Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The current health care environment has increased our focus on the cost and quality of surgical care. As a result, many hospitals and physicians are devoting significant efforts to understand and benchmark the risk of complications in surgical patients. One approach uses risk-adjusted administrative data sets (e.g., Vizient) to generate reports comparing observed to expected complications among different hospitals and departments. Another, the American College of Surgeons National Surgical Quality Improvement Program (NSQIP), collects individual patient data and reports institution-specific, risk-adjusted surgical outcomes.
While most surgeons realize that complications increase the cost of care, the incentives to reduce costs by decreasing complications have been limited in many practice environments. More recently, the Center for Medicare and Medicaid Services has linked professional reimbursement for surgical services with the Merit-Based Incentive Payment System, which links cost and quality outcomes to surgeon payments. As these programs are implemented, there should be a more direct relationship between cost, quality, and surgeon reimbursement.
Although individual surgeon judgment and technique certainly impact the risk of complications, there are many other factors that contribute to surgical outcomes, including specific aspects of the patient population, the perioperative care team, and the environment in which surgical care is provided. Suffice it to say that even the best surgical judgment and technique are only as good as the system in which they practice. Furthermore, the transition to electronic medical records (EMRs) has significantly impacted the perioperative environment. Implementing “evidence-based best practices” to improve quality requires buy-in from multiple individuals, including the patient, the surgery residents, midlevel providers, anesthesiology, nursing, information technology, infectious disease, and others, to bend the cost-quality curve. Clinical care pathways were developed in the 1980s to provide multidisciplinary, evidence-based care for specific patients or surgical procedures. However, with the transition to EMRs, order sets were developed to facilitate the use of standardized patient care. Unfortunately, despite the theoretical potential to extract high-quality data on compliance with order sets and best practices from the EMR, the practical ability to do this has been disappointing at many institutions.
In addition to developing and implementing our own evidence-based surgical practices, we must educate the next generation of surgeons on best practices and quality improvement to reduce complication rates in our patients. With this in mind, the Accreditation Council for Graduate Medical Education developed the Continuous Learning Environment Review program designed to provide teaching hospitals with feedback on their graduate medical education environment as it relates to patient safety, health care quality, care transitions, supervision, duty hours, and fatigue management and professionalism. In addition, the American College of Surgeons developed the Quality in-Training Initiative, which provides a detailed curriculum on quality improvement for surgical residents. These and other initiatives like the Milestones project that require resident involvement and engagement in the Improvement of Care practice domain should help trainees to develop the skills required to improve quality and reduce complications.
Surgical complications is a large topic that has been classified by various methods, including patient risk factors, complication type, severity (e.g., hospital readmission and/or surgical mortality), and organ system. We rely heavily on classification by complication type, severity, and organ system in this chapter.
A seroma is the collection of fluid containing fat, serum, and lymph that develops at surgical wound or dead space from surgery. Seromas are common in surgical procedures, which create dead space or interruption of lymphatic drainage. Examples of this include breast or axillary surgery and hernia surgery. The use of prosthetic mesh in hernia surgery may contribute to poor flap adherence and trigger inflammation which can increase the risk of seroma formation.
Seromas frequently present as palpable subcutaneous (SQ) fluid collections under or adjacent to a skin incision. They are more common when there is a significant dead space (e.g., SQ adiposity or large SQ hernia sac) and frequently develop after surgery when the patient is at home. Patients often complain of discomfort at the site of seroma due to pressure from the fluid collection. Some patients may experience swelling, pain, or erythema if infection is present. The diagnosis of seroma can be made clinically based on physical examination or radiologically by ultrasound or computed tomography (CT). Techniques to decrease the risk of seroma include minimization of dead space (e.g., excision of excess skin) and surgical drainage of dead space. Several techniques are used to reduce dead space, including the use of fibrin sealant, quilting sutures, and medical talc with inconsistent results. Many asymptomatic seromas will resolve spontaneously because the fluid can be resorbed by the surrounding tissues. Symptomatic seromas should be aspirated under sterile conditions and have a pressure dressing applied. Care should be taken with repeated seroma aspirations, which may introduce infection. In refractory seromas, a drain can be placed or the surgical site can be opened and packed to heal by secondary intention or treated with negative pressure therapy. Infected seromas should be drained surgically and treated with antibiotics. Prosthetic implants that are exposed should be removed in the setting of severe infection.
A hematoma is collection of blood clot and blood at the surgical site. It includes not only the SQ tissues but may also be found in a deeper tissue space where surgery was performed. Hematomas may be caused by incomplete surgical hemostasis or medical conditions that impair clotting, including coagulation disorders, platelet disorders, or other conditions that impair hemostasis (e.g., uremia, cirrhosis, or sepsis). Medications that alter blood clotting are a major factor in hematoma formation and must be managed properly in the perioperative period to reduce the risks of complications related to bleeding or clotting ( Table 12.1 ). Commonly used antiplatelet agents include aspirin, P2Y12 inhibitors (clopidogrel, prazugrel, ticagrelor), and abciximab. Although warfarin is the most commonly used anticoagulant, the requirement for frequent blood draws to monitor its effects has reduced its popularity. The development of direct-acting oral anticoagulants or novel oral anticoagulants (direct oral anticoagulants) such as rivaroxaban, apixaban, and dabigatran are as effective as coumadin in most conditions requiring anticoagulation and require less frequent testing.
| Drug | Timing of Discontinuation | ||
|---|---|---|---|
| Warfarin | 5 days | ||
| Direct thrombin inhibitors (dabigatran) | CrCl ≥50 mL/min: 1–2 days | ||
| CrCl <50 mL/min: 3–5 days | |||
| CrCl (mL/min) | Bleed risk: low | Bleed risk: high (days) | |
| ≥50 | 2–4 hours | 2–3 | |
| 30–50 | 2 days | 2–3 | |
| <30 | 2–4 days | >5 | |
| Factor Xa inhibitors | |||
| Rivaroxaban (Xarelto) | ≥24 hours | ||
| ROCKET AF: ≥3 days | |||
| Apixaban (Eliquis) | Low-bleed risk: ≥24 hours | ||
| High-bleed risk: ≥48 hours | |||
| Antiplatelet agents | |||
| Aspirin | High CV risk/minor: continue | ||
| Low CV/high-bleed risk: 7–10 days | |||
| Clopidogrel | 5 days | ||
Hematomas can occur in various spaces, and clinical presentation differs. Superficial hematomas may present with skin discoloration, SQ swelling, and pain of the affected area due to pressure. Hematomas in other locations may cause pressure effects and irritation depending on the anatomic location. For example, hematomas in the abdominal cavity may present as abdominal compartment syndrome (ACS) or ileus, compartment syndrome in the forearm or leg, and airway compromise from anterior neck hematomas. When clotting disorders are present, the bleeding may persist and patients with large hematomas can present with anemia or hypovolemia. Infected hematomas may cause fever, leukocytosis, or sepsis.
Since coagulopathy is a common cause of hematoma, the perioperative management of surgical patients must include an appropriate evaluation of bleeding disorders and anticoagulant medications. Coagulation tests should be performed selectively since global screening is neither cost effective nor recommended. A patient’s history of inappropriate bleeding or bruising, diseases related to hemostasis such as kidney or liver disease, or family history of bleeding disorder should raise the concern of hemostatic abnormality, and a screening test should be performed in these patients before surgery.
Aspirin and clopidogrel are the two most common antiplatelet medications that irreversibly inhibit cyclooxygenase 1 and P2Y12 receptor, respectively. Patients on these medications frequently have a history of coronary artery disease and/or stenting. Current guidelines recommend continuing aspirin and holding anti-P2Y12 agents before surgery, depending on timing after stenting and urgency of surgery. Warfarin is the most common anticoagulant drug used to prevent risk of thromboembolic events in many conditions such as atrial fibrillation, mechanical heart valve implantation, and hypercoagulable disorders. Warfarin use has been shown to be an independent risk factor for bleeding and hematoma in many studies. Therefore, the perioperative management of warfarin and other anticoagulants requires some consideration of the risks of bleeding and benefit of thromboembolic prevention. The risk of thromboembolism varies depending on the medical indication for anticoagulant use. The risk of surgical bleeding can be assessed by using HAS-BLED score ( Table 12.2 ), where a score of 0 to 1 has low risk of bleeding, 2 has intermediate risk, and ≥3 has high risk. Also, surgical procedures can be grouped as high risk of bleeding (2%–4%), such as cardiovascular surgery, orthopedic surgery, surgery of head and neck cancer, urologic surgery, or surgery longer than 45 minutes, or low risk (0%–2%), such as anticipated surgical time <45 minutes, abdominal hernia, or cholecystectomy.
| HAS-Bled Risk | Score |
|---|---|
| Hypertension, systolic BP >160 mm Hg | 1 |
| Abnormal renal or liver function (1 point each) | 1 or 2 |
| History of stroke | 1 |
| History of bleeding | 1 |
| Labile INRs | 1 |
| Elderly (>65 years) | 1 |
| Drugs or alcohol (1 point each) | 1 or 2 |
Despite improvements in surgical techniques, suture materials, and pre- and postoperative care, acute wound failure/or dehiscence remains a dreaded surgical complication. The sequelae of acute wound failure includes fascial dehiscence, evisceration, acute hemorrhage, incisional hernia, anastomotic leaks, and fistulas (discussed later). Abdominal wound dehiscence describes partial or total disruption of the surgical wound, which can be superficial (skin and SQ fat) or deep (fascial), with or without extrusion of abdominal viscera (evisceration). Although the process of wound healing is similar in all tissues, the rate and consequences of failed healing differ by tissue (e.g., failure of bowel healing = anastomotic leak). After an incision, the aponeurosis will never completely regain its original strength and requires relatively longer time to heal.
The physiology of wound healing includes four distinct but overlapping phases (hemostasis, inflammation, proliferation, and remodeling), which are regulated by numerous intercellular interactions (monocytes, lymphocytes, fibroblasts, etc.), the release of local and systemic factors including cytokines, chemokines, growth factors, and inhibitors. Failure of wound healing is usually caused by multiple factors. These include patient-related factors like diabetes, uremia, immunosuppression, jaundice, sepsis, hypoalbuminemia, cancer, obesity, and steroid use. Other factors contributing to wound failure include inadequate closure (technical failure), increased intraabdominal pressure (IAP), and local factors like infection or radiation.
Wound dehiscence occurs in 1% to 3% of all surgical procedures and is more common during the first postoperative week (inflammatory phase of healing) when wound strength is dependent on the suture material and holding capacity of the knot. The fascial layer provides most of the strength and anchor for abdominal incisions. Dehiscence can be caused by technical failure if the suture material breaks, knots become undone, or if the suture material cuts through the suture holding tissues. Over time (during the fibrotic phase), the intrinsic strength of the wound increases, and the integrity of wound is less dependent on the suture and suture technique.
Many factors contribute to failure of wound healing or dehiscence, including advanced age, obesity, malnutrition, cancer, steroid use, intraabdominal sepsis, and local surgical site infection or hematoma. Infection contributes to aponeurotic breakdown and failure in more than half of all cases of wound dehiscence. Infection decreases collagen formation by reducing collagen synthesis and increasing collagen breakdown (via both bacterial and neutrophil collagenase activity). The resulting scar contains dense but fragile collagen fibrils with decreased cellular proliferation, which is prone to disruption. Technical factors can also contribute to wound failure, especially suture placement (too close to the fascial edge, too far apart, or under too much tension). Excessively tight suture lines impair wound perfusion and oxygen delivery needed for adequate wound healing.
Wound dehiscence may be an early manifestation of an intra-abdominal process (e.g., abscess or anastomotic leak) presenting with serosanguineous fluid drainage from the wound or in some cases sudden evisceration with no warning. Patients with evisceration often note a sudden ripping sensation that occurs with severe coughing or retching. Wound dehiscence is associated with a mortality rate as high as 35% due to associated factors (e.g., malnutrition, sepsis, and cancer).
The optimal suturing technique for fascial closure remains a topic of debate among many surgeons. A suture:wound length ratio of more than 4:1 is recommended for proper fascial closure. The traditional technique of midline laparotomy closure uses continuous monofilament suture with sutures placed 1 cm deep on normal fascia (the bite) followed by 1 cm of progress. Incisional hernia rates of approximately 6% were reported over 5 years with this technique. The small bites versus large bites for closure of abdominal midline incisions (STITCH) trial compared large (1 cm every 1 cm) with small (0.5 cm every 0.5 cm) bite techniques for midline fascial closure. The incidence of incisional hernia was 21% in the large bite group and 13% in the small bite group, suggesting technical superiority of the small bite technique. Wound dehiscence can also occur when the sutures pull through the patient’s fascia and many surgeons are hesitant to use the “small bite” technique in obese patients or when they are concerned about tissue quality and sutures “pulling through.” General concepts for fascial closure include accurate approximation of anatomic layers, avoiding excessive tension on the suture line, and considering the use of prophylactic mesh or retention sutures in “high-risk” patients.
The management of wound dehiscence depends on the degree of dehiscence (partial or complete), its timing, the presence of evisceration or intraabdominal sepsis, and individual patient factors in many situations. When wound dehiscence presents early after surgery without evisceration, prompt reclosure of the fascia is recommended. However, if the fascial disruption is partial or presents late when the viscera are adherent to the peritoneum, urgent reoperation and fascial reclosure are not always indicated. Local wound care and abdominal binders can be used selectively in some patients if the risk of reoperation and/or bowel injury is felt to be “high.” Complete wound dehiscence with evisceration requires immediate reoperation, which is often difficult since many patients are obese or distended with distended and friable bowel due to associated inflammation or infection. The eviscerated bowel should be handled carefully to prevent injury; protection with moist towels during reexploration may help. The peritoneal cavity should be explored when there is concern for an acute intra-abdominal process (e.g., anastomotic leak, bowel obstruction, or sepsis). When wound dehiscence occurs due to local soft tissue infection, the fascial edges should be debrided back to healthy tissue and the wound reclosed without tension.
The resultant large open abdominal wound can be treated in several ways. With ongoing abdominal sepsis or massive bowel edema, leaving the abdomen open with a negative pressure dressing may help resolve intraabdominal sepsis or allow for diuresis to decrease bowel edema and facilitate subsequent abdominal closure. In many cases, the use of absorbable (polyglactin or polyglycolic acid) mesh or acellular dermal prosthesis is necessary to close the fascia without undue tension. The use of permanent mesh (polypropylene or polytetrafluoroethylene) is not recommended in most cases due to the presence of associated infection or bowel injury. In some patients, the use of component separation and unilateral or bilateral myofascial advancement flaps can facilitate fascial closure.
In many cases, the skin and SQ tissue will need to be closed over a drain or left open. In general, contaminated or actively infected wounds should be left open. Open wounds can be treated with wet-to-dry dressing changes and delayed primary closure in some cases (usually several days postoperatively). Leaving the wound open, treating with dressing changes, and allowing it to heal by secondary intention are options in cases of severe infection or when the SQ tissues would benefit from local wound care. More recently, the use of negative pressure wound therapy (NPWT) or vacuum-assisted closure (VAC) dressings can be applied. The application of negative pressure to open wounds reduces tissue edema, increases tissue perfusion, and facilitates healing by secondary intention. NPWT also regulates the inflammatory process by recruiting fibroblasts (through mechanical microdeformation) and induces cell migration, directly reducing bacterial populations through impairment of bacterial enzymatic processes. However, NPWT is not recommended in certain situations (e.g., untreated osteomyelitis, necrotic tissue with eschar, and in contact with exposed blood vessels, anastomotic sites, or nerves).
SSIs are a common complication seen after surgery or invasive procedures. It is one of the most common hospital-associated infections and contributes to increases in morbidity, length of hospital stay, readmission, and cost of care. Estimation of the average additional hospital costs is $5,000–$13,000 per SSI. Approximately half of all SSIs are potentially preventable if evidence-based best practices are followed. For this reason, SSIs are considered to be indicative of the overall quality of surgical care and are routinely monitored by most hospitals. As a result, many institutions have adopted comprehensive programs to reduce the incidence of SSIs. Although the overall incidence of SSIs are trending down, SSIs are reported in 2% to 5% of surgical patients in the United States and up to 11.8% in low-to-medium income countries. These data probably underestimate the true incidence since many infections occur after hospital discharge.
The term SSI refers to infection of the skin incision but also includes infection of the deeper anatomic tissues and organs where surgery was performed. Several standardized definitions of SSI exist, but the most commonly used definition is that of the Centers for Disease Control and Prevention (CDC), which groups SSIs into superficial, deep, and organ/space as outlined in Box 12.1 .
Infection occurs within 30 days after the operation and infection involves only skin and subcutaneous tissue of the incision and at last one of the following :
Purulent drainage, with or without laboratory confirmation, from the superficial incision.
Organisms isolated from an aseptically obtained culture of fluid or tissue from the superficial incision.
At least one of the following signs or symptoms of infection: pain or tenderness, localized swelling, redness, or heat and superficial incision is deliberately opened by a surgeon, unless incision is culture-negative.
Diagnosis of superficial incisional SSI made by a surgeon or attending physician.
Infection occurs within 30 days after the operation if no implant is left in place or within 1 year if implant is in place and the infection appears to be related to the operation and infection involves deep soft tissue (e.g., fascia, muscle) of the incision and at least one of the following :
Purulent drainage from the deep incision but not from the organ/space component of the surgical site.
A deep incision spontaneously dehisced or deliberately opened by a surgeon when the patient has at least one of the following signs or symptoms: fever (>38°C) and localized pain or tenderness, unless incision is culture-negative.
An abscess or other evidence of infection involving the deep incision found on direct examination, during reoperation, or by histopathologic or radiographic examination.
Diagnosis of deep incisional SSI made by a surgeon or attending physician.
Infection occurs within 30 days after the operation if no implant is left in place or within 1 year if implant is in place and the infection appears to be related to the operation and infection involves part of the anatomy (e.g., organs and spaces) other than the incision, which was opened or manipulated during an operation and at least one of the following .
Purulent drainage from a drain that is placed through a stab wound into the organ/space.
Organisms isolated from an aseptically obtained culture of fluid or tissue in the organ/space.
An abscess or other evidence of infection involving the organ/space that is sound on direct examination, during reoperation, or by histopathologic or radiologic examination.
Diagnosis of organ/space SSI made by a surgeon or attending physician.
Many factors contribute to SSIs, including patient factors and various aspects of surgical management ( Table 12.3 ). SSIs are usually caused by contamination of the surgical site by endogenous bacteria or a break in sterility of the surgical technique and/or instruments. Surgical wounds can be classified as clean, clean contaminated, contaminated, or dirty based on the risk of bacterial contamination by entry of the aerodigestive tract or presence of established infection at the surgical site. As one might expect, each of these categories are associated with specific risks of SSI ( Table 12.4 ). Typical pathogens depend on the procedure performed since surgery breaks the protective barrier of skin or mucosa, and endogenous microorganisms can be introduced into the tissue or organ. Infection after operations involving skin and soft tissue is predominantly gram-positive cocci ( Staphylococcus aureus , most common). Gram-positive anaerobic cocci frequently cause infections following oral cavity or pharyngeal procedures, and anaerobic bacteria are more likely with colonic surgery ( Table 12.5 ). Overall, S. aureus is the most common SSI pathogen. Other common pathogens include coagulase negative Staphylococcus , Enterococcus spp., Escherichia coli , Enterobacter spp., and Pseudomonas aeruginosa .
| Patient Factors | Factors Related to Surgery and Management |
|---|---|
| Advanced age | Duration of surgery |
| Increased BMI | Implantation of prostheses |
| High ASA score | Reoperation |
| High NNIS score | Longer hospital stay before surgery |
| Diabetic mellitus | Corticosteroid medication |
| Smoking | Inadequate sterilization, skin antisepsis |
| Dependence or frailty | Emergency procedure |
| Malnutrition | Hypothermia |
| Severe wound class | Intraoperative blood transfusion |
| Ascites | Perioperative shaving |
| Coexisting remote infection | Failure to obliterate dead space |
| Staphylococcal colonization | |
| Skin disease at surgical site | PROTECTIVE FACTORS |
| Anemia | Laparoscopic procedures |
| Increased number of comorbidities | Antibiotic prophylaxis |
| Category | Criteria | Infection Rate (%) |
|---|---|---|
| Clean | No hollow viscus entered | 1–3 |
| Primary wound closure | ||
| No inflammation | ||
| No breaks in aseptic technique | ||
| Elective procedure | ||
| Clean-contaminated | Hollow viscus entered but controlled | 5–8 |
| No inflammation | ||
| Primary wound closure | ||
| Minor break in aseptic technique | ||
| Mechanical drain used | ||
| Bowel preparation preoperatively | ||
| Contaminated | Uncontrolled spillage from viscus | 20–25 |
| Inflammation apparent | ||
| Open, traumatic wound | ||
| Major break in aseptic technique | ||
| Dirty | Untreated, uncontrolled spillage from viscus | 30–40 |
| Pus in operative wound | ||
| Open suppurative wound | ||
| Severe inflammation |
| Type of Surgery | Likely Pathogens |
|---|---|
| Placement of all grafts, prostheses, or implants | Staphylococcus aureus , coagulase-negative staphylococci |
| Cardiac | S. aureus , coagulase-negative staphylococci |
| Neurosurgery | S. aureus , coagulase-negative staphylococci |
| Breast | S. aureus , coagulase-negative staphylococci |
| Ophthalmic (limited data, however, commonly used in procedures such as anterior segment resection, vitrectomy, and scleral buckles) | S. aureus , coagulase-negative staphylococci, streptococci, gram-negative bacilli |
| Orthopedic (total joint replacement, closed fractured/use of nails, bone plates, other internal fixation device, functional repair without implant/device trauma) | S. aureus , coagulase-negative staphylococci, gram-negative bacilli |
| Noncardiac thoracic (lobectomy, pneumonectomy, wedge resection, other noncardiac mediastinal procedures), closed tube thoracotomy | S. aureus , coagulase-negative staphylococci, treptococcus pneumoniae , gram-negative bacilli |
| Vascular | S. aureus , coagulase-negative staphylococci |
| Appendectomy | Gram-negative bacilli, anaerobes |
| Biliary tract | Gram-negative bacilli, anaerobes |
| Colorectal | Gram-negative bacilli, anaerobes |
| Gastroduodenal | Gram-negative bacilli, streptococci, oropharyngeal anaerobes (e.g., peptostreptococci) |
| Head and neck (majorly procedures with incision through oropharyngeal mucosa) | S. aureus , streptococci, oropharyngeal anaerobes (e.g., peptostreptococci) |
| Obstetric and gynecologic | Gram-negative bacilli, enterococci, group B streptococci, anaerobes |
| Urologic | Gram-negative bacilli |
Methicillin-resistant S. aureus (MRSA) is a serious SSI pathogen because it is more virulent, difficult to treat, and associated with longer hospital stay, higher hospital costs, and increased mortality. MRSA infections are increased in patients with nasal colonization of MRSA, prior MRSA infection, recent hospitalization, and recent antibiotic use.
The majority of SSIs occur within 30 days of surgery and up to 1 year after implantation of a surgical prosthesis. Superficial SSIs present with localized redness, swelling, tenderness, warmth, presence of purulent discharge, or failure of wound healing. Deep SSIs may present with systemic signs and symptoms of infection, including fever, wound dehiscence, and purulent discharge from deep tissues. Organ or deep space infection can present as purulent discharge from surgical drains or with systemic signs of sepsis, including fever, tachycardia, tachypnea, and leukocytosis with associated signs of organ failure (decreased partial arterial oxygen pressure [PaO 2 ]/fraction of inspired oxygen [FiO 2 ] ratio, thrombocytopenia, hyperbilirubinemia, hypotension, delirium, or acute kidney injury [AKI]).
Patients scheduled for surgery should be managed to minimize the risk of SSI. Before performing surgery, any coexisting infection (skin, urine, and lung) should be treated and resolved. Patients who smoke cigarettes should stop for 1 to 2 months before elective surgery if possible and diabetic patients should have their blood sugar well controlled. Other conditions that may need to be “optimized” include nutritional status, anemia, and obesity. Decolonizing staphylococcal carriers with 2% mupirocin nasal ointment can reduce the risk of postoperative S. aureus infection in cardiac and orthopedic surgery. However, there is limited consensus regarding who to screen or for which operations screening should be considered. Preoperative antibiotics should be administered within 60 minutes of the skin incision to reach therapeutic concentration in serum and tissue during the surgical procedure. Vancomycin and fluoroquinolones may need to be started earlier due to their prolonged infusion times and half-lives. Redosing of antibiotics may be required if the duration of surgery exceeds 2 half-lives of the drugs or with massive blood loss. Caution should be used in patients with poor drug clearance (e.g., renal insufficiency or hepatic dysfunction) and the choice of drug should correlate with the common organisms found at the surgical site. In general, prophylactic antibiotics should not be continued after surgery and the duration of antimicrobial prophylaxis should not exceed 24 hours. Table 12.6 summarizes prophylactic antibiotic choice, dosing, and redosing, and Table 12.7 reviews the recommended antibiotic prophylaxis by surgical procedure.
| Antimicrobial | Recommended Dose for Adult | Redosing (Hours After Preoperative Dose) |
|---|---|---|
| Ampicillin-sulbactam | 3 g | 2 |
| Ampicillin | 2 g | 2 |
| Aztreonam | 2 g | 4 |
| Cefazolin | 2 g, 3 g if body weight >120 kg | 4 |
| Cefuroxime | 1.5 g | 4 |
| Cefotaxime | 1 g | 3 |
| Cefoxitin | 2 g | 2 |
| Cefotetan | 2 g | 6 |
| Ceftriaxone | 2 g | NA |
| Ciprofloxacin | 400 mg | NA |
| Clindamycin | 900 mg | 6 |
| Ertapenem | 1 g | NA |
| Fluconazole | 400 mg | NA |
| Gentamicin | 5 mg/kg ∗ | NA |
| Levofloxacin | 500 mg | NA |
| Metronidazole | 500 mg | NA |
| Moxifloxacin | 400 mg | NA |
| Piperacillin-tazobactam | 3.375 g | 2 |
| Vancomycin | 15 mg/kg | NA |
| Neomycin | 1 g | NA |
| Erythromycin-based oral antibiotic (colorectal surgery prophylaxis in conjunction with mechanical bowel preparation) | 1 g | NA |
∗ Gentamicin is calculated based on actual body weight, if the actual body weight is >20% above ideal body weight (IBW), the dosing weight (DW) can be calculated from DW = IBW + 0.4(Actual body weight - IBW)
| Type of Procedure | Recommended Agents | Alternatives For Patients with β-Lactam Allergy |
|---|---|---|
| Gastroduodenal | Cefazolin | Clindamycin or vancomycin + aminoglycoside or aztreonam or fluoroquinolone |
| Biliary tract | Cefazolin, cefoxitin, cefotetan, ceftriaxone, ampicillin-sulbactam | –Clindamycin or vancomycin + aminoglycoside or aztreonam or fluoroquinolone –Metronidazole + aminoglycoside or fluoroquinolone |
| Appendectomy for uncomplicated appendicitis | Cefoxitin, cefotetan, cefazolin + metronidazole | –Clindamycin + aminoglycoside or aztreonam or fluoroquinolone –Metronidazole + aminoglycoside or fluoroquinolone |
| Nonobstructed small bowel | Cefazolin | Clindamycin + aminoglycoside or aztreonam or fluoroquinolone |
| Obstructed small bowel | Cefazolin + metronidazole, cefoxitin, cefotetan | Metronidazole + aminoglycoside or fluoroquinolone |
| Hernia repair | Cefazolin | Clindamycin, vancomycin |
| Colorectal |
|
|
Head and neck
|
|
|
Urologic surgery
|
|
|
| Vascular | Cefazolin | Clindamycin, vancomycin |
Transplant surgery
|
|
|
| Plastic surgery | Cefazolin, ampicillin-sul bactam | Clindamycin, vancomycin |
For skin preparation, patients should shower with soap the night before surgery. If hair needs to be removed from the surgical site, a clipper should be used. Skin should be prepared with alcohol-based antiseptic solution (e.g., chlorhexidine) before incision. Perioperative glycemic control has been shown to reduce SSIs with a glucose threshold of <200 mg/dL. Avoiding perioperative hypothermia (core temperature <36°C) has been shown to reduce the risk of SSI and may be achieved with preoperative room warming, warmed intravenous (IV) fluids, etc. Use of increased FiO 2 is recommended during general anesthesia and for 2 to 6 hours postoperatively, especially after colon surgery, to ensure adequate tissue oxygen levels which are associated with reduced risk of SSI. Other considerations include the operating room environment (air handling, etc.), sterile processing of surgical instruments, use of clean instruments to close the abdomen in contaminated cases, etc. In the intraoperative period, using adhesive skin drapes (e.g., Ioban) may help to reduce SSIs in some cases and wound protection devices may help to reduce SSIs in open abdominal surgery by decreasing contamination of the SQ tissues. Antimicrobial coated suture has been developed to reduce bacterial colonization and prevent SSI, but the results remain controversial. Topical NPWT (Provena) is being used by some surgeons to reduce SSI but has not been universally adapted as best practice at this time. However, managing open wounds with NPWT is commonly used to reduce edema, increase blood flow, and decrease bacterial burden. After the operation, the wound should be routinely assessed for infection surveillance.
Infected surgical wounds should be opened to allow the infection to drain, and debridement should be considered if devitalized or infected tissue is present. IV or oral antibiotics should be given when there are signs of systemic infection, including fever >38.5°C, tachycardia >110 beats/min, and leukocytosis >12,000/μL, or when cellulitis (erythema extends >5 cm from wound edge) is present. Patients with risk factors for MRSA infection should be treated with appropriate antibiotics (e.g., vancomycin, daptomycin, linezolid, or ceftaroline). Empiric antibiotics for operations involving axillae, groin, perineum, genital tract, and gastrointestinal (GI) tract should cover gram-negative and anaerobic bacteria. Moreover, patients requiring antibiotics should have drainage or discharge from the wound or site of infection sent for culture to identify the pathogen and its antibiotic resistance profile. The wound should be wet dressed with normal saline damped sterile gauze at least daily. Antibiotics should be optimized according to the culture results when available.
Maintenance of normothermia is important physiologically as even modest deviations in core body temperature contribute to metabolic alterations, resulting in cellular and tissue dysfunction. Hypothermia is a common complication in surgical patients and is defined as core body temperature below 35 o C. It can be classified by severity into three categories: mild (32 o C–35°C), moderate (28°C–32°C), and severe (<28°C). Vasoconstriction and shivering are the body’s major thermoregulatory protective mechanisms, both of which may be impaired in the perioperative period. Risk factors for heat loss and perioperative hypothermia include elderly patients, burn injuries, open surgical procedures, cool operating rooms, prolonged surgeries (>4 hours), infusion of room-temperature fluids, cutaneous vasodilatation from anesthetic agents, and increased evaporative losses from serosal surfaces. Hypothermia can develop during any stage of surgery: preoperatively, intraoperatively, or postoperatively. Preoperatively, the use of muscle relaxants impairs shivering. Intraoperatively, heat loss occurs from large, exposed operative area, anesthetic effects on heat production, cool room temperatures, vasoconstriction, and shivering.
Hypothermia after surgery contributes to organ injury through various mechanisms: ventilation-perfusion (V/Q) mismatch; shift of oxyhemoglobin-dissociation curve to the left causes tissue hypoxia, decreases myocardial contractility and peripheral vasoconstriction, increased blood viscosity; reduced platelet function; and decreased activation of the coagulation cascade. Hypothermia is common after traumatic injury due to shock, alcohol intoxication, environmental exposure, fluid resuscitation, and loss of shivering. Hypothermia is also associated with increased risk of SSI.
Intraoperative hypothermia causes significant postoperative discomfort and shivering. Hypothermia significantly impairs cardiovascular function, blood clotting, and wound healing and increases the risk of infection. When the core temperature falls below 32°C, significant reductions in blood pressure and cardiac output occur. Cardiovascular manifestations of hypothermia include cardiac depression, myocardial ischemia, dysrhythmias, peripheral vasoconstriction, impaired tissue oxygen delivery, blunted response to catecholamines, and hypotension. The characteristic electrocardiogram finding of J point elevation, and Osborn wave (notch and deflection at the QST-ST junction), are considered pathognomonic of hypothermia. Adverse myocardial outcomes have been reported in hypothermic patients with preexisting cardiovascular disease (when compared with postoperative normothermic patients). Peripheral vasoconstriction due to shock is the most important impediment to wound oxygenation. Mild core hypothermia results in immune dysfunction by impeding granulocyte chemotaxis and phagocytosis, macrophage function, and antibody production. These changes in immune function, in combination with decreased tissue oxygen tension, abnormal collagen deposition, and poor wound healing, increase susceptibility to infection.
Hypothermia also induces coagulopathy by attenuating hemostatic enzyme function and platelet sequestration, resulting in an increased risk of bleeding. With mild and moderate hypothermia, renal perfusion and glomerular filtration are decreased, resulting in “cold-induced diuresis.” Decreased hepatic and renal blood flows, in turn, reduce drug metabolism and excretion, with resultant decreases in plasma clearance and potential prolongations in drug effects, which can lead to delays in emergence from anesthesia and prolonged postoperative anesthesia care unit stays. Also, fluid resuscitation with Ringer’s lactate in a patient with existing metabolic acidosis further worsens cardiac function. Severe hypothermia impairs cough reflex and increases the risk of a comatose surgical patient to postoperative pneumonia.
Patients at risk for hypothermia should be monitored frequently and every attempt should be made to maintain normal central core temperature. Pulmonary artery, tympanic membrane, urinary bladder, esophagus, trachea, nasopharynx, or rectum have been established as reliable sites for estimation of core temperatures. Continuous temperature monitoring and maintaining normothermia are essential during surgery as anesthesia, cool operating room environment, and significant evaporative cooling occurs during skin preparation making most surgical patients susceptible to hypothermia. Increasing the ambient room temperature, administering warmed IV fluids, covering patients with blankets, and using forced-air warming devices are commonly used techniques to prevent intraoperative hypothermia. Invasive core rewarming techniques can also be used during surgery, including intraperitoneal irrigation with warmed saline and intubation and ventilation with warmed humidified air or gases. Circulating water warmers produce faster rewarming than heat exchanging systems. Inadvertent core hypothermia is commonly seen in the immediate postoperative period. Maintenance of normal body temperature decreases blood loss, fluid requirement, length of intensive care unit (ICU) stay, organ failure, and mortality. Maintenance of intravascular volume and electrolytes is important, particularly in head injuries where mannitol can augment the effects of cold diuresis. However, in the case of major abdominal, cardiothoracic surgery, surgery involving intentional hypothermia (cardiac bypass), or prolonged surgery (>4 hours), forced-air warming, warm IV fluids, and ambient temperature alone are inadequate for maintaining normothermia. When rapid warming is needed, continuous arteriovenous rewarming is more effective. In patients with asystole, defibrillation and drugs have unpredictable efficacy, and cardiopulmonary bypass is essential for rewarming and maintaining perfusion.
Malignant hyperthermia is a life-threatening condition that develops in approximately 1:10,000 to 1:250,000 anesthetic cases, with a higher incidence in younger patients. It is an autosomal dominant pharmacogenetic disorder that presents as hypermetabolic response to inhalation anesthetic agents like halothane, isoflurane, sevoflurane, desflurane, or depolarizing muscle relaxants succinylcholine or suxamethonium.
During muscle contraction, the neuronal signal action potential is transferred to muscle cells, resulting in the release of intracellular calcium from sarcoplasmic reticulum via ryanodine receptors to initiate muscle contraction. The energy used in this process also generates heat and oxygen is consumed with carbon dioxide (CO 2 ) release. Calcium is transported back to storage and muscles are then relaxed. In genetically susceptible patients, most commonly ryanodine receptor mutations, certain triggers can stimulate continuous release of calcium, leading to persistent high levels of intracellular calcium causing constant muscle contraction or rigidity, generation of heat, increased oxygen consumption, and (CO 2 ) release, which lead to respiratory and metabolic acidosis and eventually, if left untreated, rhabdomyolysis.
Early presentations of malignant hyperthermia include an increase in end-tidal (CO 2 ) or tachypnea if the patient is not intubated and ventilated, hypoxia, tachycardia, masseter muscle spasm, or trismus. Later presentations of malignant hyperthermia include muscle rigidity, cardiac arrhythmias, respiratory and metabolic acidosis, rhabdomyolysis, and hyperthermia, as the name suggests. Complications from rhabdomyolysis include disseminated intravascular coagulation, AKI, hyperkalemia, and possible cardiac arrest.
Since malignant hyperthermia is an autosomal dominant disorder, patients with a family history of malignant hyperthermia should be carefully evaluated and consider testing before surgery. They should be carefully monitored during anesthesia and trigger-free anesthetic agents should be used. Once malignant hyperthermia develops, the initial management is to discontinue the inciting anesthetic agent and halt the operation if possible. Dantrolene is the medication of choice to treat malignant hyperthermia, and an initial dose of 2.5 mg/kg IV should be given and can be repeated according to the response: end-tidal CO 2 , tachycardia, muscle rigidity, and acidosis. Oxygen supplementation should be given with hyperventilation. Blood should be tested for electrolyte and blood gas to assess for acidosis and hyperkalemia, creatine phosphokinase, and renal function and then treated accordingly. The electrocardiogram should be continuously monitored for arrhythmias. Core body temperature should be measured and monitored. Active cooling with ice packs and 4°C normal saline IV should be initiated if the body temperature is more than 39°C but should be stopped when the body temperature decreases to 38.5°C to avoid overcooling and hypothermia. Renal function should be assessed and urine output should be closely monitored. IV hydration should be given with diuresis when rhabdomyolysis is present; hemodialysis may be needed in some cases. Clotting studies and platelet count should be checked for the possibility of disseminated intravascular coagulation. When stable (i.e., end-tidal CO 2 and temperature are decreased, tachycardia or other arrhythmia is improved, and muscle rigidity is resolved), patients should be monitored in the intensive setting for at least 24 hours with dantrolene maintenance. Muscle weakness is a side effect from dantrolene so breathing and oxygenation should be monitored and aspiration should be prevented. Other side effects of dantrolene are hepatitis, phlebitis, and drowsiness. First-degree relatives should be advised of the potential risks and provided with genetic counseling.
Fever refers to an increase in the body’s normal core temperature. Postoperative fevers can be broadly divided into infectious and noninfectious (systemic inflammatory response syndrome [SIRS]) causes ( Table 12.8 ). Fevers are most often transient increases in temperature caused by the systemic inflammatory stimuli as a normal response to injury. However, fever can also be an early sign of potentially life-threatening infection. Pyrogenic cytokines are produced in response to infection and trauma (including surgery) and play an important role in regulating host inflammation and fever. Duration and extent of tissue trauma during surgery cause a release of interleukin-1 (IL-1), a primary activator of the febrile response; IL-1 levels correlate with an increase in core temperature. Also, the timing of fever onset provides an important diagnostic clue; early postoperative fever is characterized by the release of cytokines during surgery. Immediate postoperative fever occurring within the first 48 hours after surgery is most likely due to an inflammatory response to surgery. The proinflammatory mediators (tumor necrosis factor-α [TNF-α], IL-6, and interferon γ), released in response to inflammation, cause a cascade of systemic effects that induce a febrile inflammatory response, also known as SIRS. SIRS is diagnosed when there is presence of two or more of the following criteria: temperature >38.5°C or <36°C, heart rate (HR) >90 beats/min, respiratory rate >20/min or PaCO 2 <32 mm Hg, white blood cell (WBC) count >12,000/mm 3 , or <4000/mm 3 or >10% band forms. A fever that develops 72 hours or more after surgery is more likely to be due to infection. Hence, it can sometimes be clinically challenging to delineate the precise etiology of these fevers since they can result from infectious and/or noninfectious causes.
| Infectious | Noninfectious |
|---|---|
| Abscess | Acute hepatic necrosis |
| Acalculous cholecystitis | Adrenal insufficiency |
| Bacteremia | Allergic reaction |
| Decubitus ulcers | Atelectasis |
| Device-related infections | Dehydration |
| Empyema | Drug reaction |
| Endocarditis | Head injury |
| Fungal sepsis | Hepatoma |
| Hepatitis | Hyperthyroidism |
| Meningitis | Lymphoma |
| Osteomyelitis | Myocardial infarction |
| Pseudomembranous colitis | Pancreatitis |
| Parotitis | Pheochromocytoma |
| Perineal infections | Pulmonary embolus |
| Peritonitis | Retroperitoneal hematoma |
| Pharyngitis | Solid organ hematoma |
| Pneumonia | Subarachnoid hemorrhage |
| Retained foreign body | Systemic inflammatory response syndrome |
| Sinusitis | Thrombophlebitis |
| Soft tissue infection | Transfusion reaction |
| Tracheobronchitis | Withdrawal syndromes |
| Urinary tract infection | Wound infection |
In the postoperative period, the most common infectious causes are wound infections, urinary tract infections (UTIs), and pneumonia. Prolonged IV access, bladder catheterization, or endotracheal intubation presents ongoing risks of infection that result from disruption of normal host defense mechanisms. Postoperative UTI is more common in patients with preexisting prostrate hypertrophy. Urinary tract instrumentation and indwelling urinary catheters damage the epithelial lining, eliciting an inflammatory response that facilitates bacterial adherence and the risk of UTI increases with duration of bladder catheterization.
Catheter-related bloodstream infection (CRBSI) is the most common cause of nosocomial bacteremia and septicemia. As such, early diagnosis and treatment are vital to reduce the morbidity and mortality involved. The incidence of CRBSI varies considerably by type of catheter, frequency of catheter manipulation, underlying patient-related factors, and local risk factors such as poor personal hygiene, occlusive transparent dressing, and moisture around the exit site ; administration of parenteral nutrition through intravascular catheters choice to treat malignant hyperth risk. The mode of contamination for CRBSI varies with the duration of catheterization (short vs. long). Short-term CRBSIs (<10 days) are extraluminal and are preventable as they result from contamination by normal resident flora of the skin at the insertion site. In contrast, the source of infection is endoluminal that propagates the infection in long-term CRBSI (>10 days) that results in sepsis with multiorgan failure. The organisms most commonly involved in CRBSI are Staphylococci (both S. aureus and the coagulase-negative staphylococci), enterococci, aerobic gram-negative bacilli, and fungal species (e.g., Candida albicans ). The diagnosis of CRBSI requires at least one positive blood culture obtained from a peripheral vein, clinical manifestations of infection (e.g., fever, chills, and/or hypotension), and no apparent source for the blood stream infection (BSI) except the catheter. Antibiotic therapy is often initiated empirically; Vancomycin is recommended for empirical therapy for MRSA. Factors responsible for recurrent bacteremia despite parenteral therapy include antibiotic administration through retained catheter and biofilm formation. Severe sepsis and metastatic infectious complications (e.g., infective endocarditis) prolong the course of CRBSI. Catheters should be removed from patients with CRBSI associated with any local or systemic inflammation or immunocompromised condition.
Surgical interventions (especially thoracic and abdominal) and anesthesia impact pulmonary physiology by decreasing functional residual capacity (FRC). In most patients, this is well tolerated, but patients with underlying pulmonary disease (e.g., chronic obstructive pulmonary disease, emphysema, cigarette smokers, etc.) may be prone to develop pulmonary complications. Identifying “high-risk” patients before surgery can be helpful and preoperative pulmonary function testing, tobacco cessation, or sleep studies may help the surgical team reduce the risk of complications by optimizing the patient’s condition before surgery (e.g., preoperative bilevel positive airway pressure ventilation, bronchodilator therapy, etc.). More recently, standard patient care protocols (e.g., iCough) have been developed to decrease the risk of pulmonary complications, which include incentive spirometry, coughing and deep breathing, oral care (brushing teeth and using mouthwash), elevating the head of bed, and getting out of bed three times a day. Multimodal pain control and judicious use of regional analgesia (e.g., thoracic epidurals) may also help to prevent pulmonary complications in surgical patients.
Atelectasis due to partial or complete collapse of alveoli is the most common respiratory complication in the postoperative patient. Predisposing factors for atelectasis include general anesthesia and upper abdominal or thoracic surgery with stimulation of GI viscera, which can alter diaphragmatic function for several days. The mechanisms include decreased lung compliance (due to reduced FRC), along with accumulated endobronchial secretions, resulting in V/Q mismatch and shunt, which directly correlates with the degree of atelectasis. Anesthesia, cigarettes, morbid obesity, and preexisting pulmonary disease also impair mucociliary clearance and decrease the patient’s ability to cough and clear secretions, contributing to an increased risk of atelectasis.
Atelectasis is the most common cause of postoperative fever in the early postoperative period. It may also present with tachypnea, decreased oxygen saturation ± accessory muscle use. On physical examination, breath sounds may be absent or reduced, or “bronchial” in nature. The chest radiograph may reveal loss of the left hemidiaphragm, air bronchograms, or decreased lung volume with tracheal deviation toward the collapsed side in severe cases. Atelectasis can be reversed in the first 24 to 48 hours with early mobilization, deep breathing (five sequential breaths held for 5–6 seconds), incentive spirometry, coughing, chest physiotherapy, bronchodilator therapy, hydration, and tracheal suctioning. Multimodal pain control using acetaminophen, nonsteroidal antiinflammatory agents, and opioids as needed or regional blocks represent the most commonly effective approach for optimal perioperative pain control.
Nosocomial pneumonia is the second leading cause of nosocomial infection and is more common in surgical patients. The diagnosis of postoperative pneumonia requires the absence of infiltrates prior to admission or before surgery and can be classified as either hospital-acquired pneumonia (developing 48 hours after admission) or ventilator-associated pneumonia (VAP) (pneumonia developing 48–72 hours after endotracheal intubation). Aspiration of oropharyngeal secretions, diminished humoral defense mechanisms, injury to the surface epithelium by instrumentation (endotracheal or nasogastric [NG] tube), azotemia, critical illness, duration of surgery/ventilation, advanced age, preexisting pulmonary conditions (e.g., chronic obstructive pulmonary disease), cigarette smoking within a year prior to surgery, altered sensorium, malnutrition, and prior antibiotic therapy may facilitate colonization. Stress ulcer prophylaxis (histamine 2 [H 2 ] blockers, antacids) and enteral feeding can increase gastric pH, gastric colonization, and aspiration (gastropulmonary route), which plays an important role in the pathogenesis of VAP.
Postoperative pneumonias are commonly caused by gram-negative, aerobic bacteria, S. aureus in neurosurgical patients or fungal organisms in immunocompromised patients. VAP is polymicrobial in nearly half of cases, and the most common organisms include enteric gram-negative bacilli ( Pseudomonas aeruginosa , Actinobacter species, Enterobacter species, Klebsiella species, Serratia marcescens , Escherichia coli , Proteus species, and Legionella species) or gram-positive organisms ( S. aureus ). In surgery, trauma, and critically ill patients, the use of prophylactic antibiotics can alter the microbial flora. In early-onset VAP (<4 days postintubation), the organisms are usually antibiotic (e.g., methicillin) sensitive. In contrast, late-onset VAP (>4 days postintubation) is frequently due to drug-resistant bacteria. Also, risk of VAP is greatest during the first 5 days of mechanical ventilation (3%, with a mean of 3.3 days); thereafter, between 5 and 10 days, the risk declines to 2% per day, further declining to 1% per day after 10 days. Refractory VAP is defined as VAP with failure to improve after 72 hours. Postoperative pneumonia is associated with a high mortality (50%).
A high index of suspicion is required for the diagnosis of postoperative pneumonia, especially in mechanically ventilated patients. Patients with postoperative pneumonia usually present with fever, leukocytosis, and a new pulmonary infiltrate. In intubated patients, VAP should be suspected when two or more of the following clinical features are present (purulent respiratory secretions, temp >38 o C or <36 o C, leukocytosis or leukopenia, or hypoxemia). Hypoxemia should be treated supportively by increasing the inspired oxygen level or positive end-expiratory pressure to increase the PaO 2 to 65 mm Hg, and/or the SpO 2 to 92%. If a newer or persistent chest radiographic abnormality is found, tracheobronchial secretions should be cultured (qualitative or quantitative) and empiric therapy should be started.
The management of postoperative pneumonia depends on the patient’s clinical status, the timing of pneumonia occurrence relative to admission, prior antibiotic exposure, and type of surgery. While intubation and mechanical ventilation may be required, the alternatives to this should be considered (e.g., noninvasive positive-pressure ventilation can be applied to manage hypoxic or hypercarbic failure in some patients) before intubation, which is the main risk factor for VAP. When intubation is considered, the orotracheal route is usually preferred over nasotracheal intubation, which has an increased risk of sinusitis and VAP. In patients intubated for 48 to 72 hours, continuous or intermittent endotracheal and oropharyngeal secretions should be suctioned prior to extubation. Although the use of selective oropharyngeal decontamination is controversial, chlorhexidine may be beneficial in reducing postoperative respiratory infections in cardiac surgery patients. In addition, the risk of VAP can be lowered with preventive care bundles, including hand washing, limiting sedation, elevating the head end of the bed, early mobility, spontaneous breathing trials with early extubation, and low tidal volume ventilation. Prolonged intubation results in biofilm formation and microorganism colonization of the endotracheal tube surface. Coating the endotracheal tube with antibacterial agents (silver, silver sulfadiazine) may help to reduce the risk of VAP in some patients. The use of sucralfate for stress ulcer prophylaxis is associated with a reduced incidence of pneumonia relative to prophylaxis with H 2 receptor antagonists. Patients diagnosed with postoperative pneumonia and VAP should be reevaluated for clinical response (usually marked by improvement of fever, leukocytosis, and infiltrate). Prompt initiation of appropriate antibiotics within 12 hours of diagnosis leads to improved survival, and antibiotic regimen should be modified to specifically cover the pathogens and antibiotic resistance profile of the culture results.
Aspiration of gastric contents in the perioperative period is associated with significant pulmonary morbidity and mortality. Aspiration pneumonitis (Mendelson syndrome) refers to an acute inflammatory injury of the lung parenchyma resulting from aspiration of usually sterile acidic gastric contents (critical pH is 2.5). It can also occur due to aspiration of oropharyngeal contents. Aspiration pneumonia is a common infectious complication of enteral nutrition usually resulting either from contamination of the initial aspirate or secondarily from aspiration of colonized oropharyngeal secretions. Although the underlying patient characteristics that predispose to these conditions are similar, the distinction between the two entities is important since aspiration pneumonia requires antibiotic treatment and aspiration pneumonitis is managed supportively. Surgery patients are at increased risk of aspiration pneumonia since tissue injury, hemorrhage, and anesthesia can contribute to impaired host defenses.
The factors that predispose an individual to increase risk of aspiration include emergency surgery, chronic debilitating disease, oropharyngeal or airway instrumentation (e.g., enteral feeding, prolonged intubation, upper GI endoscopy, or tracheostomy), small bowel obstruction, autonomic neuropathy with delaying gastric emptying, and impaired consciousness (e.g., from general anesthesia, epileptic seizure, trauma, alcohol, drug overdose, or cerebrovascular accident). Aspiration risk inversely correlates with the patient’s Glasgow Coma score. Older patients are at increased risk of oropharyngeal aspiration secondary due to the combination of pharyngeal dysmotility, gastroesophageal reflux, and poor oral hygiene in this population. The common infectious organisms in aspiration pneumonia are E. coli , Klebsiella , Staphylococcus, Pseudomonas, and Bacteroides species.
The pathogenesis and outcomes of gastric aspiration depend on the nature of aspirated matter, volume and pH of gastric acid, and immunologic status of the patient. The more acidic (e.g., pH <2.5) and voluminous (>20 mL) the aspirate, the greater the severity of pulmonary damage. However, independent of acidity, aspiration of gastric fluid with particulate matter causes significant and persistent pulmonary damage. The initial parenchymal inflammatory changes (airspace edema, hemorrhage, hyaline membrane) in Mendelson syndrome are similar to acute respiratory distress syndrome (ARDS) and attributed to neutrophil activation and recruitment and inflammatory cytokines (TNF and IL-8) release. Aspiration of particulate matter can result in death from airway obstruction or subsequent granulomatous reaction to the foreign particles.
Some physicians feel the risk of aspiration can be minimized by monitoring of gastric residual volumes in tube-fed patients. However, there is no consensus regarding the safe or unsafe limits of gastric residual volumes in these patients. Bolus feedings possess a higher risk of aspiration than continuous feeding. The use of small-bore NG tubes has been suggested to decrease aspiration pneumonia and reflux. Some centers prefer to use double-lumen feeding tubes that allow simultaneous postpyloric feeding and gastric decompression.
According to the American Society of Anesthesiology, the routine administration of preoperative medications to reduce gastric pH is not routinely recommended for patients with no increased risk of pulmonary aspiration. The risk-benefit ratio for using prophylactic H 2 receptor antagonists or proton pump inhibitors (PPIs) to reduce the gastric acid pH is favorable in high-risk patients. However, none of these drugs are absolutely reliable in preventing the risk of aspiration pneumonitis.
Aspiration of gastric contents in patients undergoing elective surgery rarely results in severe pulmonary manifestations. However, in critically ill patients, aspiration can present with a range of pulmonary sequelae. Gastric aspiration may cause mild, subclinical pneumonitis, or more severe progressive respiratory failure with significant morbidity and mortality. After an aspiration event, the clinical and radiographic changes begin to appear within the next 24 to 36 hours. Common signs and symptoms of gastric aspiration include fever, cough, rales, diminished breath sounds, wheezing, and infiltrates on chest radiograph. Hypoxia is the earliest and most reliable sign. Arterial blood gas analysis should be performed to determine the severity of hypoxemia. The absence of symptoms in the first 2 hours usually correlates with a benign course. The risks of hypercapnia and acidosis are increased in the presence of particulate matter, and tracheobronchial aspirate should be collected for culture and sensitivity tests. In supine patients, infiltrates are seen in the in posterior segments of the upper lobe or superior segments of the lower lobes due to aspiration. However, aspiration pneumonitis differs from other aspiration sequelae, since it frequently has a rapid onset, is self-limiting, and most radiographic changes of simple toxic aspiration usually clear within 48 hours. In contrast, the persistence of symptoms and associated signs of bacterial infection should raise concerns about the possibility of aspiration pneumonia.
The risk of aspiration is decreased by reducing the volume of gastric contents, minimizing regurgitation, and protecting the airway. Patients should be NPO (nothing by mouth) for 2 or more hours before elective procedures requiring general or regional anesthesia or deep levels of conscious sedation. In intubated patents with suspected or increased risk of aspiration, extubation can be delayed until the patient is fully awake and has protective airway reflexes. Gastric decompression and rapid sequence induction of anesthesia with cricoid pressure has been advocated as the most effective way to prevent aspiration during intubation in high-risk patents but is not 100% effective. When aspiration occurs, the treatment is usually supportive with supplemental oxygen. The airway should be suctioned, the stomach decompressed, and bronchoscopy should be considered for retrieval of aspirated particulate matter. In more severe cases, the patient requires mechanical ventilation and positive end-expiratory pressure.
Antibiotics are not required in most cases since aspirated gastric content is usually sterile. However, in certain cases (e.g., feculent small bowel contents with intestinal obstruction), antibiotics should be administered. Empiric antibiotics (fluoroquinolones, piperacillin/tazobactam, or ceftriaxone) are recommended in patients with small bowel obstruction or ileus and for pneumonitis that fails to resolve within 48 hours. The administration of corticosteroids to reduce pulmonary inflammation is controversial and not routinely recommended.
Respiratory gas exchange occurs in the alveoli of the lung parenchyma. The alveolar wall is composed of a thin alveolar fluid layer with epithelial cells, a basement membrane, interstitial space, and capillary vessels. Oxygen diffuses through this alveolar wall and is transported primarily by red blood cells (RBCs) in the circulation, while CO 2 is dissolved in the blood and passes from the circulation to the alveolar space in the opposite direction. The pulmonary vessels, their normal endothelial barrier, tissue hydrostatic and oncotic pressures, and the lymphatic drainage system maintain fluid balance in the alveolar and interstitial spaces. When normal fluid balance is disrupted, fluid accumulates in the alveolar wall and spaces, resulting in decreased lung compliance, gas exchange, and hypoxemia. Pulmonary fluid imbalance is multifactorial in etiology and can be caused by increased hydrostatic pressure or capillary leak. Examples of these causes include excess IV fluid administration, congestive heart failure, myocardial infarction, valvular dysfunction, arrhythmias, fluid overload, renal failure, systemic infection or inflammation causing increased capillary permeability, radiation pneumonitis, toxin, gastric fluid aspiration, infection, pancreatitis, trauma, burn, interruption of lymphatic drainage, and lung resection surgery or tumor. A sudden increase in pulmonary blood flow can also raise hydrostatic pressure and cause pulmonary edema. Examples of this include reexpansion pulmonary edema, negative pressure pulmonary edema, and rapid change in inspiratory force with upper airway obstruction such as laryngeal spasm after extubation or facial fracture.
ARDS is a severe form of acute lung injury caused by either direct or indirect insult to the lung parenchyma. Direct lung injury is commonly caused by pneumonia, aspiration, chest trauma, or smoke inhalation. Indirect lung injury is typically caused by severe sepsis and systemic inflammation due to pancreatitis, shock, or ischemia/reperfusion. The pathophysiology of ARDS is characterized by inflammation, increased capillary permeability, alveolar edema, and surfactant deactivation. The resulting injury to alveolar epithelium and endothelial damage results in alveolar edema and collapse with impaired gas exchange and lung compliance. Because the inflammatory pathogenesis of ARDS results in the destruction of epithelial and endothelial cells, the resolution takes longer than pulmonary edema.
Patients with clinical risk factors for ARDS may present with shortness of breath, hypoxia, and coughing. Physical examination may indicate decreased breath sounds with crackles during inspiration, tachypnea, use of accessory respiratory muscle, anxiety, agitation, cold and clammy skin, sweating, cyanosis, and arrhythmias. The differential diagnosis of ARDS includes cardiogenic pulmonary edema, which is more commonly seen in patients with preexisting heart disease as signs or symptoms of congestive heart failure (e.g., lower extremity edema, engorged neck veins, and orthopnea). Chest radiographs show perihilar haziness, Kerley B lines, septal lines, thickening of the fissures, “batwing” distribution of air space opacification, pleural effusions, and possibly cardiomegaly in cardiogenic pulmonary edema. Arterial blood gas may show hypoxia with increased PaCO 2 or respiratory acidosis.
Acute lung injury and ARDS are characterized by the acute worsening of respiratory symptoms, usually within 1 week of clinical insult. The chest radiograph in ARDS shows bilateral infiltrates. The normal PaO 2 /FiO 2 ratio is approximately 500. The severity of ARDS is categorized as mild, with a PaO 2 /FiO 2 ratio ≤300; moderate, with a PaO 2 /FiO 2 ratio <200; and severe, with a PaO 2 /FiO 2 ratio <100. In cases where the etiology of pulmonary edema is unclear, measuring central venous pressure and echocardiography can be used to differentiate between cardiogenic and noncardiogenic pulmonary edema.
Patients with pulmonary edema should be positioned upright, given oxygen and ventilatory support as clinically indicated. Oxygen is given to maintain normoxia (SpO 2 <92%, and <88% in patients with chronic obstructive pulmonary disease). Noninvasive ventilation (e.g., continuous positive airway pressure or bilevel positive airway pressure) can be beneficial in patients with hypoxemia to recruit alveoli and improve oxygenation but should not be used in patients with hypotension or possible pneumothorax. In many cases, intubation and mechanical ventilation are required. Diuretics should be given to patients with objective evidence of volume overload, and urine output as well as total fluid intake and output should be monitored. IV furosemide 40 to 80 mg is commonly used and can be given in higher doses to patients who take diuretics regularly or in patients with renal insufficiency. Associated causes of pulmonary edema should also be treated (e.g., inotropic agents or afterload reducing therapy in patients with congestive heart failure).
In patients with ARDS, treatment is focused on the underlying cause (e.g., smoke inhalation, sepsis, etc.), and the care for lung injury is supportive with mechanical ventilation to optimize oxygenation and allow time for the lungs to recover. Noninvasive ventilation (e.g., continuous positive airway pressure or bilevel positive airway pressure) can be used to support oxygenation in patients with mild ARDS who do not require mechanical ventilation. When mechanical ventilation is necessary in ARDS, care should be taken to prevent additional lung injury by minimizing ventilator-induced lung injury. The patient’s ideal or predicted body weight should be calculated and a tidal volume of 6 mL/kg of predicted body weight with plateau pressure of ≤30 mm H 2 O should be used initially according to current ARDS net protocols. There is emerging evidence that protective ventilation strategies like airway pressure release ventilation with prolonged inspiratory phase and positive end-expiratory pressure can help to prevent ventilator-induced lung injury. Because the lung injury in ARDS is frequently heterogeneous, prone positioning can be used to decrease V/Q mismatch in some patients. In severe cases, deep sedation and muscle relaxants may be necessary to facilitate patient-ventilator synchrony. Salvage therapy for refractory hypoxemia in severe ARDS includes extracorporeal membrane oxygenation in some cases. While high-frequency oscillatory ventilation has been used in some situations, a recent trial comparing it to standard ARDS net ventilation showed no survival benefit. Importantly, the cause of lung injury must be managed accordingly. Assessment of arterial blood gases can be used to monitor and adjust ventilator settings of intubated patients. Patients should be weaned from the ventilator and endotracheal tube should be removed as soon as possible.
Virchow triad (venous stasis, endothelial injury, and hypercoagulability) describes the factors contributing to venous clot formation and deep venous thrombosis (DVT). Venous thromboembolism (VTE) includes DVT and pulmonary embolism (PE). DVT describes a blood clot in the deep venous system of the upper or lower extremities. DVTs often start at venous saccules or valves where blood flow is turbulent. Clot formation slows venous blood flow, which further enhances thrombus propagation and proximal extension of the clot. Newly formed thrombi are not firmly attached to the vessel wall and can detach and embolize through the venous system, ending up in the pulmonary vasculature. These pulmonary emboli are subsequently coated with fibrin and platelets, causing mechanical obstruction of the pulmonary blood flow, pulmonary hypertension, and acute right ventricular strain. VTE is associated with increased postoperative morbidity and mortality in surgery patients but are less significant when they are detected early and properly treated. About 50% of VTE occur in current or recently hospitalized patients, especially if they are admitted for surgery. Conditions related to Virchow triad increase the risk of clot formation and VTE development, including long-distance travel (>4 hours), increased age, obesity, frailty, malignancy, nephrotic syndrome, varicose veins, inflammatory bowel disease, prolonged immobilization, history of VTE, inherited hypercoagulation conditions, pregnancy, use of contraceptive medication, trauma, and indwelling venous catheters.
The clinical symptoms of VTE are variable, and patients can be asymptomatic if the clot is small or nonobstructing. However, many patients with DVT have significant symptoms, including unilateral or bilateral swelling of the extremities, warmth, and localized tenderness. In patients with an acute PE, the most common symptoms are dyspnea or shortness of breath. However, there can be significant variability in PE symptomatology depending on the size of the clot, its location in the pulmonary vasculature, and the patient’s underlying cardiac and pulmonary function. Massive PEs can cause obstruction of pulmonary blood flow and acute right heart strain, leading to hemodynamic compromise. Consequently, patients with massive PE can present with life-threatening symptoms of severe hypotension, unconsciousness, and/or cardiac arrest. The majority of PE are not “massive” and do not cause either hemodynamic compromise or right ventricle (RV) dysfunction. Other PE-related symptoms include dyspnea, anxiety, cough, pleuritic or dull chest pain, hemoptysis, and syncope. Physical examination may show tachycardia, low-grade fever, loud P2, or poor perfusion. The arterial blood gas measurement may show hypoxemia with respiratory alkalosis from hyperventilation. Chest radiographs are frequently normal but may show the classic wedge shape opacity (Hampton hump), decreased vascularity, atelectasis, or pleural effusion. Electrocardiography usually demonstrates tachycardia but may also have nonspecific T-wave change, right-axis deviation, right bundle branch block, cor pulmonale, or S1Q3T3. Chest radiography and electrocardiography are not diagnostic, and normal results do not exclude PE. Cardiac biomarkers (troponin T [TnT], TnI, B-type natriuretic peptide [BNP], N-terminal [NT] proBNP) can be elevated due to right ventricular strain and are indicative of higher mortality.
Since clinical findings can be variable, a number of scoring systems have been developed and validated to determine the probability of VTE prior to diagnostic imaging. For example, the Wells criteria categorize patients into likely or unlikely groups and the revised Geneva score categorizes into high-, moderate-, and low-risk group of having VTE. D-dimer is a product of fibrin breakdown, which is increased in VTE but is also elevated in many other conditions and is not useful in patients with recent surgery or trauma. Although these scoring systems and D-dimer level are sensitive screening tools, they are not specific and the diagnosis of VTE should not be made based on these factors alone. In patients where VTE is suspected, imaging studies should be used to confirm or exclude the diagnosis. Moderate-risk and low-risk VTE groups should have D-dimer tested and diagnostic imaging if elevated. However, D-dimer should not be used to screen for VTE in surgery or trauma patients (who have a high incidence of elevated D-dimer) in which diagnostic imaging is preferred. In hemodynamically unstable patients suspected of having massive PE, diagnostic imaging should be done as soon as possible.
Venous duplex ultrasonography is the imaging of choice for DVT showing cross-sectional vein incompressibility, direct thrombus with vein enlargement, abnormal spectral Doppler, and color Doppler flow, which provides useful information regarding clot location and size. For many years, contrast venography was considered the gold standard for DVT diagnosis. However, it is rarely used today because of its invasive nature and its limited availability relative to duplex ultrasonography. Other diagnostic modalities for DVT include CT venography and magnetic resonance (MR) venography. CT pulmonary angiography has become the diagnostic imaging procedure of choice for PE due to the routine availability of the CT scan at most institutions. Conventional pulmonary artery angiography can be useful in select cases but is more invasive and more commonly used when endovascular or surgical intervention is being considered (e.g., pulmonary embolectomy). V/Q scan has high sensitivity in diagnosing V/Q mismatch. Unfortunately V/Q scans can also be abnormal in other pulmonary conditions (e.g., pneumonia, atelectasis, and previous PE). However, V/Q scan is sometimes preferred over CT pulmonary angiography in specific situations like pregnancy, renal insufficiency, and IV contrast allergy. Negative V/Q scans can reliably exclude clinically significant acute PEs. Transthoracic echocardiography (TTE) is commonly used when there are concerns about cardiac function and may show thrombus in the RV strain. A nondiagnostic TTE does not exclude PE, and positive findings provide indirect evidence of PE and should be carefully interpreted. However, TTE is a useful technique for bedside evaluation and follow-up and is especially useful in emergency situations when emergency thrombolytic therapy is used. Transesophageal echocardiography can give better direct visualization of pulmonary thromboembolism than TTE, and transesophageal echocardiography is the preferred over TTE if available.
Many surgery patients are at increased risk of VTE and should be risk stratified for prophylactic therapy. Many validated scoring systems have been developed to quantify the VTE risk, including the Caprini score, the Department of Health VTE risk assessment tool, the Padua score, the IMPROVE score, and the Rogers score. Although pharmacologic DVT prophylaxis is generally considered to be “low risk” for bleeding complications, certain groups are considered “high risk” for bleeding complications. Patients with active GI bleeding, intracranial hemorrhage, liver disease, bleeding disorder, thrombocytopenia, recent head trauma or spinal injury/surgery, or relative contraindication to anticoagulants should be carefully assessed for risk-benefit ratio before initiating pharmacologic VTE prophylaxis.
VTE prophylaxis modalities include pharmacologic prophylaxis with low–molecular-weight heparin (LMWH), unfractionated heparin (UFH), fondaparinux, and mechanical prophylaxis with intermittent pneumatic compression, foot pumps, and graduated compression stockings. Decisions regarding VTE prophylaxis modalities are based on assessment of VTE and bleeding risk in the specific patient or population. In high-risk VTE patients (Caprini score ≥5), pharmacologic prophylaxis is preferred, if not contraindicated. Mechanical prophylaxis is often used in low-risk groups (Rogers score 7–10, Caprini score 1–2) or when patients have high bleeding risk or if anticoagulants are contraindicated. In very low risk (Rogers score <7, Caprini score 0), frequent mobilization and early ambulation are preferred. Vena cava filters are considered on a case-by-case basis in high-risk patients with contraindications to anticoagulation.
Patients diagnosed with acute DVTs are started on therapeutic anticoagulation using LMWH, UFH, or fondaparinux for 5–10 days and usually transitioned to long-term anticoagulant therapy with LMWH, vitamin K antagonist (VKA), or direct oral anticoagulants for at least 3 months. VKA medications are usually given to overlap with parenteral anticoagulant until a target international normalized ratio (INR) of 2 to 3 is reached. Extended treatment with anticoagulants beyond 3 months is considered in specific cases (e.g., inherited hypercoagulable state or chronic medical conditions with high thrombotic risk). Vena cava filters can be considered in patients with contraindications to anticoagulants, bleeding from anticoagulant, or in patients with recurrent VTE despite anticoagulation. However, patients who have recurrent VTE despite anticoagulation and have vena cava filters placed should still be given anticoagulants if possible. In severe cases, such as impending venous gangrene, thrombectomy should be considered.
In suspected PE patients, the management depends on severity and mortality risk assessed by patient conditions: hemodynamic instability, RV failure, cardiac biomarkers, and PE severity index. Patients with hypotension, RV failure by echocardiography or CT, elevated cardiac biomarkers, and PE severity index score of class III or higher are considered high risk for mortality. The first priority is to maintain hemodynamics and ventilation and the patient care team should be prepared for cardiac life support in case of cardiac arrest. Patients may need intubation, mechanical ventilation, and vasopressors to maintain blood pressure and cardiac output. IV fluids should be administered carefully in patients with PE since increased vascular volume can worsen right heart failure. Patients should be cared for in an ICU setting with central venous catheter, arterial line, electrocardiogram monitoring, and bladder catheterization with strict intake and output. Parenteral anticoagulation should be initiated immediately unless strongly contraindicated, and echocardiography should be considered when patients are hemodynamically unstable. Levophed is commonly used as the vasopressor of choice in this situation and interventions to treat obstructing PE causing severe pulmonary hypertension and RV failure should be considered. These include fibrinolytic drugs like streptokinase, urokinase, recombinant tissue plasminogen activator (rTPA) or catheter-directed therapy, and surgical embolectomy. Fibrinolytic therapies are usually preferred over the surgical or catheter-based interventions but have a higher incidence of bleeding complications (e.g., major hemorrhage or intracranial bleeding). Catheter or surgical embolectomy is considered in patients with contraindications to fibrinolytic therapy, patients who do not improve with fibrinolysis, or patients with severe hemodynamic instability. Catheter-directed therapy allows for the administration of fibrinolytic medication at the site of the clot and is associated with fewer systemic bleeding problems. Complications of catheter-directed therapy include pulmonary artery injury, cardiac tamponade, distal embolization, hemoptysis, and groin hematoma.
Extracorporeal membrane oxygenation can both provide hemodynamic support and improve oxygenation and is considered in potentially reversible patients for stabilization prior to embolectomy. Other possible causes of hemodynamic instability should be evaluated, including acute valvular dysfunction, myocardial infarction, cardiac tamponade, and aortic dissection. However, the majority of PE cases are less severe and hemodynamically stable, and the management is similar to DVT with therapeutic anticoagulation and transition to long-term anticoagulants for usually 6 months. The routine use of fibrinolytic medication for PE outweighs the benefits in most patients and should be reserved for hemodynamically unstable patients with clinical deterioration despite anticoagulation and aggressive supportive care.
For management of anticoagulants, SQ LMWH and fondaparinux are preferred over UFH due to lesser risk of major bleeding and lower incidence of heparin-induced thrombocytopenia (HIT). Examples of LMWH are enoxaparin, tinzaparin, dalteparin, and nadroparin. However, they should not be used in patients with renal impairment due to possible accumulation and bleeding complications. IV UFH is preferred in patients whom reperfusion therapy is considered due to its shorter half-life, ease of effect monitoring, availability of rapid discontinuation, and protamine reversal, and UFH should be held during infusion of fibrinolytic medication. An initial bolus of 80 units/kg is administered, and then 18 units/kg/h. The medication effect of UFH is monitored by activated partial thromboplastin time (aPTT), with a target of 1.5 to 2.5 times control value, or plasma heparin antifactor Xa 0.3 to 0.7 IU/mL. Complications include bleeding and HIT. LMWH has better bioavailability and can be given subcutaneously, with a longer half-life. The dose is calculated based on actual body weight: enoxaparin 1 mg/kg 2 times/day or 1.5 mg/kg once daily, tinzaparin 175 U/kg once daily, dalteparin 100 IU/kg 2 times/day, 200 IU/kg once daily, nadroparin 86 IU/kg 2 times/day, 171 IU/kg once daily, and LMWH does not require laboratory monitoring. However, the dose of LMWH must be carefully titrated in patients with renal insufficiency, especially since LMWH is not reversible with protamine. In patients diagnosed with HIT, both UFH and LMWH should be avoided. Direct thrombin inhibitors, hirudin, argatroban, and bivalirudin inhibit conversion of fibrinogen to fibrin and is reserved for patients likely to develop HIT. Hirudin (0.4 mg/kg IV) is given followed by an infusion of 0.15 mg/kg/h; the dose must be adjusted to renal function, and levels are routinely monitored by aPTT to achieve 1.5 to 2.5 times the laboratory normal value. Argatroban is given, 2 μg/kg/min IV, with an aPTT target of 1.5 to 3 times the normal laboratory value. Fondaparinux is given daily at a dose of 5 mg SQ if body weight is less than 50 kg, 7.5 mg SQ if body weight is between 50 and 75 kg, and 10 mg SQ if body weight is more than 100 kg. Fondaparinux is contraindicated in renal insufficiency patients (creatinine clearance <30 mL/min). Oral VKA is started as soon as oral intake is possible until reaching a target INR of 2 to 3 for two consecutive days. Patients are followed up every 4 weeks during VKA treatment for INR testing and dose adjustment if required, or at 6 weeks in stable cases. Patients who have INR >4.5 but <10 are advised to stop taking VKA without giving vitamin K, and patients with life-threatening bleeding are admitted and should receive vitamin K and 4-factor prothrombin complex concentrates or fresh-frozen plasma if not available. Direct oral anticoagulants, dabigatran, rivaroxaban, apixaban, and edoxaban, are recommended as alternative to heparin/VKA treatment. Physicians should be aware of drug interaction in patients receiving anticoagulation.
Perioperative myocardial ischemia and infarction (PMI) are important and potentially severe complications in noncardiac surgery. They are the most common cardiac complications that increase length of hospital stay and cost of care and worsen prognosis. Normally, acute myocardial infarction is diagnosed by an elevation of cardiac enzyme with either ischemic symptoms, electrocardiographic changes attributable to myocardial ischemia, infarction, or imaging findings. However, 65% of patients with PMI do not experience ischemic symptoms, according to the Perioperative Ischemia Evaluation (POISE) trial, and only 41.8% of patients with myocardial injury after noncardiac surgery met the criteria for myocardial infarction in the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) cohort study of 15,065 patients. Elevation of cardiac troponin alone is an independent risk factor of myocardial ischemia and is associated with increased postoperative mortality. In short, if the diagnostic criteria of chest pain with associated signs and symptoms, serum markers, and change in electrocardiogram were used, we would routinely underdiagnose PMI and myocardial injury after noncardiac surgery.
The cause of PMI is from two main mechanisms. The first is the classic theory of plaque rupture and thrombosis of coronary vessels occluding the blood supply to myocardium. The second is the imbalance between oxygen demand and supply during the perioperative period and postoperative care. Therefore, patients at risk for cardiac complications are those who have underlying coronary artery disease, recent myocardial infarction or stenting, recent stroke, and other risk factors for coronary disease, including peripheral arterial disease, diabetes, hyperlipidemia, smoking, family history, or hypertension. Other risk factors are the types of surgery and perioperative events contributing to alteration of oxygen demand and supply. Major surgeries are more stressful to the patient and can impact normal homeostasis by causing inflammation, hypercoagulability, platelet activation, tachycardia, or high blood pressure, which exert stress on coronary vessels, leading to preexisting plaque rupture. The stress response to surgery activates the sympathetic nervous system, resulting in elevated catecholamines, which can lead to coronary vasoconstriction and increased myocardial oxygen demand. The examples of major surgeries with increased risk of PMI include major vascular surgery, noncardiac transplant surgery, intraperitoneal and thoracic surgery, and emergency surgery. During the perioperative period, alterations in hemodynamics, oxygenation, and ventilation related to surgery can also affect coronary blood flow and cause imbalance of myocardial oxygen demand and supply. Examples include hypoxia, hypothermia, hypotension, hypertension, tachycardia, bleeding, and anemia.
Most patients have perioperative ischemia or infarction within 48 hours after surgery. Symptomatic patients may develop substernal chest pain or pressure radiating to the left shoulder or neck. They may also experience arrhythmia, tachycardia, sweating, dyspnea, or signs of heart failure, hypoxia, acidosis, cardiogenic shock, and cardiac arrest. When these symptoms occur, cardiac biomarkers should be measured and electrocardiography should be performed. Changes in electrocardiography are another indication of cardiac ischemia and range from ST-segment elevation, ST depression, T-wave inversion, or Q waves. However, most patients with perioperative ischemia or infarction are asymptomatic, and there are still no standardized diagnostic criteria for this group. Cardiac biomarkers are associated with perioperative ischemia and infarction and may be the only abnormality detected. Elevations of TnT and TnI are associated with higher mortality since concentrations are normally very low (TnT 0.02 ng/mL, TnI 0.2 ng/mL). Also, preoperative natriuretic peptides (BNP, NTproBNP) are shown to be predictive of ischemia or infarction, and creatine kinase MB fraction is also elevated but has lesser sensitivity and specificity. Screening for PMI by routinely checking troponin levels in surgical patients remains a controversial topic according to recent clinical practice guidelines. The 2014 American College of Cardiology and American Heart Association (ACC/AHA) guidelines recommend troponin screening be considered only in patients with signs and symptoms suggestive of myocardial infarction both before and 48 to 72 hours after surgery. Their rationale is that troponin levels may be elevated in different medical conditions like renal insufficiency, heart failure, PE, and atrial fibrillation. Furthermore, most high-risk patients present with high troponin levels before surgery. There is insufficient evidence that biomarker screening reduces cardiac events; therefore, routine screening is not recommended (2014 AHA guideline).
In contrast, the 2014 European Society of Cardiology and the European Society of Anaesthesiology guidelines recommend screening every high-risk patient both before surgery and 48 to 72 hours after surgery. Consequently, the diagnosis and management of patients with perioperative ischemia who do not fulfill the standard criteria of myocardial infarction should be individualized based on the patient’s characteristics, risks, and biomarkers.
Identification of patients with increased risk of developing PMI allows their physicians to more carefully evaluate their cardiac risks before surgery. Several methods for cardiac risk stratification are available, and the most commonly used today is the revised cardiac risk index (RCRI). The RCRI assesses the perioperative risk of major cardiac complications, including myocardial infarction, pulmonary edema, ventricular fibrillation, or cardiac arrest, and complete heart block based on six patient characteristics ( Box 12.2 ). Patients with at least two risk factors are at increased risk for cardiac complications. Other common risk-calculating methods are the NSQIP-myocardial infarction or cardiac arrest ( www.surgicalriskcalculator.com/miorcardiacarrest ) and the NSQIP risk calculator ( www.riskcalculator.facs.org ) developed by the American College of Surgeons, which are commonly used in the United States. Patients are categorized as low risk (risk <1%) or elevated risk (risk >1%). If categorized by surgical procedure, it can be grouped as low, intermediate, and high risk ( Table 12.9 ).
Patients with two or more risk factors are considered elevated risk.
High-risk type of surgery (intraperitoneal, intrathoracic, suprainguinal vascular surgery)
History of ischemic heart disease
History of congestive heart failure
History of cerebrovascular disease
Insulin therapy for diabetes
Preoperative serum creatinine >2 mg/dL
| Level of Risk | Risk Factor |
|---|---|
| High (cardiac risk often >5%) | Emergency major operations, particularly in elderly patients |
| Aortic and other major vascular surgery | |
| Peripheral vascular surgery | |
| Anticipated prolonged surgical procedures associated with large fluid shifts and blood loss | |
| Intermediate (cardiac risk generally <5%) | Carotid endarterectomy |
| Intraperitoneal and intrathoracic surgery | |
| Orthopedic surgery | |
| Prostate surgery | |
| Low (cardiac risk generally <1%) | Endoscopic procedures |
| Superficial procedures | |
| Cataract surgery | |
| Breast surgery |
Before surgery, physicians should obtain and evaluate the patient’s functional class since poor functional class is associated with cardiac complications. Electrocardiography should be done within 3 months before surgery, and patients with unknown dyspnea or change in functional class should have left ventricular function examined. Functional class can be assessed by daily activity using metabolic equivalence of the task (MET), where 1 MET is equal to the resting oxygen consumption of a 40-year old, 70-kg man. Simple questions about daily activities can be asked to evaluate the patient’s conditions. Examples of activities, which patients with poor functional class (METs <4) are able to do, are walking slowly (3 mph), ballroom dancing, working on a computer, and playing a musical instrument, and activities indicating moderate or great functional class (METs ≥4) include gardening, walking up a hill, biking, and heavy work around the house. Recommendations for perioperative testing by the ACC/AHA are presented in Fig. 12.1 to guide management and reduce risk before surgery. Perioperative beta blockers have been shown to reduce the risk of myocardial infarction and are recommended before surgery; however, beta blockers may also increase the risk of stroke, bradycardia, and mortality in some patients. Therefore, beta blockers should be continued in patients who already take them and initiation of beta blockers should be considered in high-risk patients (≥3 RCRI) several days before surgery. Aspirin and statins are two commonly used medications in patients with coronary disease. Initiating aspirin before surgery is controversial due to the potential risk of bleeding. However, aspirin should be continued in patients taking aspirin with a history of coronary disease or stenting. Statins have also been shown to reduce postoperative cardiovascular events and should be continued or initiated especially in vascular surgery patients.
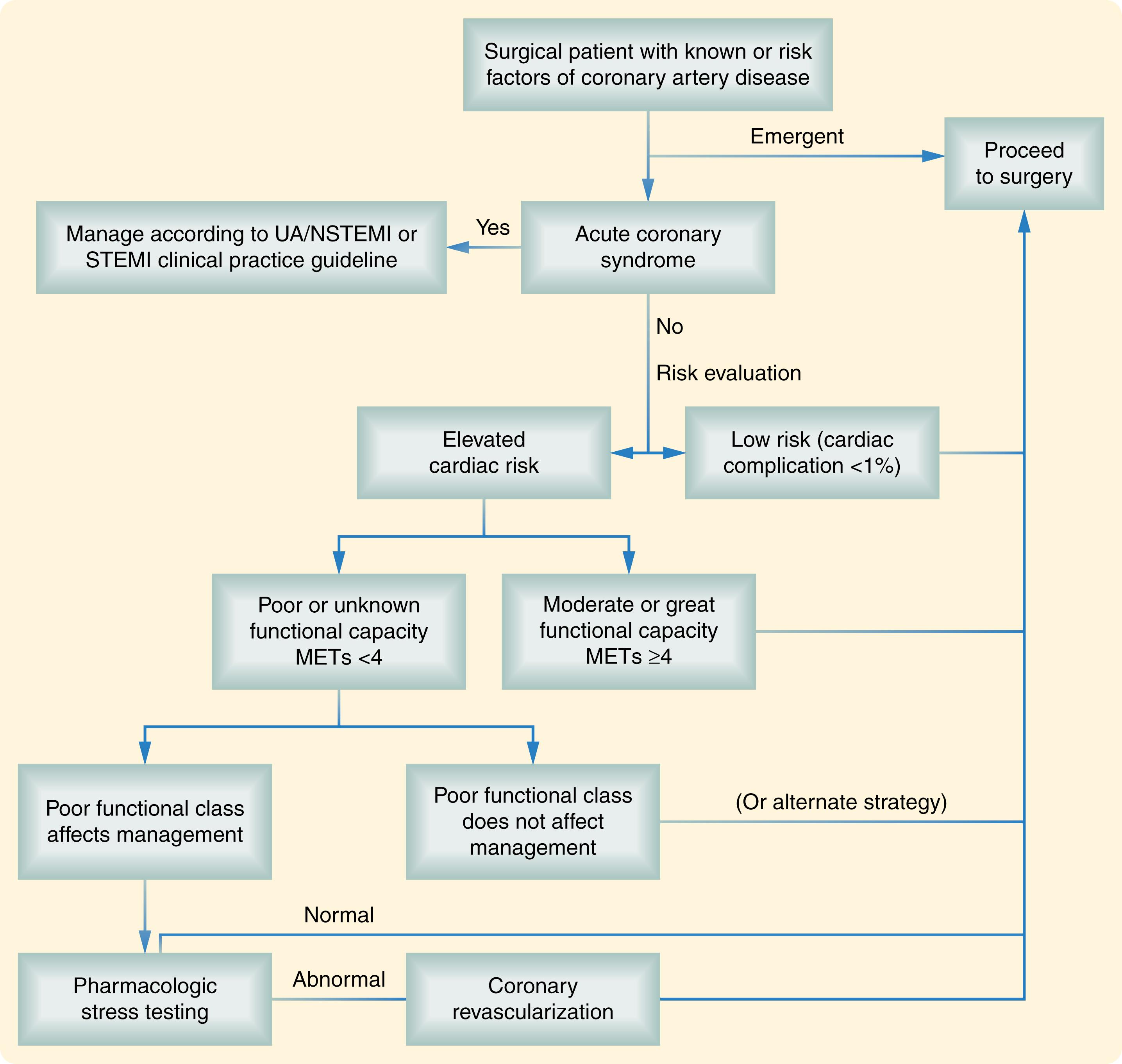
Patients with coronary disease who have been treated with percutaneous coronary intervention (PCI) ± stents are placed on dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 receptor inhibitor to prevent thrombosis. Thrombotic risk is considered low 4 weeks after balloon angiography), 6 months after bare-metal stents, and 1 year after drug-eluting stents. Elective surgery for PCI patients should be postponed if possible during the high-thrombotic-risk periods described above. Perioperative management of DAPT for noncardiac surgery patients should consider the risk of thrombosis and the risk of hemorrhage. Specific guidelines are available to guide perioperative antiplatelet therapy, but surgeon judgment is required to optimize care for individual patients. Anemia should be corrected to optimize oxygen delivery during operation.
During the operation, surgeons and anesthetists should maintain good blood pressure and oxygenation, minimize bleeding, avoid hypothermia, and provide adequate pain control. However, there is no specific evidence-based intraoperative values or goals of vital signs and measurements. Monitoring these variables should be continued in the postoperative period, and electrocardiography should be obtained if PMI is suspected.
Once myocardial tissue has been damaged, the median time from PMI to death is approximately 12 days. The challenge in management comes in determining the pathophysiology of PMI between plaque rupture/thrombosis (type I) or imbalance of oxygen demand and supply (type II), and there is no standard or international criteria for diagnosis and management. However, ST-elevated myocardial infarction and unstable angina/non-ST-elevated myocardial infarction should be managed according to clinical practice guidelines, but recent major surgery is a relative contraindication of fibrinolytic drugs. Judgment in managing patients not fulfilling the criteria for ST-elevated myocardial infarction and unstable angina/non-ST-elevated myocardial infarction should be individualized with consideration of a cardiology consult. Patients with suspected type I injury should receive aggressive aspirin therapy, with caution exerted for bleeding risk, and statin therapy with consideration of angiography. Secondary prevention using beta blockers and angiotensin-converting enzyme (ACE) inhibitors should be given when feasible. Patients suspected of type II injury should have optimal hemodynamics and oxygenation therapy with possible angiography during follow-up after surgery. The consequences of severe myocardial infarction can be life-threatening and include cardiogenic shock or cardiac arrest. Life-threatening complications of severe myocardial infarction include free rupture of the cardiac wall or septum, acute mitral valve regurgitation from rupture of chordae tendineae, and complete heart block. Aggressive management is required to prevent death and includes hemodynamic and oxygenation support in case of cardiogenic shock and cardiopulmonary resuscitation in cardiac arrest. However, the mortality in this group is as high as 70%.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here