Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Albright’s 1934 description of primary water clear cell hyperplasia, that involving all four parathyroids represents the first recognition of the concept of primary hyperparathyroidism (PHPT) due to multiple gland disease (MGD) rather than single-gland disease. Although the incidence of this now-unusual condition was to decline, the “New Entity in the Surgery of Hyperparathyroidism,” known as primary chief cell hyperplasia, was described in Cope’s landmark 1958 paper. Even then, both authors postulated that the phenomenon of hyperparathyroidism (HPT) due to MGD might be the result of an extrinsic stimulus acting on the parathyroids. Cope also commented on the histologic similarities of adenomas and chief cell hyperplasia and the diagnostic uncertainty that this occasioned. In his later description of the surgical management of 104 cases of PHPT due to hyperplasia, he demonstrated that subtotal resection of 3.5 glands (which left a remnant of 30 to 50 mg of viable tissue) was the treatment of choice for this condition.
Since then, the advent of preoperative parathyroid localization has resulted in a paradigm shift in the approach to single-gland disease: from bilateral cervical exploration (BCE) to a focused approach. The shift has resulted in fewer four-gland explorations. Conversely, patients with negative localization are more likely to have smaller parathyroid tumors and more often have MGD. The diagnosis of PHPT with mild aberration or even normal serum calcium levels means that patients with negative imaging now account for one-fifth of patients referred with PHPT. One national study that investigated the outcomes for parathyroidectomy in unlocalized disease reported a 13% negative exploration rate and persistent HPT (surgical failure) in 18%.
Therefore parathyroidectomy for MGD is complex and associated with a higher operative failure compared with single-gland disease. Surgery in this setting poses several challenges; the diagnosis is often not clear until well into the neck exploration because preoperative localization is rarely helpful. Furthermore, hyperplastic glands are typically much smaller than solitary parathyroid adenomas; therefore they are more difficult to identify. At times, it can be difficult to distinguish a small hyperplastic gland from a normal parathyroid. Lastly, subtotal resection with or without thymectomy requires more surgical experience and judgment compared with excision of a solitary adenoma. For these reasons, consideration should be given toward centralizing the management of patients predicted to have MGD, including those with negative preoperative localization, to high-volume centers.
In this chapter, we discuss the pathology and etiology of sporadic multiglandular parathyroid disease and the rationale for the different surgical approaches used in the management of this specific condition.
Although 80% to 90% of patients presenting with PHPT have disease that is due to a single adenoma, the remainder of patients will have more than one diseased gland encountered at surgery, termed MGD . A systematic review of more than 22,000 patients determined the incidence of MGD PHPT to be 9.84% with 5.74% patients affected by multiple gland hyperplasia and 4.14% affected by double adenomas. However, despite the large number of patients included in this study, its results highlight the wide variation in the reported incidence of MGD (9% to 26%, Table 60.1 ) and the varying definitions of MGD. This disparity is due, in part, to whether parathyroid glands are designated abnormal based upon their macroscopic appearance, pathologic criteria, genetic differences, or endocrine functionality. Data are further confounded by the extent to which abnormal glands are sought in the era of more limited parathyroid exploration.
| Year | Center | Operative Approach | Intraoperative PTH used? | No. of Patients Studied | Double Adenoma (%) | Hyperplasia (%) | Overall MGD | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1956–1990 | Uppsala, Sweden | BCE | No | 659 | 77 (11.7) | 54 (8.2) | 19.9% | 12 |
| 1982–1992 | San Francisco, USA | BCE | No | 416 | 49 (11.8) | 58 (13.9) | 25.7% | 9 |
| 2001–2013 | Wisconsin, USA | Focused and BCE | Yes | 1402 | 124 (8.8) | 181 (12.9) | 21.7% | 6 |
| 1999–2009 | Ann Arbor, USA | Focused | Yes | 1855 | Not stated | 13% | 8 | |
| 1990–2013 | Sydney and Melbourne, Australia | Focused and BCE | No | 4569 | 519 (11.3) | 181 (3.9) | 15.2% | 10 |
| 1993–2010 | Leuven, Belgium | BCE | No | 698 | 46 (6.6) | 17 (2.4) | 9% | 7 |
| 2004–2014 | Cardiff, United Kingdom | BCE and Focused | Yes | 552 | Not stated | 10.8% | 11 |
Visual appearance has long been the standard for identifying abnormal parathyroid glands via BCE. Although it is reliable, the technique necessitates extensive surgical experience, a sound knowledge of parathyroid embryology, and knowledge of the likely location of eutopic and ectopic glands.
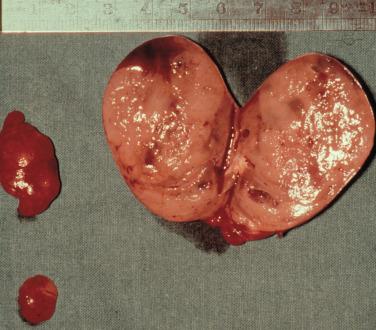
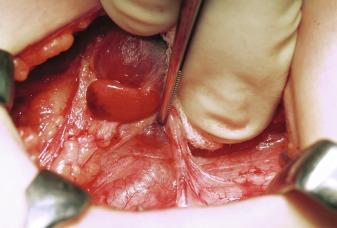
The normal parathyroid measures 6mm in length, is pale-tan in color, oval-shaped, and possesses a well-defined capsule, allowing it to “float” independently in the perithyroidal fat. The average weight is 40 to 60 mg. In contrast, pathologic, glands are enlarged (weighing ≥ 80 to 100 mg) and assume a more rounded, bilobed, or even multilobulated appearance ( Figures 60.1 and 60.2 ). The softer consistency and yellow-brown or brown-reddish color on sectioning of parathyroid tissue sets it apart from the grayish cut surface of a lymph node and the firmer consistency of the dark brick red thyroid gland ( Figure 60.3 ). The finding of one enlarged gland in the presence of three other normal glands is indicative of single adenoma or uniglandular disease. Conversely, it can usually be presumed that the presence of two, three, or four enlarged glands signifies MGD.
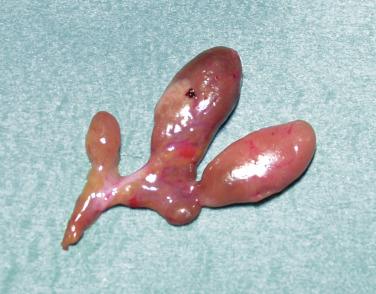
Encountered in between 3 and 12% of explorations for PHPT, there is debate as to whether this entity represents the synchronous or asynchronous occurrence of two distinct adenomas or asymmetric four-gland hyperplasia. It is argued that, because the hypercalcemia rates after double adenoma excision are similar, albeit slightly higher than those observed in patients with uniglandular disease (2% to 4% for double adenoma versus 1% to 2% for single adenoma), it is most likely that patients with double adenoma comprise a heterogenous group of patients with true adenomas and asymmetric four-gland hyperplasia.
The most frequent manifestation of MGD in primary HPT, chief cell hyperplasia affecting all 4 glands, is sporadic in approximately 75% of the patients; however, it may develop from a background of long-term lithium therapy for bipolar illness, or it may be inherited as part of a multiple endocrine neoplasia (MEN) syndrome (see Chapter 62 , Parathyroid Management in the MEN Syndromes). Secondary four-gland parathyroid hyperplasia also develops as an inevitable result of chronic renal failure (see Chapter 61 , Surgical Management of Secondary and Tertiary Hyperparathyroidism). In chief-cell hyperplasia, size-discrepancy between individual glands is usually marked; enlarged, obviously abnormal glands will commonly be found to coexist with smaller, apparently normal ones ( Figures 60.2 and 60.4 ). Nodularity and variable growth patterns are generally more obvious in the enlarged hyperplastic glands; frequently, one or several nodules appear to have acquired more pronounced growth advantage or may even occupy the entire gland. Cases with modest hypercalcemia commonly exhibit less pronounced glandular enlargement and less variability between glands (see Figure 60.4 ) but typically still show signs of micronodular hyperplasia in the smaller glands.
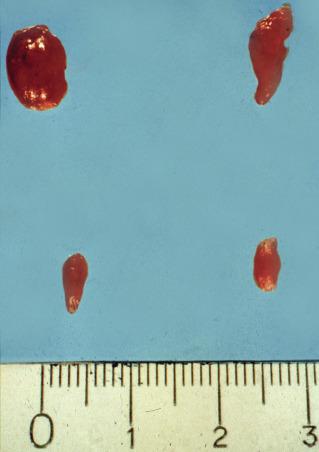
Pathologic examination of excised parathyroid tissue can determine whether or not it is abnormal. Normal parathyroids are composed of chief cells rich in cytoplasmic fat droplets, which are notable for their positive Oil-red O staining; these droplets are interspersed with stromal fat, which increases with age and patient obesity. Conversely, parathyroid adenomas excised from patients with PHPT demonstrate encapsulated nodules composed of chief cells, a prominent absence of cytoplasmic lipid droplets, and may exhibit a rim of compressed and lipid-rich parathyroid tissue, which is best seen at the vascular pole of the gland. The presence of these changes is pathognomonic of parathyroid adenoma, although the absence of a rim does not exclude adenoma.
Parathyroid hyperplasia affects multiple glands, usually all four. Typically, the glands are enlarged with diffuse, nodular or mixed, predominantly chief cell hyperplasia. They may also frequently contain foci of oxyphil cells and very occasionally clear cells. Larger nodules may exhibit reduced fat content and mimic adenomas by flattening a rim of suppressed tissue at their boundary. For this reason, distinguishing histologically between adenoma and hyperplasia may be difficult or impossible. The distinction may be particularly difficult if only one gland is sent for evaluation, and the status of the remaining glands is unknown to both surgeon and pathologist, which is often the case in focused exploration ( Figure 60.5 ).
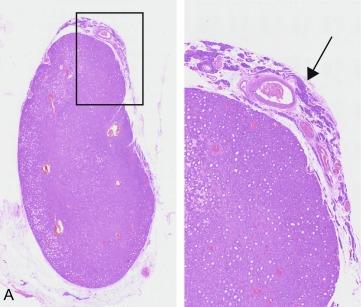
The presence of MGD can be reliably diagnosed as BCE if more than one enlarged gland is present. However, it is less clear whether all enlarged glands are hyperfunctioning. The modern-day preeminence of scan-directed parathyroidectomy has raised questions about whether functionality can be assumed based upon appearance and whether it is clinically relevant. Historically, centers that undertake BCE have tended to quote higher rates of MGD (> 20%) compared with centers undertaking focused surgery based on preoperative localization scans (i.e., minimally invasive parathyroidectomy [MIP] or unilateral exploration), where the rate of MGD is reported to be around 5% to 10%. The accepted explanation for this discrepancy is that enlarged-yet-nonfunctioning glands are left in situ during scan-directed surgery. This is borne-out by the observations that normocalcemia rates after BCE and focused surgery are approximately equivalent (2.3% and 3.7% respectively) and that the recurrence rate after focused surgery with intraoperative PTH is less than 3% during long-term follow-up of 10 years, rather than the 10% to 15% predicted by experience with BCE. These data suggest that although it is highly likely that enlarged glands remain in situ after targeted surgery, they are not the cause of recurrent disease in any clinically significant way.
Therefore the definition of MGD may implicitly depend on the extent of surgical exploration (e.g., minimally invasive, unilateral or bilateral), whether intraoperative PTH levels decline after parathyroidectomy, and the failure to obtain postoperative normocalcemia, despite removal of a pathologic gland.
The somatic mutation theory of tumorigenesis predicts that parathyroid adenomas in sporadic PHPT are monoclonal expansions derived from one transformed parathyroid cell, whereas parathyroid hyperplasia describes a reactive polyclonal expansion due to an exogenous stimulus. Therefore clonality would be expected to distinguish adenoma and hyperplasia. However, genetic studies have yielded conflicting results, with some authors determining that parathyroid adenomas are polyclonal, monoclonal and polyclonal, purely monoclonal, and predominantly polyclonal with only a minority found to be truly monoclonal. Although it is possible that the differences in results derived in these studies may in part be due to the research methodologies employed, they also involved small numbers of patients. A more recent prospective study examining the clonal status of more than 100 patients with apparent adenomas determined that up to 46% were polyclonal. The study also examined clinical, operative, and pathologic data on those with mono- and polyclonal tumors; no statistically significant differences were found in terms of age, race, body mass index (BMI), bone mineral density, preoperative biochemistry, and localization results between patients with monoclonal and polyclonal tumors. However, as might be expected, the incidence of MGD was significantly greater in the polyclonal group (23% versus 7%, p = 0.039), and patients with polyclonal tumors undergoing unilateral exploration were less likely to have MGD identified at surgery than those undergoing BCE (1 of 16 patients versus 8 of 14 patients, p = 0.025). The authors concluded that focused surgery in patients with polyclonal tumors was likely to result in abnormal glands being left in situ, but patients with polyclonal tumors were not discernably different in terms of their preoperative parameters.
Because of the preponderance for MGD in inherited PHPT, one might suppose that four-gland hyperplasia would be more common in younger patients. Indeed, in one series 465 patients, MGD was found to be twice as common in patients aged less than 40 years compared with those older than 40 years of age (22.9% versus 11% respectively). However, this difference disappeared once patients with inherited disease were removed from the analysis (12.5% versus 10%), and no gender difference was noted either. Double adenomas have been observed to occur in older patients and cited as a factor in recurrent PHPT in two large studies. The evidence on increasing age as a factor in hyperplastic MGD is mixed; in both of the latter studies on double adenoma, hyperplasia occurred in slightly younger patients (median age less than 60 years). In contrast, a more recent study reported that the rate of MGD in patients older than 65 years was double that of those patients under 65 years of age (24% versus 12%, p = 0.001). During follow-up, older patients also had a lower normocalcemia rate (93% versus 95%, p = 0.27). Again, there were no gender differences. The authors suggested that planned BCE should be considered in older patients, especially if a small gland was localized on preoperative scans. Therefore when considered as a whole, the evidence suggests that increasing age is a risk factor for MGD, particularly double adenoma.
It is well recognized that vitamin D deficiency, chronically low calcium intake, and raised BMI result in compensatory secondary HPT usually in the presence of eucalcemia. For nearly 60000 women in a prospective cohort study of nearly that lasted 22 years, the risk of PHPT was found to be inversely related to calcium intake; a high calcium intake was protective against the onset of PHPT (RR 0.56, 95% CI 0.37 to 0.86). A retrospective review of more than 1300 consecutive patients with PHPT treated surgically, of whom 15% had MGD, found raised BMI to be associated with MGD (BMI 30 to 39.9: OR 1.5, 95% CI 1.2 to 2.5 and BMI ≥ 40: OR 1.8, 95% CI 1.3 to 3.1). Furthermore, gastrointestinal malabsorption secondary to celiac disease, bariatric surgery, chronic pancreatitis, and diabetes are also risk factors for PHPT. Recently, a Scandinavian study demonstrated an independent association between diabetes and MGD (OR 2.97, 95% CI 1.4 to 6.3). However, although population-based studies suggest a causal link between these disorders and HPT, the underlying mechanisms by which a normal physiologic response to an exogenous stimulus results in dysregulated PTH secretion and hypercalcemia remains elusive. Furthermore, although one would expect MGD hyperplasia to be more prevalent in patients who develop PHPT on the background of such metabolic alterations, apart from the findings in obese patients and those with diabetes, the data are inconsistent. Similarly, there are no clear mechanistic insights into to how these disorders lead to MGD PHPT at present.
A causal link between radiation exposure to the neck and PHPT was first postulated when patients treated with radiotherapy for tuberculous adenitis in the 1930s and 1940s were followed-up with in the 1970s; up to 15% of the patients had evidence of HPT; which appeared to be dose-related and may explain why other investigators have failed to demonstrate a link. The subsequent report that 25% of the so-called “liquidators” (i.e., clean-up workers) for the Chernobyl accident developed PHPT 10 to 20 years later seems to substantiate this link. More detailed clinical studies that examined the presentation, biochemistry, and demographic characteristics of patients treated for PHPT between 1982 and 1993, found the factors to be similar regardless of prior history of radiation exposure; the incidence of MGD was similar (26% versus 28%). However, the incidence of concomitant thyroid pathology was much greater in the irradiated group (multinodular goiter 27% versus 7% and papillary thyroid cancer (PTC) 14% versus 0.3%). It seems highly likely, therefore, that radiation does induce PHPT, but the incidence of MGD does not appear to be increased and it remains to be elucidated why any putative field change brought about by radiation exposure does not appear to increase the risk of four-gland disease compared with single-gland disease.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here