Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Human cells produce three antigenically distinct forms of interferon (IFN), originally described as leukocyte (α), fibroblast (β), and immune (γ)
Recombinant DNA technology can produce large quantities of highly purified human IFN
IFNs have antiviral, antiproliferative, and immunoregulatory properties
Dermatologic FDA-approved indications for IFNs include condyloma acuminata (IFN-α-2b), melanoma (IFN-α-2b), and AIDS-associated Kaposi sarcoma (IFN-α-2a and IFN-α-2b)
Side effects include flu-like symptoms, leukopenia, nausea, vomiting, and hepatitis
Interferons (IFNs) are a family of secretory glycoproteins produced by most eukaryotic cells in response to a variety of viral and non-viral stimuli. All IFNs display antiviral activity, and they also modulate other cellular functions. IFN does not inactivate viruses directly but rather renders cells resistant to viruses.
There are three antigenically distinct IFNs, originally described as leukocyte (α), fibroblast (β), and immune (γ) ( Table 128.1 ). Utilization of recombinant DNA technology has enabled the production of large amounts of highly purified human IFN by Escherichia coli. This chapter will focus primarily on IFN-α-2a and IFN-α-2b, which are the most frequently used IFNs for patients with dermatologic diseases.
| INTERFERONS | ||||
|---|---|---|---|---|
| Generic name | Trade name® | Route | Half-life | Peak effect |
| Interferon-α-2a | Roferon-A | sc | 5.1 h | 3.8–7.3 h |
| Interferon-α-2a | Pegasys | sc | 160 h | 72–96 h |
| Interferon-α-2b | Intron A Rebetron when combined with ribavirin |
iv, il, im, sc | 2–3 h | 30 min (iv) 3–12 h (im, sc) |
| Interferon-α-2b | Peg-Intron | sc | 40 h | 15–44 h |
| Interferon-α-N3 | Alferon N | il | 2–3 h | 3–12 h |
| Interferon-β-1a | Avonex | im | 10 h | 3–15 h |
| Rebif | sc | 69 h | 16 h | |
| Interferon-β-1b | Betaseron | sc | 4.3 h | 1–8 h |
| Interferon-γ-1b | Actimmune | sc | 5.9 h | 4.7 h |
| Interferon alphacon-1 | Infergen | sc | − | 24–36 h |
There are over 30 subtypes of IFN-α, each composed of 165–172 amino acids with very similar sequences. For example, IFN-α-2a and IFN-α-2b differ in just a single amino acid. IFN-β has 29% structural homology to IFN-α, but IFN-γ has no significant structural homology to either IFN-α or IFN-β .
IFNs must be injected intralesionally or administered parenterally. Systemic absorption via the intramuscular or subcutaneous route is >80% for IFN-α; its peak effect is 3–12 hours after injection and is undetectable after 24 hours . Pegylated forms of IFN-α contain polyethylene glycol (PEG) side chains, which prolong their therapeutic effect. As a result, they can be administered less frequently with improved tolerability (see Table 128.1 ). IFNs undergo renal catabolism.
IFN activity is dependent upon its ability to bind to specific receptors on the surface of target cells. IFN-α and IFN-β (type I IFNs) share the same IFN-α/β receptor, whereas IFN-γ (type II IFN) binds to a separate receptor; both receptors signal via JAK (Janus kinase)–STAT (signal transducer and activator of transcription)-mediated pathways. The mechanisms of their antiviral, antiproliferative, and immunoregulatory activities are listed in Table 128.2 .
| MECHANISMS OF ACTION FOR INTERFERONS | |
|---|---|
| Cellular mechanism | Clinical effect |
| Induction of 2′–5′oligoadenylate synthetase Induction of ribonuclease Induction of protein kinase P1 |
Antiviral |
| Induction of 2′–5′oligoadenylate synthetase Inhibition of various growth factors Enhanced p53 tumor suppressor gene expression Downregulation of MYC, FOX and certain RAS oncogenes |
Antiproliferative |
| Induction of class I & II MHC antigens Increased number of natural killer (NK) cells Inhibit production of Th2 cytokines such as IL-4 and IL-5 |
Immunoregulatory |
| DERMATOLOGIC DISEASES TREATED WITH INTERFERONS |
| FDA-approved indications |
| Condyloma acuminata (α) AIDS-associated Kaposi sarcoma (α) Melanoma (adjuvant) (α) Chronic granulomatous disease (γ) |
| Some off-label dermatologic uses |
| Neoplasms |
| Basal cell carcinoma (α) Actinic keratoses (α) Squamous cell carcinoma (α) Giant condyloma of Buschke–Lowenstein (α) Cutaneous T-cell lymphoma (α, β, γ) Granulomatous slack skin (α, γ) Infantile hemangioma (α) Tufted angioma (α) |
| Infections |
| Verrucae vulgares (α, β, γ) Epidermodysplasia verruciformis (α) Herpes zoster (α) Herpes simplex (α) Necrolytic acral erythema (with hepatitis C) (α) Leishmaniasis (γ) Leprosy (γ) Mycobacterium avium complex infections (γ) |
| Inflammatory/other |
| Atopic dermatitis (γ) Keloids (α, γ) Behçet disease (α) Systemic sclerosis (γ) Scleromyxedema (α) |
Absolute contraindications are hypersensitivity to components of the formulations. Relative contraindications include cardiac arrhythmias, depression or other psychiatric disorders, leukopenia, coagulopathies, and previous organ transplantation.
The adverse effects of IFN are dose-dependent and generally improve or remit during continued therapy or following dose reduction. In addition, the adverse effects are usually rapidly reversible upon cessation of treatment.
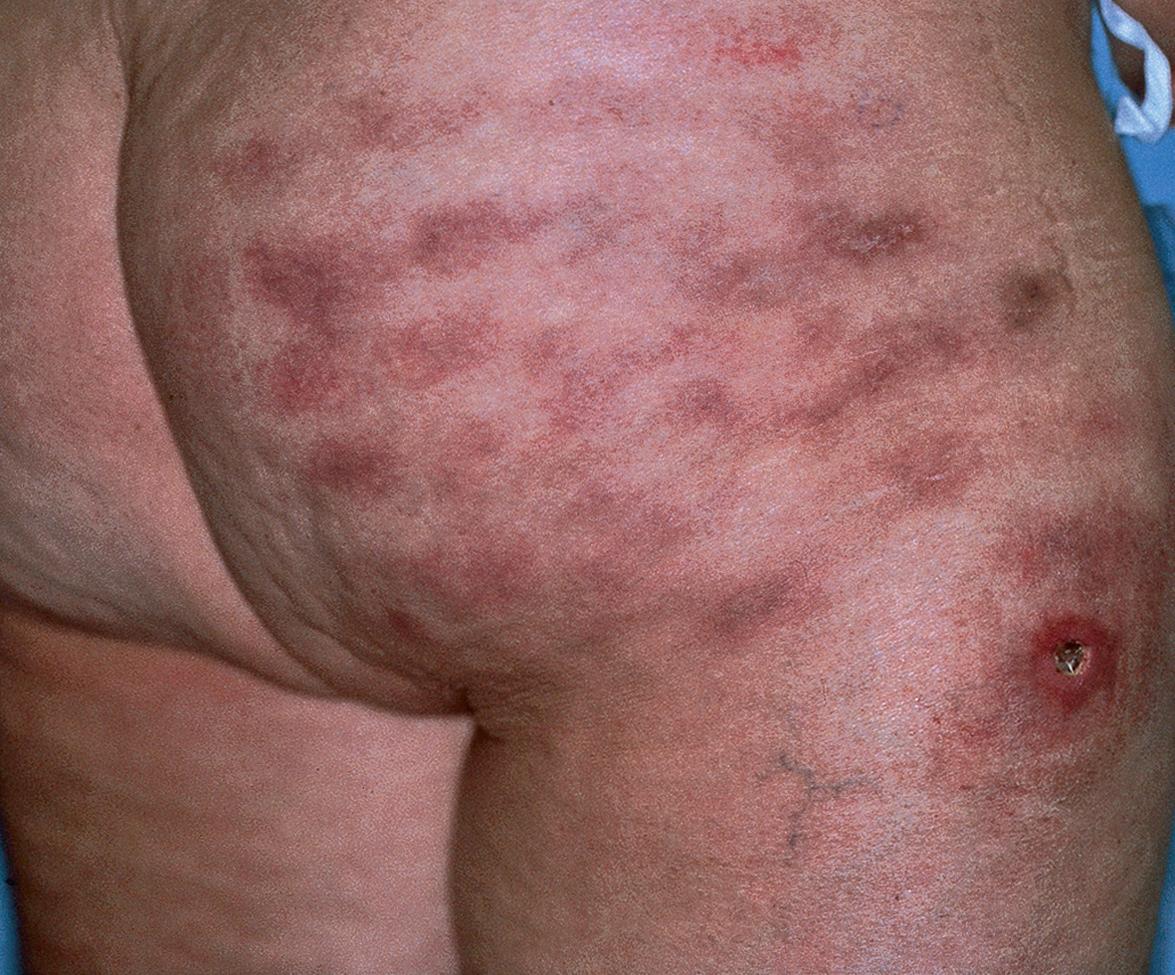
Approximately 5% of patients treated with IFN-β-1b for multiple sclerosis develop skin necrosis at the site of injection secondary to a localized vasculopathy ( Fig. 128.1 ). Plaques of psoriasis can also develop at the sites of injection of IFN, as can flares of psoriasis in patients receiving IFN ( Fig. 128.2 ). Other occasional cutaneous side effects include alopecia, xerosis, and vitiligo.
The most common adverse effects are flu-like symptoms of fever, chills, fatigue, myalgias, headache, and arthralgias. Generally, in healthy individuals, subcutaneous administration of IFN-α in doses of ≤3 million IU every other day induces tolerable flu-like symptoms or no adverse effects. Prophylactic (1–2 hours prior to injection) administration of acetaminophen (650 mg), aspirin (650 mg), or NSAIDs (e.g. ibuprofen 400 mg) helps to prevent these effects.
Spastic diplegia was reported in 5 of 26 patients with infantile hemangiomas treated with 1.0–3.6 million IU/day of IFN-α-2a ; postulated explanations included the immaturity of the infant's central nervous system and preservatives (e.g. benzyl and phenol alcohol) in the preparations used. Depression and suicidal ideation/behavior have been reported in association with IFN-α therapy .
Significant hypotension, arrhythmias, or tachycardia (heart rate ≥150 beats/minute) can occur in association with IFN therapy . These side effects usually resolve upon IFN dose reduction or discontinuation but occasionally require additional therapy. Patients with a history of a myocardial infarction or arrhythmias require close monitoring.
Rhabdomyolysis occasionally occurs and has been fatal in at least one patient treated with high-dose IFN-α-2b (20 million IU intravenously twice daily for 5 days) . Elevated serum creatine kinase levels should prompt a reduction in dose or cessation of therapy, depending on the severity of the elevation.
Gastrointestinal disturbances such as nausea, vomiting, and diarrhea can occur, as can anorexia and hepatitis. Fatal cases of hepatitis have been reported in patients receiving high-dose IFN. Bone marrow suppression may develop in patients receiving high-dose therapy or in those who have other reasons for bone marrow suppression (e.g. concomitant medications). Serial evaluation with liver function tests and complete blood counts is recommended.
Neutralizing antibodies can develop in patients receiving IFN-α-2a or IFN-α-2b, and they appear to be specific to recombinant rather than natural IFN . Thyroiditis and/or hypothyroidism occurs in up to 40% of hepatitis C patients treated with IFN-α .
IFN-α-2a may reduce the clearance of aminophylline, probably due to inhibition of the cytochrome P450 enzyme system . Caution must be taken when IFN is administered in conjunction with other potentially myelosuppressive (including zidovudine) or neurotoxic (e.g. vinca alkaloids) drugs. The concomitant use of IFN and IL-2 may increase the risk of renal failure.
IFNs are rated as pregnancy category C, although they do not appear to cross the placenta. While it is unknown whether IFN is excreted into human milk, it is excreted into mouse milk.
The indications and side effects of granulocyte–macrophage and granulocyte colony-stimulating factors (GM-CSF and G-CSF) are outlined in Table 128.4 .
| GRANULOCYTE–MACROPHAGE AND GRANULOCYTE COLONY-STIMULATING FACTORS (GM-CSF & G-CSF) |
| Properties |
| These glycoproteins regulate the survival, proliferation, differentiation and functional activation of hematopoietic cells |
| Uses |
|
| Side effects |
| GM-CSF |
|
| G-CSF |
|
| Interactions |
| Additive effects with other drugs that cause neutrophilia, e.g. corticosteroids, lithium |
| Pregnancy and lactation |
| Category C; unknown if excreted in breast milk |
Tumor necrosis factor (TNF) inhibitors such as etanercept, adalimumab, and infliximab can be effective treatments for psoriasis, psoriatic arthritis, hidradenitis suppurativa, and other dermatoses in which TNF signaling has a pathogenic role
Interleukin (IL)-12 and IL-23 signaling is important for the Th17 immune response; ustekinumab binds to the p40 receptor subunit shared by these cytokines and is approved for the treatment of psoriasis and psoriatic arthritis
The IL-17 inhibitors secukinumab, ixekizumab, and brodalumab are approved for the treatment of psoriasis and (for secukinumab) psoriatic arthritis
Dupilumab blocks IL-4 and IL-13 signaling and is approved for the treatment of atopic dermatitis
The IL-1 antagonists anakinra, canakinumab, and rilonacept are used to treat several autoinflammatory diseases, including cryopyrin-associated periodic syndromes and deficiency of the IL-1 receptor antagonist (DIRA)
Rituximab, an agent that blocks CD20 on B cells, is approved for the treatment of B-cell lymphoma, granulomatosis with polyangiitis, and microscopic polyangiitis; it is also useful in other B-cell-mediated skin diseases such as pemphigus vulgaris
Omalizumab binds to the IgE receptor on mast cells and is approved for the treatment of chronic urticaria
Janus kinase (JAK) inhibitors such as tofacitinib and ruxolitinib have efficacy in the treatment of dermatologic conditions including psoriasis and alopecia areata
Advances in molecular technology have enabled the development of drugs that target specific proteins involved in disease pathogenesis. This has resulted in effective therapies for a variety of immune-mediated skin disorders, including psoriasis, atopic dermatitis, hidradenitis suppurativa, pemphigus vulgaris, urticaria, and cutaneous B-cell lymphomas as well as the cutaneous manifestations of systemic conditions such as Crohn disease, sarcoidosis, and vasculitis. These drugs, which are referred to as “biologic” agents, work via mechanisms that include altering T-cell activation and differentiation into Th1, Th2, or Th17 cells; blocking cytokines, their receptors, or their intracellular signaling mechanisms; and eliminating pathogenic B cells.
The FDA has approved multiple tumor necrosis factor (TNF; previously known as TNF-α) inhibitors: etanercept (Enbrel®), etanercept-szzs (Erelzi TM ), infliximab (Remicade®), infliximab-dyyb (Inflectra®), adalimumab (Humira®), adalimumab-atto (Amjevita®), golimumab (Simponi®), and certolizumab pegol (Cimzia®). A review of their mechanisms of action, major side effects, and contraindications is followed by a discussion of issues that relate specifically to individual drugs.
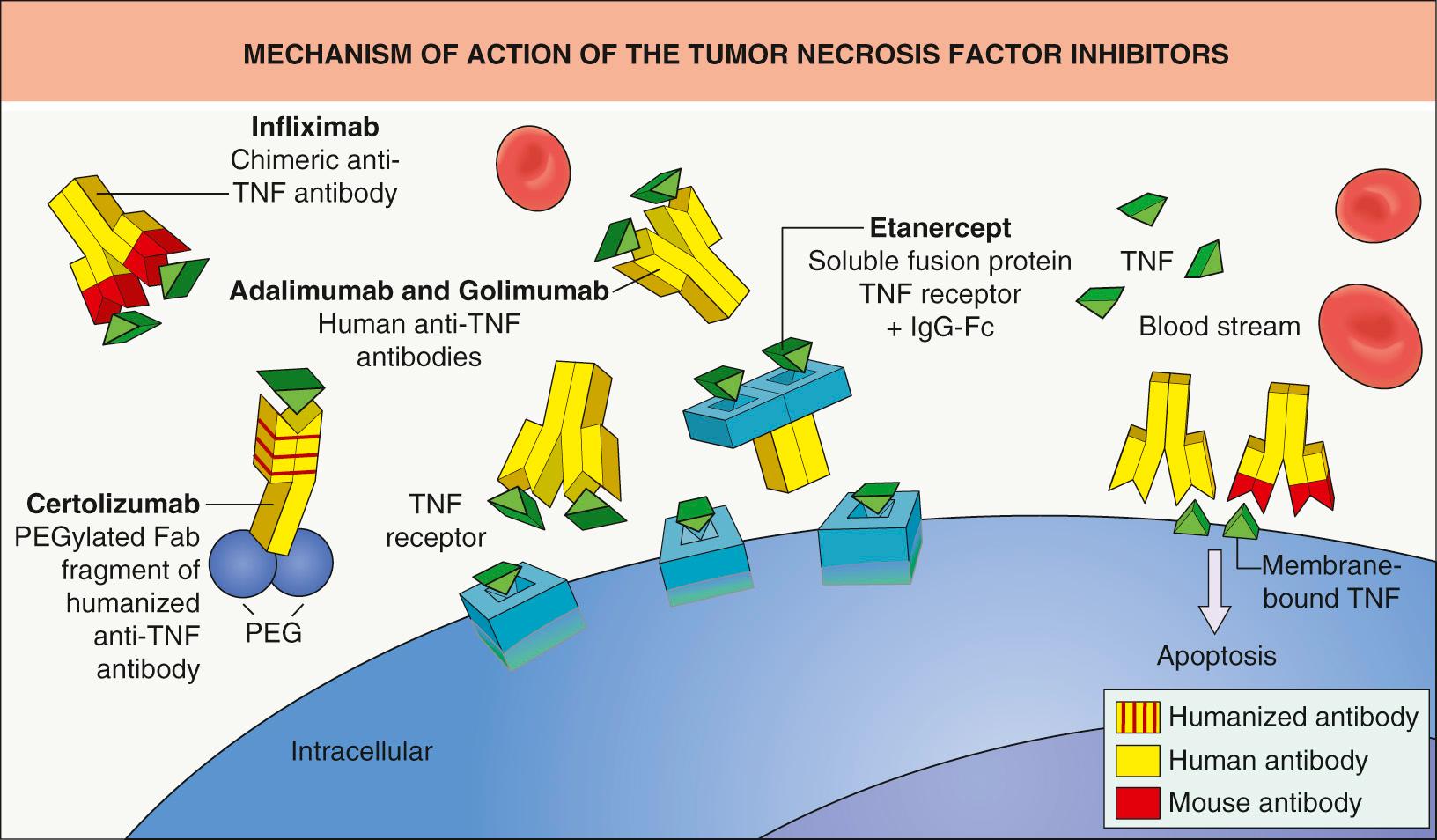
Infliximab, adalimumab, golimumab, and certolizumab pegol are monoclonal antibodies, while etanercept is a dimeric fusion protein composed of the extracellular portions of two p75 TNF receptors linked to the Fc portion of IgG1 ( Fig. 128.4 ). Although all four monoclonal antibodies target human TNF, infliximab is chimeric (human–mouse) IgG1, adalimumab and golimumab are human recombinant IgG1, and certolizumab pegol is a humanized pegylated Fab fragment (see Table 128.5 ).
| NOMENCLATURE FOR MONOCLONAL ANTIBODIES AND FUSION PROTEINS | ||||||
|---|---|---|---|---|---|---|
| Prefix | Sub-stem A: target system [previous nomenclature system] | Sub-stem B: source system | Suffix | |||
| Variable “Peg-” can be used if pegylated (or can add pegol as a second word) |
-anibi- | Angiogenesis | -a- | Rat | -mab | Immunoglobulin variable domain |
| -b(a)- [-ba(c)-] | Bacterial | -e- | Hamster | |||
| -f(u)- [-fung-] | Fungal | -i- | Primate | -cept | Receptor (takes the place of a variable domain) | |
| -k(i)- [-ki(n)-] | Interleukin | -o- | Mouse | |||
| -l(i)- [-li(m)-] | Immunomodulating | -u- | Human | -nib | Tyrosine kinase inhibitor | |
| -n(e)- [-ne(u)(r)-] | Nervous system | -xi- | Chimeric † | |||
| -tox(a)- | Toxin * | -zu- | Humanized ‡ | |||
| -t(u)- | Tumor | |||||
| -v(i)- | Viral | |||||
* If conjugated to (rather than directed at) a toxin, the suffix -tox can be used in a second word.
† Contains contiguous foreign -derived amino acids comprising the entire variable region (both heavy and light chains), linked to a constant region of human origin.
‡ Has segments of foreign-derived amino acids interspersed with human-derived amino acids in the variable region, linked to a constant region of human origin.
All of the TNF inhibitors can bind to soluble TNF and block its ability to activate TNF receptors (see Fig. 128.4 ). By interacting with membrane-bound TNF, the IgG1 monoclonal antibodies can also activate complement-dependent cytotoxicity and induce cellular apoptosis . This capacity to destroy TNF-producing cells may explain the greater efficacy of these monoclonal antibodies compared to etanercept in the treatment of granulomatous conditions as well as the potentially higher risk of infections associated with their use. In addition to the anti-inflammatory effects of TNF inhibitors, blocking TNF signaling may have an impact on the neuroendocrine system .
Each TNF inhibitor is contraindicated in patients with known hypersensitivity to that particular medication, and infliximab is contraindicated in those with an allergy to murine proteins. These agents should be avoided in patients with a significant active infection, malignancy, congestive heart failure (especially if unstable), or multiple sclerosis .
Disseminated and/or opportunistic infections such as histoplasmosis, coccidioidomycosis, listeriosis, and Pneumocystis jiroveci pneumonia may occur in patients treated with TNF inhibitors . An increased risk of symptomatic mycobacterial infections ( Fig. 128.5 ), including reactivation of latent tuberculosis, has also been observed in patients treated with these agents. However, most of the studies evaluating the risk of infection associated with the use of TNF inhibitors have been in patients with rheumatoid arthritis or inflammatory bowel disease, who often concomitantly receive additional immunosuppressive agents. A meta-analysis of 20 randomized controlled trials (total n=6810) of TNF inhibitor therapy for psoriasis or psoriatic arthritis over periods of 12–30 weeks found no association between the use of these agents and the risk of infection (overall or serious) when adjusted for different follow-up times in treatment and control groups .
| FDA WARNINGS AND PRECAUTIONS FOR TARGETED IMMUNE MODULATORS WITH DERMATOLOGIC INDICATIONS |
| TNF inhibitors: adalimumab, certolizumab, etanercept, golimumab, infliximab |
|
| Ustekinumab |
|
| IL-17 inhibitors: ixekizumab, secukinumab, brodalumab |
|
| Dupilumab |
|
| IL-1 inhibitors: anakinra, canakinumab, rilonacept |
|
| Rituximab |
|
| Omalizumab |
|
Recommended monitoring for dermatologic patients receiving TNF inhibitors is outlined in Table 128.7 . A purified protein derivative (PPD) skin test, IFN-γ release assay (e.g. QuantiFERON® TB Gold, T-SPOT®.TB), and/or chest X-ray (e.g. if immunosuppression or history of tuberculosis) is required at baseline and (typically) repeated annually during therapy. Patients with untreated latent tuberculosis should receive antituberculous therapy prior to treatment with a TNF inhibitor. Reactivation of hepatitis B virus has also been observed in patients who are chronic hepatitis B carriers (i.e. hepatitis B surface antigen positive) while receiving a TNF inhibitor, so patients should be evaluated for hepatitis B virus infection prior to therapy ( Table 128.8 ). TNF inhibitors do not generally appear to have an adverse effect on viral load or hepatitis activity in psoriasis patients with chronic hepatitis C virus infection .
| RECOMMENDED EVALUATION FOR PATIENTS RECEIVING TARGETED IMMUNE MODULATORS FOR DERMATOLOGIC DISORDERS | |
|---|---|
| Baseline history and physical examination | |
|
|
| Laboratory testing | |
| Prior to treatment | During treatment |
|
|
* Recommended vaccines should be administered prior to initiation of therapy, with live vaccines (see Table 128.10 ) given at least 2–4 weeks prior to starting the medication; the bacillus Calmette–Guérin (BCG) vaccine should not be given in the year prior to beginning or the year after completing ustekinumab therapy.
The potential of TNF inhibitors to increase the risk of malignancy is controversial and may be dependent upon the disease that is being treated. TNF inhibitor therapy in patients with rheumatoid arthritis has been associated with an approximately threefold increase in the risk of lymphoma and malignancy in general , but this patient group is known to have a heightened susceptibility to lymphoma and often receives additional immunosuppressive agents. Most studies have not demonstrated an increase in the risk of other internal malignancies in patients treated with TNF inhibitors . However, in one study there appeared to be an increased risk of solid malignancies associated with etanercept therapy for granulomatosis with polyangiitis . In addition, non-melanoma skin cancers and melanoma may be more frequent in patients with rheumatoid arthritis who are treated with TNF inhibitors than those who do not receive these agents . A meta-analysis of 20 randomized controlled trials (total n=6810) of TNF inhibitor therapy for psoriasis or psoriatic arthritis over periods of 12–30 weeks found no significant association between the use of these agents and the development of malignancy .
Adolescents and young adults with inflammatory bowel disease who receive infliximab and concomitant azathioprine or 6-mercaptopurine appear to have an increased risk of a rare, aggressive hepatosplenic T-cell lymphoma. In an analysis of 48 malignancies in children and adolescents treated with TNF inhibitors that were reported to the FDA , it was noted that half were lymphomas, almost two-thirds were in patients taking infliximab, and 88% of the patients were concomitantly receiving another immunosuppressive agent. Although the incidence of malignancy was higher than the background rate in the general pediatric population, the potential role of other medications as well as the underlying disorders, primarily inflammatory bowel disease and juvenile idiopathic arthritis (JIA), precluded establishing a causal relationship with TNF inhibitor therapy.
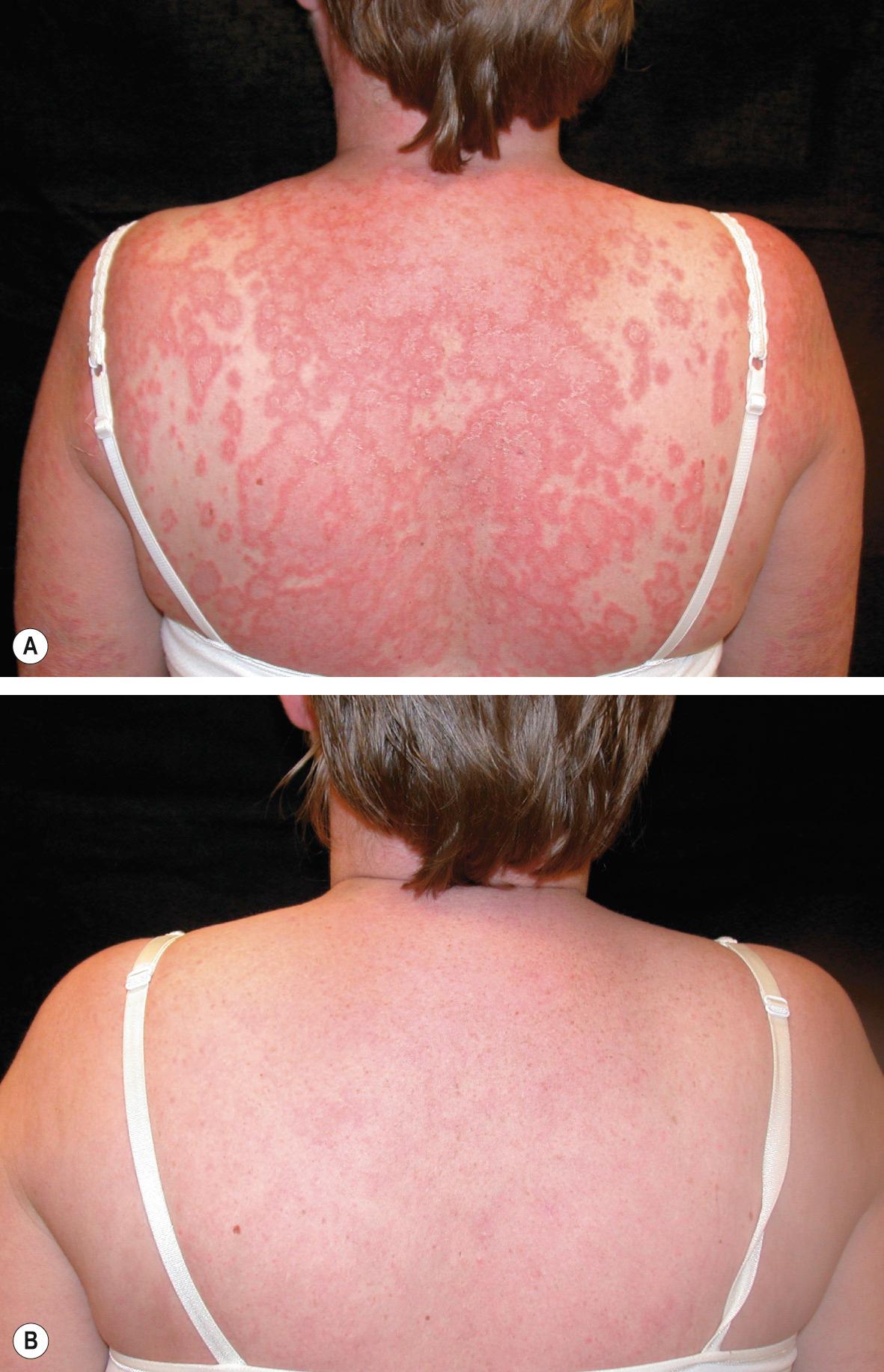
Treatment with TNF inhibitors is associated with an increased likelihood of developing antinuclear and anti-dsDNA antibodies. Signs and symptoms of cutaneous and systemic lupus erythematosus (SLE) occasionally arise in patients receiving TNF inhibitors, but in general these manifestations resolve upon cessation of treatment . Conversely, several small case series have reported marked improvement of subacute cutaneous lupus erythematosus by etanercept ( Fig. 128.6 ). It is not necessary to evaluate patients for autoantibodies before or during therapy with TNF inhibitors. However, the development of antinuclear antibodies may be linked to loss of TNF inhibitor efficacy .
Although etanercept was originally utilized as a therapy for multiple sclerosis, new onset or exacerbation of multiple sclerosis or other demyelinating diseases represents is a potential complication of TNF inhibitor treatment . In some patients, the symptoms of multiple sclerosis have abated despite continued therapy. It seems reasonable to avoid using TNF inhibitors in patients with a history of multiple sclerosis or other demyelinating diseases.
There have been reports of congestive heart failure worsening or developing in patients receiving TNF inhibitors. As a result, it is recommended that therapy with TNF inhibitors be avoided in patients with unstable cardiac disease.
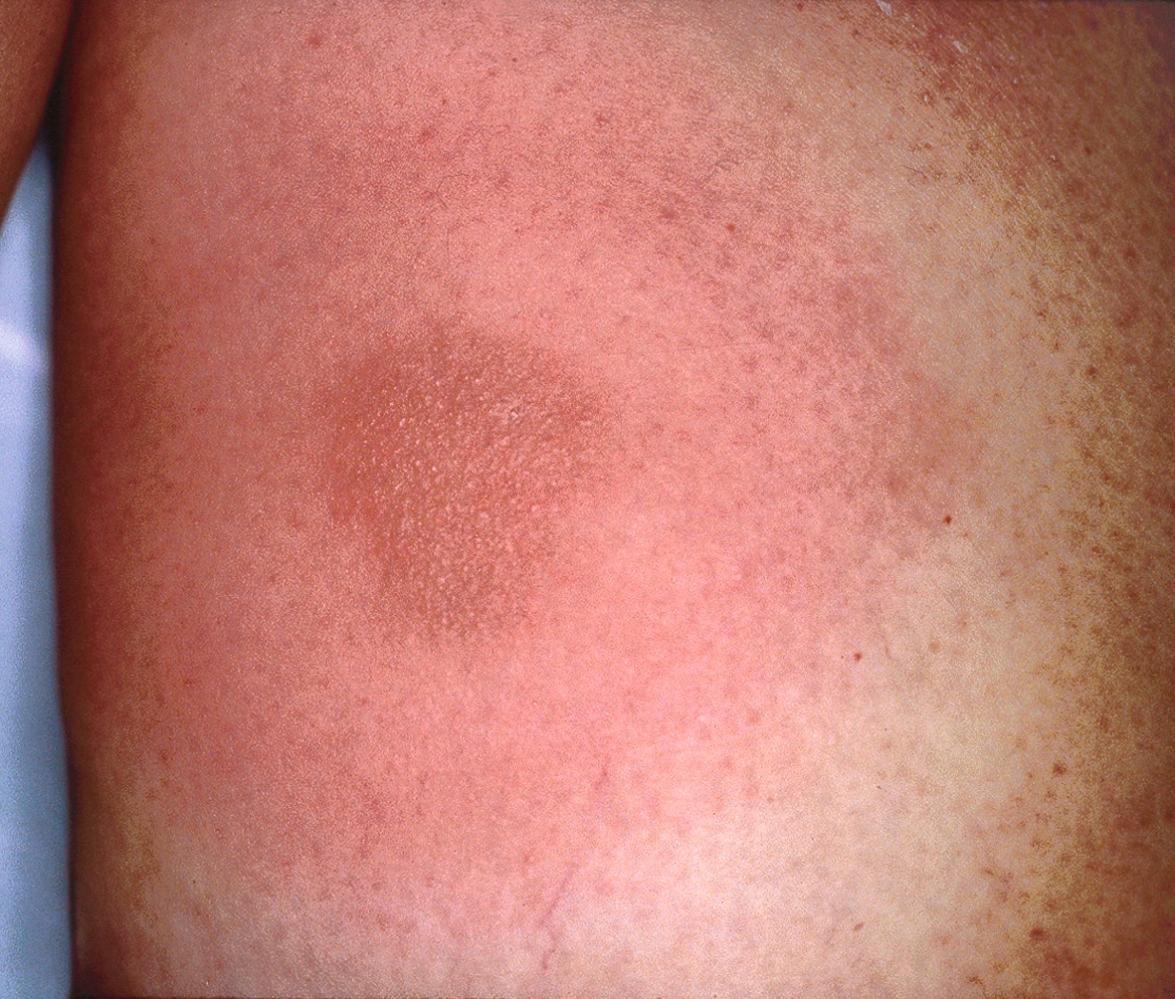
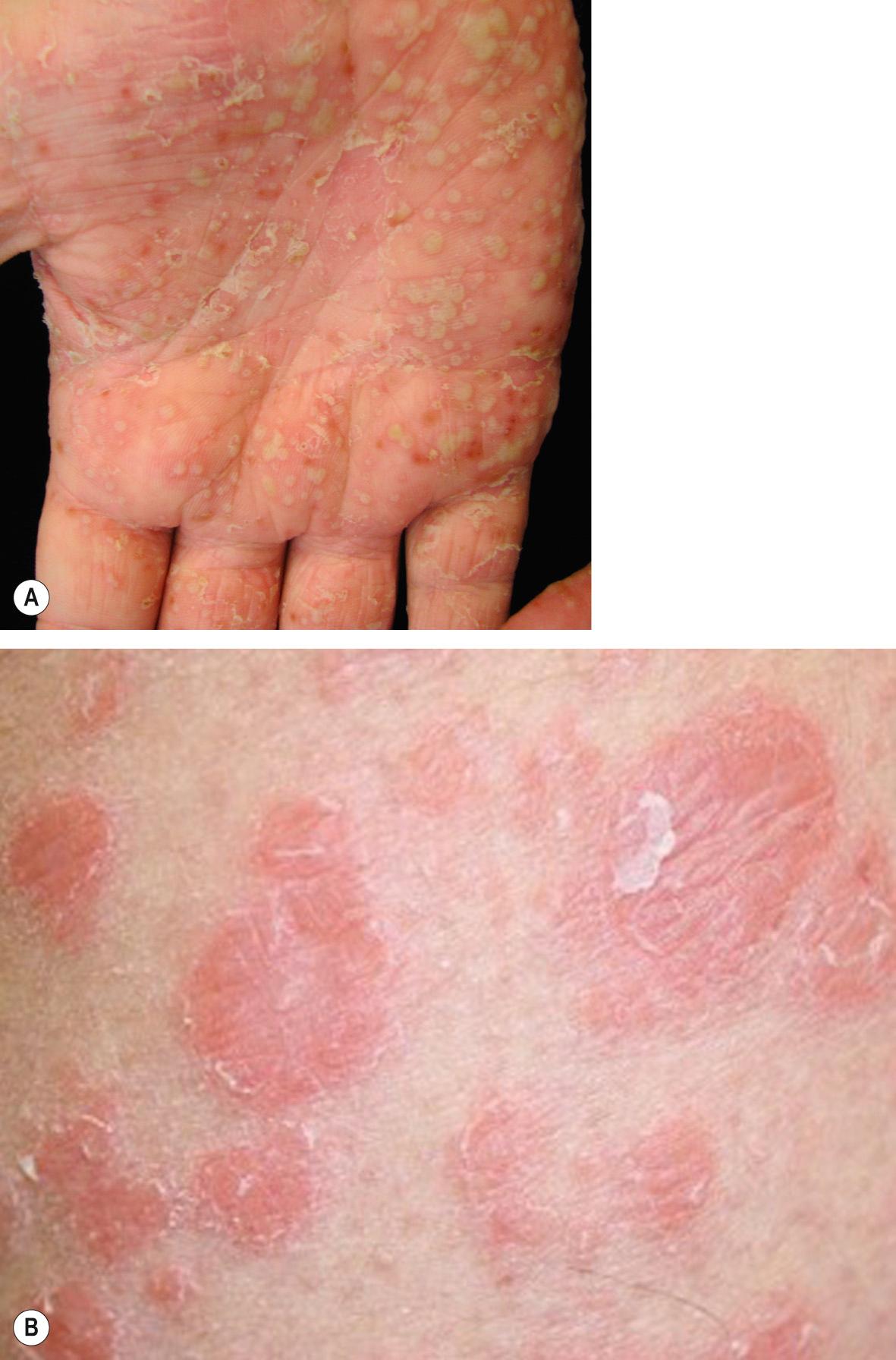
A variety of adverse skin reactions to TNF inhibitors have been described ( Table 128.9 ). There are reports of new-onset psoriasis in patients with no previous history of psoriasis, often manifesting as palmoplantar pustulosis ( Fig. 128.7 ) . The exact mechanism for this reaction is not known, and it is possible that some patients with presumed rheumatoid arthritis actually had psoriatic arthritis. Cutaneous small vessel vasculitis and interstitial granulomatous dermatitis (IGD) can also develop in patients undergoing TNF inhibitor therapy , but a subset of these patients have rheumatoid arthritis, which itself can be associated with vasculitis and IGD . Additional cutaneous reactions that have been described in patients receiving TNF inhibitors include eczematous eruptions, lichenoid dermatitis, and other granulomatous conditions .
| CUTANEOUS SIDE EFFECTS OF TARGETED IMMUNE MODULATORS |
| Injectable agents |
|
| Tumor necrosis factor inhibitors |
|
| Epidermal growth factor receptor inhibitors |
|
| Other kinase inhibitors and blocking antibodies – see Table 21.16 |
|
Live vaccines are contraindicated in patients receiving TNF inhibitors ( Table 128.10 ). The effectiveness of vaccines in patients receiving these agents is unknown, but an immune response to influenza and pneumococcal vaccines has been documented . It is recommended that patients (especially children) be brought up-to-date with all immunizations prior to initiating treatment with TNF inhibitors.
| VACCINES THAT CONTAIN LIVE ATTENUATED VIRUSES OR BACTERIA |
|
Etanercept (Enbrel®) and etanercept-szzs (Erelzi™) are fusion proteins consisting of the extracellular domain of the TNF receptor fused with the Fc portion of human IgG1 .
Etanercept has been approved for the treatment of moderate to severe plaque-type psoriasis in adults since 2004 and in children ages 4–17 years since 2016. Depending on the regimen (see below), ~30–60% of patients achieve a 75% improvement in their Psoriasis Area and Severity Index (PASI) score; significant decreases in fatigue and depression have also been described . Other approved indications for etanercept include rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis in adults as well as JIA in children ≥2 years of age.
Etanercept has been used to treat a variety of other mucocutaneous disorders, including dermatomyositis, cutaneous lupus erythematosus, lichen planus, autoimmune bullous diseases (especially mucous membrane [cicatricial] pemphigoid), neutrophilic dermatoses (including pyoderma gangrenosum), hidradenitis suppurativa, multicentric reticulohistiocytosis, relapsing polychondritis, toxic epidermal necrolysis, and GVHD . Although successful treatment of sarcoidosis has been described in case reports and small series, etanercept therapy failed to improve systemic manifestations of sarcoidosis in randomized controlled trials . Presumably, all of the disorders for which etanercept is effective are characterized by elevated levels of TNF in the circulation and/or affected tissues.
For most of its indications, etanercept was initially approved to be administered subcutaneously at a dose of 25 mg twice weekly in adults. Shortly after its approval for psoriatic arthritis, it was demonstrated that 50 mg weekly gave equivalent results to 25 mg twice weekly. The approved dose for moderate to severe plaque psoriasis is 50 mg twice weekly for the first 3 months, followed by 50 mg weekly. Some physicians choose to initiate therapy at a dose of 50 mg weekly and only escalate the dose if the patient fails to respond after 6 to 12 months. Etanercept is available in 25 and 50 mg prefilled syringes, 50 mg autoinjectors, and 25 mg multiple-use vials. The recommended dose for pediatric psoriasis is 0.8 mg/kg (maximum 50 mg) weekly.
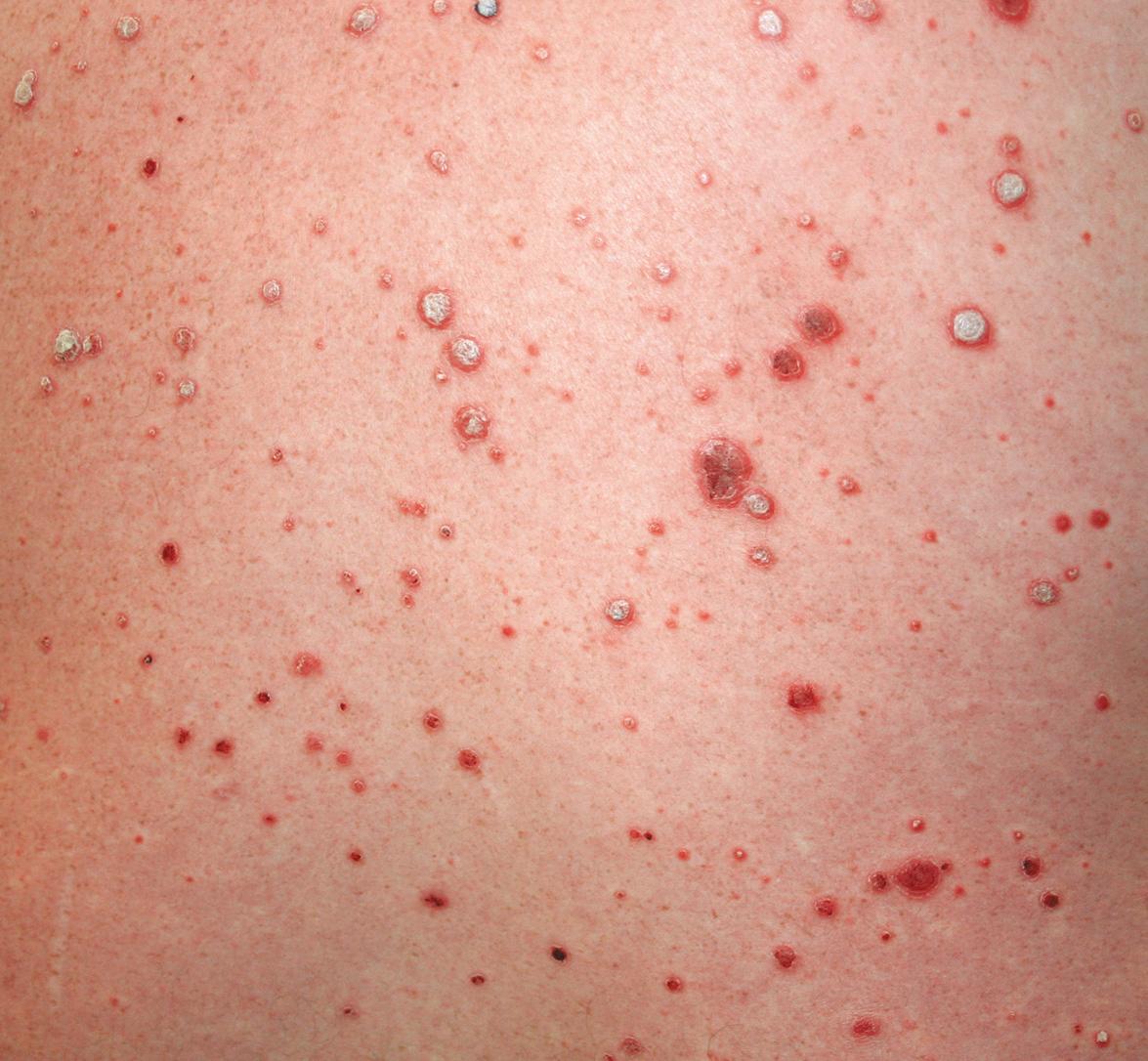
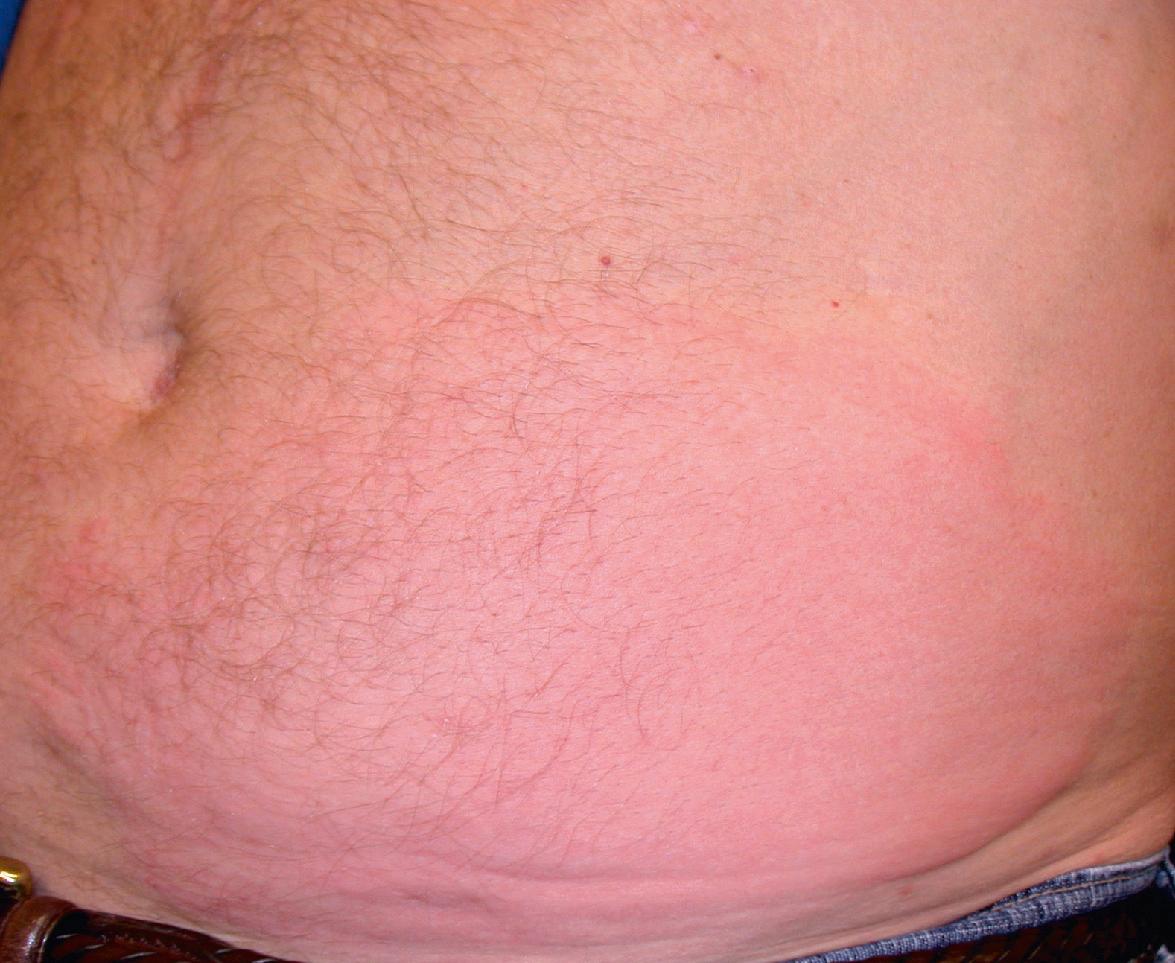
Side effects shared with other TNF inhibitors are discussed above and summarized in Tables 128.6 and 128.9 . Injection site reactions are the most common side effect of etanercept ( Fig. 128.8 ) but in general are mild to moderate and diminish in frequency after the first month of treatment. Erythema, pruritus, pain, and swelling have been described, and “recall” reactions at sites of previous injections have been reported. These reactions do not progress to anaphylaxis.
The concomitant use of etanercept and methotrexate is approved for the treatment of rheumatoid arthritis and psoriatic arthritis. Due to the potential for increased immunosuppression, combination of etanercept with other targeted immune modulators should generally be avoided. A study of anakinra plus etanercept for patients with rheumatoid arthritis found no increase in efficacy but a substantial increase in the frequency of infection . Etanercept has been used together with narrowband UVB without an increase in adverse events and with added therapeutic benefit .
Etanercept is pregnancy category B, with no evidence of harm to the fetus in animal studies. Studies in pregnant women have not been conducted. It has been suggested that etanercept and other TNF inhibitors be avoided after the first trimester . It is not known whether etanercept is secreted in breast milk; however, in general, this drug is not absorbed via an oral route.
Infliximab (Remicade®), infliximab-dyyb (Inflectra®), and infliximab-abda (Renflexis®) are chimeric human–mouse monoclonal IgG1 antibodies, and their target is human TNF .
Infliximab is approved for the treatment of adults with chronic severe plaque-type psoriasis. Compared with the other TNF inhibitors, its onset of action tends to be faster and a higher proportion of patients (~75–85%) achieve a 75% reduction in the PASI score . Infliximab is also approved for the treatment of adults with psoriatic arthritis, Crohn disease (including associated fistulas), ulcerative colitis, rheumatoid arthritis and ankylosing spondylitis, as well as children ≥6 years of age with Crohn disease and ulcerative colitis. Infliximab appears to be useful for pyoderma gangrenosum, whether or not it is associated with inflammatory bowel disease . It has been effective as a treatment for sarcoidosis, granulomatous cheilitis, Behçet disease, various vasculitides, pityriasis rubra pilaris, reactive arthritis, subcorneal pustular dermatosis, GVHD, Sjögren syndrome, multicentric reticulohistiocytosis, and hidradenitis suppurativa .
In patients with plaque psoriasis, the typical infliximab regimen is 5 mg/kg administered by slow intravenous infusion at 0, 2 and 6 weeks, and then every 8 weeks. However, doses ranging from 3 to 10 mg/kg have been utilized, and the frequency of administration as well as the dose can be adjusted when the response is inadequate. For Crohn disease, rheumatoid arthritis, and psoriatic arthritis, it is approved for concomitant administration with methotrexate, azathioprine, or low-dose prednisone. Loss of efficacy over time may be related to the development of neutralizing anti-chimeric antibodies and has been associated with the presence of antinuclear antibodies ; the concurrent administration of low-dose weekly methotrexate may help to prevent the formation of anti-chimeric antibodies.
Side effects shared with other TNF inhibitors are discussed above and summarized in Tables 128.6 and 128.9 . Infusion-related reactions are the most commonly reported side effect of infliximab, occurring in ~15% of patients. The incidence of severe reactions, in particular anaphylaxis, is ~1%. Associated symptoms include fever, chills, pruritus, urticaria, chest pain, hypotension, hypertension, and shortness of breath. Infusion reaction risk has been linked to the presence of human anti-chimeric antibodies. Risk of reactions may be lessened by a slower infusion rate or concomitant use of methotrexate, azathioprine, or corticosteroids.
Infliximab should not be administered together with other targeted immune modulators due to the potential for additive immunosuppression; concomitant use with anakinra or abatacept has been associated with the development of severe infections.
Infliximab is rated pregnancy category B. However, because it crosses the placenta and can lead to an increased risk of infection in the infant, use in pregnancy should be avoided unless the benefits outweigh the risks. It is not known whether infliximab is secreted in breast milk, and its administration to nursing mothers is not recommended.
Adalimumab (Humira®) and adalimumab-atto (Amjevita®) are human recombinant IgG1 monoclonal antibodies with specificity for human TNF .
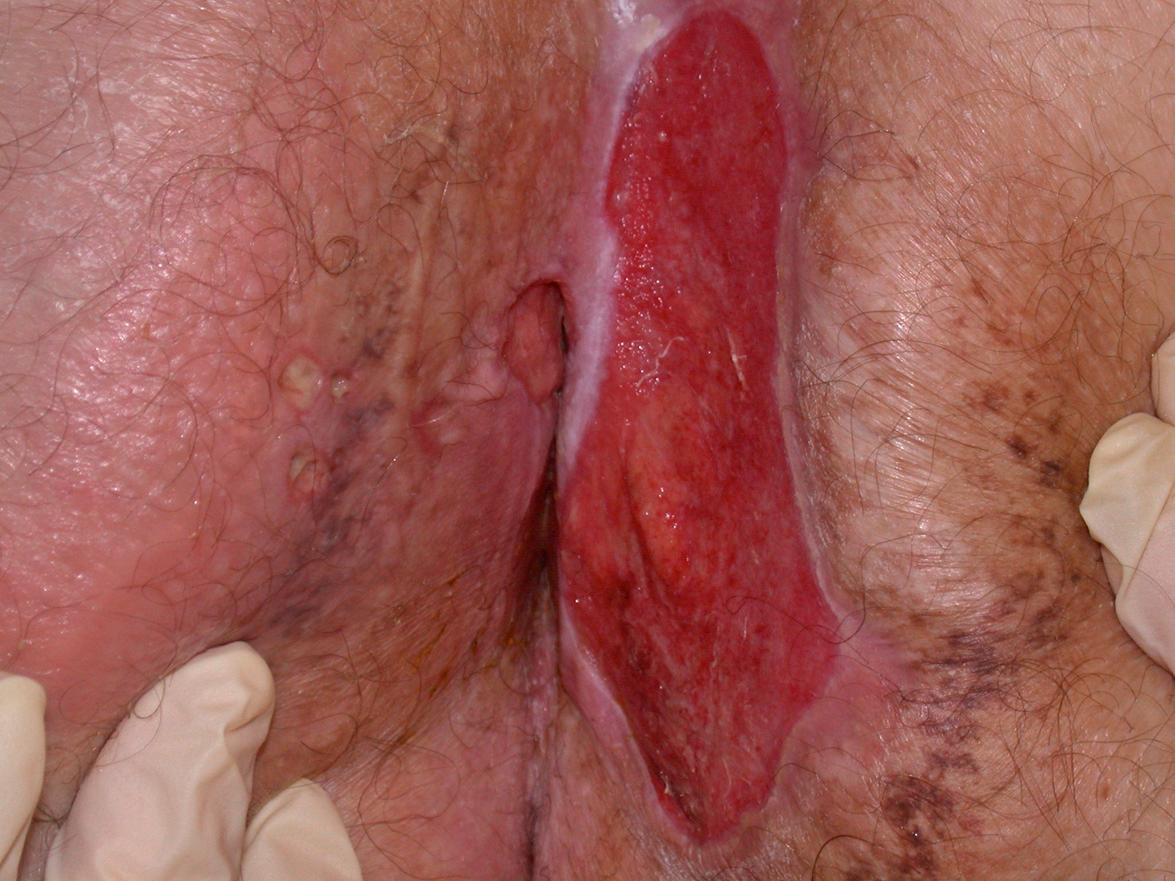
Adalimumab is approved for the treatment of moderate to severe plaque-type psoriasis in adults, and ~50–80% of patients achieve a 75% improvement in their PASI score . It is also approved for the treatment of moderate to severe hidradenitis suppurativa in adults, with ~40–60% of patients achieving a 50% reduction in the abscess/inflammatory nodule count with no increase in the draining fistula count . Additional approved indications include psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, uveitis, and Crohn disease in adults as well as JIA and Crohn disease in children ≥2 years and ≥6 years of age, respectively. There have also been reports of successful adalimumab therapy for pustular psoriasis , sarcoidosis, pyoderma gangrenosum, Behçet disease, other neutrophilic dermatoses, and dermatomyositis.
Adalimumab is supplied in 10–80 mg prefilled syringes and a 40 mg autoinjector; it is administered subcutaneously. For the treatment of psoriasis, an initial loading dose of 80 mg is typically given, followed by 40 mg on day 8 and then 40 mg every other week. The approved regimen for hidradenitis suppurativa is a loading dose of 160 mg, 80 mg on day 15, and then 40 mg weekly starting on day 29. The pediatric dosing approved for JIA is every-other-week administration of 10 mg, 20 mg, or 40 mg for children who weigh 10–14 kg, 15–29 kg, or ≥30 kg, respectively. Loss of efficacy of adalimumab over time related to the production of anti-adalimumab antibodies can occur and may be prevented by the concurrent administration of low-dose weekly methotrexate; loss of efficacy has also been associated with the development of antinuclear antibodies . The concomitant use of adalimumab and methotrexate is approved for the treatment of psoriatic arthritis, rheumatoid arthritis, and JIA.
Side effects shared with other TNF inhibitors, including risk of infection with mycobacteria (see Fig. 128.5 ) and fungi, are discussed above and summarized in Tables 128.6 and 128.9 .
The concomitant administration of other targeted immune modulators with adalimumab may increase the risk of infection and should be avoided .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here