic ducts |
| Stomach | ↓ Gastric acid secretion | ||
| Somatostatin | D cells of stomach and duodenum, δ cells of pancreatic islets | Stomach Intestine |
↓ Gastrin release ↑ Fluid absorption ↓ Secretion |
| ↑ Smooth muscle contraction | |||
| Pancreas Liver |
↓ Endocrine/exocrine secretions ↓ Bile flow |
||
| Tachykinins: substance P; neurokinin A | Sensorimotor neurons; enteric neurons | Longitudinal and circular layer smooth muscle | ↑ Contraction |
| Enteric nervous system | Stimulation of inhibitory and excitatory pathways | ||
| Blood vessels | ↑ Vasodilatation | ||
| Small and large intestinal mucosa | ↑ Fluid secretion | ||
| Vasoactive intestinal peptide | Enteric neurons | Gastrointestinal smooth muscle | ↑ Relaxation |
| Small intestinal mucosa | ↑ Fluid secretion | ||
| Pancreatic neurons | Pancreas | ↑  and fluid secretion by pancreatic ducts and fluid secretion by pancreatic ducts |
The mouth or oral cavity consists of the lips, tongue, teeth, gums, and hard and soft palate. Stratified squamous epithelial cells, kept moist by saliva, cover the surface of the oral cavity and tongue. Sensory nerves sensitive to pain, touch and temperature are present in the lining of the mouth together with taste buds in the dorsal surface of the tongue (see Ch. 8 ). The temperature and taste of ingested food and drink are sampled by the sensory receptors before chewing or drinking begins.
In adults, there are 32 permanent teeth that have different functions.
Incisors – chisel-shaped teeth for cutting and biting
Canines – for puncturing and holding food
Molars – for grinding and crushing food.
The adult teeth are preceded by 20 deciduous or milk teeth. The deciduous teeth begin to appear at 6 months; the set is complete at 6–8 years and is replaced by permanent teeth at 10–12 years.
The anterior two-thirds of the tongue are composed of skeletal muscle fibres, covered by a layer containing sero-mucous glands and stratified epithelium. The secretions of these glands, together with saliva, ensure the tongue is moist to facilitate movement during chewing and swallowing of food.
The mastication, or chewing, process physically breaks food into smaller pieces. This voluntary and reflex behaviour serves to
Dissolve chemicals in saliva so that they can stimulate the taste buds
Lubricate the food to ease swallowing
Mix starch-containing food with salivary α-amylase
Increase the surface area of food to facilitate digestion in the stomach and duodenum.
The absence of teeth and neuromuscular defects in the elderly after strokes can severely interfere with mastication.
Saliva is an exocrine secretion that enters the mouth from salivary glands by a duct system. There are two types of salivary secretion produced by different cells:
Serous cells produce a watery secretion
Mucous cells produce a thick mucus-rich secretion.
In humans, there are three pairs of salivary glands: parotid, submandibular and sublingual ( Fig. 15.9 ) . The glands vary in the type of saliva they produce.
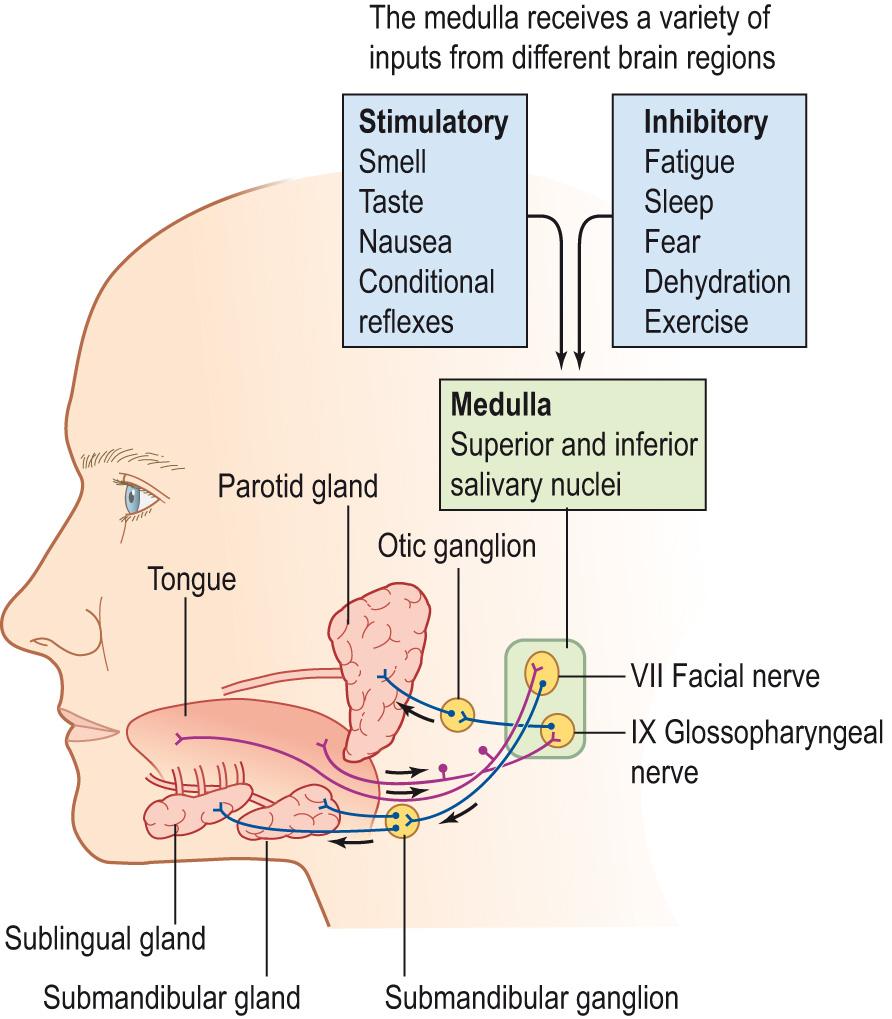
The parotid glands lie in the cheeks anterior to the ear, with their excretory ducts opening into the mouth opposite the second upper molar teeth. They are serous glands producing a watery secretion rich in amylase. These are the largest glands, but they produce only 25% of the daily saliva.
The submandibular glands lie under the mandible, with their excretory ducts entering the mouth under the tip of the tongue behind the central incisor teeth. They secrete 70% of the daily saliva production. These are mixed glands that contain serous and mucous cells, producing a more viscous secretion than the parotid glands.
The sublingual glands lie in the floor of the mouth just posterior to the mandible and have many small ducts opening into the mouth, some of them entering the submandibular duct. The cells of the sublingual glands are predominantly mucous cells; therefore, the secretion is thick and viscous owing to the presence of mucus. These glands produce 5% of the total daily output. In addition, there are smaller salivary glands scattered over the tongue, in the buccal cavity and on the inner side of the lips ( Clinical box 15.5 ).
Disease of the salivary glands may lead to impaired secretion of saliva, giving rise to xerostomia (dry mouth). This interferes with both chemical and physical digestion of food and also increases the risk of oral infections and ulceration.
Mumps is a common childhood infection of the salivary glands, characterised by pain and swelling, which temporarily affects salivation.
Salivary duct calculi (stones) obstruct the flow of saliva and may cause pain and swelling of the gland.
Salivary gland tumours are usually benign but may be malignant.
Drugs with anticholinergic action inhibit saliva secretion.
Sjögren syndrome is an autoimmune condition in which saliva-secreting cells are destroyed. There may be associated parotid gland enlargement, with ensuing dry mouth. Dry eyes, skin and vagina are also clinical features. This condition can occur in association with other systemic autoimmune diseases (e.g. rheumatoid arthritis, thyroid disease, etc.). Difficulty with swallowing because of the lack of lubrication and mouth ulcers and oral infections may be a manifestation of systemic disease (see earlier).
‘Water brash’ is a symptom in which there is a sudden inflow of saliva into the mouth, but without the stimulation of food. This is sometimes associated with peptic ulceration and inflammatory bowel disease.
Saliva has three functions. These are
Lubrication
Protection
Digestion.
The water and mucus in saliva act as lubricants that aid the movement of the tongue to facilitate speech and swallowing of food. You may have noticed that it is difficult to talk when your mouth is dry. Swallowing a number of dry biscuits is also difficult unless they are accompanied by a drink.
The presence of food debris in the warm and wet environment of the mouth provides ideal conditions for bacterial growth and division. The mouth is protected from metabolic acids produced by bacteria by the buffering action of bicarbonate, phosphate and mucus in saliva. The saliva is saturated with calcium salts at neutral pH, so that the calcium in the teeth does not dissolve. However, calcium from the teeth will dissolve and the formation of dental caries will occur if the pH falls to 5.5, once saliva flow has been reduced after a meal. The presence of protein in saliva forms a protective coat on the teeth surface to reduce the adherence of bacteria and to act as a barrier to acid. In addition, bacterial growth is limited by
Lysozyme, an enzyme responsible for breaking down bacterial cell walls
Lactoferrin, an iron-chelating agent that binds iron to prevent its use by some bacteria requiring iron for growth
Secretory immunoglobulin A, derived from plasma cells, which provides immunity against bacteria and viruses.
Large volumes of alkaline saliva are produced before vomiting. The alkali neutralises the gastric acid and inactivates the enzyme pepsin when it enters the oesophagus and mouth during vomiting.
The digestive function of saliva is to dissolve food so that the chemical constituents can stimulate the taste buds and to begin chemical digestion of carbohydrate and fat. Saliva contains an α-amylase or ptyalin, which starts the digestion of starch by hydrolysing α-1,4 glycosidic bonds to produce maltose, maltotriose and oligosaccharides known as α-limit dextrans (see Fig. 15.3 ). Salivary amylase is active over the pH range of 4–11 and has an optimum pH of 7. Chewing mixes the saliva with food. The amylase present inside a bolus of swallowed food continues its activity in the stomach until the acid in the gastric secretions penetrates the bolus. Up to 75% of starch digestion may occur in the stomach before amylase is destroyed by gastric acid.
Salivary glands present on the tongue secrete a lingual lipase that digests fat. This enzyme is also active in the stomach and intestine.
Saliva consists of water (99%) and dissolved chemicals (1%). In humans, it is less concentrated (hypotonic) than the plasma. The solid materials include organic and inorganic constituents.
These consist of
Amylase (ptyalin)
Lipase
Kallikrein
Lysozyme
Mucus
Immunoglobulin A
Lactoferrin
Blood group antigens, e.g. A and B.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here