Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The systemic circulation carries blood from the systemic ventricle through a network of arteries and arterioles to the tissue capillaries and drains it via the systemic venous system to the systemic venous atrium.
The systemic arterial system serves two important functions. First, it acts as a low-resistance conduit through which blood is distributed to different parts of body. Second, the arterial tree buffers the pulsatile pressure to convert the systemic ventricular pulsatile blood into a steady stream of capillary flow. Additionally, the endothelium, which lines the vascular lumen, exerts important vascular homeostatic effects through the production of a variety of substances. Hence alterations of the mechanical properties of the arterial wall and function of the endothelium have significant implications for normal functioning of the systemic arterial system and in the development of cardiovascular disease. Furthermore, optimal performance of the systemic ventricle depends on its favorable interaction with the systemic circulation. In the setting of congenital heart disease, the systemic ventricle may be a morphologic left ventricle, morphologic right ventricle, or single functional ventricular chamber of right, left, or indeterminate morphology.
This chapter discusses the systemic circulation from the structural, physiologic, and mechanical perspectives. Assessment of arterial function and structure and pediatric conditions associated with systemic arterial dysfunction are highlighted. Finally, the concept of ventriculoarterial interactions and their relevance in congenital and acquired heart disease in the young is described. The systemic venous system is discussed in Chapter 28 .
The systemic arterial tree begins with the aorta, which ramifies into tributaries to perfuse all parts of body with the exception of hair, nails, epidermis, cartilages, and cornea. The large central arteries are protected within the thoracic and abdominal cavities, while peripheral conduit arteries run along the flexor surfaces in the upper and lower limbs, where they are less exposed to injury. The ascending aorta arises at the base of the left ventricle and gives off its first branches, the right and left coronary arteries. It continues as the aortic arch, from which the brachiocephalic, left common carotid, and left subclavian arteries arise. The thoracic descending aorta begins as a continuation of the aortic arch and penetrates the diaphragm to continue as the abdominal descending aorta. The celiac trunk and superior and inferior mesenteric arteries arise from the abdominal descending aorta to supply the liver and gastrointestinal tract, while the renal arteries branch off at right angles to perfuse the kidneys. The descending aorta bifurcates at its distal end into the right and left common iliac arteries, the latter bifurcating into the internal iliac artery to supply the pelvic organs and the external iliac artery, which continues as the femoral artery to supply the lower limbs. The aorta tapers from its origin to its termination at the iliac bifurcation, and branched daughter vessels are always narrower than the parent vessel, which has implications on wave reflection. The arterial ramifications end in arterioles, which then usually continue as capillaries. Beyond the major arterial branches, the total cross-sectional area increases progressively to the capillary bed. The proportion of cellular and structural components also varies along the arterial tree. Nevertheless, the arterial wall is made up of three constant layers: an internal tunica intima, a tunica media, and an external tunica adventitia.
The intima comprises the endothelium, a subendothelial layer, and an elastic membrane. The endothelium consists of a monolayer of cells that line the vascular lumen. Apart from forming a physical barrier between the circulating blood components and the vascular wall, the endothelial cells play a pivotal role in vascular homeostasis. The subendothelial layer is made up of fibroblasts and variable amount of collagen. The internal elastic membrane consists of a network of elastic fibers and forms a boundary with the media.
The media, usually the thickest layer of the arterial wall, is responsible for the mechanical properties of the vessel. Its structural components are vascular smooth muscle cells and extracellular matrix, the latter consisting of elastic lamellae, collagen fibers, structural glycoproteins, and ground substance. Vascular smooth muscle cells maintain vascular tone through contraction and relaxation, while the extracellular matrix of the media provides a structural framework for optimal functioning of the blood vessels.
The elastic fibers in the media, arranged in concentric lamellae that form the boundaries between layers of vascular smooth muscle cells, are 90% composed of elastin. Cross-linking of elastin confers elasticity to the arteries. In addition, elastin has been implicated in the control of proliferation and phenotype of smooth muscle cells. Elastin has an estimated half-life of more than 40 years in humans; its rate of synthesis is thought to be negligible in adulthood. Elastin, damaged by degenerative and pathologic processes, is unlikely to be replaced. Other constituents of elastic fibers include microfibrillar-associated glycoproteins and fibrillin. Fibrillin forms a microfibrillar network that serves as scaffolding for the deposition of elastin and assembly of elastic fibers. Fibulin-5, through its interactions with elastin and integrins, plays a critical role during elastic fiber development and is a potential therapeutic agent for the treatment of elastinopathies. Other structural glycoproteins in the arterial wall include fibronectin, vitronectin, laminin, entactin/nidogen, tenascin, and thrombospondin.
Collagens are composed of three polypeptide α chains arranged to form a triple helix, which confers tensile strength to the vessel wall. Types I and III collagen are the major fibrillar collagens in blood vessels, constituting about 90% of vascular collagens. Collagen is the stiffest component of the arterial wall, with an elastic modulus of 10 8 to 10 9 dyne/cm 2 . By contrast, the elastic modulus of elastin is in the order of 10 6 dyne/cm 2 . Hence the absolute and relative quantities of elastin and collagen contribute significantly to the stiffness of the arterial wall. Elasticity of the arterial wall is a nonlinear function of transmural pressure. Proposed models of this nonlinear function take into account the contribution of vascular smooth muscle cells, viscoelastic properties of the matrix proteins, residual stresses due to growth and remodeling, and gradual recruitment of collagen fibers with increasing pressure.
The ground substance is filled by proteoglycans. Proteoglycans are macromolecules that possess one or more linear glycosaminoglycan chains linked to a core protein. The proteoglycans in the vessel wall are hyaluronan, versican, biglycan, decorin, lumican, syndecans, fibroglycan, and glypican. The proteoglycans have diverse roles in the organization of connective tissue structure, regulating cellular activities and metabolism, permeability, filtration, and hydration, and controlling cytokine bioavailability and stability. Matrix metalloproteinases play a fundamental role in the degradation of vascular extracellular matrix during physiologic and pathologic vascular remodeling.
The distribution of structural components within the media varies along the arterial tree. With increasing distance from the heart, the elastin-to-collagen ratio falls and smooth muscle cells increase. Alterations of structural components of the media as a result of degeneration, genetic mutations, or imbalance between the synthesis and degradation of extracellular matrix have a significant impact on the mechanical properties of the vessels.
The adventitia contains mainly fibroblasts and collagen fibers and some elastic fibers. It contributes also to the elastic properties of arteries. Nutrient vessels, vasa vasorum, arise from a branch of the artery or from a neighboring vessel to ramify and distribute to the adventitial layer.
The endothelium comprises a monolayer of endothelial cells lining the vascular lumen. It is strategically located between circulating blood components and vascular smooth muscle cells to exert a pivotal role in vascular homeostasis. By producing a wide variety of substances, the endothelium regulates vascular tone, inhibits smooth muscle cell proliferation and migration, controls cellular adhesion, regulates inflammation, and exerts fibrinolytic and antithrombotic actions. The concept of endothelial function is also extended from the vascular lumen to the vascular wall and adventitia, which are supplied by vasa vasorum, considered to be an active intravascular microcirculation.
Nitric oxide, initially identified as the endothelium-derived relaxing factor, is the major vasodilating substance released by the endothelium. Nitric oxide is synthesized from L-arginine by the action of endothelial nitric oxide synthase, primarily in response to shear stress produced by blood flow. Cofactors including tetrahydrobiopterin and nicotinamide adenine dinucleotide phosphate are involved in nitric oxide production. Apart from shear stress, endothelial nitric oxide synthase is also activated by bradykinin, adenosine, vascular endothelial growth factor, and serotonin. Asymmetric dimethylarginine, on the other hand, is an endogenous inhibitor of nitric oxide synthase and may mediate the adverse effects of traditional risk factors on endothelial vasodilator function. Nitric oxide has a half-life of a few seconds in vivo. It diffuses from endothelial cells to exert its relaxation effects on vascular smooth muscle cells by activating guanylate cyclase, which in turn increases the production of cyclic guanosine monophosphate and leads to a reduction of the intracellular calcium concentration. Apart from regulating vascular tone through vasodilation, nitric oxide also mediates other important vascular homeostatic functions by exerting inhibitory effects on the proliferation of vascular smooth muscle, counteracting leukocyte adhesion to the endothelium, and inhibiting platelet aggregation.
The endothelium also mediates hyperpolarization of the vascular smooth muscle to cause relaxation. Although the identity of the endothelium-derived hyperpolarizing factor remains elusive, its hyperpolarizing mechanism is considered to be mediated by calcium-activated potassium channels on vascular smooth muscle. Candidates include epoxyeicosatrienoic acids, potassium ion, gap junctions, hydrogen peroxide, and C-type natriuretic peptide. It has been suggested that endothelium-derived hyperpolarizing factor might play a compensatory role for the loss of nitric oxide–mediated vasodilation in patients with heart failure. Other endothelium-derived vasodilators include prostacyclin and bradykinin. Prostacyclin is produced via the cyclooxygenase pathway and acts independently of nitric oxide to cause vasodilation. It also acts synergistically with nitric oxide to inhibit platelet aggregation. Prostacyclin appears to have a limited role in humans in the control of vascular tone. Bradykinin stimulates the release of nitric oxide, prostacyclin, and endothelium-derived hyperpolarizing factor.
Regulation of vascular tone by the endothelium is also accomplished by the control of vasoconstrictor tone through the release of endothelin and the conversion of angiotensin I to angiotensin II at its surface. Endothelin-1, the predominant endothelin isoform in the cardiovascular system, binds to ET A receptors on vascular smooth muscle cells to cause vasoconstriction. At lower concentrations, however, endothelin-1 causes transient vasodilation in the human forearm circulation, probably owing to the release of nitric oxide and prostacyclin via ET B receptors located on endothelial cells.
Contraction of vascular smooth muscle cells reduces vessel diameter, increases vascular tone, and regulates blood flow by shortening the cells. This contractile phenotype is modified by the expression of genes that encode contractile proteins, ion channels, and other molecules involved in contraction. Smooth muscle contraction is regulated in vivo primarily by pharmacomechanical and electromechanical activation of the contractile proteins myosin and actin. Pharmacomechanical coupling refers to the activation of contraction by ligands of cell surface receptors without an obligatory change in the plasma membrane potential. The phosphoinositide signaling cascade is the common second-messenger system utilized by the surface receptors. Electromechanical coupling, on the other hand, involves alterations in the plasma membrane potential. Receptor activation may induce an activation of receptor-operated or voltage-dependent channels and lead to the passive influx of calcium down its concentration gradient. The balance between force generation and release is responsible for the maintenance of vascular tone. The vascular tone is influenced by local metabolic substances, humoral factors, and activity of the autonomic nervous system. A detailed discussion of the molecular mechanisms of smooth muscle contraction is beyond the scope of this chapter; however, interested readers are referred to recent published reviews.
Apart from a contractile phenotype, vascular smooth muscle cells exhibit other phenotypes. This phenotypic diversity plays an important role in the normal development, repair of vascular injury and in vascular disease process. After vascular injury, phenotypic modulation of vascular smooth muscle cells causes the upregulation of genes required for their proliferation and the production of extracellular matrix and suppression of genes that characterize the contractile phenotype. On the other hand, inappropriate pathologic differentiation into other mesenchymal lineages—such as osteoblastic, chondrocytic, and adipocytic ones—may contribute to vessel calcification, altered matrix production, and abnormal lipid accumulation, respectively. Studies have focused on the understanding of mechanisms that underlie the physiologic control and pathologic alterations of phenotypic switching of vascular smooth muscle cells.
The regulation of circulation aims to adjust the blood flow precisely to meet the needs of tissue and to maintain an adequate driving pressure to perfuse the various body tissues. Such control is achieved through local mechanisms, humoral factors, and neural regulation.
Autoregulation refers to the ability to maintain a relatively constant blood flow in response to acute changes in perfusion pressure. The coronary, renal, and cerebral circulations exhibit autoregulation. Two theories have been proposed for this autoregulatory mechanism. The metabolic theory suggests that elevated perfusion pressure increases blood flow, and hence oxygen delivery and removal of vasodilators, thereby leading to vasoconstriction and reduction of blood flow and vice versa. The myogenic theory proposes that stretching of vascular smooth muscle cells by the elevated perfusion pressure increases their tension, which in turn causes vasoconstriction to reduce blood flow. Conversely, less stretching at lower perfusion pressure causes smooth muscle relaxation and increases blood flow. However, the exact mechanisms that link intraluminal pressure generation to myogenic constriction remain uncertain.
Metabolic mechanisms also contribute to the control of local blood flow. Two theories have likewise been proposed. The vasodilator theory proposes that vasodilator substances are formed and released from tissues when metabolic rate increases or oxygen and other nutrient supplies decrease. Possible vasodilator substances include adenosine, carbon dioxide, potassium ion, hydrogen ion, lactic acid, histamine, and adenosine phosphate. The nutrient theory suggests that blood vessels dilate naturally when oxygen or other nutrients are deficient. Hence increased utilization of oxygen and nutrients increases metabolism to cause local vasodilation, a phenomenon referred to as active hyperemia. Reactive hyperemia is another phenomenon related to the local metabolic flow-control mechanism. In reactive hyperemia, a brief interruption of arterial blood flow results in a transient increase in blood flow that exceeds the baseline, after which the flow returns to baseline level. Both the deprivation of tissue oxygen and accumulation of vasodilating substances probably account for this phenomenon. The duration of reactive hyperemia depends on the duration of flow cessation and usually lasts long enough to repay the oxygen debt.
Autoregulation and metabolic mechanisms control blood flow by dilation of the microvasculature. The consequent increase in blood flow dilates the larger arteries upstream via the mechanism of flow-mediated dilation. The pivotal role of endothelial cells in the transduction of shear stress secondary to increased blood flow and the release of the vasodilators has been alluded to earlier. Flow-mediated dilation occurs predominantly as a result of local endothelial release of nitric oxide. The mechanisms of shear stress detection and subsequent signal transduction are unclear but probably involve opening of calcium-activated potassium channels that hyperpolarizes endothelial cells and calcium activation of endothelial nitric oxide synthase. Flow-mediated dilation increases flow with a negligible increase in pressure gradient, thus optimizing energy losses within the circulation. The phenomenon of flow-mediated dilation as induced by reactive hyperemia has commonly been used as an assessment of endothelial function in vivo. All of the aforementioned mechanisms represent relatively acute responses to regulate local blood flow. Long-term local mechanisms involve changes in tissue vascularity, the release of angiogenic factors, and the development of collateral circulations.
Humoral control refers to regulation by hormones or locally produced vasoactive substances that act in an autocrine or a paracrine fashion. These humoral substances act either directly via receptors on vascular smooth muscle cells or indirectly by stimulating the endothelium to release vasoactive substances.
Circulating catecholamines, noradrenaline and adrenaline, are secreted by the adrenal medulla, which is innervated by preganglionic sympathetic fibers. Sympathetic activation stimulates the release of catecholamines, about 80% being noradrenaline, from the adrenal gland. The adrenal gland and the noradrenergic sympathetic vasoconstrictor fibers provide dual control of the circulation by catecholamines. The adrenergic receptors in the blood vessels are α 1 , α 2 , and β 2 receptors. Noradrenaline causes vasoconstriction by acting on α-receptors, while adrenaline causes vasodilation at physiologic concentrations through its β-agonist effect. At higher concentrations, adrenaline also causes vasoconstriction by activating α-receptors.
The regulatory role of the renin-angiotensin system in the circulation is well known. The final effector of the system, angiotensin II, mediates its effects classically in an endocrine fashion. In response to decreased renal perfusion pressure or extracellular fluid volume, renin is secreted from the juxtaglomerular apparatus of the kidney and cleaves angiotensinogen, released from the liver, to form angiotensin I. By action of the angiotensin converting enzyme, which is predominantly expressed on the surface of endothelial cells in the pulmonary circulation, angiotensin I is converted to angiotensin II. Angiotensin II is a potent vasoconstrictor and acts directly by stimulating the angiotensin II type I (AT 1 ) receptor and indirectly by increasing sympathetic tone and the release of vasopressin. A local paracrine renin-angiotensin system also exists in the vasculature. Vascular production of angiotensin II has been shown to be mediated by the endothelium. The tissue renin-angiotensin system has dual effects on vessel function, being mediated through opposing effects of two receptors. Stimulation of AT 1 receptor causes contraction of vascular smooth muscle by directly increasing intracellular calcium and indirectly stimulating synthesis of endothelin-1 and other vasoconstrictors. Furthermore, promotion of oxidative stress via the AT 1 receptor may possibly reduce nitric oxide bioavailability. On the other hand, stimulation of angiotensin II type 2 receptor appears to mediate vasodilation by activating the nitric oxide pathway. The local tissue angiotensin II hence also plays an important role in maintaining vascular homeostasis. Other biologically active aminopeptides of the circulating renin-angiotensin system, such as angiotensins III and IV, may act in the central nervous system to raise blood pressure through the AT 1 receptor.
Three peptides of the natriuretic peptide family—atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide—also participate in the control of circulation. Atrial natriuretic peptide is primarily produced by the atrial myocardium, while brain natriuretic peptide is synthesized by the ventricular myocardium. The main stimulus for their release is stretching of the myocardium. Other stimuli include endogenous vasoactive factors, neurotransmitters, proinflammatory cytokines, and hormones. Atrial and brain natriuretic peptides reduce sympathetic tone through suppression of sympathetic outflow from the central nervous system, reduction of release of catecholamines from autonomic nerve endings, and probably damping of baroreceptors. The consequence is decrease in vascular tone and increase in venous capacitance. Both of these peptides also inhibit the activities of the renin-angiotensin system, endothelins, cytokines, and vasopressin. The renal hemodynamic effects include the induction of diuresis secondary to increased glomerular filtration due to vasodilation of afferent renal arterioles and vasoconstriction of the efferent arterioles and the promotion of natriuresis. Despite preload reduction, reflex tachycardia is suppressed as these peptides lower the activation threshold of vagal afferents. C-type natriuretic peptide is a more potent dilator of veins than the other natriuretic peptides and acts in an autocrine or paracrine fashion.
Adrenomedullin was first isolated from human pheochromocytoma cells. It is produced in a wide range of cells, including vascular endothelial and smooth muscle cells, and plays a significant role in the control of circulation. Infusion of adrenomedullin via the brachial artery in humans induces dose-dependent vasodilation to increase blood flow. Furthermore, blockade of the vasodilating effect of adrenomedullin by inhibition of nitric oxide synthase suggests that nitric oxide may be an important mediator for adrenomedullin.
The endothelium-derived vasoactive substances and their role in the control of vascular tone and homeostasis have already been discussed. The three classes of eicosanoids, prostaglandins, thromboxanes, and leukotrienes are generated by metabolism of arachidonic acid present in the phospholipids of cell membranes. Endothelial cells produce predominantly prostacyclin and lesser amounts of prostaglandin E 1 , also a vasodilator, and prostaglandin F 2α , a vasoconstrictor. Nonetheless prostacyclin appears to have a limited role in humans in the control of basal vascular tone. Thromboxane A 2 , although predominately generated by platelets, is also synthesized by the endothelium and induces vasoconstriction and platelet aggregation. Under normal physiologic conditions, eicosanoids—primarily prostacyclin, produced by the cyclooxygenase pathway—induce vasorelaxation. Furthermore, the cyclooxygenase-dependent vasodilators can compensate for the deficiency of other vasorelaxants. By way of the lipoxygenase pathway, leukotrienes are produced from arachidonic acid. Leukotrienes C 4 , D 4 , and E 4 cause arteriolar constriction, whereas leukotrienes B 4 and C 4 induce pulmonary vasoconstriction by activating cyclooxygenase to produce thromboxane A 2 .
Several other endogenous substances affect the systemic circulation. Vasopressin, produced in the supraoptic and paraventricular nuclei of the hypothalamus, is probably the most potent known endogenous constrictor. It is released in quantities sufficient to exert a pressor effect when volume depletion is significant but has little role in normal vascular control. Serotonin exists in large amounts in the enterochromaffin cells of the gastrointestinal tract. Although serotonin exerts vasoconstrictor and vasodilator effects, depending on the vasculature, its function in regulating the circulation is unknown. Kinins are among the most potent endogenous vasodilators; examples include bradykinins and kallidin. Bradykinin is believed to play a role in the control of blood flow in the skin, gastrointestinal glands, and salivary glands. Histamine is released from mast cells and basophils upon stimulation by injury, inflammation, or allergic reaction to induce vasodilation and increase capillary permeability.
Neural control of the systemic circulation involves feedback mechanisms that operate in both the short and long term through the autonomic, primarily the sympathetic, nervous system. Short-term changes in sympathetic activity are triggered either by reflex mechanisms involving peripheral receptors or by a centrally generated response. Long-term changes, on the other hand, are evoked through modulation of sympathetic nervous system by other humoral factors and possibly by central mechanisms involving the hypothalamus.
Peripheral receptors constitute the afferent limb of the reflex. These include arterial baroreceptors, arterial chemoreceptors, and cardiac stretch receptors. Arterial baroreceptors are located in the walls of the carotid sinus and aortic arch. Afferent fibers run in the glossopharyngeal and vagal nerves and terminate within the nucleus of the solitary tract. The neurons at the nucleus then excite neurons within the caudal and intermediate parts of the ventrolateral medulla to cause inhibition of the sympathoexcitatory neurons in the rostral ventrolateral medulla. Hence stretching of arterial baroreceptors increases afferent input and results in the reflex slowing of heart rate, a decrease in cardiac contractility, and vasodilation, thereby providing a negative feedback mechanism for the homeostasis of arterial pressure.
Peripheral chemoreceptors are located in the carotid and aortic bodies and are stimulated primarily by decreased arterial partial pressure of oxygen. Their afferent fibers also run in the glossopharyngeal and vagus nerves. Activation of peripheral chemoreceptor results in hyperventilation and sympathetically mediated vasoconstriction of vascular beds with the exception of those of the heart and brain. Hence oxygen conservation is attempted by increasing oxygen uptake and reducing tissue oxygen consumption. These chemoreflexes are subjected to negative feedback interaction, with inhibition of the chemoreflex-mediated sympathetic activation through the stimulation of baroceptors and thoracic afferents.
Atrial receptors are located in the walls of the right and left atria and in pulmonary venous and caval-atrial junctions. Two types of atrial receptors are described based on their discharge pattern in relation to atrial pressure changes. Type A receptors signal atrial contraction and hence respond to an increase in central venous pressure. These receptors send impulses via myelinated fibers in the vagus nerve, and the efferent portion consists of sympathetic activation. The tachycardia in relation to stimulation of sinuatrial node caused by atrial stretch is termed the Bainbridge reflex. Type B baroreceptors are stretch receptors stimulated by volume distension of the atria and their firing during ventricular systole. The afferents are unmyelinated vagal fibers. Atrial distension decreases sympathetic activity. Receptors that respond to stretch and contractility are also present in the ventricles. These receptors provide afferent input to the medulla via unmyelinated C fibers. Stimulation of these fibers decreases sympathetic tone and causes bradycardia and vasodilation. Stretching of the atrial and ventricular myocardium also leads to the release of natriuretic peptides, as discussed earlier.
Apart from the reflex-triggered short-term control of the circulation, the central pathways responsible for the central command responses—such as those occurring at the onset of exercise or evoked by a threatening stimulus—are now better understood. Evidence suggests the existence of a supramedullary integrative loop that connects the brain stem and paraventricular nucleus of the hypothalamus. The loop is composed of ascending noradrenergic projections from the nucleus of the solitary tract and caudal ventrolateral medulla and descending oxytocinergic and vasopressinergic neurons in the paraventricular nucleus of the hypothalamus projecting to brain stem areas. It is likely that reflex-triggered control interacts with the central command responses to regulate the cardiovascular response during exercise. Groups of neurons in the hypothalamus can project to synapse directly with sympathetic preganglionic fibers in the spinal cord, implying that the medullary vasomotor center is perhaps not the only region that directly controls sympathetic outflow.
The autonomic nervous system represents the efferent component of the neural control of the circulation. Up to three types of fibers may innervate blood vessels: sympathetic vasoconstrictor fibers, sympathetic vasodilator fibers, and parasympathetic vasodilator fibers. As the size of vessel decreases, the density of autonomic innervation increases. The small arteries and arterioles are therefore the most richly innervated arteries.
Sympathetic vasoconstrictor fibers release noradrenaline upon nerve stimulation and constitute the most important components in the neural control of the circulation. Postsynaptically the α 1 -adrenoceptor is the predominant receptor mediating vasoconstriction. Although noradrenaline is the principal neurotransmitter in the sympathetic nervous system, it coexists with adenosine triphosphate and neuropeptide Y in sympathetic neurons. Sympathetic vasoconstriction of arterioles increases vascular resistance, while constriction of capacitance vessels alters the circulating blood volume. In larger arteries, contraction of vascular smooth muscle in response to sympathetic activation causes less significant change in arterial caliber but alters vascular tone and hence arterial stiffness.
Sympathetic vasodilator fibers are scarce and not tonically active. Evidence suggests that sympathetic vasodilator fibers regulate skeletal vascular tone in many animal species. Both cholinergic and nitric oxide–dependent mechanisms contribute to the vasodilator effect. Parasympathetic vasodilator fibers are found in blood vessels of the salivary gland, cerebral arteries, and coronary arteries. The vasodilator effect is mediated via release of acetylcholine with hyperpolarization of the vascular smooth muscle.
Long-term neural regulation of the circulation is modulated by humoral and other factors. Angiotensin II is an important facilitator of sympathetic transmission. It may enhance neurotransmitter release at sympathetic nerve terminals, sympathetic transmission through sympathetic ganglia, and perhaps central activation of sympathetic nervous activity. Nitric oxide interacts with the autonomic nervous system at both the central and peripheral levels. Centrally, nitric oxide decreases sympathetic vasoconstrictor outflow. Peripherally, augmented vasoconstriction to nitric oxide synthase inhibition has been demonstrated in denervated forearm in humans. Interaction between nitric oxide and cholinergic vasodilator fibers is also evidenced by significant pressor response to nitric oxide synthase inhibition with cholinergic blockade. Finally, the hypothalamic paraventricular nucleus, which plays a role in the central command responses as discussed earlier, may mediate sustained increases in sympathetic nerve secondary to a variety of stimuli. Stress, anxiety, or pathologic conditions such as heart failure may hence exert a long-term influence on neural control of the circulation through the tonic activation of sympathoexcitatory neurons located in the paraventricular nuclei of the hypothalamus.
From the mechanical perspective, the systemic arterial system can be envisaged as a network of elastic tubes that receive pulsatile blood flow from left ventricular ejection and transmit it distally as a steady stream into capillaries. Hence, apart from acting as a low-resistance conduit, the systemic arterial tree functions as a cushion to smooth out pressure and flow pulsations generated by cycles of left ventricular contraction. Although the success of the conduit function depends primarily on a low peripheral vascular resistance, the efficiency of cushioning function depends on the elastic properties, described in terms of stiffness, of the arterial system.
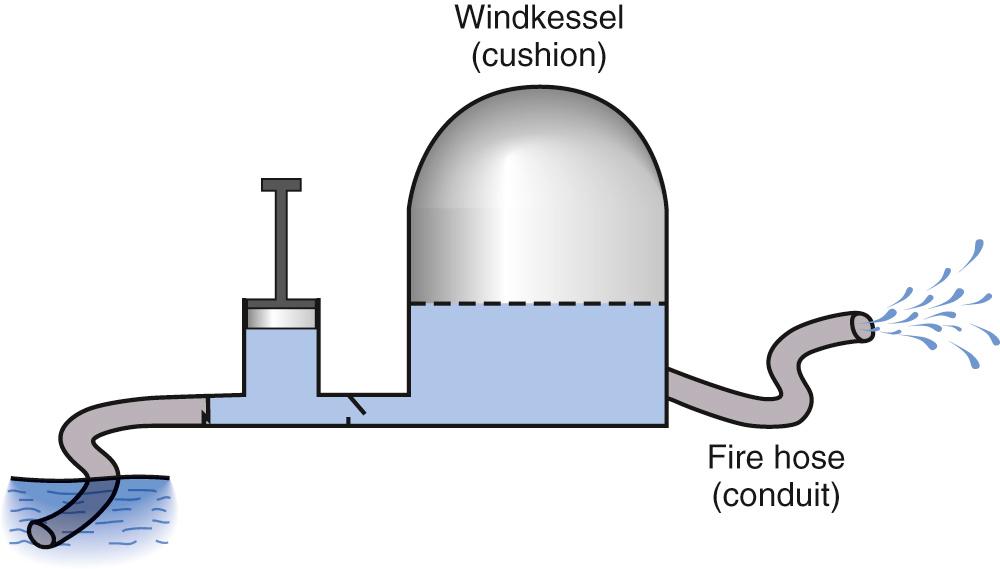
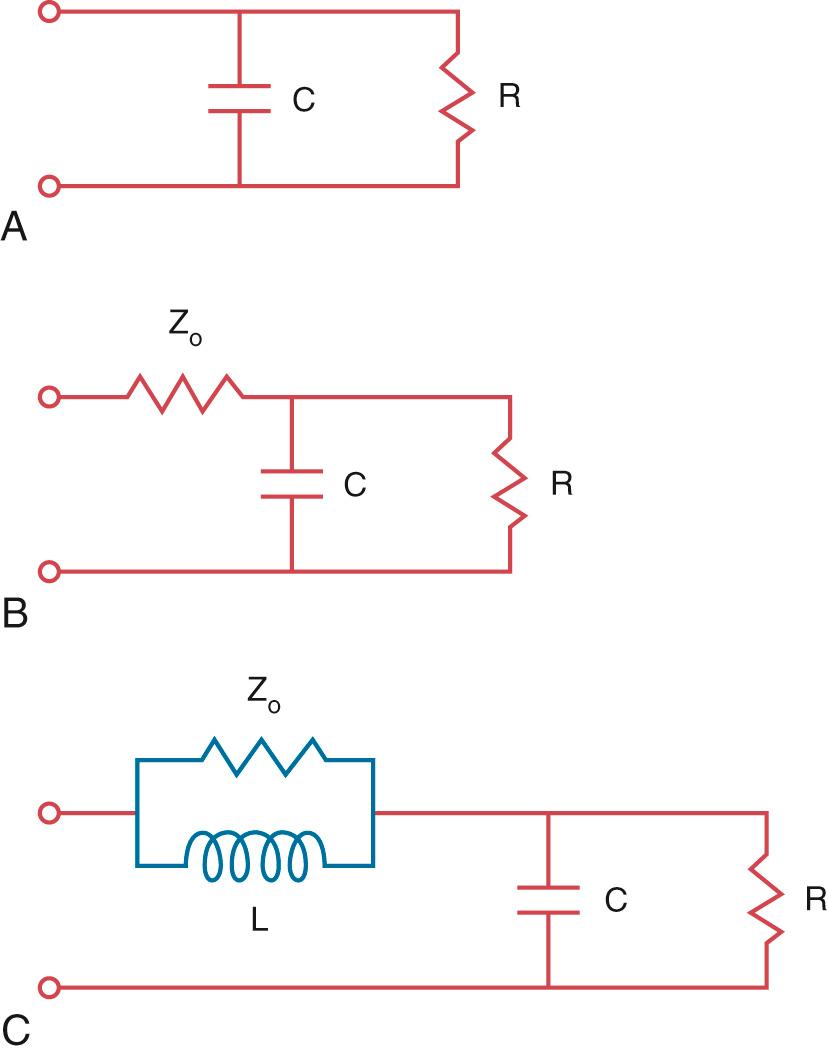
Modeling of the arterial circulation has contributed significantly to the understanding of the behavior of the arterial system and the effects of arterial load on the systemic ventricle. The lumped model of arterial circulation, commonly termed the Windkessel model, was first described in the 18th century. In his book Haemastaticks , Hales drew an analogy between the arterial system and an air-filled dome of the fire engine compression chamber (Windkessel) ( Fig. 74.1 ). The cushioning function of the dome smooths out the pulsatile blood flow and protects the peripheral vascular beds from exposure to large fluctuations in pressure. The electrical analogues of the systemic arterial system are shown in Fig. 74.2 . The two-element electrical analogue of the Windkessel model comprises a capacitor, which represents the arterial compliance, and a resistor, the total peripheral resistance. The modified Windkessel model takes into account the input impedance (see later) of the proximal aorta by the addition of a resistor proximal the two-element capacitance-resistance model. A four-element Windkessel model, with the addition of an inertial term, has further been proposed and shown to be superior to the three-element Windkessel as a lumped model of the entire systemic tree. Inertance is due to the mass of the fluid and, physiologically, it can be regarded as the inertial effect secondary to simultaneous acceleration of the blood mass within the vessel. However, intrinsic shortcomings of the Windkessel models include the limitation of vessel elasticity to one site, lack of a finite velocity of propagation of the pulse wave, and failure to consider the significance of wave reflection.
The combination of the cushioning and conduit functions of the arterial tree results in two phenomena: (1) traveling of a pulse wave at a finite speed along the arterial wall and (2) wave reflection at arterial terminations and other discontinuities. A more realistic model of a distensible tube with one end receiving pulsatile ejection of blood from the left ventricle and with the other end representing the peripheral resistance has therefore been proposed. The pressure wave at any point along the tube represents the result of the incident and reflected waves. Elasticity of the tube determines the velocity at which the pulse travels and the timing of arrival of the reflected wave. When the tube is distensible, the wave velocity is slow and the reflected wave returns late in diastole. With stiffening of the tube, the pulse velocity increases and the reflected wave arrives earlier to merge with the systolic part of the incident wave and results in a higher systolic pressure and a lower diastolic pressure. Vascular stiffness is therefore an important mechanical property of the arterial tree and contributes to left ventricular afterload.
Ventricular afterload can be conceptualized as all the external factors that oppose ventricular ejection and contribute to myocardial wall stress during systole. The hydraulic load of the systemic arterial system has therefore been taken to represent the afterload presented to the systemic ventricle. The total arterial hydraulic load comprises three components: resistance, stiffness, and wave reflection, all of which can be obtained from impedance spectra based on analysis in the frequency domain.
Vascular resistance is commonly used in the clinical setting as an index of systemic ventricular afterload. The electrical analogue for vascular resistance is described by the Ohm's law, which applies to direct electric current circuit. For a steady flow state, the vascular resistance is derived by dividing pressure gradient by volume flow. As the systemic venous pressure is very small when compared with the mean aortic pressure, the systemic arterial resistance can be approximated as mean aortic pressure divided by cardiac output. Nonetheless, as arterial blood flow is pulsatile in nature, the use of vascular resistance alone to describe afterload is deemed inadequate.
For pulsatile flow, the corresponding pressure-flow relationship is vascular impedance. This is analogous to the voltage-current relationship of an alternating current electrical circuit. To analyze the mathematical relationship between pressure and flow waves, Fourier analysis is used to decompose these complex nonsinusoidal waves into a set of sinusoidal waves with harmonic frequencies that are integral multiples of the fundamental wave frequency.
Vascular input impedance is defined as the ratio of pulsatile pressure to pulsatile flow. The aortic input impedance is particularly relevant as it characterizes the mechanical property of the entire systemic arterial circulation and represents the hydraulic load presented by the systemic circulation to the left ventricle. To obtain the aortic input impedance spectrum, the ascending aortic flow is measured by an electromagnetic flow catheter, while the pressure is measured by a micromanometer mounted onto the catheter. Noninvasive determination of aortic input impedance involves the use of Doppler echocardiography to measure flow and tonometry to obtain a carotid, subclavian, or synthesized aortic pressure waveform, the latter based on the radial arterial waveform. An example of the human aortic input impedance spectra is shown in Fig. 74.3 . For a heart rate of 60 beats/min, the fundamental frequency is 1 Hz, the second harmonics is 2 Hz, and so forth. The vascular impedance modulus at different harmonics is the ratio of pressure amplitude to flow amplitude. The phase difference is the delay in phase angle between the pressure and flow harmonics, which is analogous to time delay in the time domain.
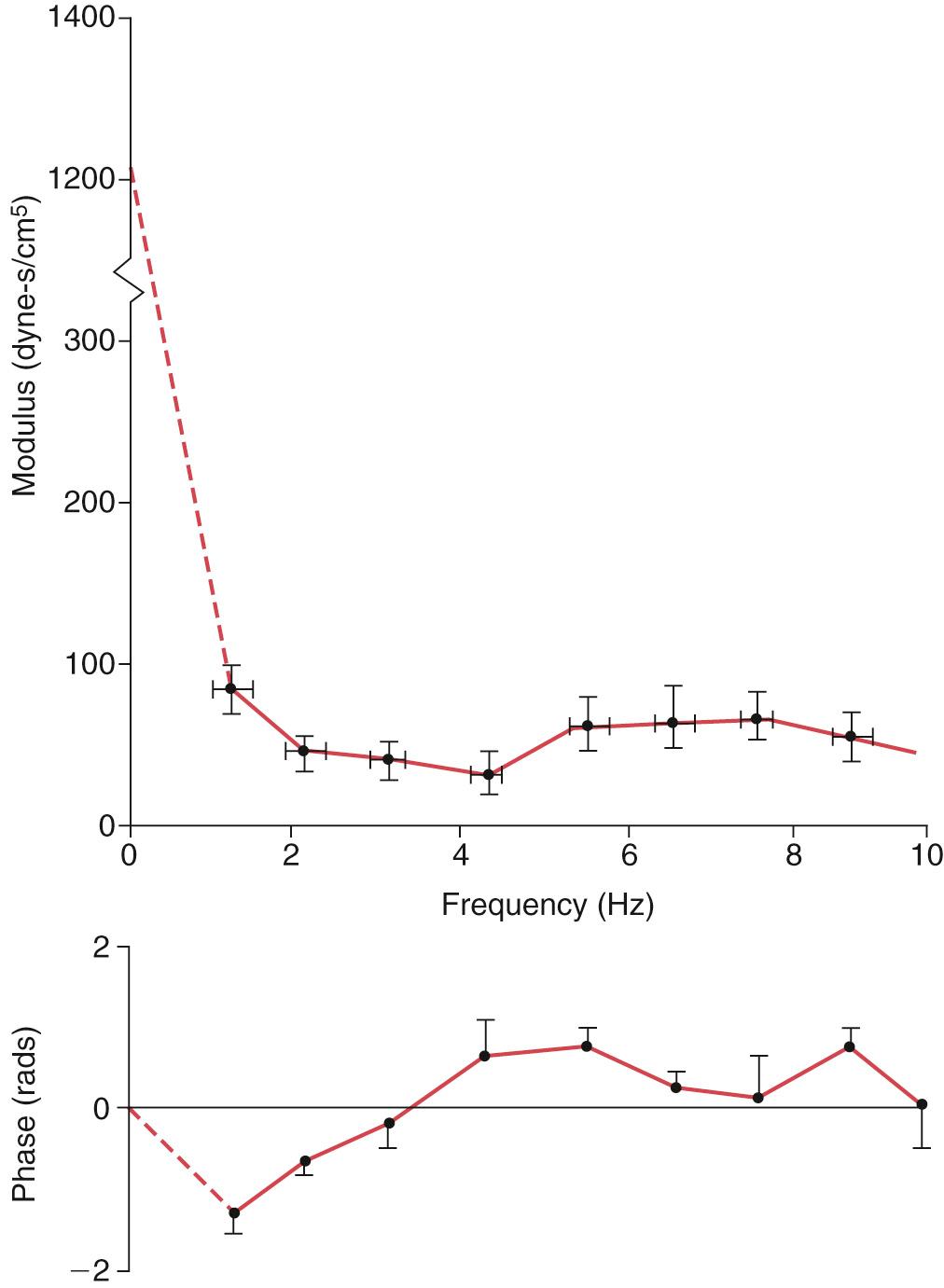
The impedance at zero frequency is equivalent to resistance in the steady-flow state. Characteristic impedance is the ratio of pulsatile pressure to pulsatile flow at a site where pressure and flow waves are not influenced by wave reflection. The concept of characteristic impedance is important as it is related directly to stiffness of the major arteries distal to the site of measurement. Hence it represents the pulsatile component of the hydraulic workload presented to the left ventricle when measured at the ascending aorta. As wave reflection is always present, characteristic impedance cannot be measured directly. It is usually estimated by averaging impedance moduli over a frequency range where fluctuations due to wave reflection above characteristic impedance are expected to cancel out those below. Hence characteristic impedance has been estimated as the average value of moduli between 2 and 12 Hz, above 2 Hz, or above the frequency of the first minimum.
As the velocities of pressure and flow waves transmitted in the arteries are in the order of meters per second, it is obvious that the waves have sufficient time to travel to the periphery and be reflected back before the next cardiac cycle. The terminations at where low-resistance conduit arteries terminate in high-resistance arterioles are usually regarded as the principal sites for reflection. Possible reflecting sites include branching points in major arteries, areas of alterations in arterial stiffness, and high-resistance arterioles.
The pressure and flow waves measured at any site in the arterial system can be envisaged as a summation of a forward or incident wave and a reflected wave. Wave reflection exerts opposite effects on pressure and flow. Reflected pressure wave increases the amplitude of the incident pressure wave, whereas a reflected flow wave decreases the amplitude of the incident flow wave. In most experimental animals and in young human subjects who have elastic arteries, wave reflection returns to the ascending aorta from the periphery after ventricular ejection. Such timing is desirable, as the reflected pressure wave augments early diastolic blood pressure and contributes to aortic valve closure, thereby boosting the perfusion pressure of the coronary arteries without increasing left ventricular afterload. Stiffening of the systemic arteries due to aging or disease processes, however, increases pulse-wave velocity and causes an earlier return of the reflected wave to augment aortic blood pressure in late systole rather than in diastole. The implications of this pressure augmentation are discussed in the section on ventriculoarterial interaction further on.
Arterial stiffness describes the rigidity of the arterial wall. It is primarily determined by the structural components of the arterial wall, elastin and collagen in particular, vascular smooth muscle tone, and transmural distending pressure. The endothelium also plays a role in the regulation of arterial stiffness through the release of vasoactive substances to alter the smooth muscle tone. The significance of arterial stiffness stems from its direct relationship to characteristic impedance, hence the pulsatile component of the arterial afterload, and its effect on the timing of return of the reflected waves from peripheral sites.
In adults, the role of arterial stiffening in the development of cardiovascular disease is recognized. Associations between increased arterial stiffness and various pathophysiologic conditions, which are themselves also associated with increased cardiovascular risk, in adults has been extensively reviewed. Importantly, stiffness of central arteries, as assessed by aortic pulse-wave velocity and carotid distensibility, has been shown to have independent predictive value for cardiovascular events in the general adult population, in the elderly and adults with hypertension, end-stage renal disease, and impaired glucose tolerance.
Although stiffness of the central arteries has been the focus of adult studies, the contribution of stiffness of the smaller peripheral arteries to total vascular impedance cannot be ignored. Structural remodeling occurs also in smaller arteries and branching points, and changes in the mechanical properties of conduit and resistive arteries influence wave reflections and contribute to augmentation of late systolic blood pressure in the aortic root. Associations between increased small artery stiffness, as assessed by pulse contour analysis, and aging, hypertension, smoking, diabetes, and cardiovascular events have also been reported. Indeed, the mapping of arterial stiffness at multiple sites may provide a holistic approach to the prediction of cardiovascular events.
The increasing application of noninvasive methods to determine systemic arterial stiffness in the clinical and research arenas has significantly increased the understanding of its pathophysiologic significance. With adoption of these noninvasive methodologies for use in children and adolescents, the significance of arterial stiffening in the young is also being increasingly understood.
Noninvasive methods for the determination of local, regional, and systemic arterial stiffness and the quantification of wave reflections in vivo are available. For meaningful interpretation of these indexes, their fundamental limitations have to be taken into account. First, the relationship between pressure and arterial diameter is nonlinear due to progressive recruitment of the stiffer collagen as transmural pressure increases. Second, modulation of smooth muscle tone by sympathetic nervous activity, hormones, or endothelium-derived vasoactive substances as previously mentioned can alter arterial stiffness. Finally, spontaneous vasomotor changes in the muscular arteries can alter arterial diameter and stiffness.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here