Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Several inflammatory, infectious, and neoplastic disorders can present with nonspecific sinonasal symptoms requiring a high index of suspicion for timely diagnosis by the otorhinolaryngologist.
Granulomatosis with polyangiitis (GPA) frequently presents with sinonasal involvement, including crusting, bloody discharge, septal perforation, saddle nose deformity, and epiphora.
Sarcoidosis can manifest with various head and neck manifestations, including nasal subcutaneous nodules, lupus pernio, supraglottic nodules, and salivary gland enlargement.
Eosinophilic granulomatous with polyangiitis is characterized by a necrotizing eosinophilic vasculitis affecting small vessels that can present with chronic rhinosinusitis, nasal polyposis, and asthma.
Cocaine-induced midline destructive lesion presents with chronic nasal obstruction, hyposmia, epistaxis, and severe facial pain and shares histologic features with GPA.
Extranodal natural killer/T-cell lymphoma is a rare clinicopathologic entity characterized by a rapidly progressive process with the potential for massive destruction of midfacial structures. It requires timely diagnosis with definitive management with radiotherapy with or without chemotherapy.
Cholesterol granuloma is a benign expansile cystic lesion most commonly involving the frontal sinus and may be associated with prior trauma or surgery.
A wide array of inflammatory, infectious, and neoplastic disorders can present with sinonasal involvement ( Table 51.1 ). Given that the presentation may be initially indolent with nonspecific sinonasal symptoms, a high index of suspicion is required to arrive at a timely and accurate diagnosis to prevent serious patient sequela. The following list presents some potential clinical scenarios that should alert the otorhinolaryngologist that a more careful patient investigation may be required to rule out an underlying systemic disease presenting with sinonasal symptoms.
Chronic rhinosinusitis (CRS) with significant crusting and bleeding.
Unexplained septal perforation and/or tissue loss.
Atypical nasal or sinus mass.
Extrasinus manifestations.
Systemic symptoms (fevers, joint pains, etc.) with CRS.
| I nflammatory | ||
| Rheumatologic | Granulomatosis with polyangiitis | |
| Sarcoidosis | ||
| Eosinophilic granulomatosis with polyangiitis | ||
| Other | Cholesterol granuloma | |
| N eoplastic | ||
| Lymphomas | T-cell lymphoma | |
| B-cell lymphoma | ||
| I nfectious | ||
| Bacterial | Tuberculosis | Mycobacterium tuberculosis |
| Leprosy | Mycobacterium leprae | |
| Rhinoscleroma | Klebsiella rhinoscleromatis | |
| Syphilis | Treponema pallidum | |
| Actinomycosis | Actinomyces israelii | |
| Fungal | Aspergillus | Aspergillus fumigatus, flavus, niger |
| Zygomycosis | Rhizopus | |
| Rhinosporidiosis | Rhinosporidium seeberi | |
| Blastomycosis | Blastomyces dermatitidis | |
| Histoplasmosis | Histoplasma capsulatum | |
| Sporotrichosis | Sporotrichum schenckii | |
| Coccidioidomycosis | Coccidioides immitis | |
| Protozoa | Leishmaniasis | Leishmania |
| M iscellaneous | Cocaine-induced midline destructive lesion | |
| Intranasal acetaminophen/opioid abuse | ||
The first case of granulomatosis with polyangiitis (GPA) was reported by Klinger in 1931, though Friedrich Wegener is often credited with discovering the disease in 1936. Carrington described the more limited form in 1966 in 16 patients with extensive pulmonary lesions, including extrapulmonary lesions in 7 of these cases. GPA is defined with two key features: granulomatous inflammation involving the upper and lower respiratory tract and necrotizing vasculitis affecting small- to medium-sized vessels (e.g., capillaries, venules, and arteries). The exact pathophysiology of GPA is unknown but likely represents a complex, immune-mediated disorder resulting from the interplay of an initiating inflammatory event and a highly specific immune response. Part of this response is directed against previously shielded epitopes of neutrophil granule proteins, leading to high-titer autoantibodies known as antineutrophil cytoplasmic autoantibodies (ANCA).
The prevalence of GPA is approximately 3 per 100,000 in the United States. There is no sex predilection, and the mean age at time of diagnosis is 40 to 55 years, although the range can be wide. Most of those affected are Caucasians (30% to 97%). GPA typically presents as a multisystem disease process characterized by otorhinolaryngologic, pulmonary, and renal involvement (the ear, lung, and kidney, or ELK, triad). Pulmonary involvement, including alveolar, bronchial, or pleural extension, is noted in 94% of cases. Renal involvement is seen in 10% to 85% of cases, most commonly presenting as necrotizing glomerulonephritis. However, GPA can present as more limited disease with single organ system involvement. These patients present at an earlier age, often have longer disease duration with more frequent exacerbations, and a higher rate of destructive upper airway disease.
Overall, 63% of patients present with ENT manifestations, including rhinologic, otologic, and laryngeal (subglottic)/pharyngeal in 41%, 16%, and 6%, respectively. Previous work has demonstrated that patients undergoing rhinologic assessment have sinonasal involvement in 89% of cases. Presenting symptoms include crusting, nasal obstruction, bloody discharge, and epiphora in 69%, 58%, 52%, and 13% of patients, respectively. At the time of presentation, 61% met the diagnostic criteria for CRS, and septal perforation and saddle nose deformity were present in 33% and 23%, respectively. Endoscopic evaluation commonly demonstrates crusting, mucosal hyperemia, and ulceration ( Fig. 51.1 ). Over time, chronic changes cause extensive scarring of the nasal airway or destruction of the paranasal sinus structures resulting in a “common cavity” configuration ( Fig. 51.2 ).
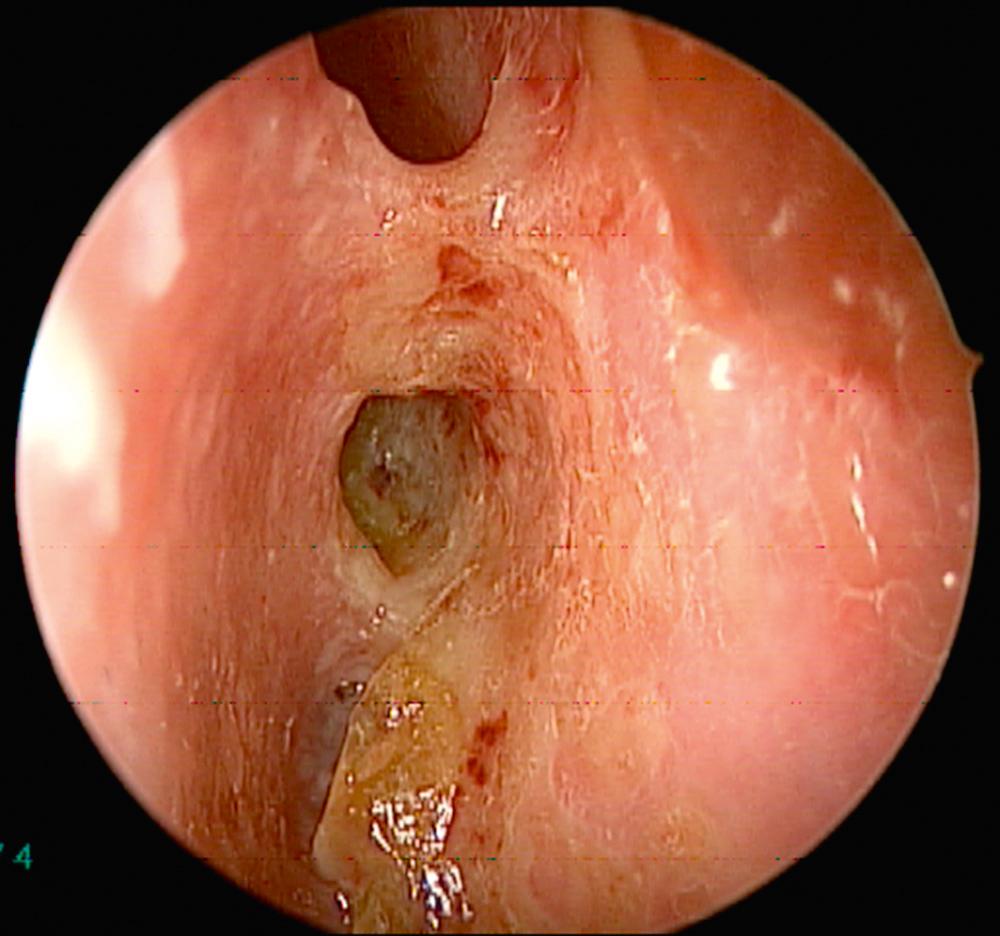
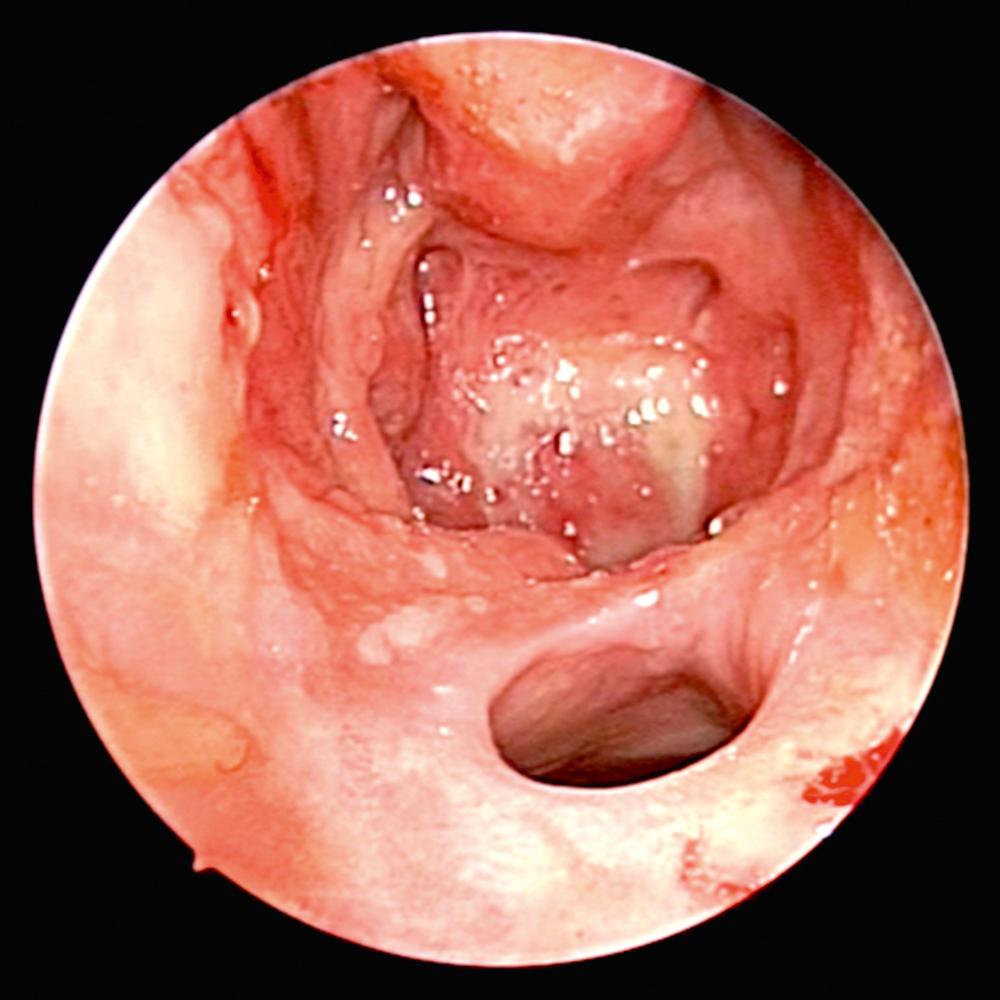
Diagnosis of GPA is based on both clinical and laboratory data outlined by the American College of Rheumatology. The laboratory workup includes urinalysis, complete blood count, metabolic panel, erythrocyte sedimentation rate (ESR), and serum markers of GPA (cytoplasmic-ANCA [c-ANCA] and perinuclear-ANCA [p-ANCA]). The c-ANCA test has high specificity and sensitivity in systemic GPA, but is less helpful in localized forms of the disease. Positive c-ANCA is seen in active and localized forms in 95% and 60% over the course of the illness, respectively. Furthermore, it is recognized that approximately 10% can be positive for p-ANCA. Otorhinolaryngologic findings can be supportive but not pathognomonic for the disease. Computed tomography (CT) imaging of the sinuses may demonstrate extensive destruction of normal paranasal structures with active osteoneogenesis and bony erosion. The role of nasal biopsy is limited, and often leads to low yield in support of the diagnosis of GPA, with one study reporting only 12 of 51 biopsies (24%) demonstrating histologic findings suggestive of GPA.
Prior to the introduction of immunosuppression, mean survival among patients with untreated active GPA was less than 6 months, and more than 80% of patients died within 3 years of onset of symptoms. Management is divided into two phases, an induction phase and a remission maintenance phase. The aim of induction treatment is to rapidly reduce inflammation to control signs and symptoms of disease and prevent permanent tissue damage. In the remission maintenance phase, lower-dose immunosuppression is used to prevent relapse. Agents used for the induction phase include methotrexate (minor disease only), cyclophosphamide, or rituximab, in conjunction with systemic steroids. Maintenance phase agents include azathioprine, methotrexate, or rituximab. With treatment, 85% to 90% of patients will go into remission, but the disease follows a relapsing-remitting course, with a 50% relapse rate within 5 years.
Sinonasal involvement portends worse overall quality of life in patients with GPA; therefore active management of sinonasal symptoms is imperative. Medical treatment with topical therapies is the mainstay of long-term management, including saline irrigations, antibiotic irrigations, and topical nasal steroids. Culture-directed antibiotics for defined infectious exacerbations can also help control symptoms. Staphylococcus aureus colonization has been shown to be an independent risk factor for relapses, and co-trimoxazole prophylaxis has been demonstrated to reduce the number of respiratory tract infections. Given the high prevalence of CT abnormalities during active or quiescent GPA, the mere presence of radiographic changes is not an indication for surgery. Endoscopic sinus surgery should only be considered in exceptional cases after exhaustive medical measures to potentially address severely symptomatic CRS, expanding mucoceles, and epiphora/recurrent dacryocystitis. Surgery can be a significant challenge for many reasons, including drastic alterations due to previous surgery, extensive scarring due to GPA, and risk of activation of the underlying systemic disease. Careful counseling is crucial prior to embarking on sinus surgery as “cures” are uncommon, and revision surgery may be required for recurrent symptoms. Nasal reconstruction may be considered after the disease has been inactive for at least a 6- to 12-month period.
Sarcoidosis is a systemic granulomatous disease that can affect multiple major organ systems, including the lungs, liver, muscles, bones, kidneys, and central nervous system. The disease can also involve head and neck sites at any stage; commonly affected sites include the salivary glands, larynx, nose and sinuses, orbit, ear, and lymph nodes. The exact etiology of sarcoidosis is unknown. Most studies suggest that it results from an exaggerated immune response in genetically susceptible individuals to an undefined antigen, such as certain environmental factors, microbes (e.g., Mycobacterium tuberculosis and Propionibacterium acnes ), or partially degraded antigens. Furthermore, there is strong evidence of a genetic predisposition to developing sarcoidosis. The classic histopathologic finding on biopsy is noncaseating epithelioid granuloma formation. However, definitive diagnosis requires exclusion of diseases with similar pathologic findings, such as tuberculosis, fungal infection, malignancy, and vasculitis.
Sarcoidosis afflicts approximately 6 per 100,000 people in the United States, typically adults between 20 and 40 years of age. There is a predilection for women, with higher prevalence among African Americans and Hispanics. Many patients may be asymptomatic at the time of diagnosis. Lungs are the most commonly affected organ system in 90% of cases, while there is variable involvement of mediastinal lymph nodes (95% to 98%), liver (50% to 80%), spleen (40% to 80%), eyes (20% to 50%), and musculoskeletal region (25% to 39%). Sinonasal symptoms are common, with 61% of patients reporting nasal symptoms by careful history. The most common presenting ear, nose, and throat (ENT) symptom is nasal obstruction, followed by crusting, dysphagia, hoarseness, and epistaxis. Nasal endoscopic findings include nasal crusting, mucosal thickening, and submucosal nodules. The nasal mucosa is described as having a “strawberry skin” appearance due the granulomas on an erythematous background ( Fig. 51.3 ). Other head and neck manifestations include lupus pernio, supraglottic nodules, and salivary gland enlargement. Septal perforation and saddle nose deformity, although less common than GPA, can also be seen in sarcoidosis, together with infiltration of the nasal bones forming a soft tissue mass.
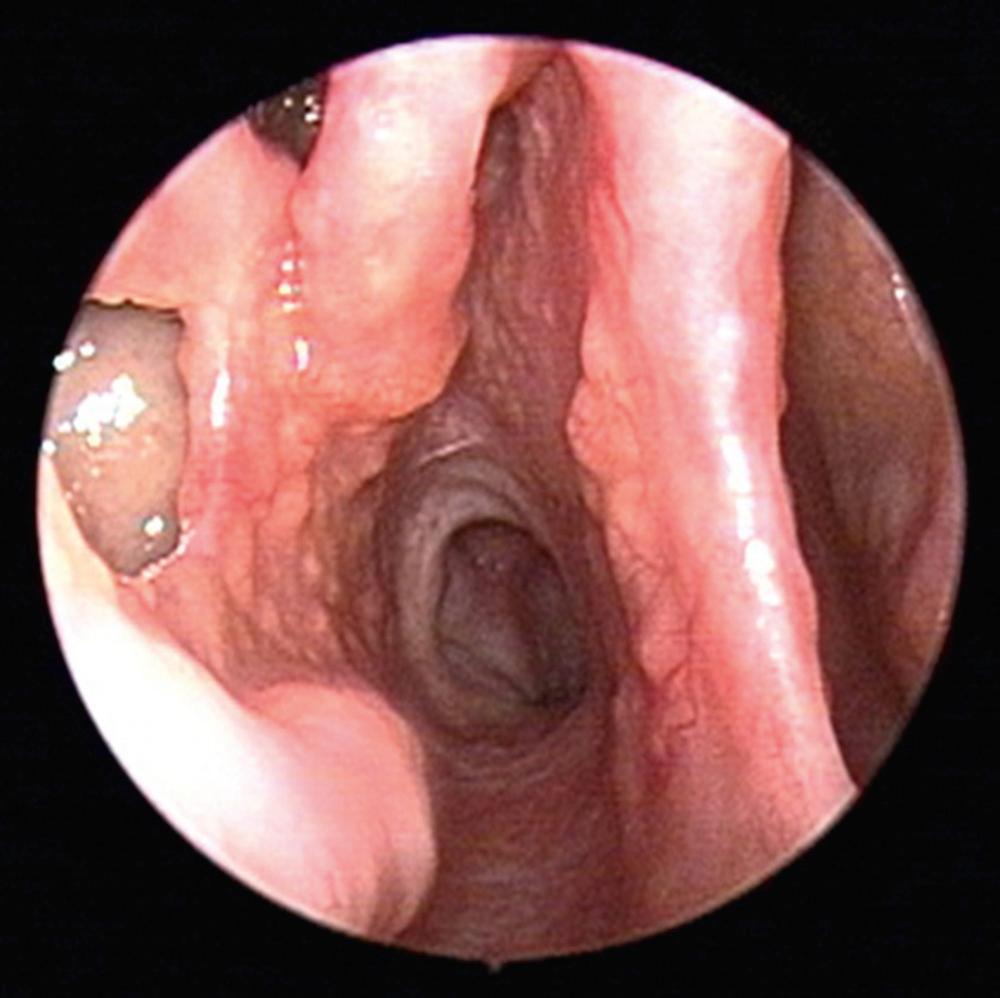
Typically, sinonasal involvement in sarcoidosis presents as a component of the multisystem disease process. Workup includes a thorough history and physical examination, peripheral blood counts, serum chemistries, urinalysis, chest x-ray, pulmonary function tests, ophthalmologic exam, and tuberculin skin test. The angiotensin converting enzyme (ACE) level is elevated in 80% of cases. Directed biopsies of subcutaneous nasal nodules often demonstrate a high yield for noncaseating granulomas, further supporting diagnosis of sarcoidosis.
Treatment is not required for asymptomatic or early-stage sarcoidosis. Systemic corticosteroid therapy remains the mainstay of therapy for those with significantly symptomatic pulmonary disease or serious extrapulmonary disease. The exact duration of treatment depends on the clinical response and disease activity, factoring in long-term complications of systemic steroids. Long-term management of sinonasal sarcoidosis is best performed with topical therapies. Saline irrigations and topical nasal steroids clear mucus and crusting and reduce nasal inflammation, respectively. Sinus surgery is reserved for selected cases only, typically directed by symptoms resulting from obstruction by sarcoid deposits, mucocele formation, or extension into contiguous structures like the orbit.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here