Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Thymus-derived lymphocytes (T cells) play an essential role in the immune response to pathogens and against host cells that have undergone malignant transformation. T cells are critical regulators of the immune system that act through production of soluble mediators and direct interactions between ligands on the T-cell surface and receptors on other immune cells. This chapter reviews T-cell activation after engagement with specific antigens and describes how signals delivered by the antigen receptors shape the repertoire of mature T cells in secondary lymphoid organs. The mechanism by which different populations of mature T cells exert their effector functions is then discussed. Because homeostasis of the immune system requires not only that T cells become activated under appropriate conditions but also that their activity be curtailed once the pathogenic challenge has been met, the processes by which T-cell activation is terminated are also described. Finally, we review how the molecular understanding of T-cell activation has resulted in important advances in the treatment of human diseases.
T-cell activation begins when T cells encounter a specific antigen that engages and then initiates signal transduction through the T-cell antigen receptor (TCR). Unlike B cells that respond to soluble antigens, T cells are stimulated by small peptides presented on the surface of other cells. These peptides are incorporated into the binding groove of proteins of the major histocompatibility complex (MHC, known in humans as human leukocyte antigen [HLA] complexes) through a process called antigen presentation . Thus, the ligand for the TCR is a peptide surface generated from both amino acids in the antigenic peptide and residues found in the MHC molecules themselves. Engagement of peptide–MHC complexes by the TCR induces a series of intracellular biochemical events that culminate in T-cell activation. Although T cells make use of many of the same biochemical pathways used by other cells for activation, there are a number of molecules unique to immune cells that are critical for T-cell activation. This section discusses TCR signal transduction, focusing on immune cell-specific molecular events.
Invading pathogenic bacteria and viruses use different strategies to survive within infected hosts. Many bacteria, such as the pathogens Staphylococcus, Streptococcus , and various enteric Gram-negative bacilli, survive in the extracellular milieu, whereas viruses and other bacteria, such as Listeria , survive inside host cells. Successful elimination of pathogens in each of these locations requires distinct responses from the host immune system. T cells play a central role in the control of extracellular and intracellular pathogens; however, the responding subset of T cells differs for each type of pathogen, with subsets of T cells expressing the cell surface marker CD4 most important for protection against extracellular pathogens and those expressing the CD8 marker essential for control of intracellular organisms. Stimulated CD4 + T cells act on other cells of the immune system by producing cytokines, soluble mediators that elicit a variety of cellular responses important for clearance of extracellular pathogens, whereas CD8 + T cells function largely by directly lysing host cells that have become infected with an intracellular organism. It is therefore critical for antigens derived from extracellular sources to stimulate CD4 + T cells and for intracellular antigens to stimulate CD8 + T cells. Whether a particular antigenic peptide activates a CD4 + versus a CD8 + T cell is determined by which MHC proteins present the peptide to the TCR.
Class II MHC proteins are found on cells of the innate immune system known as “professional” antigen-presenting cells (APCs) as well as B cells and the thymic epithelium. Professional APCs include dendritic cells (DCs) and various tissue macrophages, which engulf extracellular organisms (often after these are coated with host antibodies), host cells that have undergone apoptosis (programmed cell death), and cellular debris through an endocytic pathway that brings the ingested material into contact with degradative enzymes. The peptides that are formed in these reactions are bound to the MHC class II proteins for presentation to T cells. The MHC class II complex is a dimer consisting of a single α chain and a single β chain. Both α and β chains contribute to peptide binding and interaction with the TCR. During synthesis within the cell, MHC class II complexes bind invariant chain (Ii), a protein that directs the newly formed MHC proteins into an acidic vesicle. During this trafficking event, a portion of the Ii occupies the peptide-binding site. Once the MHC class II protein reaches the acidic vesicle, Ii is proteolyzed by cathepsin S, leaving behind a small fragment that remains lodged within the peptide-binding cleft of the MHC class II complex. This fragment is termed the class II–associated invariant chain peptide (CLIP) . The MHC class II–containing vesicles then fuse with other vesicles containing the peptide fragments from the endocytosed particles. There, CLIP is replaced with a peptide, thus stabilizing the MHC class II complex and allowing it to be transported to the cell surface, where it interacts with the TCR on CD4 + T cells ( Fig. 24.1 ).
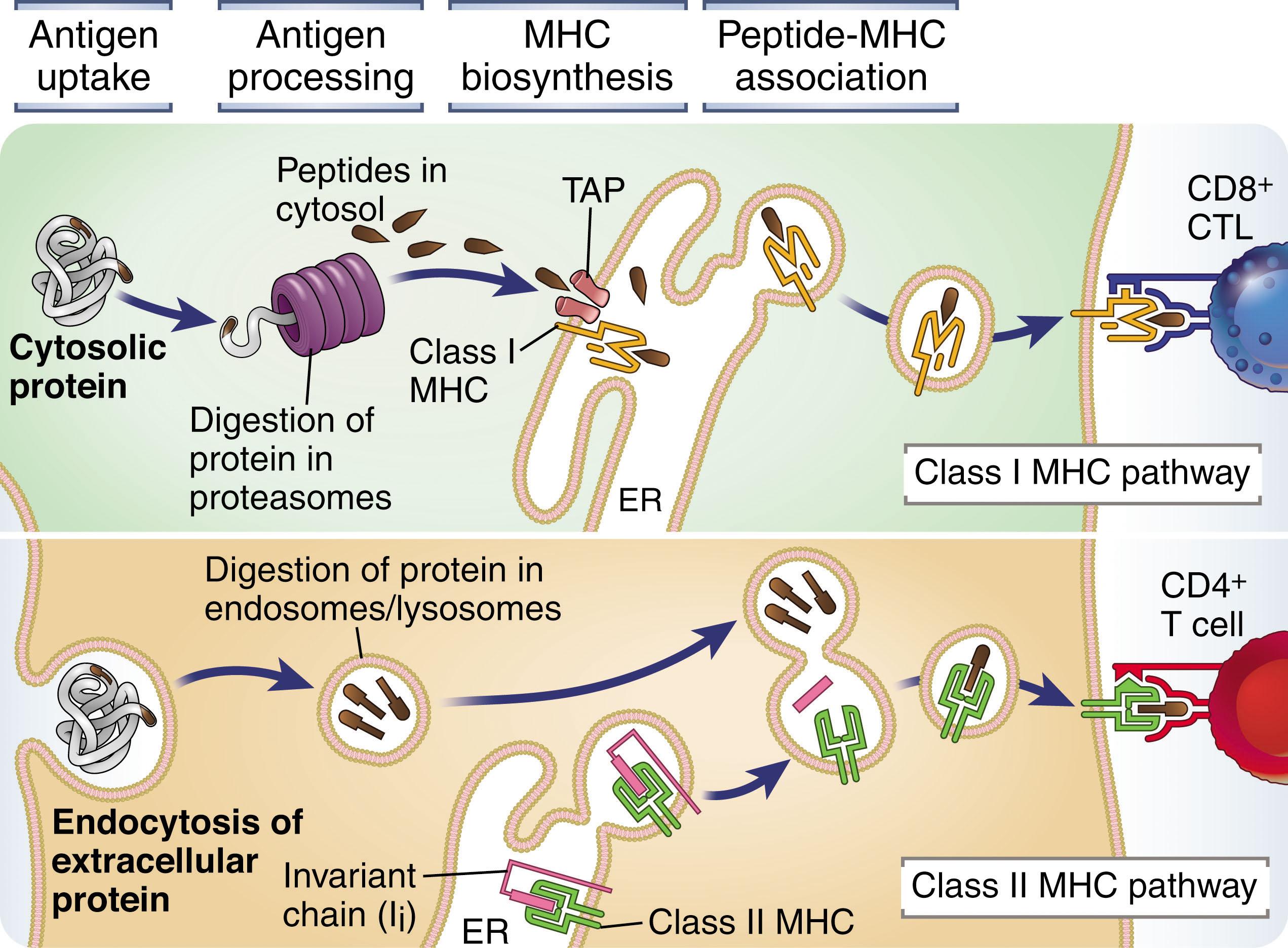
All cells of the body are at risk of being infected with intracellular pathogens or becoming transformed. Because protection against such challenges requires a CD8 + T-cell response, all nucleated cells in the body express class I MHC, the protein complex that presents antigen to CD8 + T cells. Like class II MHC, class I MHC is a protein dimer. However, in contrast to class II, only the α chain of class I MHC is variable. This α chain is associated with β 2 microglobulin, which stabilizes the complex but plays no direct role in antigen presentation. During its assembly in the endoplasmic reticulum (ER), the MHC class I complex comes into contact with peptides derived from proteins undergoing translation within the cell. During protein synthesis, small amounts of protein are modified by ubiquitinylation. This serves as a targeting sequence, directing the modified protein to the proteasome, where it is degraded into small peptide fragments. These fragments are transported back into the ER by the transporters associated with antigen processing (TAP-1 and TAP-2), where they become available for binding to the newly synthesized MHC class I complexes. Peptide association completes the folding and assembly of MHC class I, which is then transported to the cell surface, where it can be recognized by CD8 + T cells ( Fig. 24.1 ).
T cells can respond to antigenic peptides only if these peptides fit into the binding pocket of either MHC class I or MHC class II. Although a large number of peptides are able to bind to a specific MHC complex, the diversity of antigen presentation is enhanced through expression of three different MHC class I alleles (in humans, HLA A, B, and C) and class II alleles (in humans, HLA DR, DP, and DQ). To further increase the spectrum of peptides any particular cell may present, MHC alleles are always codominantly expressed. Thus, any individual expresses a large number of different class II dimers on its APCs and class I dimers on all nucleated cells, providing broad protection against potential pathogenic organisms. It is possible, however, that even with this degree of potential for antigen presentation, pathogens may evolve that do not possess unique proteins with sequences that can be efficiently presented by MHC. To circumvent this problem, the MHC locus has evolved to be highly polymorphic, thus providing enormous diversity within the population for antigen presentation, ensuring that some individuals will express MHC dimers that can present antigens from virtually any pathogen. Interestingly, predominant MHC alleles exist in different parts of the world, suggesting that there is local pressure, perhaps based upon prevailing microorganisms, that shapes selection of MHC expression.
Neither MHC class I nor MHC class II distinguishes foreign from host peptides as they fill their peptide binding grooves. Because MHC class II are capable of presenting any ingested antigen and MHC class I present peptides produced within the cell, the majority of the MHC complexes are filled with self-peptides. The T cell must distinguish self from non-self to ensure that a response is directed only against peptides generated from foreign peptides. Control over which antigens elicit a T-cell response is accomplished through selection of a population of T cells expressing appropriate TCRs (see T-Cell Development section later).
The TCR is a multimolecular complex with separate components able to bind ligand or to transduce an activating signal to the cell. The peptide–MHC binding regions of the TCR consist of an α/β heterodimer in the majority of T cells, and the related γ/δ heterodimer in a smaller subset of T cells. α and β as well as γ and δ consist of variable and constant regions. Similar to antibodies (see Chapter 22 ), the variable regions of the TCR antigen-binding proteins arise from rearranging gene segments that are imprecisely joined during T-cell development. This process allows for an extraordinarily diverse repertoire of potential antigen reactivity, although there are in total only several hundred genes that make up the α, β, γ, and δ loci. The germline configurations of the α and β loci are different, such that the α-chain locus comprises about 70 variable (V) segments, 60 joining (J) segments, and 1 constant (C) segment, whereas the β-chain locus comprises 50 V regions, 2 diversity (D) segments, 13 J segments, and 2 C regions. Greater diversity is generated by the addition of nucleotides between the V and J gene segments on α chains and the V, D, and J segments in β chains during the formation of the mature TCR. In total, it has been calculated that approximately 10 18 different TCRs can be created from these segments, although the functional population is much smaller because of the requirements for selection during maturation in the thymus (see T-Cell Development section later). Thus, once it has completed its developmental program, an individual T cell expresses a unique TCR encoded by a combination of gene segments that have been altered and rearranged ( Fig. 24.2 ). The T cells circulating through the lymphatics, lymph nodes (LNs), and spleen possess sufficient diversity for nearly all pathogens encountered to express an antigenic sequence recognized by a circulating T cell, which will then expand in number to combat that pathogen.

Soon after identification of the genes encoding TCR α and β, it became apparent that although they were sufficient to bind peptide–MHC, the α/β heterodimer was not capable of transmitting an intracellular signal once ligand was bound. A series of studies demonstrated that the signal transduction function of the TCR complex resides in a protein complex that noncovalently associates with the α/β dimer. This complex, CD3, is required both for stable expression of the ligand-binding components of the TCR and for signal transduction. CD3 is composed of three subunits, δ, ε, and γ, expressed as heterodimers (γ/ε and δ/ε) along with the ζ subunit, which is present as a homodimer. Each subunit contains immunoreceptor tyrosine-based activation motifs (ITAMs), a stretch of amino acids with discretely placed tyrosine residues: one ITAM in δ, ε, and γ and three ITAMS in ζ. The ITAM tyrosines are inducibly phosphorylated upon engagement of the α/β TCR chains by peptide–MHC and become docking sites for other proteins that initiate the signaling cascade for T-cell activation. Notably, the CD4 or CD8 protein also plays a role in mediating signal transduction. These coreceptors bind both the appropriate MHC complex (MHC I for CD8, MHC II for CD4) and, via their cytoplasmic tails, the signaling molecule Lck, one of the kinases capable of phosphorylating the ITAMs ( Fig. 24.3 ).
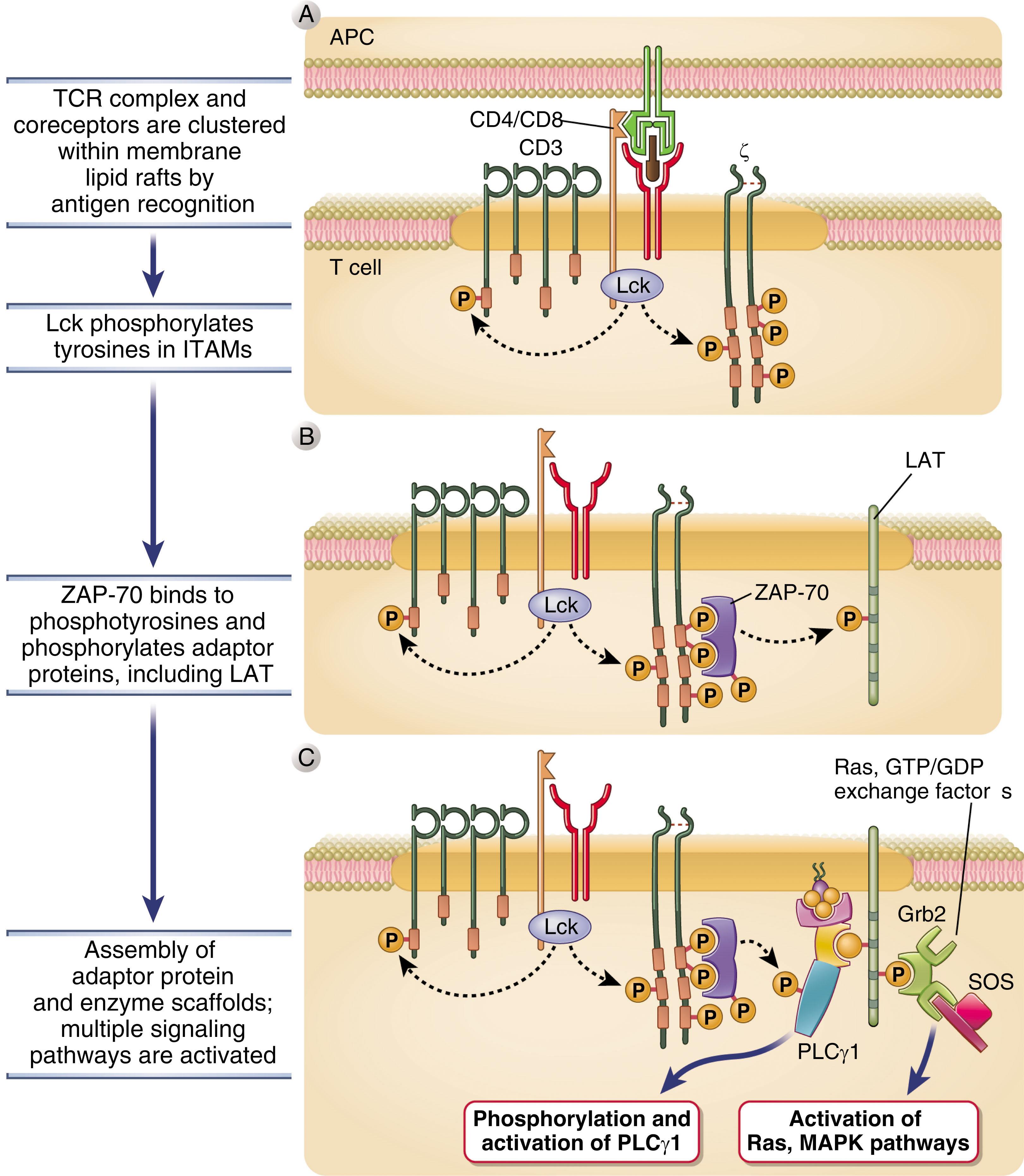
Once the genes were cloned for each TCR complex component, it became clear that, unlike many other cell surface receptors that transduce activating signals, neither the ligand-binding domains nor the CD3 proteins of the complex have intrinsic enzymatic function. Engagement of the TCR by the peptide–MHC was found to result in the rapid activation of protein tyrosine kinases (PTKs) within the T cells. Exactly how TCR engagement initiates PTK activation remains unclear; however, clustering of TCRs on the cell surface with resultant conformational changes in the CD3 proteins appears critical in the process. Src family (Lck and Fyn) PTKs are activated first following TCR stimulation, and the tyrosines within the CD3 and ζ ITAMs are substrates of these kinases. Phosphorylation of the ITAM tyrosines makes these residues able to bind to Src homology 2 (SH2) domains of other proteins. The most important SH2 domain-containing protein that is recruited to the ITAMs is ζ-associated protein of 70 kDa (ZAP-70), a PTK itself and a member of the Syk family of proteins. Thus binding of the TCR by ligand converts an enzymatically inactive receptor complex into an active PTK through recruitment and activation of cytosolic proteins.
Activation of ZAP-70 leads to tyrosine phosphorylation of a number of substrates, including enzymes that catalyze reactions generating second messengers important for T-cell activation. Phospholipase Cγ1 (PLCγ1) is activated by its tyrosine phosphorylation to cleave phosphatidylinositol-(4,5)-bisphosphate (PIP 2 ) into the second messengers diacylglycerol (DAG) and inositol-(1,4,5)-triphosphate (IP 3 ). DAG is a membrane-bound lipid second messenger that binds to and activates downstream signaling components, including protein kinase C θ (PKCθ) and the Ras guanine exchange factor RasGRP. PKCθ, a serine/threonine kinase, regulates numerous effectors of gene transcription and T-cell effector function development, including the transcription factors nuclear factor κB (NFκB) and activator protein 1 (AP-1). RasGRP is responsible for activating the small molecular weight guanosine triphosphate (GTP)-binding protein Ras by enhancing Ras release of GDP, allowing it to assume its activated GTP-bound form. Active Ras collaborates with PKC family members to stimulate transcription of new genes by activating mitogen-activated protein kinase (MAPK) family members. IP 3 mobilizes calcium stores from the ER. This increase in calcium is important for enzyme function, most notably the phosphatase calcineurin that dephosphorylates nuclear factor of activated T cells (NFAT), allowing it to translocate to the nucleus and transactivate genes necessary for T-cell proliferation, such as the gene encoding interleukin 2 (IL-2).
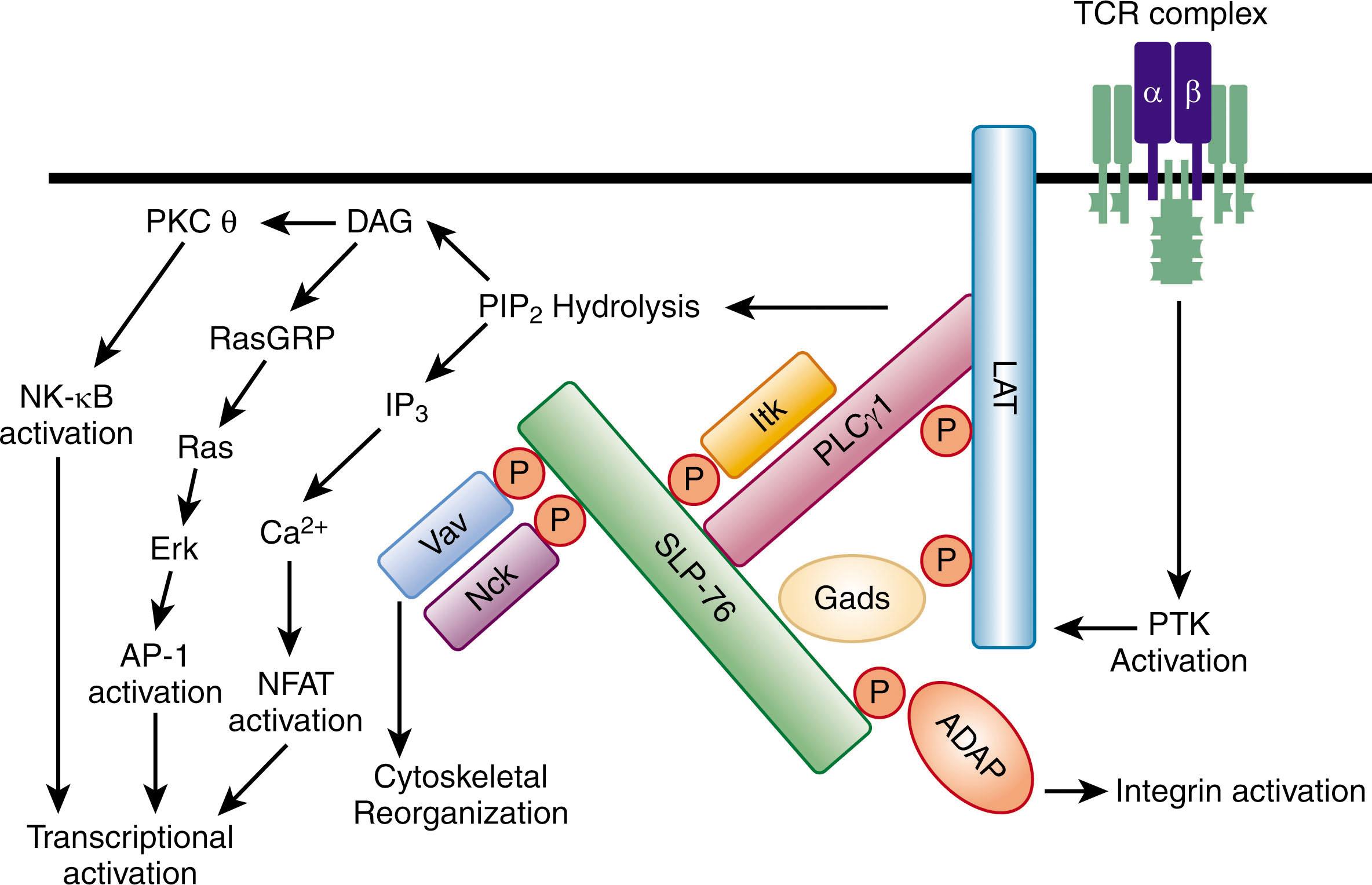
Although early TCR signal transduction studies demonstrated the importance of TCR-initiated PTK activity for T-cell activation, the mechanistic basis by which PTK activation drove the many critical second-messenger cascades required more precise study. These findings were elucidated with the identification and characterization of adapter proteins, which possess modular domains important for intermolecular interactions. Two central adapters in the TCR signaling pathway are linker of activated T cells (LAT) and SH2 domain-containing leukocyte protein of 76 kDa (SLP-76). LAT is a transmembrane protein with seven cytoplasmic tyrosines that are phosphorylated by the PTKs activated by the TCR. SLP-76 is a cytosolic adapter protein that is also phosphorylated by these PTKs. Because these tyrosine phosphorylation events create docking sites for other proteins with SH2 domains, once the TCR is engaged, SLP-76 and LAT nucleate a large complex of signaling molecules at the membrane, in the vicinity of the activated TCR. This cluster of molecules initiates the signaling cascades that are integrated to result in T-cell activation. Key proteins in this complex are Vav1, a guanine nucleotide exchange factor important for cytoskeletal reorganization; inducible T-cell kinase (ITK), a member of the Tec family of PTKs (a third family of PTKs essential for T-cell activation); adhesion and degranulation-promoting adapter protein (ADAP), an adapter that is a key regulator of integrins to promote T-cell interactions with other cells; PLCγ, the enzyme described earlier that initiates both the calcium and Ras/MAPK pathways in T cells; and growth factor receptor-bound protein 2 (Grb2) and Son of Sevenless (SOS), two proteins, like RasGRP, capable of activating Ras ( Fig. 24.4 ).
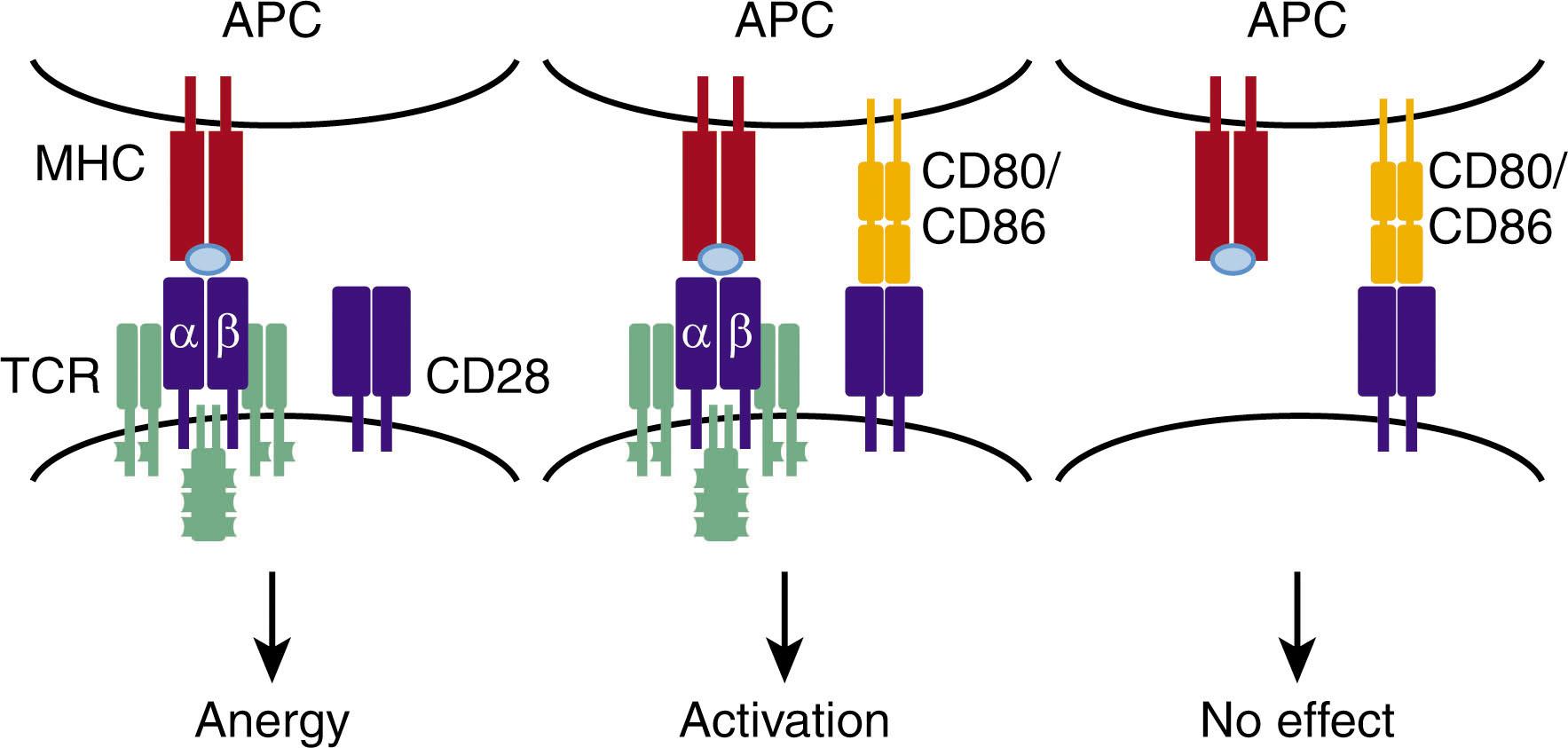
For T-cell immunity to be effective, T cells must possess TCRs that are exquisitely sensitive to specific antigen. Because the TCR is generated through random reassortment and alteration of gene segments, it is impossible to prevent generation of TCRs that have the potential to respond to self-antigens. Although the developmental program of T cells in the thymus provides a mechanism to eliminate most potentially self-reactive T cells (see T-Cell Development section later), this process is not 100% effective. Hence mechanisms exist to prevent mature T cells from responding against normal host tissues. One such mechanism is the requirement for T cells to receive two signals to become activated, one mediated by the TCR and the second through a costimulatory receptor. Although several different T-cell molecules can provide this costimulatory function, the best studied is the surface protein CD28. This additional requirement for T-cell activation helps to prevent autoimmunity because the ligands for CD28 (CD80 and CD86) are upregulated on APCs only in the presence of pathogen-associated molecular signals generated largely by bacterial and viral components or in the setting of cellular stress. (The mechanism of how bacterial and viral components signal through Toll-like receptors to activate APCs is described in Chapter 20 .)
For CD28 engagement to provide the second signal for T-cell activation, it must also initiate signal transduction pathways ( Fig. 24.5 ). CD28 signaling not only augments those signals stimulated by the TCR (described earlier) but also delivers independent signals. The interaction between CD28 and its ligands triggers the activation of phosphatidylinositol 3-kinase (PI3K), a protein that phosphorylates PIP 2 to form phosphatidylinositol-(3,4,5)-trisphosphate (PIP 3 ). Although the formation of PIP 3 induces broad changes within cells, the PIP 3 effector pathways that have been studied most intensively include two serine/threonine kinases: Akt, a PIP 3 -binding protein responsible for regulating the T-cell metabolism to favor cell division, and PKCθ, a protein required for cytokine production in T cells that is dependent upon PIP 3 generation for full activation. The importance of CD28 costimulation of T cells goes beyond its requirement for T-cell activation because engagement of the TCR in the absence of CD28 signaling in naive (i.e., antigen-inexperienced) T cells induces an impaired functional state within T cells termed anergy (see Anergy section later in this chapter).
Although CD28 is the prototypical and best studied costimulatory receptor, a multitude of other costimulatory molecules are expressed on T cells and regulated in a spatiotemporal manner in response to environmental cues, including CD27, inducible costimulator (ICOS), and 4-1BB. These costimulatory molecules bind an array of ligands on other cells (primarily APCs). Stimulation of individual costimulatory molecules can influence T-cell activation, effector function, and survival. One of the mechanisms by which distinct costimulatory molecules play unique roles in T-cell responses is likely due to the differential activation of discrete signaling pathways through their intracellular signaling domains. For instance, ICOS contains a binding motif that recruits the more active subunit of PI3K, leading to enhanced AKT signaling relative to CD28 activation. These differences have been exploited in building chimeric antigen receptor constructs to enhance T-cell-based adoptive cellular therapy (see Therapeutic Manipulation of T-Cell-Mediated Immunity section below and Chapter 26 ).
As the biochemical signaling events that occur following TCR engagement by peptide–MHC became known, investigators sought to define the topography of the activation events. Sophisticated imaging technologies were applied to visualize the contact site between the APC and the T cell, and this interaction was modeled by visualizing the contact between key receptors on T cells and ligands fixed to a solid support. These studies revealed a stepwise reorganization of the T-cell membrane at the contact site called the immunologic synapse (IS). The first step in IS formation is an interaction between integrins on the surface of the T cell and their ligands on the APC that brings the T cell and APC into close proximity. If a productive interaction occurs between the TCR and peptide–MHC, the next event is clustering of TCRs in the central portion of the developing IS (the so-called central supramolecular activation complex [cSMAC]) with the activated integrins forming the peripheral supramolecular activation complex (pSMAC), a ring around the clustered TCRs. Although ligands on the APC initially direct the formation of the IS, changes within the T cell, including reorganization of the actin cytoskeleton, are also critical for stabilization of this structure. As sophistication of imaging in real time has advanced and with the advent of tools to visualize smaller and smaller numbers of molecules, it has become clear that the IS is a dynamic structure that includes not only the TCR and integrins but also many of the signaling molecules essential for T-cell activation. Functionally, the IS has been demonstrated to regulate TCR signaling and polarized secretion of effector molecules, including cytokines and lytic granules.
The number of naïve T cells potentially responsive to any particular peptide antigen (the precursor frequency of the responding population) is quite small, yet a large number of antigen-specific T cells are required to combat pathogens. Accordingly, a consequence of TCR plus costimulatory receptor engagement is the clonal expansion of an activated T cell. One outcome of the second-messenger cascades stimulated by the TCR and CD28 is the production of IL-2, an essential cytokine for T-cell proliferation. Another outcome of TCR signaling is upregulation of the high-affinity receptor for IL-2, thus making the activated T cell able to respond to local concentrations of this cytokine. Signaling through the IL-2 receptor is necessary for the proliferative response. Similar to the TCR, the IL-2 receptor makes use of cytoplasmic PTKs (in this case members of the Janus kinase [JAK] family) to initiate a cascade of second messengers that lead ultimately to T-cell proliferation. Additionally, other cytokines such as IL-7 and IL-15 also regulate T-cell proliferation in antigen-experienced (i.e., memory) T cells. The details of IL-2 and other cytokine receptor signaling are provided in Chapter 11 .
Protective T-cell immunity requires populating the secondary lymphoid organs with a large number of mature T cells. Unlike most hematopoietic cells that complete the transition from progenitors to mature cells in the bone marrow, T cells develop primarily in the thymus. This population collectively must possess a diverse TCR repertoire capable of recognizing the enormous number of foreign antigens that will be encountered over the lifetime of an individual. Because the TCR binds antigenic peptide plus amino acid residues of self-MHC molecules, it is essential that only cells with a TCR able to recognize self-MHC, albeit with limited affinity, be exported from the thymus to the periphery. It is also critical, however, that the population of peripheral T cells be restricted to those that respond to foreign antigens, and cells possessing TCRs recognizing self-peptides plus MHC must not be allowed to complete their developmental program. Ensuring that only those cells with an appropriate TCR mature in the thymus relies heavily on many of the same TCR signal transduction events described earlier.
Of the T-cell lineages, those expressing the α/β TCR cells are the best studied and most numerous. However, γ/δ T cells, another population that possesses an antigen receptor generated through combinatorial rearrangement of gene segments, as well as natural killer (NK) T cells, a subtype of lymphocytes that has characteristics of both T and NK cells (see Chapter 21 ), are also generated in the thymus. It has become clear recently that additional small populations of T cells possessing unique characteristics are also produced in the thymus. This chapter focuses primarily on α/β T cells and touches briefly on γ/δ T-cell development.
Identifying T-cell progenitors is an area of intense investigation because developing tools to manipulate these cells has great therapeutic potential for increasing the speed at which T-cell repopulation may occur following hematopoietic stem cell transplant. A population of bone marrow-derived thymic settling progenitors (TSPs) that can give rise to mature T-cell populations has been identified. Understanding the steps that arise following settling of these cells in the thymus will be key to advancing approaches to quickly reconstitute T-cell function after bone marrow ablation.
The thymus is composed of developing T lymphocytes, DCs, epithelial cells, and mesenchymal components. Histologically, it is divided into two principal zones, the cortex and the medulla. TSPs enter the thymus at the corticomedullary junction, transition into early thymic progenitors (ETPs), which then develop into double-negative (DN) T cells, characterized by lack of expression of the CD4 or CD8 coreceptors ( Fig. 24.6 ). As these early T cells progress though the DN stage, they are further subdivided into DN1, DN2, DN3, and DN4 stages on the basis of the cell surface receptors they express. During DN1, developing thymocytes lose the ability to differentiate into non-T lineages and begin to proliferate in the deep cortex of the thymus. As these early thymocytes progress to the DN2 phase, they begin to express T-cell–specific markers, such as Thy-1 (CD90), CD24, and CD25, and initiate TCR gene rearrangement at the TCR-γ, TCR-δ, and TCR-β loci. Throughout the DN1 to DN3 stages, as the cells migrate from the cortex to the subcapsular zone, interactions between Notch receptors on the developing T cells and specific Notch ligands collaborate with signaling through the IL-7 cytokine receptor to regulate lineage commitment and developmental progression.

During the DN3 stage, rearrangement of TCR-γ, TCR-δ, and TCR-β loci occurs with maximal efficiency, and initial expression of the TCR proteins these genes encode occurs. From this time onward in T-cell development, the proliferation and survival of the developing thymocytes depend on TCR signals. Two key checkpoints must be passed for full T-cell development to occur. First, upon productive rearrangement of the TCR-β locus, the TCR-β protein forms a “pre-TCR” complex with an invariant cytosolic protein designated pre-Tα . This complex engages the TCR signaling machinery, including the PTKs Lck, Fyn, and Syk (a ZAP-70-related PTK) and the adapters SLP-76 and LAT, to initiate the TCR signaling cascade. The resultant biochemical second messengers suppress rearrangement of the other β allele resulting in “allelic exclusion,” or silencing of the non-rearranged allele, to ensure each T cell expresses only one TCR specificity. These signals also induce continued T-cell development by promoting rearrangements at the α locus, maintaining cellular survival, initiating a proliferative burst, and inducing expression of CD4 and CD8. For effective signaling to occur, the rearranged β locus must encode a protein that folds correctly and pairs with pre-Tα. Because the rearrangement of the genes that eventually make up the β chain is a random process, it is often the case that the rearranged allele encodes a dysfunctional protein. In this circumstance, signaling does not occur, and the cell initiates rearrangement at the other β chain allele. If this again does not result in a functional protein, no signaling occurs, and the cell undergoes apoptosis. In other cells, a similar rearrangement process occurs in the γ and δ loci. Productive rearrangements of these gene families create a functional, mature γ/δ TCR that also associates with the TCR signaling complex to propagate signals to trigger further cellular development.
Although the determining factors that result in either γ/δ or α/β T-cell development have not been fully elucidated, several molecular events are thought to contribute. The expression of a TCR gene rearrangement product likely plays a role in lineage determination because there is evidence suggesting that developing thymocytes with a functional γ/δ TCR are often excluded from the α/β cell fate. However, TCR expression is not the only factor in determining lineage fate; cytokine signals and TCR signal strength may also play a role. Experiments have shown that DN2 thymocytes distinguished according to IL-7 receptor expression differentiate into α/β or γ/δ T cells, with DN2 cells expressing high IL-7 receptor levels preferentially developing into γ/δ T lymphocytes and those with lower expression more likely to differentiate into the α/β lineage. Other studies have suggested that the strong signals propagated by the γ/δ TCR in comparison to those of the pre-TCR complex may promote γ/δ lineage commitment.
Developing α/β T cells that have passed the first checkpoint demonstrating functional β-chain rearrangement transition into the double-positive (DP; CD4 + CD8 + ) stage and complete TCR-α rearrangement to produce a mature α/β TCR heterodimer. The stochastic nature of TCR gene rearrangements guarantees that a significant proportion of cells expressing TCR-α/β complexes will not be able to interact with self-MHC proteins and hence would not be stimulated by peptide–MHC complexes in the periphery. DP thymocytes therefore undergo a series of tests, collectively known as positive and negative selection , to determine TCR fitness. If the TCR is not stimulated via peptide–MHC complexes presented by thymic APCs, the developing cell undergoes “death by neglect” through apoptosis. Approximately 90% of developing α/β DP thymocytes express a TCR that cannot weakly recognize self-peptide–MHC and thus die by neglect. In contrast, those DP thymocytes that interact with self-peptide–MHC complexes on thymic cortical epithelial cells with sufficient strength pass this “positive selection” test and are protected from apoptosis.
The MHC specificity of the TCR on a positively selected DP thymocyte influences lineage fate. Cells signaled through a MHC class I–restricted TCR develop into CD8 single-positive (SP; CD4 − CD8 + ) cells, and those that receive signals via MHC class II–restricted TCRs develop into CD4 SP T cells. The underlying molecular mechanisms governing CD4/CD8 lineage choice is much debated. Most recently, a kinetic signaling model has emerged. It proposes that CD4 or CD8 lineage fate is determined by TCR signal duration.
Among the many proteins that are involved in CD4 or CD8 lineage choice are key transcription factors. T-helper-inducing POZ/Krüppel-like factor (Th-POK), a zinc finger protein that is expressed exclusively in CD4 + T cells and not in CD8 + T cells, and RUNX3, a member of the Runx transcription factor family, are required for CD4 and CD8 lineage commitment, respectively. Additional studies have identified a network of key transcription factors and signaling proteins important for lineage choice in the thymus, underscoring the complexity of this stage of T-cell development.
Although positive selection ensures that the random combinatorial rearrangement of gene segments results in a TCR that recognizes antigen presented by self-MHC proteins, until this point in T-cell development, there is no guard against the emergence of T cells that possess TCRs with high reactivity against self-peptides in the MHC binding pockets. Thus, to prevent autoimmunity, there must also be a mechanism to eliminate developing T cells with TCRs expressing these potentially autoreactive specificities. This process is called negative selection . Negative selection occurs primarily in the thymic medulla, where thymocytes serially interact with medullary thymic epithelial cells (mTECs) and other thymic APCs including DCs. At this stage, if thymocytes with TCRs engage peptide–MHC complexes with high affinity, the strong TCR signal initiates apoptosis. Whereas it is easy to see how this model allows for deletion of developing thymocytes with reactivity against self-antigens generated within the thymus itself, it was difficult to imagine how cells with reactivity against antigens known to be expressed outside the thymus would also be deleted. An explanation for how this occurs came from the discovery of the autoimmune regulator (AIRE) protein. Initially identified as the gene product mutated in a rare human autoimmune disorder, autoimmune polyendocrinopathy-candidiasis-ectoderm dystrophy (APECED), AIRE was later found to be essential for the expression of peripheral tissue-specific antigens by mTECs. Although AIRE does not regulate thymic expression of all peripheral antigens, its contribution to the elimination of autoreactive cells is highlighted by the widespread, multiorgan autoimmunity seen in patients with APECED. Identifying additional mechanisms responsible for thymic expression of tissue-specific genes is an area of active investigation.
Negative selection is one mechanism for development of “tolerance” or immune unresponsiveness to self-antigens; however, the process is not perfect in eliminating all self-reactive T cells. Hence, other means exist to promote self-tolerance after T cells leave the thymus. One such mechanism relies on development of regulatory T cells (Tregs), which actively interfere with effector T-cell function. Like conventional αβ T cells, a subset of Tregs (previously known as natural or nTregs and more recently designated thymic or tTregs ) also develops in the thymus. Tregs are characterized by the surface expression of CD4 and CD25 (the high affinity α chain of the IL-2 receptor) and depend on the transcription factor forkhead box protein 3 ( FoxP3 ) for their lineage commitment. The gene encoding FoxP3 was originally identified as the causal mutation in a rare, and frequently fatal, human autoimmune disease called immunodysregulation, polyendocrinopathy, and enteropathy, X-linked (IPEX) syndrome . A mutation in the mouse gene for FoxP3 causes a similar disease ( scurfy mice). These naturally occurring loss-of-function mutations demonstrate the necessity for Tregs in maintaining self-tolerance. In the thymus, development into a tTreg is enhanced in cells that have high-affinity TCR-peptide-MHC interactions, suggesting that these cells develop specifically to counter autoreactive responses. The exact mechanism that drives these cells to adopt a Treg fate and avoid negative selection during development is being investigated.
The path of developing γ/δ thymocytes contrasts with that of α/β T-cell development, which is likely related to the function of mature γ/δ T cells. In the periphery, γ/δ T cells reside in secondary lymphoid organs with conventional α/β T cells but also are enriched in epithelial tissues of various organs, such as the skin, intestinal epithelium, reproductive tract, and lung. In these distinct settings, the TCR diversity of the γ/δ T cells is more restricted, suggesting that these subsets may preferentially recognize ligands expressed at these anatomic locations during times of infection or tissue damage.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here