Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Body temperature is a critical part of homeostasis, a process that biological systems use to preserve a stable internal state that allows for normal functioning and survival. Humans use thermoregulation to maintain a somewhat consistent internal body temperature to survive (37°C [98.6°F]), even when the external environment changes. Alterations in body temperature can affect a wide array of physiologic processes, from vital chemical reactions catalyzed by enzymes to the optimal functioning of white blood cells and platelets.
Humans maintain body temperature by balancing heat production, primarily by metabolism, with heat loss through a variety of physiologic mechanisms, as well as environmental factors. During anesthesia, many of these temperature-regulating pathways are interrupted or inhibited, underscoring the need for temperature monitoring during anesthesia.
The goal of the anesthesia provider is to minimize deviations in body temperature – unless indicated for specific reasons, such as organ protection. While malignant hyperthermia syndrome, a potentially fatal pharmacogenetic disorder of anesthesia, had a marked influence on how the anesthesia community viewed the importance of temperature measurement and regulation. Studies have shown that even mild hypothermia, not uncommon during anesthesia, carries physiologic risks. As documented in detail below, core temperature in the range of 35 to 36°C triples the incidence of morbid cardiac outcomes, triples the risk of surgical wound infection, and significantly increases blood loss and the need for allogeneic transfusion.
This chapter discusses perianesthetic thermoregulation in terms of effects of anesthetic agents and techniques, use of monitoring devices, and techniques for maintenance of normothermi. Recommendations for management of core temperature during surgery and in the recovery period are also discussed.
Normal core body temperature varies by at least 1°C based on circadian and menstrual cycles. Temperature regulation has three components: (1) an afferent input, (2) a central control, and (3) an efferent response. The afferent component is composed of both heat and cold receptors, which are widely distributed in the body, with transient receptor potential proteins being the most important. Among them, transient receptor potential vanilloid (TRPV) receptors 1–4 are activated by heat whereas transient receptor potential melastatin (TRPM8) and transient receptor potential ankyrin (TRPA1) are activated by cold. Heat and warmth receptors travel primarily through unmyelinated C fibers, whereas cold receptors travel along A-δ nerve fibers, although some overlap occurs.
Ascending sensory thermal input is then transmitted to the hypothalamus, which is the primary thermoregulatory control center of mammals, via the spinothalamic tracts in the anterior spinal cord. Although most thermal information is integrated by the hypothalamus, some processing and response occurs within the spinal cord itself. Central thermoregulatory control is based on thermal input from structures throughout the body; mean skin temperature contributes about 50% to thermal comfort, with the upper chest and face contributing more than other regions.
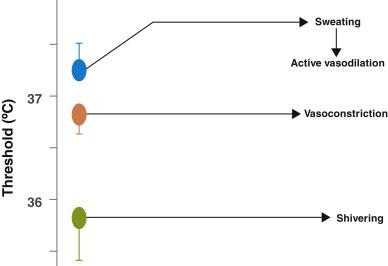
Each thermoregulatory response can be characterized by the threshold, gain, and response. The threshold is the temperature at which a response will occur. The gain represents the intensity of that response ( Fig. 13.1 ). The threshold for responses to warmth (sweating and vasodilation) normally exceeds the threshold for the first response to cold (vasoconstriction) by 0.2 to 0.4°C. Temperatures within this interthreshold range—the range between the threshold for response to cold and the threshold for response to warmth (0.2°C to 0.4°C)—do not trigger any thermoregulatory responses. However, temperatures outside the interthreshold range do trigger a response ( Fig. 13.2 ).
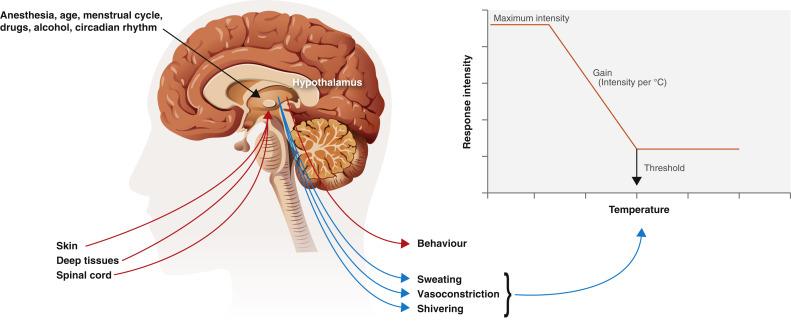
Efferent responses are the activation of effector mechanisms, which either increase metabolic heat production or alter environmental heat loss and can be broadly categorized into behavioral and autonomic responses. Quantitatively, the most effective responses are behavioral ones: dressing appropriately, moving voluntarily, or adjusting ambient temperature, which are irrelevant for patients under general anesthesia.
The major autonomic responses to heat are sweating and active cutaneous vasodilation. Sweating is mediated by postganglionic cholinergic (acetylcholine) nerves, and sweat essentially is an ultrafiltrate of plasma. Sweating is the only mechanism by which the body can dissipate heat in an environment in which ambient temperature exceeds core temperature. The body is highly efficient at doing so: 0.58 kcal of heat is dissipated per gram of evaporated sweat. Cutaneous vasodilation, a unique effector mechanism of humans mediated by the cholinergic system, diverts blood to the periphery where heat can be dissipated by the environment more easily and ultimately can lower core temperature. Several specific vasodilators including nitric oxide, vasoactive intestinal peptide, substance P, histamine, and prostaglandins appear to be involved in this mechanism.
The primary autonomic thermoregulatory responses to cold are cutaneous arteriovenous shunt vasoconstriction, mediated by α1- and α2-adrenergic receptors, and shivering. Cutaneous vasoconstriction reduces the amount of blood vulnerable to heat loss from the skin surface through convection and radiation. Shivering, an involuntary muscular activity, increases metabolic heat production by 50% to 100%. The response of shivering is absent in the newborn and probably not fully functional for several years. Infants therefore rely on nonshivering thermogenesis, an important thermoregulatory response that doubles heat production in infants, although it raises temperature only slightly in adults. Nonshivering thermogenesis is mediated by β 3 -adrenergic receptors via activation of brown fat by an uncoupling protein, thermogenin, which allows the direct transformation of substrate into heat.
The thermoneutral zone is defined as the range of ambient temperature where healthy adults can maintain normal body temperature without using energy above normal basal metabolic rate. The range is 25–30°C (77–86°F) for a naked adult man, standing upright, in still air.
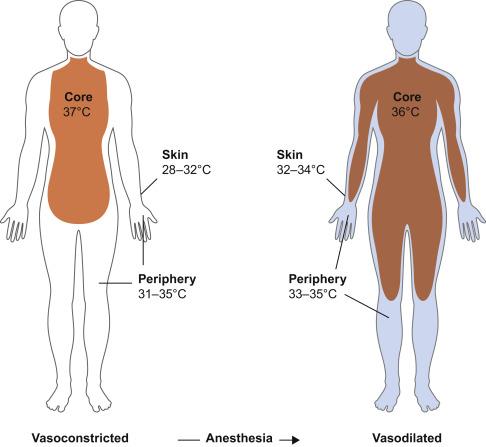
Both general and neuraxial anesthesia are associated with impaired thermoregulatory control. The fall in temperature during general anesthesia follows a characteristic pattern and has three phases: an initial rapid decrease of approximately 0.5 to 1.5°C over approximately 30 minutes, a slow linear reduction of about 0.3°C per hour, and a plateau phase as shown in Fig. 13.3 . The initial decrease in temperature reflects increased heat loss due to redistribution of body heat from the core to cooler peripheral tissues due to anesthesia-induced vasodilation ( Fig. 13.4 ). General anesthesia decreases the metabolic rate by 20%–30%; and, as a result, heat loss exceeds metabolic heat production causing the slower linear decrease in temperature. General anesthesia also eliminates any behavioral responses to temperature change. Autonomic responses also are markedly impaired under anesthesia; the interthreshold range increases up to 10-fold, from 0.2–0.4°C to about 2–4°C, making anesthetized patients poikilothermic (cold-blooded) over a wide range of core temperatures. When core temperature exceeds the sweating threshold or decreases to below the vasoconstriction threshold, thermoregulatory defenses will activate in anesthetized patients.

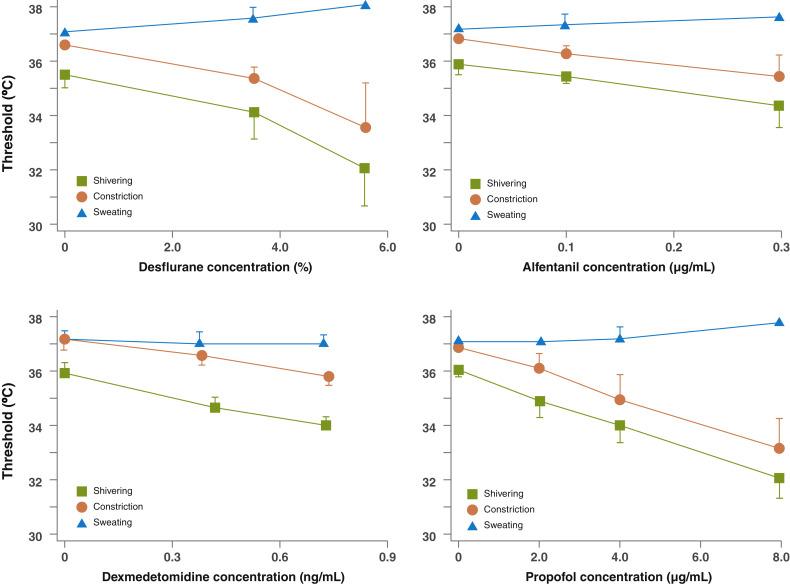
Volatile anesthetics such as sevoflurane, isoflurane, or nitrous oxide, and intravenous (IV) anesthetics such as propofol and opioids, all substantially impair thermoregulatory control ( Fig. 13.5 ). They all have minimal effects on sweating thresholds, but significantly reduce the vasoconstriction and shivering thresholds. Sedatives such as midazolam have little effect on thermoregulatory control. While volatile anesthetics directly inhibit TRPV1 receptors, the mechanism by which anesthetics impair thermoregulatory control remains unknown.
Neuraxial anesthesia affects thermoregulation to the same extent as general anesthesia. This impairment in thermoregulatory control occurs via three mechanisms:
In the presence of neuraxial blocks, hypothermia does not provoke as much thermal discomfort and consequently patients do not complain of feeling cold even when hypothermic. This might be due to misinterpretation of the lack of tonic cold signals as relative warmth by the central controller.
Central thermoregulatory control is inhibited by neuraxial anesthesia, reducing the vasoconstriction and shivering thresholds. This impairment is a linear function of the dermatomal block height and may be related to the anesthetic blocking tonic cold signals from the lower body.
Neuraxial anesthesia also impairs autonomic thermoregulatory defenses, including active vasodilation, sweating, vasoconstriction, and shivering.
In addition, behavioral responses are impaired by neuraxial anesthesia. Like general anesthesia, an initial decrease in core temperature is caused by neuraxial blockade-induced vasodilation and a slow linear decline since heat loss exceeds heat production. In contrast to general anesthesia, the decrease may not necessarily plateau since peripheral vasoconstriction is inhibited by neuraxial anesthesia ( Fig. 13.6 ) and the vasoconstriction threshold is centrally altered, decreasing by about 0.6°C.
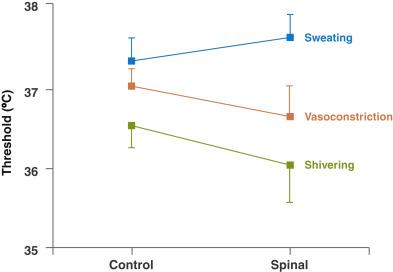
The effects of general and regional anesthesia on thermoregulation are mainly additive. Core temperature during regional/general anesthesia continues to decrease throughout the surgery. Peripheral nerve blocks, in contrast, have minimal thermoregulatory effects.
Intraoperative heat loss occurs through four physical processes: radiation, convection, evaporation, and conduction ( Fig. 13.7 ).
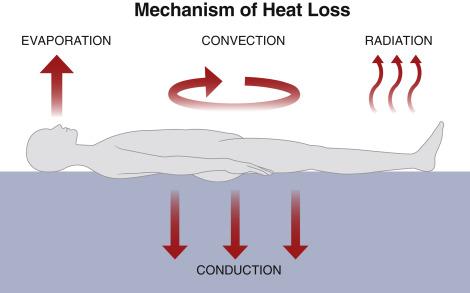
Radiation accounts for most of the heat loss to the environment, approximately 40%. It is a function of the body surface area exposed to the environment and is proportional to the fourth power of the absolute temperature difference between the surfaces. Owing to the high surface area/body mass ratio, infants are especially vulnerable to heat loss by radiation.
Convection is due to loss of heat to air immediately surrounding the body and is the second most significant route for heat loss intraoperatively, contributing to approximately 30% of heat loss. Convective heat transfer is proportional to the square root of the air speed. Clothing or drapes are effective in decreasing heat loss due to convection. Conversely, convective heat loss may be greater in rooms with laminar air flow.
Evaporation accounts for 8%–10% of heat loss, due to the latent heat of vaporization of water from open body cavities and the respiratory tract. Sweating is the main pathway of evaporative heat loss but is uncommon during anesthesia.
Conductive heat loss is due to direct contact of body tissues or fluids with a colder material. Examples include contact between the skin and the operating table or between the intravascular compartment and an infusion of cold fluid and is directly proportional to the temperature differences between the two surfaces. For example, infusion of 1 L of crystalloid fluid at 21°C to a body temperature of 37°C decreases mean body temperature by 0.25°C, and the infusion of two units of cold blood can result in a core temperature decrease of 1°C.
Unintended perioperative hypothermia is common during anesthesia. Defined as unintended core body temperature less than 36°C in the perioperative period, unintended perioperative hypothermia has a 70% incidence (varying from 6%–90%) , and depends on the surgical procedure and the demographic characteristics of the patient. Prolonged surgeries, extremes of age, major intraoperative fluid shifts, severe trauma, and extensive burns are associated with higher risk of unintended perioperative hypothermia. Many studies have shown that even mild intraoperative hypothermia causes various complications.
The most well-studied complication of hypothermia is coagulopathy. Hypothermia decreases release of thromboxane A3, leading to reversible impairment of platelet aggregation, and impairs activity of enzymes involved in the coagulation cascade. Numerous trials summarized in a meta-analysis have shown that a 16% increase in blood loss and 22% higher relative risk of transfusion are associated with mild hypothermia.
Mild intraoperative hypothermia increases the risk of wound infection. Cold-induced vasoconstriction decreases oxygen delivery to the wound site, and immune function is directly impaired by hypothermia. In addition, hypothermia decreases tissue healing leading to an increased incidence of wound infection and prolonged hospital stay. Mild intraoperative hypothermia triples the risk of surgical wound infection compared to normothermic patients (19% vs 6%) undergoing colorectal surgery.
Hypothermia is associated with a threefold increase in morbid myocardial outcomes. Hypothermia is associated with increased blood pressure and heart rate and increased plasma catecholamine levels, which might lead to myocardial injury.
Postanesthetic shivering occurs in up to 40% of patients. Although benign shivering is uncomfortable for patients, it is potentially harmful because it can increase oxygen demand by 135% to 468%, which can be problematic in the elderly and in patients with preexisting cardiac disease. Postanesthetic shivering can be treated pharmacologically or with skin surface warming. The most commonly used treatments, such as meperidine (25 mg IV), clonidine (75μg IV), dexmedetomidine, and ketamine, reduce shivering and the vasoconstriction threshold. Other effective drugs include magnesium (30 mg/kg IV), doxapram, and ondansetron, though their mechanism of action is unknown.
Hypothermia affects hepatic and other enzymes thereby decreasing drug metabolism rates. For example, the duration of action for vecuronium is doubled by a 2°C reduction in core temperature. At 34 to 35°C, hypothermia increases plasma propofol concentrations by 28% and prolongs the duration of atracurium by 60% compared to normothermic patients. The consequence of this decreased drug metabolism is delayed postanesthetic recovery in hypothermic patients.
Intraoperative hypothermia also causes postoperative thermal discomfort. Thermal discomfort is typically intense and should be treated like postoperative pain and nausea. Active cutaneous warming greatly improves thermal comfort in hypothermic patients and simultaneously speeds rewarming.
A 1 to 3°C decrease in temperature (mild hypothermia) protects against cerebral ischemia in animals. The protective effect of hypothermia is the result of the 8% per °C linear reduction in cellular metabolic rate and the decreased release of excitatory amino acids. Therapeutic hypothermia has shown improvement in outcomes from out-of-hospital cardiac arrest. , Rapid induction of hypothermia is now indicated for patients after return of spontaneous circulation following cardiac arrest and in asphyxiated neonates. , Mild hypothermia is also at times employed in neurosurgery when brain tissue ischemia is expected.
Therapeutic temperature modulation has long been used as a treatment option in the care of patients after traumatic brain injury. Multiple prospective trials have been published with conflicting results. , While therapeutic cooling can lower intracranial pressure, no convincing evidence supports an improvement in functional outcome. Currently, use of mild hypothermia is advised in the setting of super-refractory intracranial hypertension when other therapies have failed. Some studies have suggested that mild hypothermia could improve the outcome from stroke. Mild hypothermia markedly decreased infarct size in experimental acute myocardial infarction in pigs; however, a major outcome trial in humans failed to demonstrate convincing benefit.
Acute malignant hyperthermia is more difficult to trigger during mild hypothermia, but once triggered was shown to be less severe in mildly hypothermic swine. , Active warming should therefore be avoided in patients known to be susceptible to malignant hyperthermia.
Hyperthermia denotes a core temperature above normal and is more dangerous than similar degrees of hypothermia. Hyperthermia causes discomfort, increases metabolic demand and cardiovascular stress, and places the patient at risk of coagulopathies, renal and liver dysfunction, neuropathies, and seizures. Excessive heating, excessive heat production, inadequate heat loss, or hypothalamic set-point elevation (as occurs in fever) can lead to perioperative hyperthermia.
Intraoperative hyperthermia is uncommon since anesthesia tends to lower body temperature and volatile anesthetics and opioids blunt the febrile response to interleukins. The most common cause of intraoperative hyperthermia is iatrogenic overwarming, especially during long procedures, particularly in infants and children since sweating under anesthesia is less effective in these patients. More common in head and neck surgery (due to minimal skin exposure), it can easily be treated by discontinuing active warming and removing excessive insulation. Allergic reactions and mismatched blood transfusions may also be accompanied by intraoperative hyperthermia.
Excessive heat production is one hallmark of malignant hyperthermia syndrome, one of the most feared anesthetic complications. This syndrome is associated with muscle rigidity, hypercarbia, hyperkalemia, and acidosis. The treatment is to remove the triggering anesthetic agent and begin immediate parenteral administration of dantrolene, starting with 2.5 mg/kg. More information about malignant hyperthermia is available from the Malignant Hyperthermia Association of the United States ( www.mhaus.org ) and in standard anesthesia textbooks. Many drugs also may provoke fever and hyperthermic syndromes such as serotonin syndrome and neuroleptic malignant syndrome.
In cases of marked hyperthermia in the postanesthesia care unit, the cause generally is infection. Marked hyperthermia may therefore develop postoperatively in patients undergoing urologic procedures, dental rehabilitation, or drainage of an abscess. Postoperative febrile reactions may also be associated with preexisting pneumonia or intraoperative aspiration. Such fevers respond promptly to antipyretics, antibiotics, and cautious active surface cooling.
Hyperthermia frequently complicates epidural analgesia used for labor and delivery, especially when labor is prolonged. The mechanism for this association is unknown. As a clinical consequence, women given epidural analgesia are more often given antibiotics, and their infants are more commonly treated for sepsis, compared with women who did not receive epidural analgesia for labor.
Intraoperative hyperthermia can also occur during hyperthermic intraperitoneal chemotherapy. Hyperthermic intraperitoneal chemotherapy is a technique that involves infusing chemotherapy heated to 42 to 43°C into the peritoneal cavity following extensive cytoreductive surgery, which tends to increase core body temperature.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here