Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The adrenal glands were first described by the Italian anatomist Bartolomeo Eustachi in 1563. The German comparative anatomist Albert von Kölliker (1817–1905), who noted the presence of the adrenals in a number of vertebrate species, is credited with first identifying two distinct portions of the adrenal gland, the cortex and the medulla. Although Thomas Addison described the clinical features of primary adrenal failure in 1855, it was not until nearly a century later that the adrenal hormones were fully isolated and characterized. Adrenaline (or epinephrine) was first isolated from adrenal extract at the turn of the century. Steroid hormones were crystallized from cortical extract (“cortin”) by Swiss and American investigators in the 1930s, but their highly similar chemical structures made isolation of the individual compounds challenging. Edward Kendall, Tadeus Reichstein, and Philip Hench jointly received the 1950 Nobel Prize in Physiology or Medicine for their groundbreaking work on the adrenocortical hormones. The Austrian-born endocrinologist Hans Selye first described the stress response in mammals in 1936 and made major contributions to the understanding of the hypothalamic-pituitary-adrenal (HPA) axis. Roger Guillemin, Andrew Schally, and Rosalyn Yalow were awarded the Nobel Prize in 1977 for characterizing the peptide hormones of the brain that underlie the HPA axis as we now understand it. ,
The adrenal glands are paired, mustard-colored structures that are positioned superior and slightly medial to the kidneys in the retroperitoneal space ( Fig. 40.1 ). They are flattened and roughly pyramidal (right) or crescent shaped (left), weighing approximately 4 g each. The adrenals are among the most highly perfused organs in the body, receiving 2000 mL/kg/min of blood, after only the kidney and thyroid. In most respects, the cortex and medulla can be considered two completely distinct organs that happen to colocalize during development. The two portions have disparate embryologic origins. The primordial cortex arises from the coelomic mesodermal tissue near the cephalic end of the mesonephros during the fourth to fifth week of gestation. Biosynthetic activity can be detected as early as the seventh week. Cortical cell mass dominates the fetal adrenal at 4 months of development, and steroidogenesis is maximum during the third trimester. The adrenal medulla arises from the ectodermal tissues of the embryonic neural crest. It develops in parallel with the sympathetic nervous system, beginning in the fifth to sixth week of gestation. From their original position adjacent to the neural tube, neural crest cells migrate ventrally to assume a para-aortic position near the developing adrenal cortex. There, they differentiate into chromaffin cells that make up the adrenal medulla.
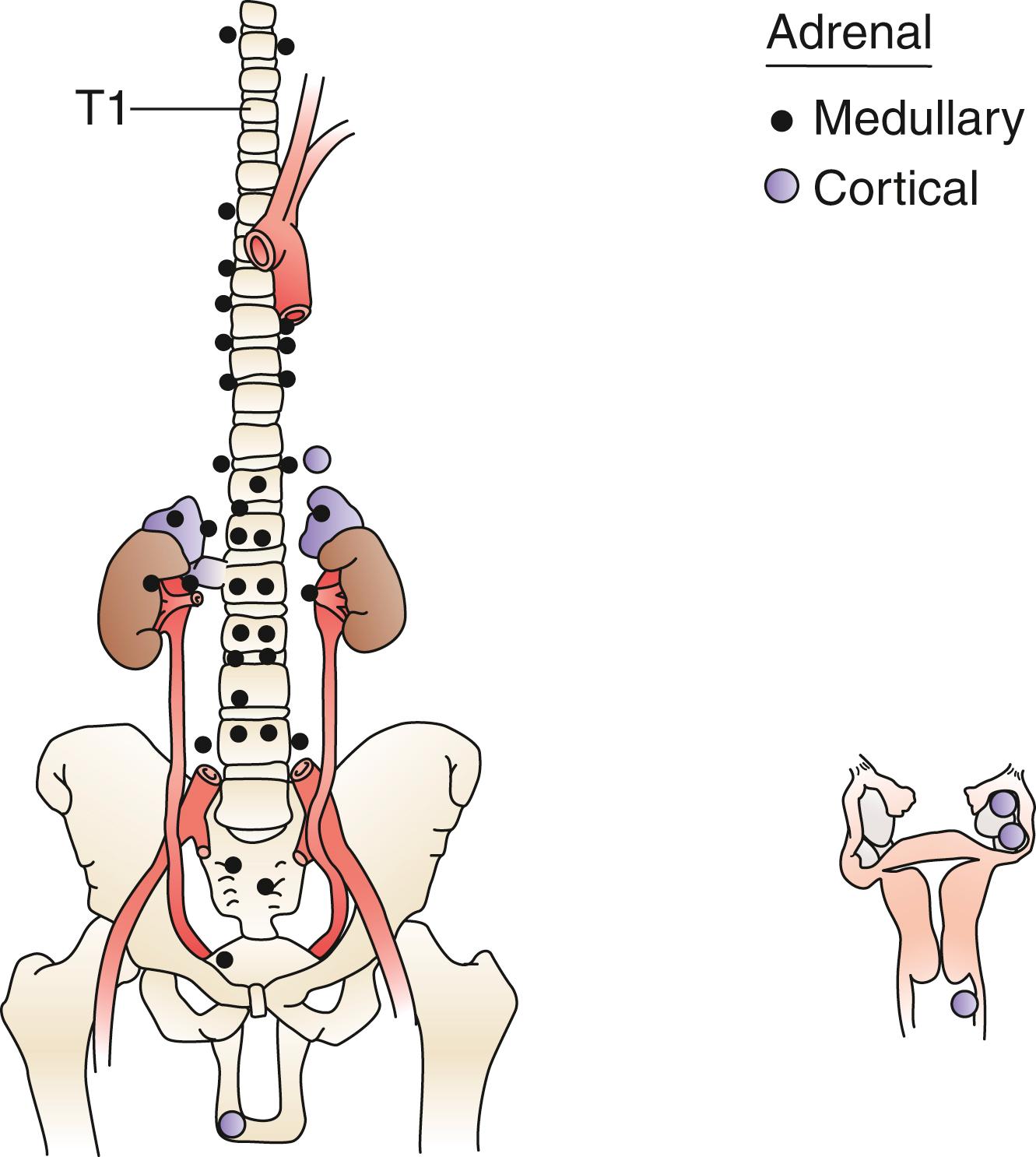
This course of embryologic development yields certain surgically relevant sequelae. Both cortical and medullary tissue can be found at extra-adrenal sites ( Fig. 40.2 ). The range of potential sites is wider for chromaffin tissue than for cortical tissue. Pheochromocytomas may arise in extra-adrenal sites more commonly than previously believed. When they are extra-adrenal, pheochromocytomas are also termed “paragangliomas.”
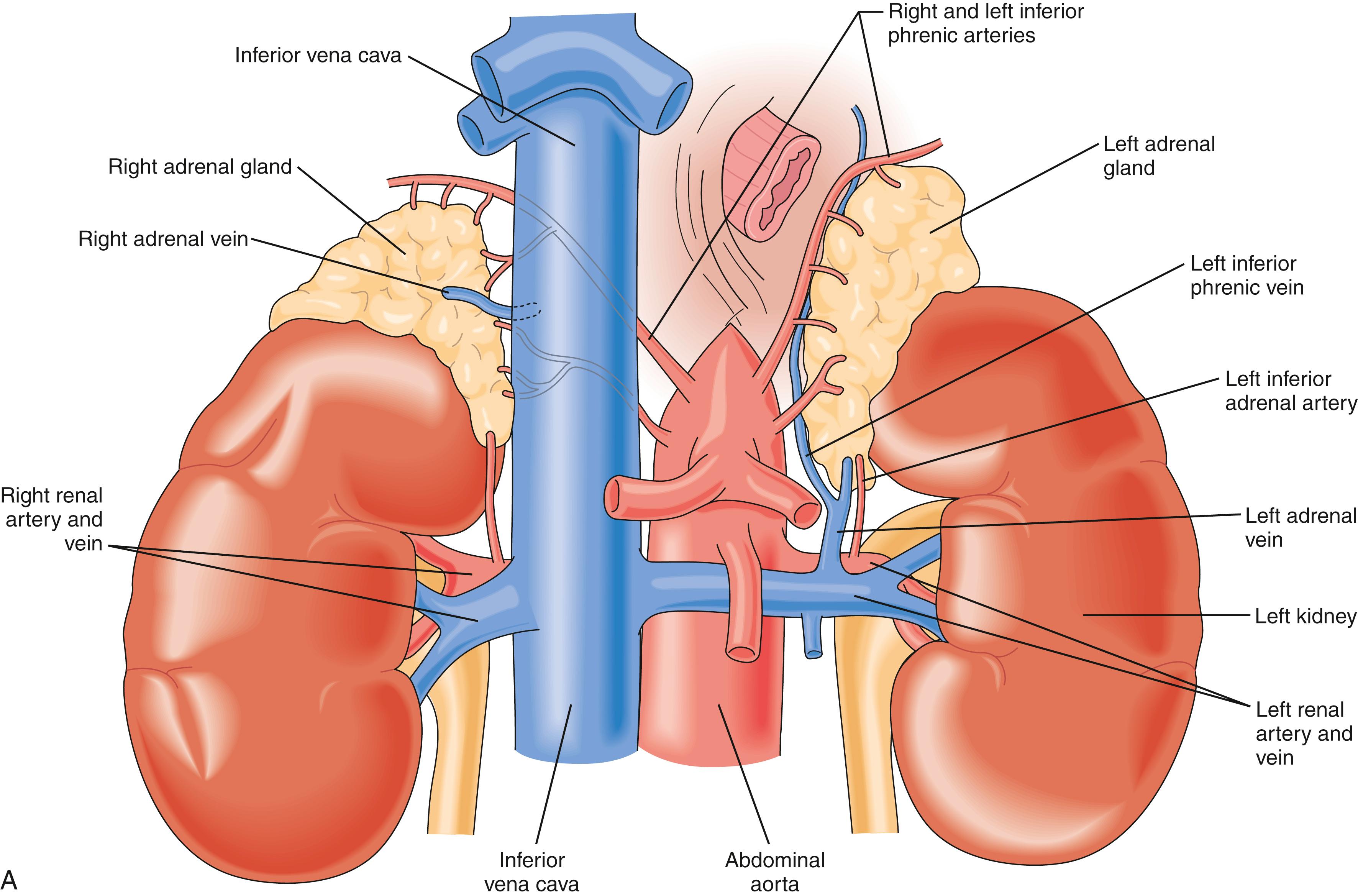
The right adrenal gland abuts the posterolateral surface of the retrohepatic vena cava. The right adrenal fossa is bounded by the right kidney inferolaterally, diaphragm posteriorly, and bare area of the liver anterosuperiorly. The left adrenal gland lies between the left kidney and aorta, with its inferior limb extending farther caudad toward the renal hilum than the right adrenal. The other relationships of the left adrenal gland are the diaphragm posteriorly and the tail of the pancreas and splenic hilum anteriorly. Each adrenal gland is enveloped by its proper capsule, in addition to sharing Gerota fascia with the kidneys. The adrenal capsules are immediately associated with the perirenal fat.
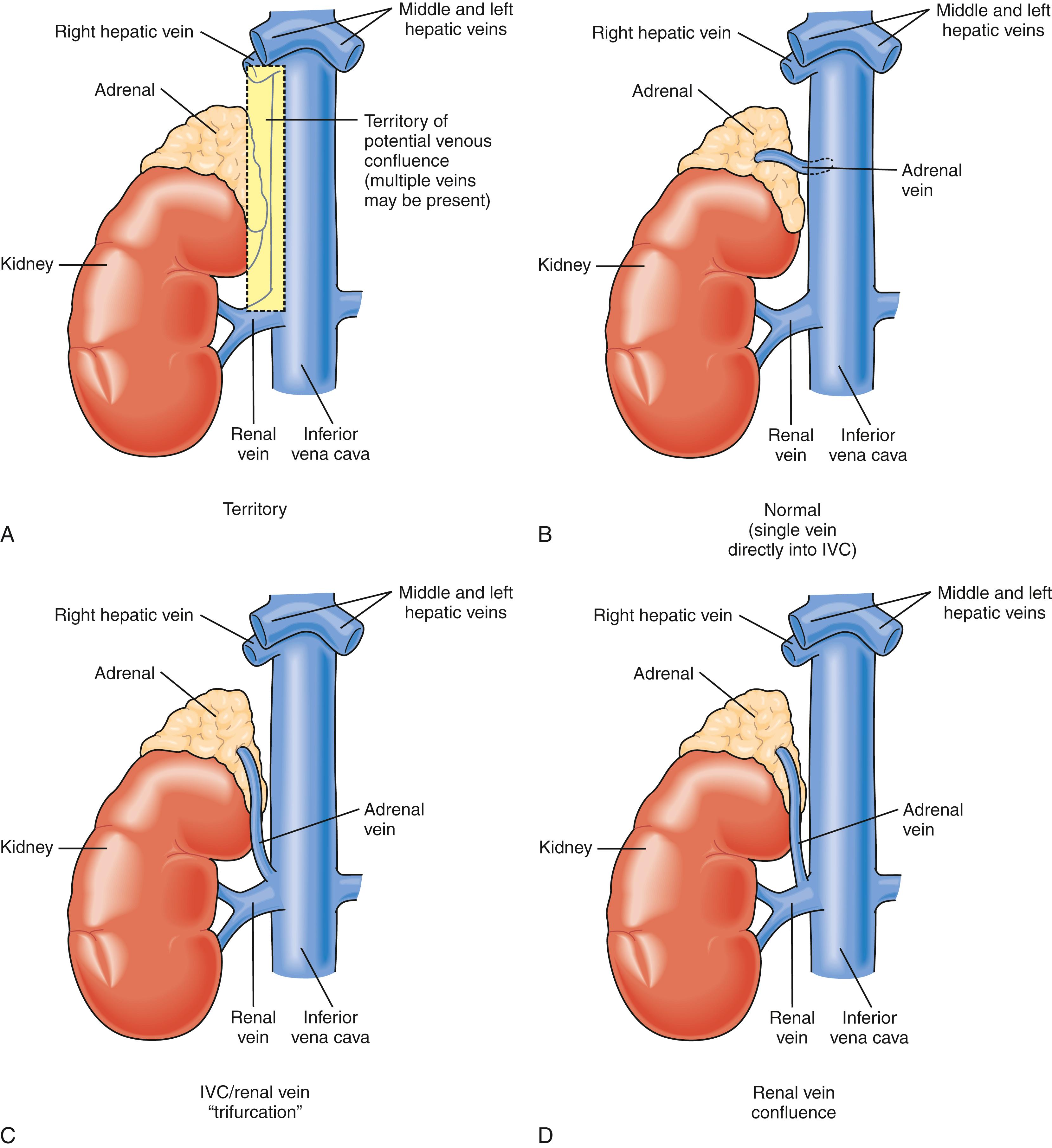
Knowledge of the macroscopic vascular anatomy of the adrenal glands is essential to proper surgical management. It is important to conceptualize that although the arterial supply is diffuse , the venous drainage of each gland is usually solitary . The arterial supply arises from three distinct vessels—superior adrenal arteries from the inferior phrenic arteries, small middle adrenal arteries from the juxtaceliac aorta, and inferior adrenal arteries from the renal arteries. Of these, the inferior is the most prominent and is commonly a single identifiable vessel. The left adrenal vein is approximately 2 cm long and drains into the left renal vein after joining the inferior phrenic vein. The right adrenal vein is typically as short as it is wide (0.5 cm) and drains directly into the vena cava. This configuration presents a surgical challenge that is discussed in more detail later in this chapter. In up to 20% of individuals, the right adrenal vein may drain into an accessory right hepatic vein or into the vena cava, at or near the confluence of such a vein. Vigilance about this variant and others ( Fig. 40.3 ) may reduce the likelihood of intraoperative venous hemorrhage during right adrenalectomy.
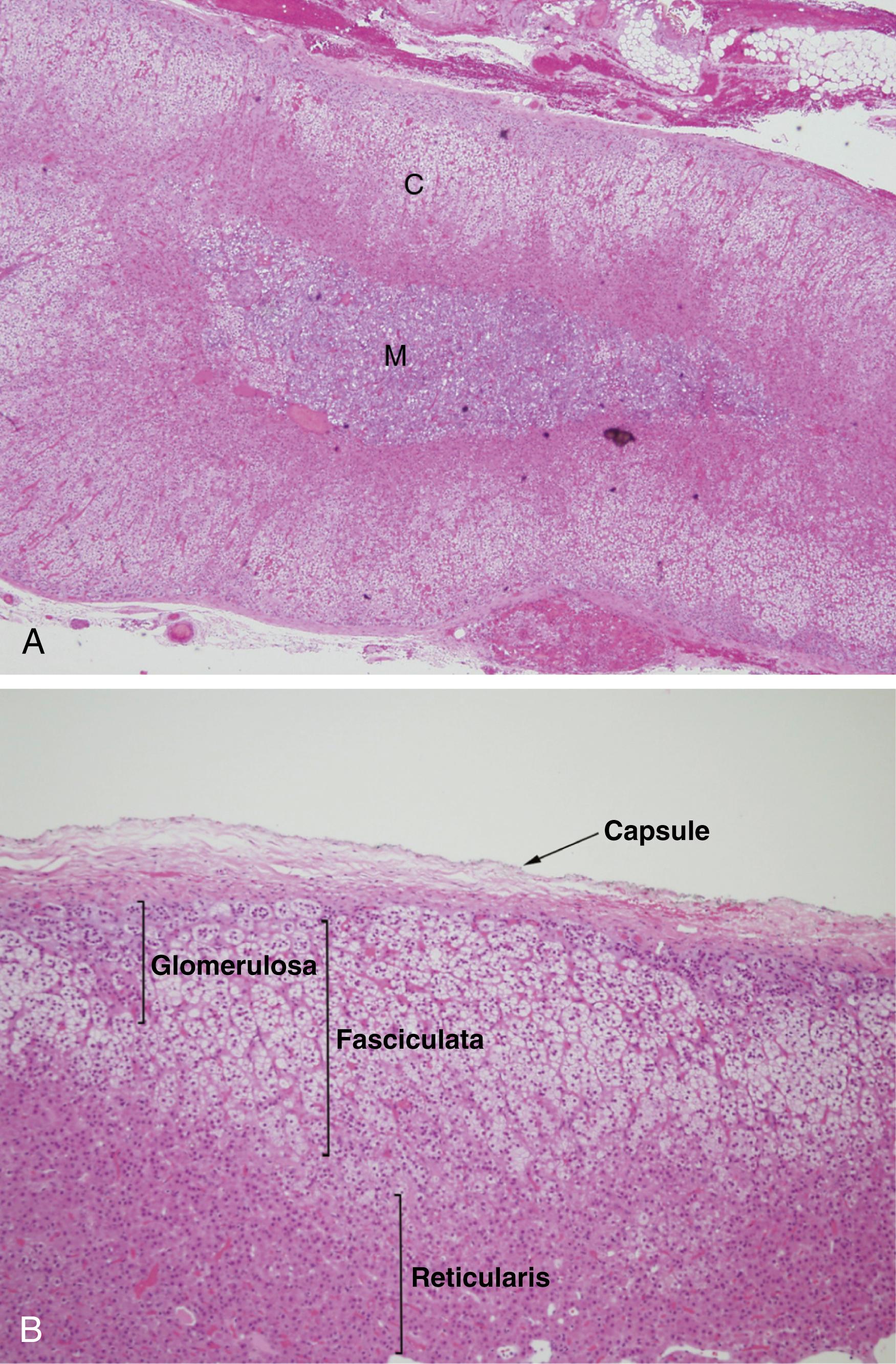
The cortex is approximately 2 mm thick and composes more than 80% of the mass of the gland. It is made up of three layers ( Fig. 40.4 ). The outer zona glomerulosa is a thin layer of relatively small cells with moderately eosinophilic, lipid-poor cytoplasm. It has an undulating inner border and normally does not form a complete circumferential layer. Most of the adrenal cortex is formed by the zona fasciculata , a middle layer composed of long radial columns of large, clear, lipid-laden cells. The inner zona reticularis is made up of small nests of compact, eosinophilic cells. The adrenal medulla consists of clusters and short cords of chromaffin cells, which are large, polyhedral, and packed with basophilic secretory granules. Catecholamines within these granules yield a brown reaction when treated with chromium salts, giving the cells their name. In contrast to the cortex, the adrenal medulla is richly endowed with autonomic nerve fibers and ganglion cells. Sympathetic fibers synapse directly with the chromaffin cells, constituting an interface between the nervous and endocrine systems.
The microvasculature of the adrenal gland functionally unifies the cortex and medulla. The adrenal arteries arborize extensively before entering the capsule to form a subcapsular plexus. Blood flows centripetally through capillaries in the zona glomerulosa and zona fasciculata before forming a deep plexus within the zona reticularis. From there, steroid-enriched postcapillary blood enters the medulla, where cortisol drives the expression of phenylethanolamine N -methyltransferase. Phenylethanolamine N -methyltransferase is responsible for the conversion of norepinephrine to epinephrine. This microvascular arrangement is essentially a portal system between the cortex and medulla.
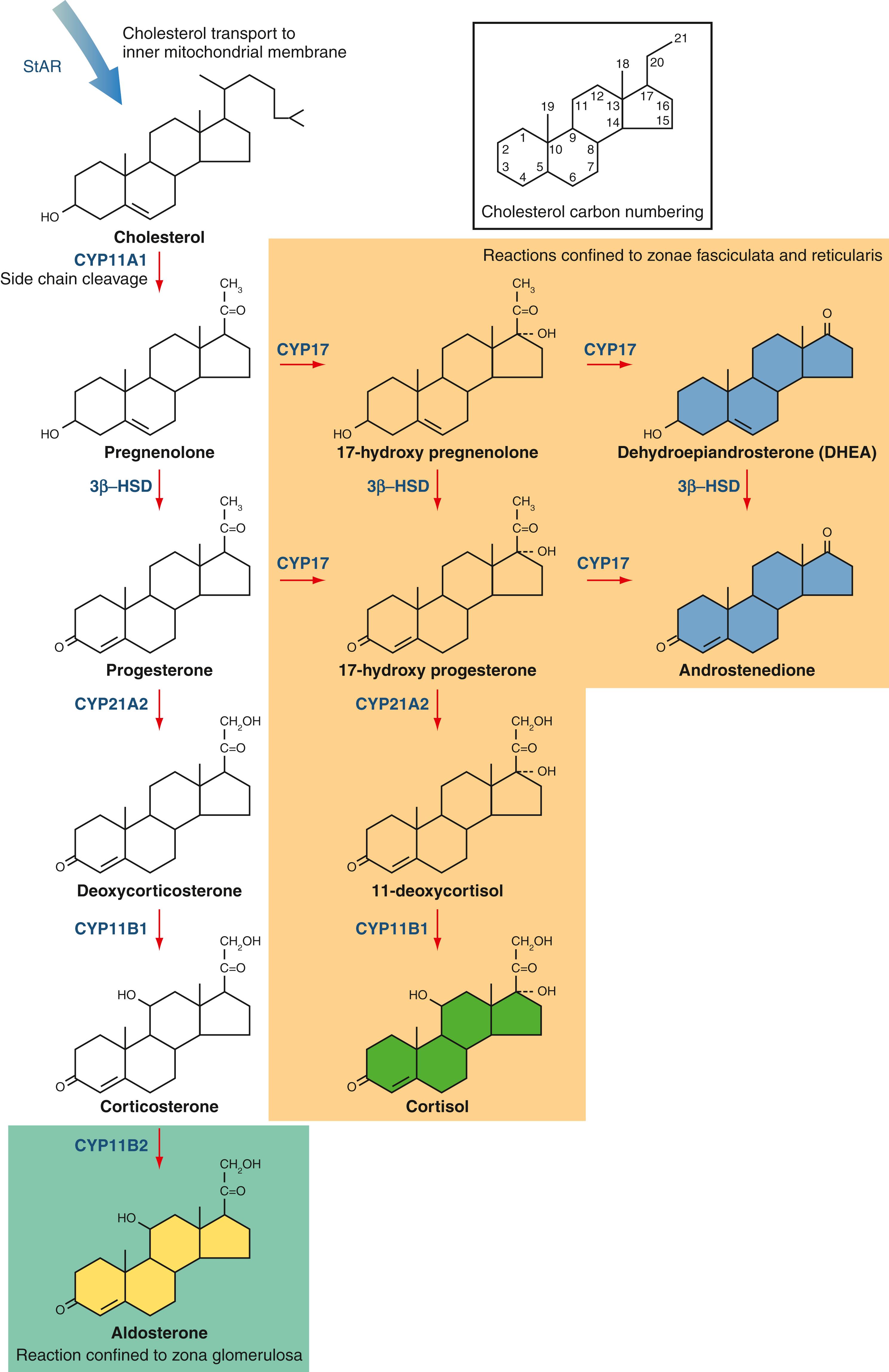
Adrenal steroid biosynthesis begins with the transport of cholesterol to the inner mitochondrial membrane by the steroidogenic acute regulatory protein ( Fig. 40.5 ). Cholesterol then undergoes a series of oxidative reactions catalyzed predominantly by membrane-associated enzymes belonging to the cytochrome P450 (CYP) family. Cleavage of the cholesterol side chain yields the hormonally inactive compound pregnenolone, the immediate precursor to the adrenal steroid hormones. Serial oxidation by CYP17 converts pregnenolone and progesterone into the major adrenal sex steroids dehydroepiandrosterone (DHEA) and androstenedione. Additional enzymatic steps confined to the gonads generate testosterone, estrone, and estradiol from androstenedione. Oxidation of 17-hydroxypregnenolone by 3β-hydroxysteroid dehydrogenase followed by action of CYP21A2 and CYP11B1 yields cortisol, the active glucocorticoid hormone in humans. Aldosterone is generated by the oxidation of corticosterone by CYP11B2 within the zona glomerulosa. CYP17 expression is confined to the zona fasciculata and zona reticularis, accounting for the synthesis of glucocorticoids and adrenal sex steroids in these regions.
Steroid hormones belong to a general class of low-molecular-weight, lipophilic signaling molecules that act by entering cells and binding to intracellular receptors. This group of hormones also includes thyroid hormone, retinoids, and vitamin D. Hormone binding results in alterations in gene expression that show a delayed and prolonged response compared with changes induced by peptide hormones, which act by binding to cell surface receptors. In the circulation, endogenous steroid hormones are largely bound to highly specific binding globulins. Serum levels of these proteins—and hence free hormone levels—can be altered by certain physiologic and disease states, such as pregnancy, nephrotic syndrome, and cirrhosis. Metabolism of both endogenous and pharmacologic steroids generally proceeds through hydroxylation, sulfonation, or conjugation to glucuronic acid in the liver, followed by urinary excretion. The regulation and physiologic actions of individual steroid hormones are discussed here.
The release of corticotropin-releasing factor into the hypothalamic-pituitary portal system by hypothalamic neurons results in adrenocorticotropic hormone (ACTH) secretion by the anterior pituitary. ACTH binds to a G protein–coupled receptor on the adrenocortical cell surface and stimulates glucocorticoid secretion. Steroidogenesis is acutely upregulated by increased steroidogenic acute regulatory protein-mediated cholesterol transport and pregnenolone synthesis by CYP11A1. ACTH is released in a pulsatile fashion that normally displays a circadian rhythm. The highest levels of ACTH and, thus, of cortisol are generally detected on waking, with levels gradually declining throughout the day to reach a nadir in the early evening. This pattern must be considered in evaluating patients for glucocorticoid deficiency or excess.
Glucocorticoid hormones have broad-ranging effects on almost all organ systems in the body. As a rule, they generate a catabolic state that characterizes the body’s response to stress. The hormones are so named because they cause alterations in carbohydrate, protein, and lipid metabolism that have the net effect of increasing blood glucose concentrations. Hepatic glucose output is elevated by the upregulation of gluconeogenesis, and net glycogen deposition occurs. Glucose uptake by peripheral tissues is directly inhibited. Glucocorticoids stimulate lipolysis with release of free fatty acids into the circulation, and a general state of insulin resistance is induced, resulting in protein catabolism. Fatty acids and amino acids serve as energy sources and substrates for gluconeogenesis. In the cardiovascular system, glucocorticoids exert a permissive and enhancing effect on catecholamine signaling by sensitizing arterial smooth muscle cells to β-adrenergic stimulation and increasing catecholamine concentrations in neuromuscular junctions. Cardiac contractility and peripheral vascular tone are thus maintained, explaining why the hemodynamic collapse that accompanies acute adrenal insufficiency can be remedied by glucocorticoid administration.
Glucocorticoids are potent antiinflammatory and immunosuppressive agents. Acutely, glucocorticoids reduce circulating lymphocyte and eosinophil counts while increasing neutrophil counts. Lymphocyte apoptosis is promoted, cytokine and immunoglobulin production is decreased, and histamine release is suppressed. Glucocorticoids also reduce prostaglandin synthesis through inhibition of phospholipase A2.
Aldosterone release from the zona glomerulosa is principally regulated by angiotensin II and the blood potassium level. The renin-angiotensin-aldosterone axis is responsive to sodium delivery to the distal convoluted tubule of the kidney. Low sodium delivery, which occurs in states such as hypovolemia, shock, renal artery vasoconstriction, and hyponatremia, stimulates the release of renin from the juxtaglomerular apparatus. The prohormone angiotensinogen is synthesized by the liver and is cleaved to inactive angiotensin I by renin. Further cleavage of angiotensin I by angiotensin-converting enzyme in the lungs and elsewhere yields angiotensin II, a potent vasoconstrictor and stimulator of aldosterone release. Hypokalemia reduces aldosterone release by suppressing renin secretion and also by acting directly at the zona glomerulosa. Hyperkalemia has the opposite effect.
Aldosterone regulates circulating fluid volume and electrolyte balance by promoting sodium and chloride retention by the distal tubule. Potassium and hydrogen ions are secreted into the urine. Acutely, expansion of the extracellular fluid volume and a rise in blood pressure are observed after aldosterone infusion. Negative feedback occurs primarily through an increase in sodium delivery to the distal tubule, suppressing renin release.
Secretion of the adrenal androgens androstenedione, DHEA, and DHEA-S (the sulfonated derivative of DHEA, synthesized in the adrenal and liver) is regulated by ACTH and other incompletely understood mechanisms. Of the three, androstenedione is produced in the smallest quantities. The physiologic effects of adrenal sex steroids are generally weak in comparison to the gonadal sex steroids, particularly in males. In females, peripheral conversion of DHEA and DHEA-S to more potent androgens, including androstenedione, testosterone, and dihydrotestosterone, supports normal pubic and axillary hair growth and may play a role in maintaining libido and a sense of well-being.
Synthesis of catecholamines in the adrenal medulla begins with the hydroxylation of tyrosine, a rate-limiting step that generates dihydroxyphenylalanine ( l -dopa) in the cytosol ( Fig. 40.6 ). Decarboxylation of l -dopa generates dopamine, which is then β-hydroxylated to form norepinephrine. Epinephrine is created by the action of phenylethanolamine N -methyltransferase, which, unlike the other enzymes involved in catecholamine synthesis, is localized to the chromaffin cells of the adrenal medulla and organ of Zuckerkandl. Sympathetic stimulation of the adrenal medulla results in the release of stored catecholamines into the circulation. Basal levels of adrenal catecholamine secretion are normally low, although large (up to fifty fold) increases in levels may be observed in response to major physiologic or psychological stressors. Target tissue responses are mediated by α- and β-adrenergic receptors. α-Adrenergic receptors display greater affinity for norepinephrine compared with epinephrine, and the opposite is true for β-adrenergic receptors. Stimulation of β 1 -adrenergic receptors in the myocardium results in increased heart rate and contractility. Stimulation of β 2 -adrenergic receptors results in smooth muscle relaxation in tissues such as the uterus, bronchi, and skeletal muscle arterioles. α 1 -Adrenergic receptors mediate vasoconstriction in tissues such as the skin and gastrointestinal tract. α 2 -Adrenergic receptors exist in presynaptic locations in the central nervous system, where they mediate attenuation of sympathetic outflow. The net effect of adrenal catecholamine release is to augment blood flow and oxygen delivery to the brain, heart, and skeletal muscle, which are essential to the fight-or-flight response, at the expense of other organ systems.
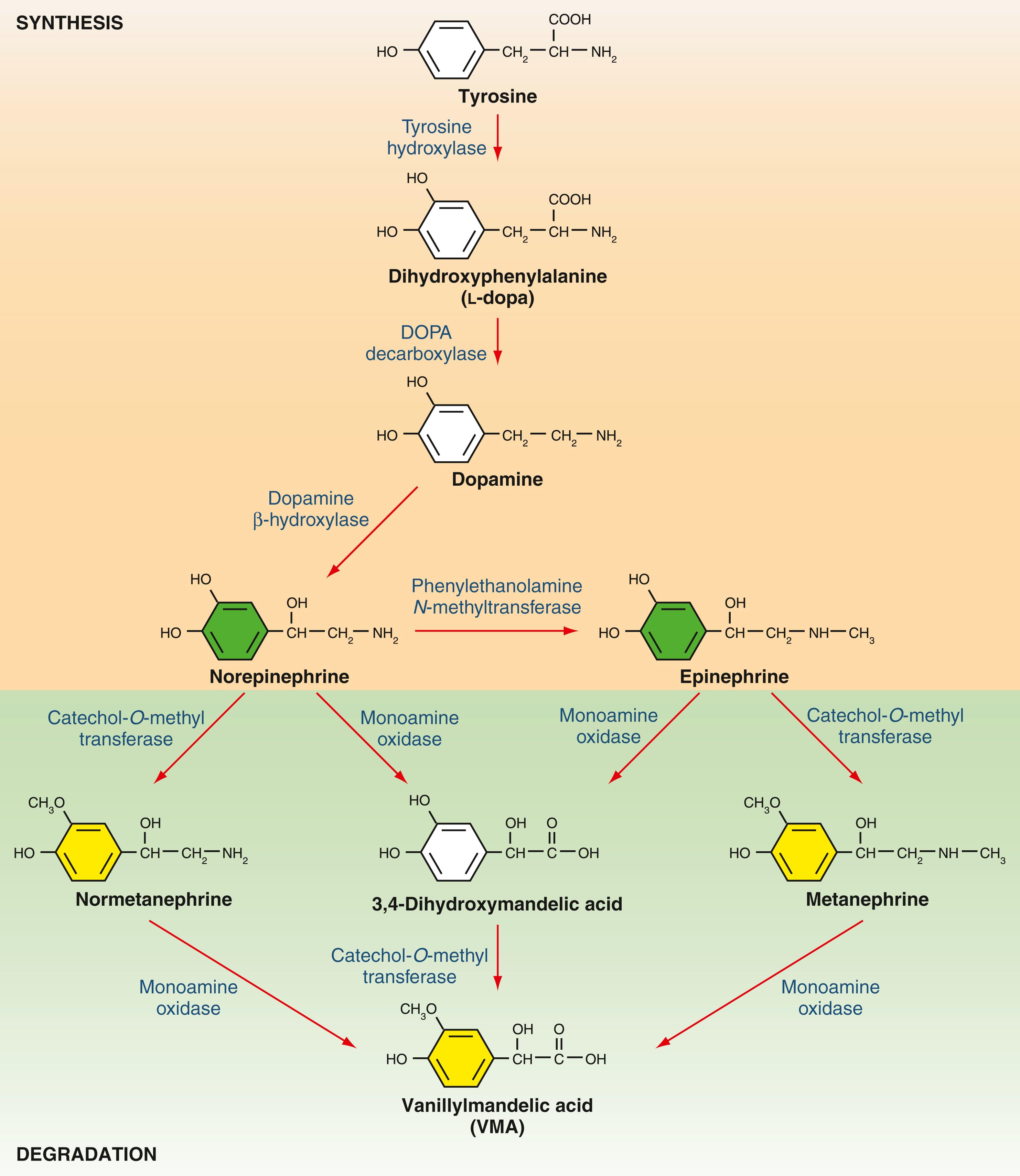
Catecholamines are potent and short-acting compounds, with a plasma half-life on the order of 1 minute. Their presence in synapses and the circulation exhibits tight negative regulation by both reuptake and degradation. Degradation pathways merit some discussion because they generate the metabolites commonly measured in the biochemical evaluation of pheochromocytoma (see later). Epinephrine and norepinephrine are inactivated by one or both of the enzymes monoamine oxidase and catechol- O -methyltransferase (see Fig. 40.6 ). Initial methylation by catechol- O -methyltransferase yields metanephrine and normetanephrine, which can be detected in plasma and urine. Their relatively stable plasma levels, which contrast with the high-amplitude fluctuations seen in plasma epinephrine and norepinephrine levels, make them attractive diagnostic markers. The sequential action of monoamine oxidase and catechol- O -methyltransferase generates the major final product, vanillylmandelic acid. Catecholamine metabolites are excreted in the urine, sometimes after sulfonation or conjugation to glucuronic acid in the liver.
Originally described in patients with tuberculous destruction of the adrenal glands, Addison disease is a rare disease that is manifested with weakness and fatigue, anorexia, nausea or vomiting, weight loss, hyperpigmentation, hypotension, and electrolyte disturbances (hyponatremia and hyperkalemia). Hyperpigmentation, previously believed to be caused by elevated levels of pro-opiomelanocortin and its cleavage product α-melanocyte-stimulating hormone, is now believed to result from ACTH-induced melanogenesis. Hormonal insufficiency caused by intrinsic adrenal disease arises from three general mechanisms—congenital adrenal dysgenesis/hypoplasia, defective steroidogenesis, and adrenal destruction. Of these, adrenal destruction from autoimmune causes is the most common, followed by infectious adrenalitis (e.g., tuberculous, fungal, viral), adrenal replacement by metastatic tumor, and adrenal hemorrhage (Waterhouse-Friderichsen syndrome). The last occurs in the setting of septicemia caused by meningococcal or other organisms and is more common in pediatric and asplenic patients.
Secondary adrenal insufficiency is a relatively common disorder resulting from ACTH deficiency, often occurring in the setting of pharmacologic steroid withdrawal. Patients receiving high supraphysiologic doses of glucocorticoids (more than the equivalent of 20 mg of prednisone daily; Table 40.1 ) for more than 5 days and those receiving low supraphysiologic doses for more than 3 weeks are at risk for HPA axis suppression. Surgical cure of Cushing syndrome likewise results in glucocorticoid withdrawal. The rate of recovery from HPA axis suppression varies in accordance with the duration and severity of previous glucocorticoid excess, and the need for glucocorticoid supplementation may last several years. Other less common causes of secondary adrenal insufficiency include panhypopituitarism caused by neoplastic or infiltrative replacement, granulomatous disease, and pituitary hemorrhage or infarction. Pituitary infarction may occur in the setting of severe postpartum hemorrhage (Sheehan syndrome).
| Compound | IV/PO ∗ | Common Trade Name | Relative Potency | Daily Physiologic Dose | Dosing Interval |
|---|---|---|---|---|---|
| Cortisol = hydrocortisone | Both | Cortef (PO) | 1× | 20 mg | q8–12h |
| Solu-Cortef (IV) | |||||
| Cortisone | PO | — | 0.8× | 25 mg | q8–12h |
| Prednisone | PO | — | 4× | 5 mg | q24h |
| Prednisolone | PO | — | 4× | 5 mg | q24h |
| Methylprednisolone | Both | Medrol (PO) | 5× | 4 mg | q24h |
| Solu-Medrol (IV) | |||||
| Dexamethasone † | Both | Decadron | 25× | 1 mg | q24h |
Studies have suggested that critically ill patients with sepsis or systemic inflammatory response syndrome may be affected by acute reversible dysfunction of the HPA axis. The incidence of the disorder is approximately 30% in critically ill patients, although this figure may be higher in those with septic shock. Whether these patients incur increased mortality because of adrenal insufficiency remains to be defined. Proposed mechanisms of reversible HPA axis dysfunction include adrenal ACTH resistance and decreased responsiveness of target tissues to glucocorticoids. Glucocorticoid supplementation in septic patients has been the topic of at least 42 randomized controlled trials. Among these studies, corticosteroid administration was associated with a small reduction in 30-day mortality (1.8% absolute risk reduction) and faster shock reversal. There appears to be an inverse relationship between survival benefit and glucocorticoid dose, with physiologic (i.e., replacement) doses yielding a possible benefit and high supraphysiologic doses demonstrating significant harm. Adverse effects of corticosteroid administration in this patient population include hypernatremia, hyperglycemia, and muscle weakness. Earlier trials suggested that patients with severe septic shock, particularly those requiring vasopressors, may benefit from 5- to 7-day courses of glucocorticoids up to 300 mg/day or less of hydrocortisone or equivalent. As more recent data have not confirmed a significant survival benefit, glucocorticoid use should be individualized in critically ill patients.
Acute adrenal insufficiency, or adrenal crisis, is a life-threatening condition that typically occurs in individuals with already marginal adrenocortical function who are subjected to a significant acute physiologic stressor, such as infection or trauma. Sudden and complete loss of adrenal function, as occurs with Waterhouse-Friderichsen syndrome and certain hypercoagulable states, can also be manifested with adrenal crisis. Clinical findings include shock, abdominal pain, fever, nausea and vomiting, electrolyte disturbances, and occasionally hypoglycemia. Mineralocorticoid deficiency, resulting in an inability to maintain sodium and intravascular volume, is the primary pathogenic mechanism, although diminished cardiovascular responsiveness to catecholamines caused by glucocorticoid deficiency also plays a role. Treatment for suspected adrenal crisis should not be delayed while awaiting the results of diagnostic testing. The treatment of adrenal crisis centers around large-volume (1–2 L) intravenous resuscitation with isotonic saline and glucocorticoid administration in the form of hydrocortisone (100 mg intravenous bolus followed by 75 mg every 8 hours) or dexamethasone (4 mg intravenously every 24 hours). Dexamethasone is long acting and carries the advantage of not interfering with biochemical assays of endogenous glucocorticoid production. Ironically, mineralocorticoid replacement is not an early priority because the sodium- and fluid-retaining effects of mineralocorticoids are not manifested until several days after administration. Fluid and electrolyte balance can be rapidly achieved by saline infusion.
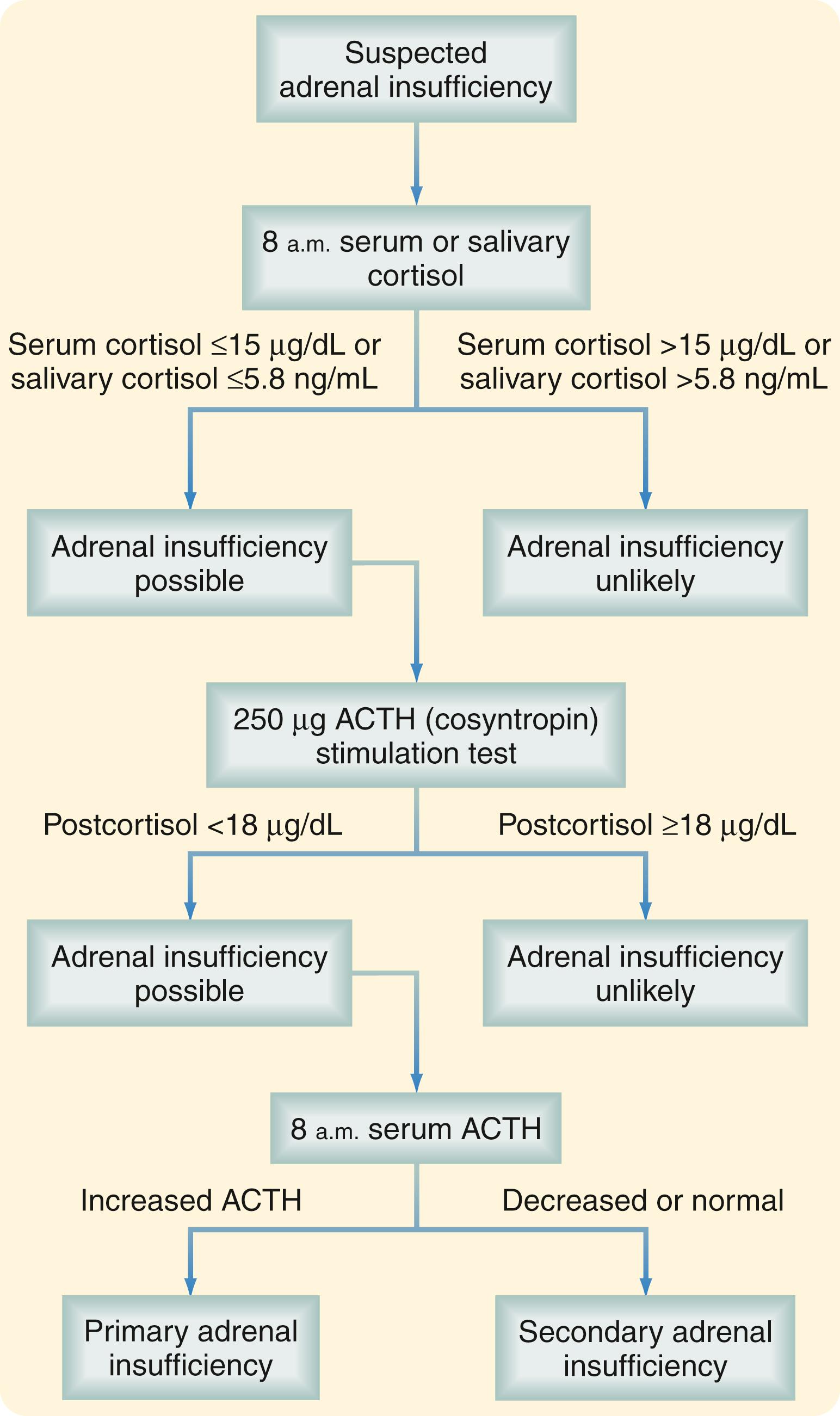
As is true for most endocrine disorders, the diagnosis of adrenal insufficiency depends on maintaining sufficient clinical suspicion for the disease. The clinical manifestations have been discussed earlier. Surgeons are most likely to encounter patients with adrenal insufficiency in the intensive care unit, trauma suite, or operating room when treating patients with steroid-dependent chronic illnesses. Routine and provocative biochemical testing is necessary to confirm the diagnosis ( Fig. 40.7 ). The first step is to document inadequate cortisol production, which can be done by measuring morning levels of cortisol in the serum or saliva. In most patients, morning serum cortisol concentration higher than 15 μg/dL or morning salivary cortisol concentration higher than 5.8 ng/mL effectively excludes adrenal insufficiency. Patients whose levels fall below these thresholds should undergo provocative testing. A high-dose cosyntropin stimulation test is performed by administering 250 μg cosyntropin and measuring serum cortisol levels 30 to 60 minutes later. A positive test result (i.e., a stimulated cortisol level less than 18 μg/dL) is strongly suggestive of adrenal insufficiency. After the diagnosis of adrenal insufficiency has been made, a morning ACTH level is determined to differentiate between primary and secondary adrenal insufficiency.
The treatment of adrenal crisis has been discussed. The goal of maintenance therapy for chronic adrenal insufficiency is to replace physiologic glucocorticoid and mineralocorticoid levels. Daily adult cortisol production is in the range of 10 to 20 mg, which can be replaced by the long-acting, orally bioavailable agent prednisone at a dosage of 5 mg/day. Typical mineralocorticoid replacement consists of fludrocortisone, 0.1 mg/day. Commensurate increased dosages of glucocorticoids are needed during periods of minor and major physiologic stress, such as mild infections (minor) and trauma, significant infections, burns, or elective surgery (major).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here