Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Additional content is available online at Elsevier eBooks for Practicing Clinicians
Additional content is available online at Elsevier eBooks for Practicing Clinicians
Primary hypertension is a key contributor to premature morbidity and mortality in the United States and consistently ranks among the top two risk factors worldwide for global disease burden in 2015. After tobacco use and diabetes, uncontrolled primary hypertension is the most important risk factor for peripheral vascular disease (the second leading cause of loss of limbs in the United States).
Uncontrolled primary hypertension is the most important modifiable risk factor for stroke, the leading contributor to all common forms of heart failure, the second most common cause of end-stage kidney disease, and also contributes to memory loss.
Blood pressure (BP) is the phenotypic expression of the genetically predisposed disease hypertension and is a continuous variable. As more data became available, the guideline-based definition of hypertension has evolved over the past 40 years. The traditional “threshold BP value” to secure a diagnosis of hypertension comes from large epidemiologic studies demonstrating a higher mortality at levels above 140/90 mm Hg. More recent guidelines, however, define the level at which one is considered hypertensive based on level of cardiovascular (CV) risk rather than just the BP number. This is true of the most recent US guidelines that define hypertension as ≥130/80 mm Hg and the European Guidelines which use a slightly different approach to risk and define hypertension as ≥140/90 mm Hg ( Table 26.1 ). Thus, in most people up to age 80 years and those with comorbidities, a BP ≥130/80 mm Hg is considered hypertension requiring treatment in the United States. However, medical treatment is not accepted in everyone with low risk and around the world for BP 130 to 140/80 to 90 mm Hg ( Table 26.1 ). ,
| Guideline Differences | American College of Cardiology/American Heart Association (ACC/AHA) | European Society of Cardiology/European Society of Hypertension (ESC/ESH) | ||
|---|---|---|---|---|
| Level of Blood Pressure (BP) Defining Hypertension | Systolic and/or Diastolic | Systolic and/or Diastolic | ||
| (mm Hg) | (mm Hg) | (mm Hg) | (mm Hg) | |
| Office/clinic BP | ≥130 | ≥80 | ≥140 | ≥90 |
| Daytime mean | ≥130 | ≥80 | ≥135 | ≥85 |
| Nighttime mean | ≥110 | ≥65 | ≥120 | ≥70 |
| 24-hr mean | ≥125 | ≥75 | ≥130 | ≥80 |
| Home BP mean | ≥130 | ≥80 | ≥135 | ≥85 |
| BP targets for treatment | <130/80 | Systolic targets | <140 and close to 130 | |
| Initial combination therapy | Initial single-pill combination therapy | Initial single-pill combination in patients >20/10 mm Hg above BP goal therapy in patients ≥ 140/90 mm Hg | ||
| Hypertensive requiring | >130/80 mm Hg | ≥140/90 mm Hg intervention | ||
| Guidelines Similarities | ||||
| Importance of home | Take BP at home, twice in the morning and twice in the evening, in the week before clinic BP monitoring | Bring the BP machine in annually for validation | ||
| Therapy | Restrict beta blockers to patients with comorbidities or other indications | Initial single-pill combination as initial therapy | ||
| Follow-up | Detect poor adherence and focus on improvement | BP telemonitoring and digital health solutions recommended | ||
The global burden of hypertension is estimated at approximately 1.4 billion individuals, and by 2025 at the current rate will exceed 1.6 billion. CV risk attributable to elevated BP dates back to 1948 and the origin of the Framingham Heart Study. , The continuous relationship between BP level and risk of events in the brain, heart, and kidney are well documented. A natural history study involving almost 12,000 veterans, followed over 15 years, noted that BP level correlated with risk for end-stage kidney disease ( Fig. 26.1 ). Note that, in this study, the highest risk for end-stage kidney disease was found at levels above the renal autoregulatory range (i.e., a systolic BP >180 mm Hg). A natural history study of over a million people demonstrates that CV risk becomes most pronounced above levels of 140/90 mm Hg ( Fig. 26.2 ).
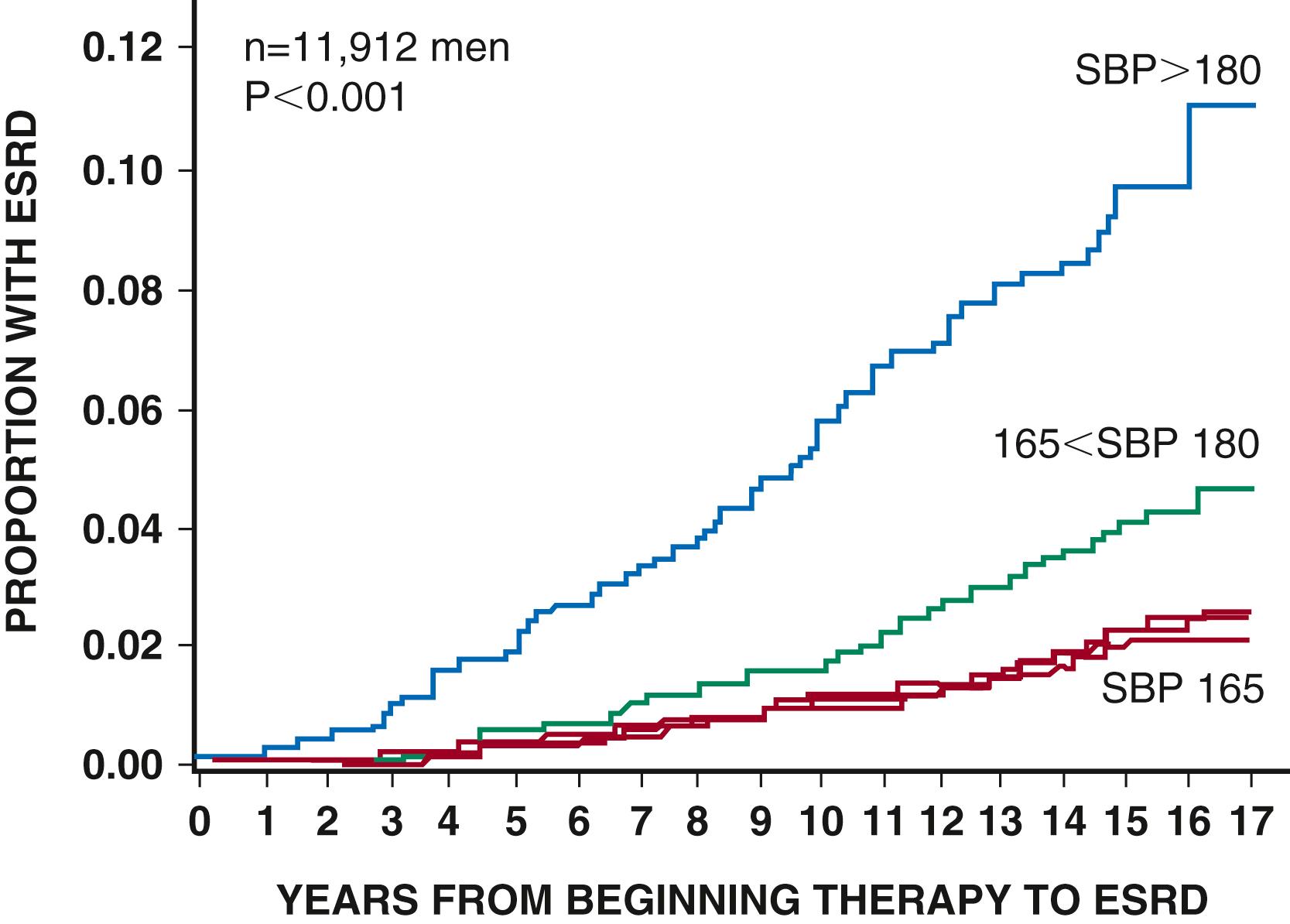
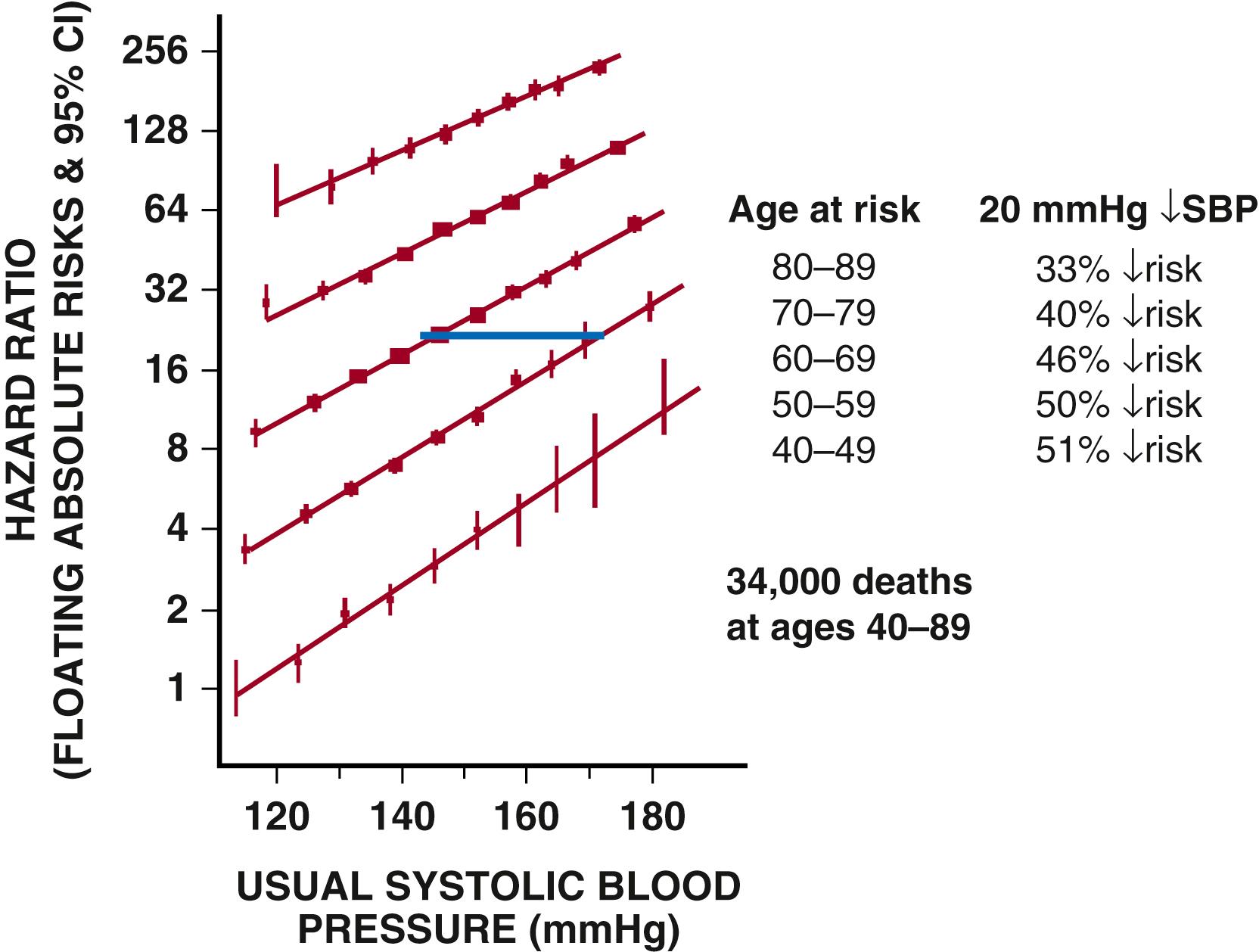
Increasing age is a major risk factor for developing hypertension, as well as a very strong confounder of its independent influence on CV and renal events due to increased arterial stiffness and reduced nitric oxide (NO) release (see Fig. 26.2 ). In an analysis of 61 epidemiologic studies followed for an average of 13.3 years, those with BP levels in the highest decile had approximately the same risk for death from either ischemic heart disease or stroke as people who were 20 years older but had BP levels in the lowest decile. In the Framingham study the lifetime risk of 55- to 65-year-old men or women for developing hypertension was above 90%. In this study, those who survived to ages 65 to 89 years, systolic BP (SBP) elevations were found in 87% of the hypertensive men and 93% of the hypertensive women. In an analysis of the Framingham data set, classification of people with hypertension older than age 60 years into the appropriate BP stages was done correctly in 99% of the cases using SBP rather than diastolic BP (DBP).
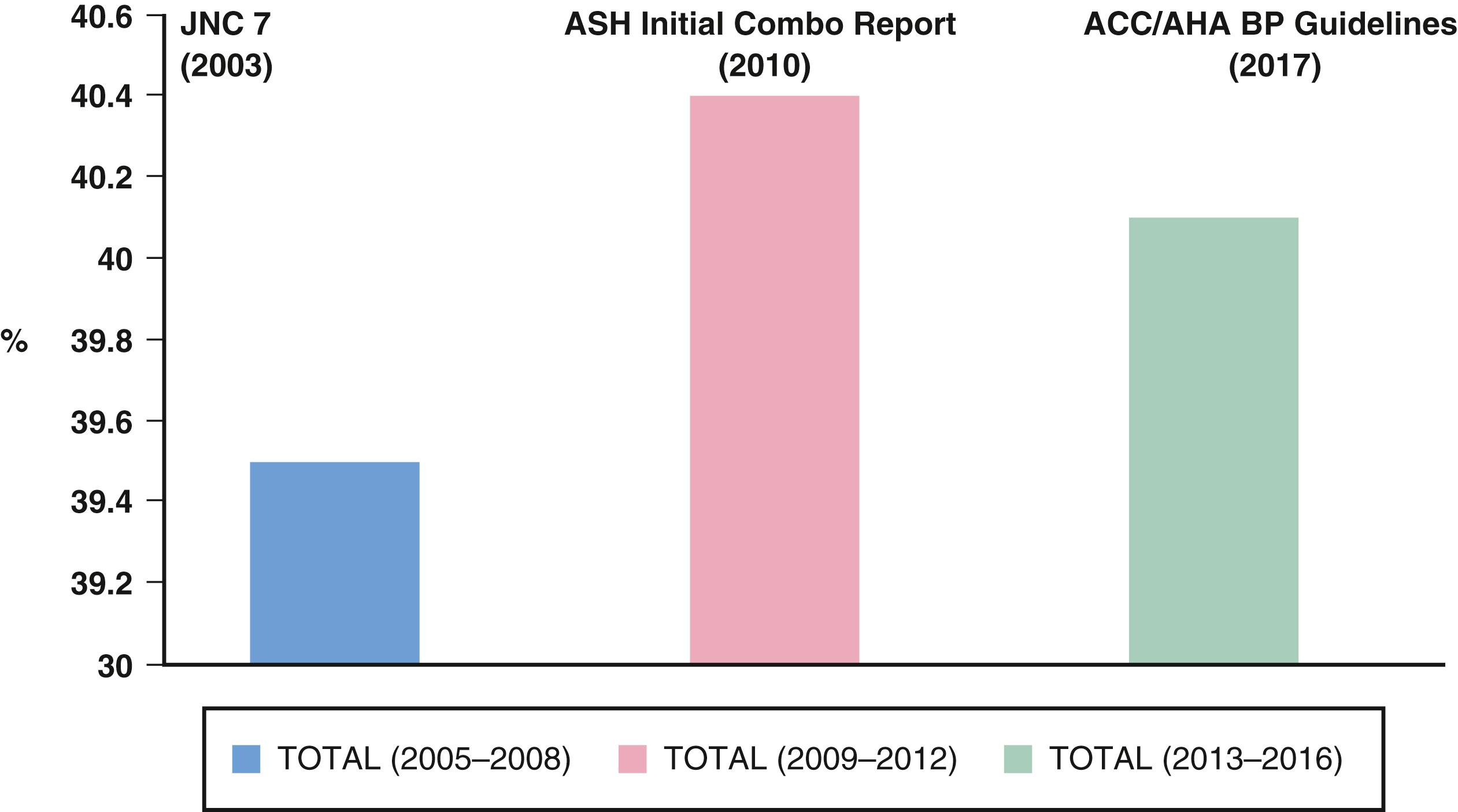
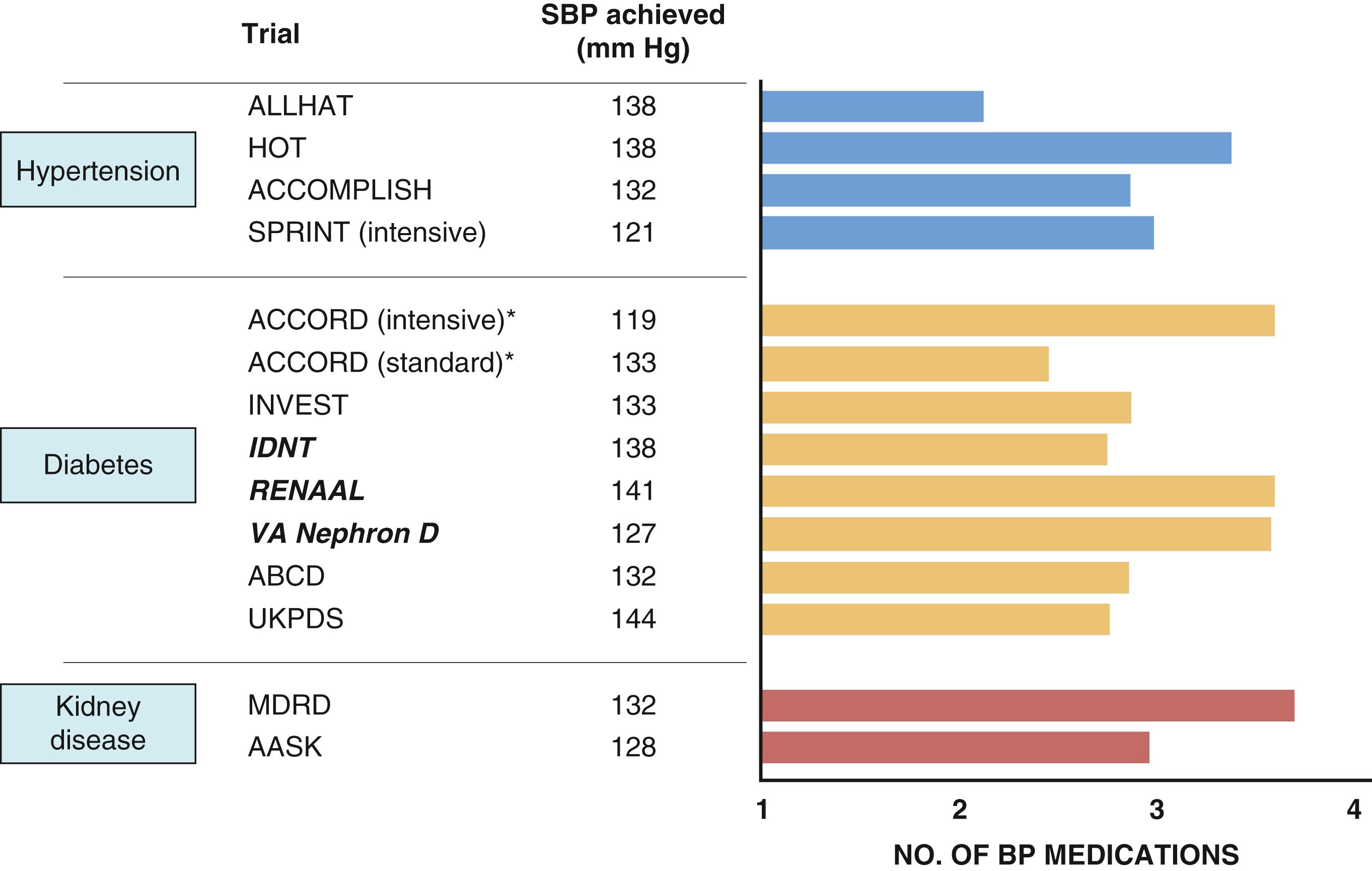
These data highlight the public health importance of SBP, particularly among people older than 50 years of age. In such individuals, SBP is a much better predictor of hypertensive target-organ damage and future CV and renal events than is DBP. , , Overall, each 20–mm Hg increase in SBP doubled the risk for CV death. Although uncontrolled hypertension in the United States in 2014 was noted in nearly 54% of people 60 years of age and older, antihypertensive drug therapy reduces CV events with its greatest absolute benefit in older people, including individuals older than 80 years of age. Moreover, data clearly show that people with sustained BP control have fewer CV events than others; however, in spite of multiple international guidelines including US guidance for initial dual therapy to help achieve BP control, initial dual therapy fails to be adopted to achieve this benefit ( Fig. 26.3 ) . ,
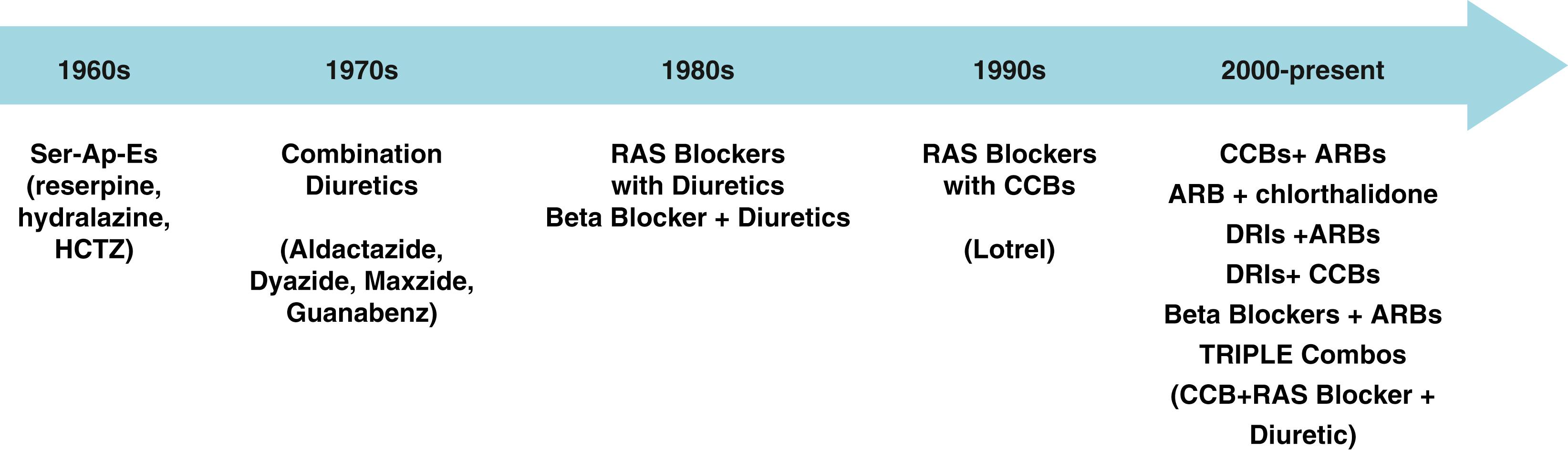
Currently there are more than 125 different medications, almost all of which are generically available, from eight different antihypertensive drug classes to help lower BP ( Fig. 26.4 ). Moreover, there are more than 15 fixed-dose single-pill combination agents ( Table 26.2 ). Note that all clinical trials over the past 30 years have used more than one medication for BP control ( Fig. 26.5 ). In spite of this, BP control remains suboptimal in many parts of the world. , , , What is missing is a focus on obesity reduction and wide availability of single-pill two-drug combinations to control BP as is strongly advocated by the European hypertension guidelines (see Table 26.1 ).
| Preferred
Acceptable
Less effective
|
The diagnosis of hypertension in children and adolescents is becoming more important, due to the epidemic of obesity in young Americans. Current US guidelines recommend BP measurement in children at least annually, but “normative values” depend on sex, age, and height of the child. As a result, interpretation of BP levels in children and adolescents usually involves comparison of a child’s average BP (from three visits) to a comprehensive table that provides threshold values for “elevated” (traditionally, BP between the 90th and 95th percentiles), “hypertension” (BP between the 95th and 99th percentiles), and “severe hypertension” (99th percentile or higher).
Non-Hispanic Blacks have approximately a 50% higher prevalence of hypertension than non-Hispanic Whites, even after age adjustment (41.2% versus 28.0%) (see Chapter 93 ). The prevalence of hypertension is geographically heterogeneous, with the highest prevalence in both Blacks and Whites in the southeastern United States. However, non-Hispanic Blacks had the highest awareness (at 85.7%) and treatment (at 77.4%) of hypertension in National Health and Nutrition Examination Survey (NHANES) 2011to 2012, but their BP control rate in 2011 to 2014 lagged that of non-Hispanic Whites (48.5% to 55.7%, age adjusted). This pattern has been consistent over the past decade.
The prevalence of hypertension has increased since 1988, with the greatest increase for non-Hispanic Blacks, compared with either Mexican Americans or non-Hispanic Whites. In contrast to non-Hispanic Whites and Mexican Americans, a slightly higher prevalence of hypertension was observed in NHANES 2011 to 2014 for non-Hispanic Black women, compared with men (41.5% versus 40.8%). In all three racial/ ethnic groups, women had higher rates of awareness, treatment, and control of BP than men in NHANES 2011 to 2014.
In 2014, the age-adjusted death rate from heart disease was 24% higher in Blacks, as was stroke (by 41%) and hypertension or hypertensive renal disease (by 111%). Incident end-stage renal disease (ESRD) was 3.1 or 1.2 times more common in 2014 in Blacks or Native Americans, compared with whites.
Although the prevalence of hypertension in Hispanics is lower than in non-Hispanic Blacks and whites, hypertension is a concern. Mexican Americans continue to have the lowest age-adjusted prevalence of controlled hypertension in both men and women (25.6% and 31.9%, respectively, in NHANES 1999 to 2006, compared with 37.0% and 49.2% in NHANES 2007 to 2014).
BP control rates (to <140/90 mm Hg) have improved substantially in the United States since 1974 and have stabilized at just over 50% in the last four biennial NHANES reports. Successful national efforts to increase hypertension treatment and control rates have been associated with significant reductions in CV hospitalizations or death in both Canada and the United Kingdom. With new hypertension goals, those with apparent treatment-resistant hypertension and the prevalence of uncontrolled hypertension are greater for undiagnosed, untreated, or older individuals and for SBP (rather than DBP).
The factors that generate BP comprise the integration of cardiac output (CO) and systemic vascular resistance (SVR): BP = CO × SVR. Note that CO = heart rate × stroke volume; SVR = 80 × (mean arterial pressure − central venous pressure)/CO.
To fully understand BP regulation, one has to appreciate that the kidney is a regulatory organ that tries to maintain a homeostatic environment under a variety of changes not only in BP but electrolytes and acid-base balance. Hence, because sodium conservation is one of its principle jobs, any large increase in pressure will lead to pressure natriuesis.
Pressure natriuresis is defined as the increase in renal sodium excretion due to mild increases in BP, typically because of extracellular fluid volume expansion, allowing BP to remain in the normal range. This concept is essential to the understanding of the sustainability of hypertension. If one understands the “set-point BP” as the BP at the point when extracellular volume and pressure natriuresis are in equilibrium, it necessarily follows that an increase in BP can be sustained only if pressure natriuresis is abnormal. Pressure natriuresis occurs over hours to days and is modulated by both biophysical and humoral factors.
In the normal state, increased sodium intake causes an increase in extracellular volume and BP. Because of the steep relationship between volume and pressure, small increases in BP produce natriuresis that restores sodium balance and returns BP to normal ( Fig. 26.6A ). Expansion of extracellular fluid volume and increased BP result in a rise in blood flow through the vasa recta, which stimulates the production of paracrine factors such as NO and ATP, which can inhibit tubular sodium reabsorption at multiple sites of the nephron. NO blunts the myogenic response of arteriolar autoregulation, thus allowing increased blood flow that is necessary to increase renal blood flow and interstitial pressure ( Fig. 26.6B ). ,
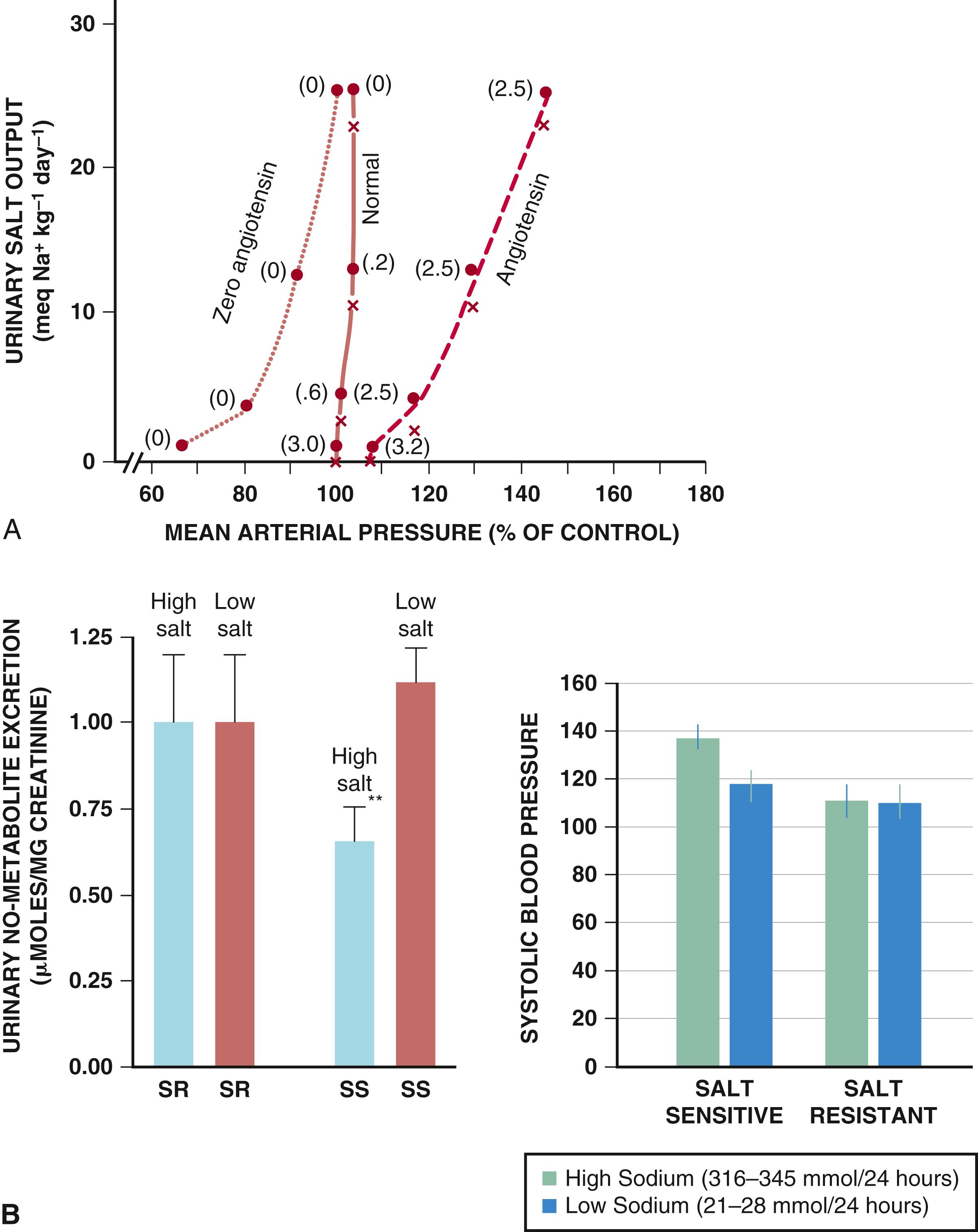
The kidneys adapt to sodium loading quickly, adapting to fluctuations in sodium intake as high as 50-fold, but this response is markedly blunted in the setting of chronic hypertension, resulting in a need for much higher BP levels to promote natriuresis. These states of abnormal sodium handling lead to sodium-sensitive hypertension, such as in conditions of reduced glomerular filtration rate (GFR) or high levels of angiotensin II. In such situations the change in extracellular fluid volume is relatively small (3% to 5%), but a state of chronic high BP develops resulting from increased SVR. The mechanisms responsible for this vascular effect are not completely understood but likely involve increased activity of the renin-angiotensin-aldosterone system (RAAS) (high angiotensin II levels) and several other vasoconstricting substances. Because abnormalities in pressure-sodium relationships are essential to maintaining chronic elevations in BP, they represent a fundamental step in the pathogenesis of any type of hypertension, not only primary, but also in the maintenance phase of most secondary causes, such as renal and renovascular hypertension, hyperaldosteronism, glucocorticoid excess, coarctation of the aorta, and pheochromocytoma.
The interplay between renal sodium retention and hypertension involves changes in sodium handling throughout the nephron. A theory with substantial experimental support proposes that increased renal vasoconstriction due to a variety of possible mechanisms (e.g., increased levels of angiotensin II, catecholamines, uric acid, or progressive aging) induces a preglomerular (afferent) arteriolopathy that results in impaired sodium filtration. , In addition, renal vasoconstriction results in tubular ischemia, another mediator of increased sodium avidity.
The RAAS has wide-ranging effects on BP regulation. Figure 26.7 summarizes the most relevant elements of the RAAS and its role in the pathogenesis of hypertension and its complications. The different elements of the RAAS have key roles in mediating sodium retention, pressure natriuresis, salt sensitivity, vasoconstriction, endothelium dysfunction, and vascular injury; and use of RAAS blockers is an effective means of treating hypertension. Taken together, the RAAS has an important role in the pathogenesis of hypertension. However, there are a number of unanswered issues about this relationship. A very large genome-wide association study (GWAS) of 2.5 million genotyped or imputed single-nucleotide polymorphisms (SNPs) in 69,395 individuals of European ancestry from 29 studies showed that the majority of SNPs associated with BP involved issues with natriuretic peptides. Thus natriuretic peptides play a prominent role in the pathogenesis of hypertension and may be more important than the RAAS system, which did not have prominent SNPs associated with hypertension in this analysis. Another meta-analysis evaluating the relationship between polymorphisms in key RAAS genes (angiotensin-converting enzyme [ACE], angiotensinogen gene [AGT], and CYP11B2) and salt sensitivity also found no significant role of RAAS polymorphisms.
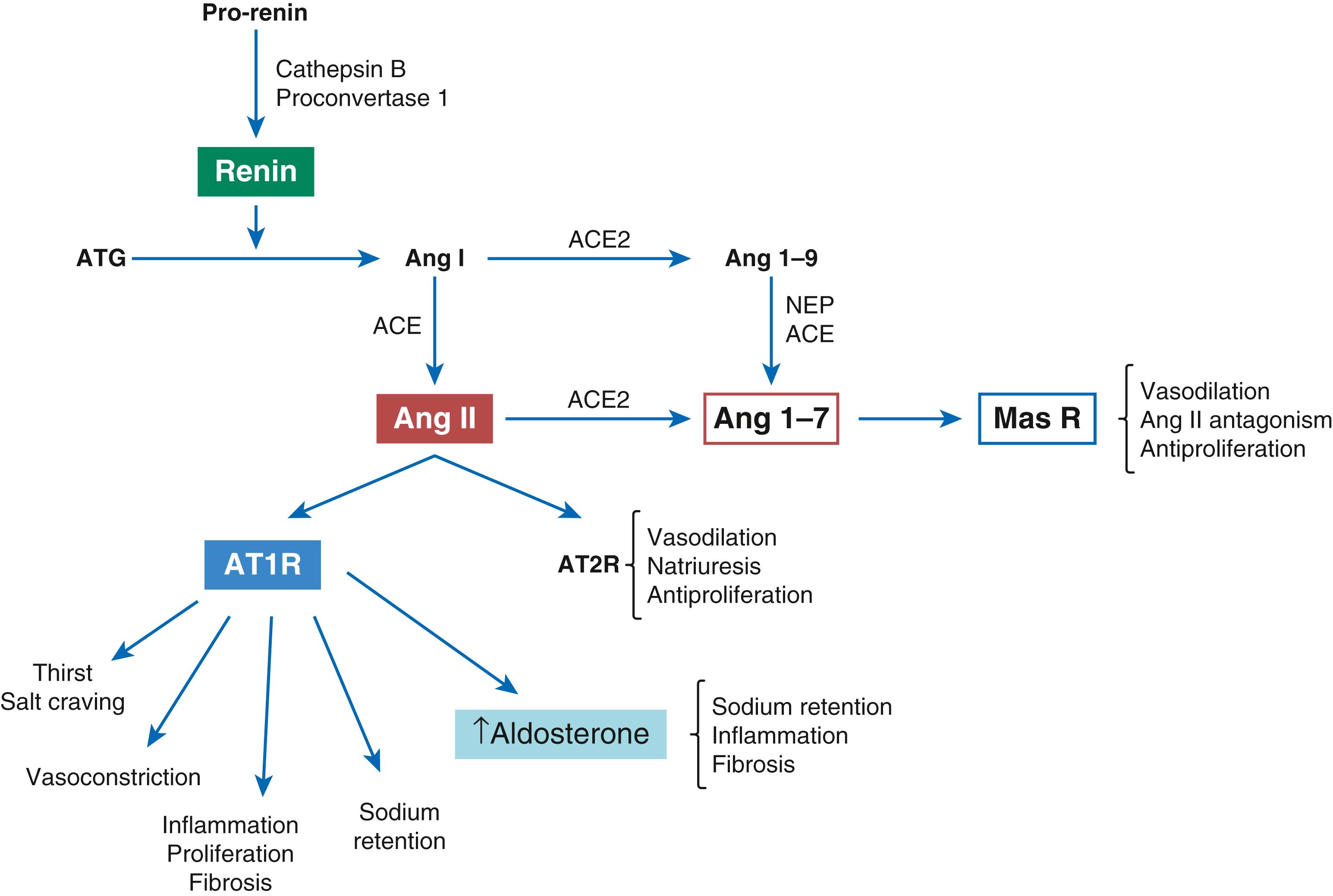
Despite these genetic inconsistencies, a wealth of experimental evidence links the RAAS to hypertension. Tissue expression of different elements of the RAAS is also important, including in the protection of animals from the development of hypertension after targeted elimination of renal ACE activity. Paradoxically the same experiments indicate that the absence of ACE in other tissues may also be protective from hypertension caused by triggers that do not involve the RAAS (such as NO inhibition), therefore raising the possibility that other (extrarenal) sites may also be relevant.
The enzyme renin and prorenin are synthesized and stored in the juxtaglomerular cell apparatus located in the kidney adjacent to the afferent arteriole and distal tubule. Renin is released in response to decreased renal afferent perfusion pressure, decreased sodium delivery to the macula densa, and activation of renal nerves (via β 1 -adrenergic receptor stimulation) and by a variety of metabolic products, including prostaglandin E 2 and several others. The main function of renin is to cleave angiotensinogen into angiotensin I.
Prorenin, previously viewed as an inactive substrate for renin production, is known to stimulate the (pro)renin receptor (PRR). This receptor leads to more efficient cleavage of angiotensinogen and activates downstream intracellular signaling through the mitogen-activated protein (MAP) kinases extracellular signal–regulated kinases 1 and 2 (ERK1/2) pathways that have been associated with profibrotic effects in some, but not all, experimental models. , It is still uncertain if the PRR is involved in the genesis or complications of hypertension in a manner that is independent of the effects of angiotensin II (see Fig. 26.7 ).
Angiotensin II, formed by the cleavage of angiotensin I by ACE, is at the center of the pathogenetic role of the RAAS in hypertension. Primarily through its actions mediated by the angiotensin II type 1 receptor (AT 1 R), angiotensin II is a potent vasoconstrictor of vascular smooth muscle, causing systemic vasoconstriction as well as increased renovascular resistance and decreased medullary flow, which is a mediator of salt sensitivity. Angiotensin II produces increased sodium reabsorption in the proximal tubule by increasing the activity of the sodium:hydrogen exchanger (NHE3), the sodium-bicarbonate exchanger, and Na + -K + -ATPase and by inducing aldosterone synthesis and release from the adrenal zona glomerulosa. Angiotensin II is associated with endothelial cell dysfunction and produces extensive fibrotic and inflammatory changes, largely mediated by increased oxidative stress, resulting in renal, cardiac, and vascular injury, thus giving angiotensin II a tight link to target-organ injury in hypertension. In contrast, stimulation of the angiotensin II type 2 receptor (AT 2 R) is associated with opposite effects, resulting in vasodilation, natriuresis, and antiproliferative effects.
The relative importance of the renal and vascular effects of angiotensin II was evaluated in classic cross-transplantation studies using both wild-type mice and mice lacking the AT 1 R. , By cross-transplanting the kidneys of wild-type mice into AT 1 R knockout mice and vice versa, investigators were able to generate animals that were selective renal AT 1 R knockouts or selective systemic (nonrenal) AT 1 R knockouts. In physiologic conditions, renal, systemic, and total knockout animals had lower BP than wild-type animals, indicating a role of both renal and extrarenal AT 1 R in BP regulation. The systemic AT 1 R absence was associated with approximately 50% lower aldosterone levels, but the lower BP observed in this group was independent of this lower aldosterone production, as BP remained low despite aldosterone infusions to supraphysiologic levels following adrenalectomy in the systemic knockout animals. In addition, the BP reduction in kidney knockout animals occurred despite normal aldosterone excretion, again confirming the importance of aldosterone-independent renal angiotensin II effects.
When hypertension is present, the presence of renal AT 1 R mediates both hypertension and organ injury. When animals were infused with angiotensin II for 4 weeks, animals lacking renal AT 1 R did not develop sustained hypertension, whereas wild-type and systemic knockout mice had a significant increase in BP. In addition, only animals with elevated BP developed cardiac hypertrophy and fibrosis. This indicates that cardiac injury is largely dependent on hypertension and not on the presence of AT 1 R in the heart, because the (hypertensive) systemic knockout animals developed significant cardiac abnormalities despite the absence of AT 1 R in the heart. In summary, these experiments indicate that both systemic and renal actions of angiotensin II are relevant to physiologic BP regulation, but in hypertension, the detrimental effects of angiotensin II are mediated via its renal effects.
Aldosterone, the adrenocortical hormone synthesized in the zona glomerulosa, plays a critical role in hypertension through its effects on sodium reabsorption largely mediated by transcriptional effects, via activation of the mineralocorticoid receptor, leading to increased expression of the epithelial sodium channel (ENaC). An extensive body of literature has identified other genomic and nongenomic effects of aldosterone with relevance to hypertension. Extensive nonepithelial effects include vascular smooth muscle cell proliferation, vascular extracellular matrix deposition, vascular remodeling and fibrosis, and increased oxidative stress leading to endothelial dysfunction and vasoconstriction.
Several other elements of the RAAS have potentially important roles in hypertension. The importance of ACE2 and angiotensin (1 to 7) to BP regulation and angiotensin II–associated target-organ injury has become apparent. ACE2 is expressed largely in the heart, kidney, and endothelium; it has partial homology to ACE and is unaffected directly by ACE inhibitors (ACEIs). It has a variety of substrates, but its most important action is the conversion of angiotensin II to angiotensin (1 to 7). Angiotensin (1 to 7) is formed primarily though the hydrolysis of angiotensin II by ACE2, and its actions are opposite to those of angiotensin II, including vasodilatory and antiproliferative properties that are mediated by the Mas receptor, a G protein–coupled receptor that, upon activation, forms complexes with the AT 1 R, thus antagonizing the effects of angiotensin II.
The vasodilatory effects are mediated by increased cyclic guanosine monophosphate, decreased norepinephrine release, and amplification of bradykinin effects. Studies have identified ACE2 and angiotensin (1 to 7) as protective factors in the development of atherosclerosis and cardiac and renal injury, , and administration of recombinant ACE2 or its activator, xanthenone, has resulted in improved endothelial function, decreased BP, and improved renal, cardiac, and perivascular fibrosis in hypertensive animals. However, a phase 1 study of recombinant ACE2 in healthy humans did not show any BP-lowering effects despite appropriate modulation of the RAAS, including sustained increase in angiotensin (1 to 7) levels. Therefore, the clinical value of the manipulation of any of the elements of this vasodepressor component of the RAAS remains to be determined.
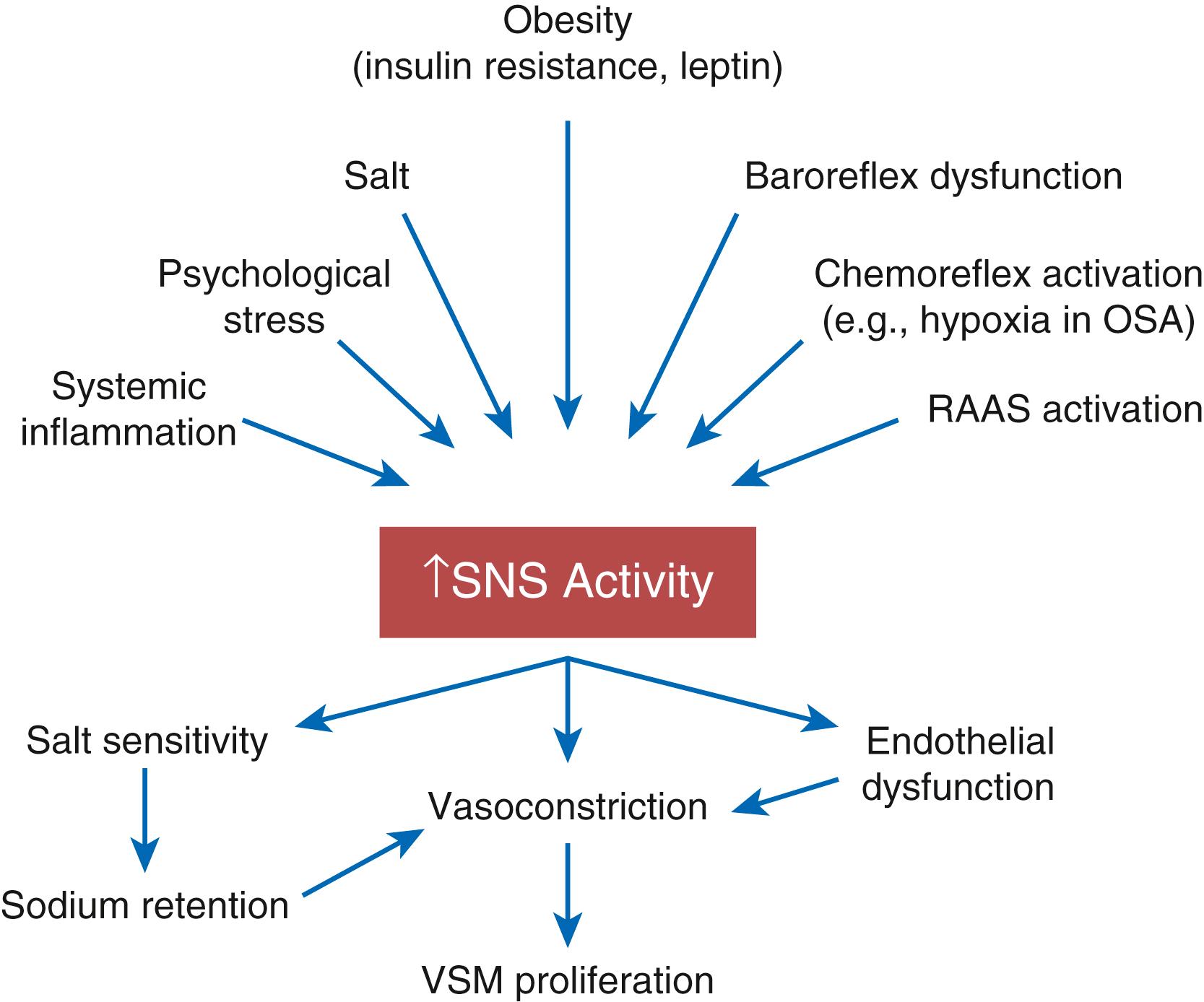
The sympathetic nervous system (SNS) is consistently activated in patients with hypertension compared with normotensive individuals, particularly in the obese. Many patients with hypertension are in a state of autonomic imbalance that encompasses increased sympathetic and decreased parasympathetic activity. , SNS hyperactivity is relevant to both the generation and maintenance of hypertension and is observed in human hypertension from the very earliest stages. Studies in humans have identified markers of sympathetic overactivity in normotensive individuals with a family history of hypertension. Among patients with hypertension, increasing severity of hypertension is associated with increasing levels of sympathetic activity measured by microneurography. , In human hypertension, plasma catecholamine levels, microneurographic recordings, and systemic catecholamine spillover studies have consistently found elevation of these markers in obesity, the metabolic syndrome, and hypertension complicated by heart failure or kidney disease. In addition, SNS hyperactivity is observed in most hypertensive subgroups, although it appears more pronounced in men than in women, and in younger than in older patients.
Several experimental models have outlined the importance of the SNS in generating hypertension. Different models of obesity-related hypertension indicate that the SNS is activated early in the development of increased adiposity, and the key factor in the maintenance of sustained hypertension is increased renal sympathetic nerve activity and its attendant sodium avidity.
SNS-mediated induction of salt sensitivity is a key element to sustaining high BP in other models of hypertension as well. For instance, rats receiving daily infusions of phenylephrine for 8 weeks developed hypertension during the infusions, but BP normalized under a low-salt diet after discontinuation of phenylephrine. However, once exposed to a high-salt diet, the animals again became hypertensive. The degree of BP elevation on a high-salt diet was directly related to the degree of renal tubulointerstitial fibrosis and decrement of GFR. These findings can be interpreted within the paradigm that catecholamine-induced hypertension causes renal interstitial injury that associates with a salt-sensitive phenotype even after sympathetic overactivity is no longer present. In addition, enhanced SNS activity results in α 1 -receptor–mediated endothelial dysfunction, vasoconstriction, vascular smooth muscle proliferation, and arterial stiffness, all of which contribute to the development of hypertension. Finally, evidence indicates that sympathetic overactivity results in salt sensitivity due to a reduction in the activity of serine/threonine-protein kinase WNK4. This results in increased sodium avidity through the thiazide-sensitive sodium chloride symporter NCC. Figure 26.8 summarizes the causes and consequences of SNS activation in the genesis of hypertension.
In a meta-analysis of hypertension trials, heart rate reduction during treatment with beta blockers was paradoxically associated with increased risk for death and CV events in patients with hypertension. In contrast, in a very large ( n = 10,000) patient outcome trial, a post hoc analysis of heart rate at baseline demonstrated that those with a resting heart rate above 80 beats/min even with a BP below 140/90 mm Hg had a higher mortality rate. Therefore, although apparent that SNS activation is deleterious to patients with CV disease, and presumably with hypertension, a cause for the overactivity should be sought and an attempt made to affect that mechanism.
Natriuretic peptides (atrial [ANP], brain [BNP], and urodilatin) play an important role in salt sensitivity, heart failure, and hypertension. These peptides have important natriuretic and vasodilatory properties that allow maintenance of sodium balance and BP during sodium loading. Upon administration of a sodium load, atrial and ventricular stretch leads to release of ANP and BNP, respectively, which result in immediate BP lowering due to systemic vasodilation and decreased plasma volume, the latter caused by fluid shifts from the intravascular to the interstitial compartment. All natriuretic peptides directly increase GFR, which in volume-expanded states is mediated by an increase in efferent arteriolar tone and increased filtration coefficient (K f ). Natriuretic peptides also inhibit renal sodium reabsorption through both direct and indirect effects. Direct effects include decreased activity of Na + -ATPase and the sodium-glucose cotransporter in the proximal tubule and inhibition of the ENaC in the distal nephron. The inhibitory effects of natriuretic peptides on renin and aldosterone release mediate indirect effects. Unfortunately, understanding the contribution of natriuretic peptides to the development of hypertension in humans is complicated by the elevation of their levels in association with increased BP (due to increased afterload) and hypertensive heart disease.
Some studies have tested whether polymorphisms in ANP or BNP genes resulting in higher levels of these peptides would be associated with lower BP; results of these studies have been inconsistent, and effects have been small. There are no published studies evaluating sequential changes in natriuretic peptides and risk for incident hypertension.
The endothelium is a major regulator of vascular tone and thus plays a key role in BP regulation. Endothelial cells produce a host of vasoactive substances, of which NO is the most important to BP regulation. NO is continuously released by endothelial cells, especially in response to flow-induced shear stress in arteries and arterioles, leading to vascular smooth muscle relaxation through activation of guanylate cyclase and generation of intracellular cyclic guanosine monophosphate. , Interruption of NO production via inhibition of the constitutively expressed nitric oxide synthase 3 (eNOS) causes BP elevation and development of hypertension in both animals and humans. Using brachial artery flow-mediated vasodilation and measurement of urinary excretion of NO metabolites as methods to evaluate NO activity in humans, several studies have demonstrated decreased whole-body production of NO in patients with hypertension compared with normotensive controls.
Several elements are responsible for endothelial dysfunction in hypertension. Normotensive offspring of patients with hypertension have impaired endothelium-dependent vasodilation despite normal endothelium-independent responses, thus suggesting a genetic component to the development of endothelial dysfunction. In addition to direct pressure-induced injury in the setting of chronically elevated BP, a mechanism of major importance is increased oxidative stress. Reactive oxygen species are generated from enhanced activity of several enzyme systems, reduced nicotinamide adenine dinucleotide phosphate-oxidase (NADPH-oxidase), xanthine oxidase, and cyclooxygenase in particular, and decreased activity of the oxygen free radical detoxifying enzyme superoxide dismutase. ,
Angiotensin II is a major enhancer of vascular NADPH-oxidase activity and plays a central role in the generation of oxidative stress in hypertension, although several other factors are also involved, including cyclic vascular stretch, endothelin-1 (ET-1), uric acid, systemic inflammation, norepinephrine, free fatty acids, and tobacco smoking.
ET-1 is the endothelial cell product that counteracts NO to maintain balance between vasodilation and vasoconstriction. ET-1 expression is increased by shear stress, catecholamines, angiotensin II, hypoxia, and several proinflammatory cytokines such as tumor necrosis factor-α, interleukins 1 and 2, and transforming growth factor-β. ET-1 is a potent vasoconstrictor through stimulation of ET-A receptors in vascular smooth muscle. In hypertension, increased ET-1 levels are not consistently found. However, there is a trend of increased sensitivity to the vasoconstrictor effects of ET-1. ET-1 therefore is considered a relevant mediator of BP elevation because ET-A and ET-B receptor antagonists attenuate or abolish hypertension in several experimental models of hypertension (angiotensin II–mediated models, deoxycorticosterone acetate–salt hypertension, and Dahl salt-sensitive rats) and are effective in lowering BP in humans.
NO is not the only gas relevant to vascular biology. Hydrogen sulfide (H 2 S) has received recent attention due to potential treatment relevance. H 2 S is a vasodilating gas produced from sulfated amino acids by action of one of two key enzymes, cystathione gamma-lyase (CSE) and cystathione beta-synthase. CSE homozygous knockout mice have approximately 80% decreased H 2 S expression in heart and aorta and approximately 60% reduction in serum, and both homozygous and heterozygous animals demonstrate impaired endothelial function and develop age-dependent hypertension. The mechanisms underlying the BP effects of H 2 S are multiple, including enhanced NO-mediated vasodilation, activation of potassium-ATP channels, activation of protein kinase G1α, inhibition of phosphodiesterase type 5, and inhibition of SNS activity. Modulation of H 2 S levels with the administration of gaseous H 2 S or H 2 S donors (sodium hydrosulfide or sodium thiosulfate) results in lower BP and decreased CV and renal injury in several experimental models. If delivery systems permit and successful oral use of these agents are developed, it is possible that H 2 S may become a therapeutic target in hypertension and vascular disease.
Taken together, the net result observed in patients with hypertension is one of endothelial dysfunction. In cross-sectional analyses, the greater the extent of endothelial dysfunction as measured by the lower the degree of forearm flow-mediated vasodilation, the greater the prevalence of hypertension. , Prospective cohort studies have used flow-mediated vasodilation as a measure of endothelial dysfunction (regardless of specific mechanism) to evaluate its relationship with hypertension and test whether endothelial dysfunction is cause or consequence of hypertension, or both. These studies have shown conflicting results, but the larger of them was unable to demonstrate an association between endothelial dysfunction and incident hypertension among 3500 patients followed for 4.8 years. Furthermore, endothelial dysfunction carries a genetic predisposition that is independent of BP and may be improved by agents that have little or no impact on BP (e.g., some antioxidants). Therefore, as it stands, the evidence is stronger for endothelial dysfunction as a consequence, not a cause, of hypertension. ,
Arterial stiffness is an important factor in the pathogenesis of hypertension, particularly the syndrome of isolated systolic hypertension with aging, because it is a common accompaniment of elevated SBP and pulse pressure. High-sodium/lower-potassium diets over time predispose to increased arterial stiffness. Cullin-3 mutations in vascular smooth muscle appear to be responsible for arterial aging. Arterial stiffness develops as a result of structural changes in large arteries, particularly elastic arteries. , These include loss of elastic fibers and substitution with less distensible collagen fibers. Factors strongly associated with arterial stiffening include aging, hypertension, diabetes mellitus, chronic kidney disease (CKD), smoking, and high-sodium intake.
A commonly used measure to assess arterial stiffness in humans is carotid-femoral pulse wave velocity (cf-PWV). The traditional view linking arterial stiffness (measured as increased cf-PWV) to hypertension invoked that faster PWV produced faster reflection of the incident pulse wave, which resulted in an earlier reflected wave that returned to the central circulation before the end of systole, resulting in increased SBP.
What is the clinical importance of arterial stiffness? Increased arterial stiffness predicts the onset of ESRD in adults with polycystic kidney disease. Basically, increased arterial stiffness should be interpreted as a poorer and reduced response of NO to any vasoconstricting stimuli, resulting in more labile hypertension and increased BP variability.
Evidence from several studies indicates that arterial stiffness may precede and predispose to hypertension. For example, in the Framingham Heart Study, markers of arterial stiffness (cf-PWV and amplitude of the forward pressure wave) were associated with a 30% to 60% increased risk for incident hypertension (per standard deviation of each variable) during 7 years of follow-up in a cohort with a baseline mean age of 60 years. Conversely, baseline BP levels did not associate with future changes in arterial stiffness. Certain studies corroborate these findings, but other studies suggest a bidirectional relationship such that arterial stiffness is also a consequence of chronic hypertension. In contraposition, a recent cohort study of younger adults (baseline age 36 years) indicated that higher BP was associated with higher large artery stiffness, not the opposite. These differences in results between younger and older adult populations may indicate that earlier in life, hypertension is mediated by factors that are largely independent of large vessel stiffness, whereas later in life, arterial stiffness has a more important causal role in the development of hypertension.
Arterial stiffening is relevant to target-organ damage in hypertension. Increased PWV is associated with increased mortality and CV events as well as with a variety of subclinical CV injury markers, such as coronary calcification, cerebral white matter lesions, abnormal ankle-brachial index, and albuminuria. A relationship with cardiac complications has been suggested: increased impedance to left ventricular ejection results in left ventricular hypertrophy (LVH), diastolic dysfunction, and subendocardial myocardial ischemia.
Immune responses, both innate and adaptive, participate in several of the mechanisms discussed earlier, including the generation of reactive oxygen species, mediation of the afferent arteriolopathy thought important to maintain salt sensitivity, and participation in the inflammatory changes noted in the kidneys, vessels, and brain in hypertension. , Innate responses, especially those mediated by macrophages, have been linked to hypertension induced by angiotensin II, aldosterone, and NO antagonism. Reductions in macrophage infiltration of the kidney or the periadventitial space of the aorta and medium-sized vessels lead to improvements in BP and salt sensitivity in several experimental models ( Fig. 26.9 ). Adaptive responses via T cells have been linked to the genesis and complications of hypertension. T cells express AT 1 R and mediate angiotensin II–dependent hypertension, as demonstrated by the observations that adoptive transfer of T cells restored the hypertensive phenotype in response to angiotensin II infusion that was absent in mice without lymphocytes. Abnormalities in both proinflammatory T cells and regulatory T cells alike are implicated in complications of hypertension, because they appear to regulate vascular and renal inflammation that underlies target-organ injury.
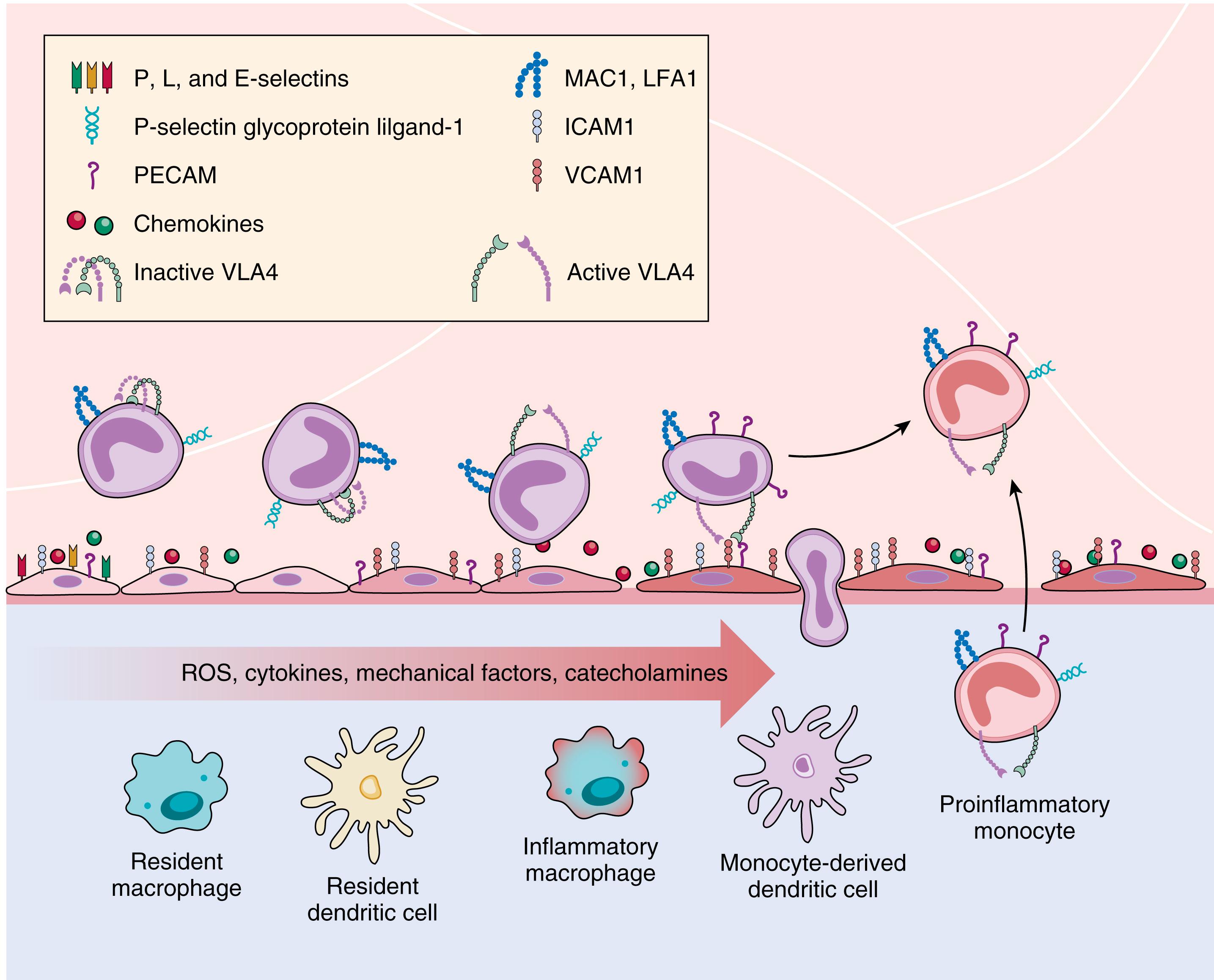
Suppression of these inflammatory responses can improve BP control. , , B lymphocytes may also play a causative role in hypertension as suggested by reports of several autoantibodies, including agonistic antibodies against adrenergic receptors, vascular calcium channels, and AT 1 R, and antibodies against endothelial cells causing endothelial dysfunction, or heat shock proteins (hsp70) causing salt-sensitive hypertension. Further research will determine if manipulation of immune targets is of value in the prevention and treatment of hypertension.
Hypertension clusters in families; an individual with a family history of hypertension has a fourfold greater chance of developing hypertension, and it is estimated that the heritability of hypertension ranges from 31% to 68%. GWASs in several multinational cohorts have identified a large number of SNPs associated with hypertension. However, these individual SNPs are responsible for only minor BP effects (0.5 to 1 mm Hg), and the overall impact of these identified SNPs on the overall BP variance is only approximately 1% to 2%. Among these genes, FOS (fos protooncogene) and PTGS2 (COX-2) have been replicated in a number of studies. The shortcomings of the use of GWAS and other large population approaches are multiple and discussed elsewhere. To advance progress toward personalized medicine in hypertension, a GWAS based on the large United Kingdom Biobank Study cohort performed functional and transcript expression analyses of candidate genes in target tissues in vitro (vascular smooth muscle cells, aortic fibroblasts, and endothelial cells) with the goal of identifying potential therapeutic targets. The study developed and validated an unbiased genetic risk score that included clinical and genotype information including data on a total of 107 independent risk loci to generate estimates of risk of hypertension and risk of specific hypertension-related outcomes (stroke, coronary disease, and any CV outcome). These analyses showed a sex-adjusted SBP difference of 9.3 mm Hg between the lowest and highest risk quintile (higher in the high-risk group, which also had 2.3-fold greater odds of hypertension and a 1.35-fold increase in the odds of any CV outcome). These results indicate the potential value for genetic risk-based clinical scoring.
With the improvement in techniques that allow expeditious, cheaper whole-exome or whole-genome analyses and the expansion of precision medicine, it is possible that greater mechanistic insights on the genetics of hypertension will become available. Unfortunately, compared with other clinical phenotypes, the heavy influence of lifestyle and environmental factors in hypertension makes it unlikely that the simple analysis of genome sequence will have a great impact in hypertension. Further in-depth discussion is beyond this chapter, and the reader is referred elsewhere.
Monogenic causes of hypertension, although quite rare, have provided substantial insight into the pathogenesis of hypertension. Of the monogenic forms of hypertension with well-described molecular mechanisms, all have one thing in common: a defect in renal sodium handling. Table 26.3 summarizes the well-recognized syndromes of familial hypertension.
| Specific Conditions | Possible Causes of Familial Hypertension | Clinical Clues |
|---|---|---|
| Catecholamine-Producing Tumors | ||
| Pheochromocytoma, paraganglioma | Familial cases are responsible for approximately 30% of cases, including MEN2A and MEN2B, von Hippel-Lindau disease, neurofibromatosis, and familial paraganglioma syndromes (SDH complex mutations) | Paroxysmal palpitations, headaches, diaphoresis, pale flushing; syndromic features of any of the associated disorders |
| Neuroblastomas (adrenal); aortic or renovascular lesions | 1%–2% of neuroblastomas are familial | |
| Coarctation of the aorta | Overrepresented in families but no familial distribution | Asymmetry between upper- and lower-extremity BP, radial-formal pulse delay; associated with Turner syndrome, Williams syndrome, and bicuspid aortic valve |
| Renal artery stenosis caused by fibromuscular dysplasia or inherited arterial wall lesions | <10% familial with AD pattern | Abnormal renal vascular imaging results; vascular disease in the carotid territory at an early age; common in neurofibromatosis and Williams syndrome; also present in tuberous sclerosis, Ehlers-Danlos syndrome, and Marfan syndrome |
| Parenchymal kidney disease GN | Alport disease (X-linked, AR, or AD), familial IgA nephropathy (AD with incomplete penetrance) | Proteinuria, hematuria, low eGFR |
| PKD | AD PKD type 1 or 2, AR PKD | Multiple renal cysts (as few as three in patients <30 years) |
| Adrenocortical disease; glucocorticoid-remediable aldosteronism (familial hyperaldosteronism type I) | AD chimeric fusion of the 11β-hydroxylase and aldosterone synthase genes | Cerebral hemorrhages at young age, cerebral aneurysms; mild hypokalemia; high plasma aldosterone, low renin |
| Familial hyperaldosteronism II | AD; unknown defect | Severe type 2 hypertension in early adulthood; high plasma aldosterone, low renin; no response to glucocorticoid treatment |
| Familial hyperaldosteronism type III | AD; unknown defect | Severe hypertension in childhood with extensive target organ damage; high plasma aldosterone, low renin; marked bilateral adrenal enlargement |
| Congenital adrenal hyperplasia | AR mutations in 11β-hydroxylase or 21-hydroxylase | Hirsutism, virilization; hypokalemia and metabolic alkalosis; low plasma aldosterone and renin |
| Monogenic Primary Renal Tubular Defects | ||
| Gordon syndrome | AD mutations of KLHL3 , CUL3 , WNK1 , and WNK4; AR mutations of KLHL3 | Hyperkalemia and metabolic acidosis with normal renal function |
| Liddle syndrome | AD mutations of the epithelial sodium channel | Hypokalemia and metabolic alkalosis; low plasma aldosterone and renin |
| Apparent mineralocorticoid excess | AD mutation in 11β-hydroxysteroid dehydrogenase type 2 | Hypokalemia and metabolic alkalosis; low plasma aldosterone and renin |
| Geller syndrome; hypertension-brachydactyly syndrome | AD mutation in the mineralocorticoid receptor AD mutations in the phosphodiesterase E3A enzyme |
Hypokalemia and metabolic alkalosis; low plasma aldosterone and renin; increased BP during pregnancy or exposure to spironolactone; short fingers (small phalanges) and short stature; brain stem compression from vascular tortuosity in the posterior fossa |
| Essential hypertension | Polygenic | When obesity or metabolic syndrome is present, likelihood of essential hypertension is higher |
Obesity-related hypertension is characterized primarily by impaired sodium excretion and endothelial dysfunction, both of which are dependent on SNS overactivity, activation of the RAAS, and increased oxidative stress. , Fat tissue in obesity is hypertrophied, meaning larger cells versus more cells, and marked by increased macrophage infiltration in the adipose tissue. As it is now well understood, adipose tissue is not inert and secretes a wide variety of cytokines and chemokines with abnormal profiles in obesity, marked by increased levels of leptin, resistin, interleukin-6, and tumor necrosis factor-α secretion, elevated free fatty acid release, and reduced adiponectin level. Decreased adiponectin levels results in insulin resistance, decreased induction of eNOS, and possibly increased sympathetic activity.
Resistin impairs NO synthesis (eNOS inhibition) and enhances ET-1 production, shifting the vasodilation/vasoconstriction balance toward vasoconstriction. Hyperleptinemia directly stimulates the SNS through complex mechanisms that involve central leptin receptors as well as activation of the pro-opiomelanocortin system (via the melanocortin 4 receptor).
Lastly, visceral adipocyte mass is directly correlated with aldosterone secretion by the zona glomerulosa, a process mediated by angiotensinogen production by adipocytes as well as increased secretion of Wnt signaling molecules that modulate steroidogenesis. In addition, despite yet unclear mechanisms, there is consistent evidence that obese individuals tend to have lower natriuretic peptides than lean individuals, and this relative deficiency is amplified in obese hypertensives. All of these factors compound the tendency toward sodium retention and shifting the pressure-natriuresis curve to the right. Activation of these systems leads to a proinflammatory state related to increased reactive oxygen species, factors directly associated with endothelial dysfunction and vascular proliferation. Therefore, multiple mechanisms contribute to the development and maintenance of hypertension in obese individuals.
The evaluation of patients with hypertension focuses on six key components: (1) the confirmation that the patient is indeed hypertensive through careful measurements of BP; (2) an assessment of clinical features that might suggest specific remediable causes of hypertension; (3) the identification of comorbid conditions that confer additional CV risk, or that may impact treatment decisions; (4) the discussion of patient-related lifestyle factors and preferences that will affect management; (5) the systematic evaluation of hypertensive target-organ damage; and (6) shared decision making about the treatment plan. To accomplish this, the clinician often needs multiple visits, a targeted clinical examination, and selected laboratory and imaging tests.
The medical history and physical examination are essential to uncovering possible secondary causes of hypertension, identifying symptoms suggestive of hypertensive target-organ damage, and diagnosing comorbid conditions that may affect treatment decisions. Although the focus is traditionally on the CV, neurologic, and renal systems, a complete review of systems is recommended when the patient is first evaluated, to identify comorbid conditions that may influence the BP. Some patients will present with hypertension because of sleep apnea (snoring, witnessed apneas/gasping), hyperthyroidism or hypothyroidism (each with their litany of possible symptoms), hyperparathyroidism (symptoms of hypercalcemia), Cushing syndrome (symptoms of cortisol excess), pheochromocytoma or paraganglioma (symptoms of catecholamine excess), or acromegaly with its distinctive physical findings. These conditions are discussed in detail later in this chapter.
High BP is typically asymptomatic, but some symptoms are common among patients with very high BP levels, such as headaches, epistaxis, dyspnea, chest pain, and faintness, all of which were present in more than 10% of patients presenting with DBP levels above 120 mm Hg. Other common symptoms include nocturia and unsteady gait, whereas treated patients often complain of fatigue in addition to symptoms related to specific side effects of medications. In patients with lower BP levels, the occurrence of symptoms is often difficult to tie to observed BP, as demonstrated in a study evaluating the relationship between headaches and BP levels, where the frequently observed headaches in patients with hypertension did not correlate well with office or ambulatory BP levels.
When evaluating for target-organ damage, symptoms are elicited that may suggest a previous stroke or transient ischemic attack, previous or ongoing coronary ischemia, heart failure, peripheral arterial disease, or a past history of kidney disease or current symptoms such as hematuria or flank pain.
Obtaining a detailed family history as it pertains to hypertension is essential. Focus should be on the development of hypertension at a young age or clustering of endocrine (pheochromocytoma, multiple endocrine neoplasia [MEN], primary aldosteronism) or renal problems (polycystic kidney disease or any inherited form of kidney disease). The young patient with hypertension and a family history of hypertension poses a particular challenge and should be evaluated in detail. Table 26.3 provides a guide to possible familial causes to be considered.
Knowledge of several conditions with potential relevance to treatment is important. For example, issues related to CV risk management such as diabetes mellitus, hypercholesterolemia, inflammation (C-reactive protein), obesity, and tobacco smoking need to be evaluated. Patients with established CV disease will need treatment for both their hypertension and their underlying disorder (e.g., beta blockers for angina pectoris), so knowledge of specific CV diagnoses is essential. Lastly, some non-CV conditions may have an impact on treatment options. For example, patients with reactive airways disease (asthma) probably should not receive nonspecific beta blockers, patients with prostatic hyperplasia may benefit from a regimen that includes an alpha blocker, and patients with attention-deficit/hyperactivity disorder or anxiety may benefit from a central sympatholytic (e.g., guanfacine), whereas those with major depression should probably not be treated with this drug class.
When obtaining the history, the clinician should explore issues related to lifestyle, cultural beliefs, and patient preferences that will be essential in designing an effective treatment plan. It is important to define dietary and physical activity patterns and, when problems are identified, to determine if the patient is willing and/or able to modify them. Cultural beliefs related to the treatment of hypertension, health illiteracy, and mistrust in physicians and the pharmaceutical industry are several items that can affect the relationship with the patient and that should be openly raised. This is critical for patients to participate in shared decision making about their treatment, an essential tenet of patient-centered care.
The physical examination is designed to complement the items discussed in the history. One should pay attention to syndromic features of cortisol excess (moon face, central obesity, frontal balding, cervical and supraclavicular fat deposits, skin thinning, abdominal striae), hyperthyroidism (tachycardia, anxiety, lid lag/proptosis, hypertelorism, pretibial myxedema), hypothyroidism (bradycardia, coarse facial features, macroglossia, myxedema, hyporeflexia), acromegaly (frontal bossing, widened nose, enlarged jaw, dental separation, acral enlargement, carpal tunnel syndrome), neurofibromatosis (neurofibromas, café au lait spots, as neurofibromatosis is associated with pheochromocytoma and renal artery stenosis), or tuberous sclerosis (hypopigmented ash leaf patches, facial angiofibromas, as tuberous sclerosis is associated with renal hypertension, usually related to angiomyolipomas). Many other even rarer associations exist but fall beyond the scope of this chapter.
A coarctation of the aorta should be considered in younger patients with unexplained, difficult-to-treat hypertension and is evaluated by measurement of BP in both arms and in one thigh. If present, there will be a significantly lower BP in the thigh (typically by more than 30 mm Hg). Sometimes, in case of a lesion proximal to the left subclavian, there may be a significant interarm BP difference, lower on the left. In addition, there is significant decrease in intensity of the femoral pulses and a palpable radial-femoral pulse delay.
A funduscopic examination is recommended to evaluate for vascular changes associated with hypertension, especially if present for a long period of time (i.e., greater than 5 to 10 years). The retinal changes are associated with severity of both acute and chronic BP elevation. Acute changes can happen quite abruptly (hours to days) and range from arteriolar spasm in most patients with uncontrolled BP to retinal infarcts (exudates) and microvascular rupture (flame hemorrhages), to papilledema once the protection afforded by vasoconstriction is overcome. Chronic changes take much longer to develop and include vascular tortuosity (arteriovenous nicking) due to perivascular fibrosis, followed by progressive arteriolar wall thickening that prevents visualization of the blood column, thus leading to the appearance of copper wiring, then silver wiring. Several studies have demonstrated a relationship between severity of hypertensive retinopathy and risk for LVH and stroke.
Although bedside ophthalmoscopy is not commonly performed, it can provide a valuable insight into the vasculature. An important recent development is the availability of smartphone-based technology that allows use of a condensing lens coupled with the smartphone’s camera for video and photography of the retina in place of a conventional ophthalmoscope. In a study of hypertensive patients seen in the emergency room, use of this technology resulted in improved identification of abnormal retinal findings (exudates, hemorrhages, and papilledema) by an observer with very little clinical experience compared with conventional bedside ophthalmoscopy, while requiring about half the time to complete the exam (74 versus 130 seconds). However, one has to purchase this device.
The CV examination focuses on the identification of volume overload (jugular venous distension, lung crackles, edema), cardiac enlargement (deviated cardiac impulse), and the presence of a third or fourth heart sound as markers of impaired left ventricular compliance. Subclinical atherosclerosis can be identified by the presence of bruits over the carotid arteries, as the prevalence of carotid atherosclerosis is increased in patients with hypertension, as well in the abdomen, primarily looking for renal arterial bruits heard over the epigastrium and/or flanks. These bruits are of greater significance if occurring on both systole and diastole. Finally, the detailed palpation of the peripheral pulses of the arms and legs is important to look for signs of peripheral arterial disease.
To wrap up the examination, a focused neurologic examination looks for obvious cranial nerve abnormalities, motor deficits, or speech or gait abnormalities. Any further testing is based on specific symptoms or on focal findings on the screening examination.
Because treatment decisions are based largely on BP levels, accurate BP measurement is essential. Cuff-based brachial BP is the most used method to measure BP, typically in the office setting. Table 26.4 lists the proper method for measuring BP. However, a rapidly growing body of evidence points to the value of out-of-office BP methods, such as 24-hour ambulatory BP monitoring (ABPM) and home BP monitoring, as superior methods to evaluate BP burden and evaluate BP-related risk in patients with hypertension. , Additionally, the most recent guidelines point to much more careful assessment of BP in the office setting. ,
|
|
| Arm circumference (cm) 22–26 27–34 35–44 45–52 |
Usual cuff size Small adult Adult Large adult Adult thigh |
Office BP measurement is the time-honored method for the diagnosis and management of hypertension. It is strongly associated with hypertension-related outcomes based on more than 50 years of observational and clinical trial data. Accordingly, guidance provided to clinicians for the diagnosis and treatment of hypertension by most major guidelines is based on office BP values. The most recent American College of Cardiology (ACC)/American Heart Association (AHA) guidelines on hypertension assessment strongly recommend the following approach for BP assessment in the office setting (see Table 26.4 ).
Because attention to measurement technique is essential, it is important to follow the techniques outlined in Table 26.4 when checking BP. Most patients should have their BP measured in the arm while in the seated position. Once an arm is selected it should always be used for subsequent BP readings. In selected situations, such as malformations, injuries, or extensive vascular disease of the upper extremities, or when comparing BP levels in the upper and lower extremities, it may be necessary to use thigh measurements with an appropriately sized thigh cuff, which should be obtained in the prone position to allow the cuff to be at the level of the heart. Mercury sphygmomanometers are now seldomly available in clinical practice because of environmental concerns. Aneroid and electronic oscillometric manometers are accurate but should have periodic maintenance (every 12 months) to ensure that they are properly calibrated, as well as any time poor function is suspected.
The phenomenon of masked hypertension is defined as a clinical condition in which a patient ’ s office BP level is normal but ambulatory or home BP readings are in the hypertensive range. This phenomenon, the opposite of white-coat hypertension (WCH), would suggest the necessity for measuring out-of-office BP in persons with apparently normal or well-controlled office BP.
When assessing BP on the initial visit, orthostatic BP should be obtained, especially among older patients, in whom it occurs in 8% to 34% of patients. Additionally, people with neurologic disorders such as Parkinson disease and other neurologic disorders such as baroreceptor dysfunction have orthostatic hypotension as a common problem. Some guidelines now provide specific recommendations for measurement of standing BP to screen for orthostatic hypotension in older patients with hypertension, as well as in patients at increased risk for autonomic dysfunction, such as those with diabetes and kidney disease. ,
Orthostatic vital signs (heart rate and BP) are best obtained after at least 5 minutes in the supine position followed by immediate assumption of the standing position, when sequential measurements are taken for up to 3 minutes. The difficulties of following this protocol in a busy clinical practice are recognized, so it is acceptable to compare values in the seated position with those after standing for 1 minute; this approach results in decreased sensitivity for the detection of orthostatic hypotension but is better than no measurement at all. To account for this fact, a fall of 15/7 mm Hg may be used for the definition of orthostatic hypotension when the test is performed using the seated BP as baseline as compared with the generally accepted definition of orthostatic hypotension as a drop in BP of more than 20/10 mm Hg that occurs after 3 minutes of standing.
Office BP measurement is the time-honored method to evaluate hypertension. It is easy to perform and is widely available at low cost. Home BP is also widely available, although accessibility to low-income patients is still a problem despite the availability of low-cost devices. ABPM, on the other hand, is less widely available due to costs and limited reimbursement by third-party payers in the United States. Both home BP monitoring protocols and ABPM include larger numbers of readings, thus decreasing variability and improving reproducibility.
In the past 30 years, ABPM and home BP have become accepted as better markers of hypertensive target-organ damage and adverse clinical outcomes. ABPM has stronger associations with LVH, albuminuria, kidney dysfunction, retinal damage, carotid atherosclerosis, and aortic stiffness than office BP, although this is not consistent among studies. Likewise, home BP is a better marker than office BP for LVH and proteinuria, though it is not consistently superior for other measures of target-organ damage.
In the assessment of hard CV endpoints, out-of-office BP has consistently outperformed office BP in studies that account for the values observed in the office; in other words, no matter what the office BP, it is the out-of-office BP that decisively drives outcomes. In a systematic review by the National Institute for Health and Care Excellence (NICE) clinical guidelines group in the United Kingdom of nine cohort studies comparing ABPM with office BP; ABPM was superior in eight and equal to office BP in one of the studies. For home BP, three studies compared similarly with office BP; home BP was superior in two and equal in one. Lastly, two studies compared ABPM, home BP, and office BP; of these, one showed superiority of both ABPM and home BP, while the other study did not show differences among any of the three methods.
In meta-analyses of studies that evaluated both office and ABPM on outcomes, only ABPM values retained significance and was useful in masked hypertension. , Likewise, in the largest home BP cohort study that included simultaneous use of office and home BP to predict CV events and mortality, only home BP remained significantly associated with adverse outcomes. Similar observations of the superior prognostic performance of out-of-office methods exist for patients with resistant hypertension, CKD, hemodialysis, and the general population.
In summary, evidence from prospective cohort studies convincingly demonstrates the superiority of out-of-office BP measurements as predictors of hypertension outcomes. Treated patients with hypertension who retain a white coat effect have the same overall risk as treated patients whose BP was controlled both in the office and at home. Moreover, an updated meta-analysis of 14 observational ABPM studies revealed an increase in risk of CV events and CV mortality among WCH patients compared with normotensive controls, but no statistically significant increase in stroke or all-cause death. As in previous analyses, sustained normotension was associated with lower risk. ,
Because WCH and masked hypertension afflict a substantial number of patients and have diametrically different impact on outcomes, their identification improves the outcome prediction in patients with hypertension.
The ability to evaluate BP during sleep was a characteristic until now restricted to ABPM, although newer home BP monitors can be programmed for activation during sleep. In some, but not all studies, nighttime BP is a better marker of CV disease than daytime or 24-hour-average BP. The importance of nighttime BP (compared with daytime levels) appears greater among treated patients, perhaps because antihypertensive treatment, often taken in the morning, might result in better BP control during the day than during the night.
The pattern of BP fluctuation between day and night also associates with prognosis. The normal circadian BP pattern includes a fall in BP of approximately 15% to 20% during sleep. Patients who lack this normal BP dip during sleep are called “nondippers” (arbitrarily defined as a sleep BP that falls by less than 10% compared with awake levels) and have increased target-organ damage and overall CV risk. In large observational studies, patients whose SBP falls by 20% or more during the night have lower fatal and nonfatal CV event rates than those whose BP decreases by less than 20%, whereas those whose BP does not fall at all during the night have significantly worse CV outcomes than all other patients.
ABPM also provides information on BP variability throughout the day. This may add further prognostic information. Increased BP variability (measured as the standard deviation of BP) has been associated with increased event rates, although these findings are of small magnitude when taken independently from BP values.
Despite these observations, objective evidence demonstrating that outcomes are better when patients are managed using an out-of-office method is lacking. Three randomized clinical trials have compared management of hypertension with office or out-of-office BP, one using 24-hour ABPM and two using home BP. All of these studies showed that more patients managed with out-of-office methods could have treatment stopped or de-escalated, thus resulting in marginal cost savings. However, none of them could demonstrate the superiority of ABPM or home BP in achieving better BP control (the primary outcome of all three trials) or less LVH (evaluated in all studies as a secondary outcome).
ABPM has been in clinical use for almost 50 years. In the United States, problems related to limited reimbursement have significantly limited its expansion compared with other parts of the world. Despite this limitation, there is general agreement on its value in several clinical circumstances. ,
Although not feasible in many clinical settings, 24-hour ABPM is recommended for all newly diagnosed individuals with hypertension to eliminate the diagnosis of WCH or masked hypertension and to evaluate dipping status while sleeping. ,
ABPM is performed, typically, over a period of 24 hours, although it can be extended for longer periods (e.g., 48 hours) to provide information covering more than one wake/sleep cycle, or to cover a specific period in detail, such as a 2-day interdialytic period for a patient undergoing hemodialysis. Clinicians should use an independently validated monitor (for a list, refer to www.dableducational.org ). A typical measurement interval is every 20 minutes during the daytime (7 am to 11 pm ) and every 30 minutes at night (11 pm to 7 am ), although the frequency and time windows can be adjusted based on clinical needs, such as the need to identify frequent BP swings, atypical sleep patterns, etc. Patients should keep a log of activities during the day, the time of retiring to bed and waking up, and time of taking vasoactive medications (if applicable). It is preferred that the periods designated as “night” and “day” reflect the actual periods of sleep and wakefulness obtained from the patient ’ s diary. Most patients tolerate the procedure well, although sometimes sleep is compromised (<10% of cases), and, rarely, patients have excessive bruising or discomfort from the frequent cuff inflations. Instructions on how to perform and interpret ABPM studies are available in guideline format from the European Society of Hypertension and the ACC/AHA Blood Pressure Guidelines. ,
Home BP is performed by the patient in the home (or sometimes work) environment. It is used commonly in clinical practice and is associated with improved adherence to therapy. It has been used successfully for self-titration of BP medications and is amenable to telemedicine approaches, in which the patient can upload BP values via telephone or direct entry to a Web server so that clinicians can inspect the BP logs and make treatment decisions remotely.
Just as with office BP, it is important that the equipment fits the patient’s arm well and that measurements are obtained using the same technique as outlined earlier for office BP. Independently validated devices are listed at www.dableducational.org ; unfortunately, many of the marketed devices have not been independently validated. The preferred devices use arm cuffs. Finger cuffs are inaccurate and should not be used . Wrist cuffs often provide incorrect readings because of inappropriate technique but, if used correctly, can be convenient and accurate, and particularly useful in obese patients.
Smartphone applications are often marketed to obtain biologic information from users, including BP, and it is likely that in the near future their use will become important in the care of hypertensive patients. However, currently, none of the available technologies has been adequately validated, and a clinical validation study of the best sold BP application (“Instant Blood Pressure” [IBP]) showed wildly unreliable performance, leading to immediate removal of the application from the market upon publication of the article.
To allow management decisions, home BP monitoring is best performed using specific periods of monitoring. For most patients, a BP log obtained over 7 days before each office visit suffices because it retains excellent reproducibility. We recommend that the patient obtain readings in duplicate (approximately 1 minute apart), twice daily (in the morning before taking medications and in the evening before dinner). In selected situations, more frequent or more prolonged monitoring may be needed. For example, patients with hypotensive symptoms may benefit from BP measurements during peak action of medications, such as in the mid-to late morning or late evening, depending on the time when medications are taken. Likewise, patients with labile BP can be monitored more often to capture the overall BP variability, although ABPM is preferred in such patients. As for ABPM, detailed home BP guidelines are available from the European Society of Hypertension and the ACC/AHA.
Normative values for the interpretation of ABPM and home BP results are available based on observed outcomes in longitudinal studies. For ease of use, these thresholds were matched to specific office BP levels at which the observed rate of CV events was the same, thus allowing clinicians to relate to office values that have historically driven clinical decisions. For ABPM, other measures such as the nocturnal dip, early morning surge (magnitude of BP rise during the first hours post awakening), BP load (percentage of time BP remains above a certain threshold, such as 140/90 mm Hg during the day and 120/80 mm Hg during the night), and overall BP variability (standard deviation of the 24-hour BP or awake BP), were not studied in relationship to hard outcomes for precise normative results.
All current hypertension guidelines recognize the value of out-of-office BP in the diagnosis of hypertension, while the UK NICE guidelines, the US Preventive Services Taskforce, and the ACC/AHA guidelines formally recommend its use to confirm the diagnosis of hypertension in patients with elevated office BP prior to initiating treatment.
The ACC/AHA guidelines recommend the use of out-of-office BP to evaluate patients who are receiving treatment for hypertension but remain above goal in the office, with the explicit caveat that the recommendation is based on expert opinion. The high prevalence (approximately 40% to 51%) of a white coat effect in patients with resistant hypertension supports this recommendation. Patients with office BP above 160/100 mm Hg do not need further confirmation of hypertension and should be treated.
Similar to the history and physical examination, laboratory tests, imaging, and other complementary tests focus on the evaluation of comorbid conditions, established target-organ damage, and possible secondary causes. In the absence of worrisome signs or symptoms during the initial evaluation, a basic set of tests include renal function; electrolytes, calcium, glucose, and hemoglobin; a lipid profile; urinalysis; and an electrocardiogram.
Further testing may be required if any of these initial test results are abnormal or if specific symptoms or physical findings suggest a diagnosis (see Secondary Hypertension section). Patients who are resistant to treatment during follow-up have higher rates of secondary causes of hypertension, in particular sleep apnea, hyperaldosteronism, and renovascular disease, thus deserving a more dedicated search for secondary causes in their evaluation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here