Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The majority of tumors of the lateral and third ventricles are benign or low-grade lesions. Because of their relatively slow growth rate, these lesions may reach several centimeters in size before patients present with neurologic dysfunction. The most common clinical manifestations of these tumors include headaches, memory loss, gait disorders, and cognitive changes.
There are multiple surgical approaches to these tumors, all designed to minimally displace or disturb normal anatomy. Before embarking on an approach, the surgeon should be familiar with both the ventricular anatomy and the options for optimally accessing these lesions. The lateral and third ventricles can be reached by transcallosal, transcortical, and, in some cases, supracerebellar, subfrontal, pterional, and transtentorial approaches. Endoscopic techniques are a valuable addition to the surgeon’s options for operative management. In recent literature, there has been an emergence of minimally invasive transsulcal resection of intraventricular lesions through white matter–splitting tubular retractors.
Because intraventricular surgery requires manipulation deep within the hemispheres, proper patient positioning, adequate tumor exposure, and brain relaxation are fundamental requirements for successful tumor removal. Intraoperative morbidity can be minimized by careful attention to presurgical planning and contingency strategies when required. Because cognitive deficits are the most commonly encountered preoperative sign of an intraventricular lesion, persistent postoperative cognitive liabilities and hydrocephalus merit close attention. For this reason, baseline preoperative neuropsychometric testing can be useful when feasible. There are several published alternative surgical approaches that have been used for accessing the ventricular system (interhemispheric-transcortical, transsylvian fissure) that this chapter will not present. Although these alternative approaches may have some merit, the authors consider them to be of limited value for the vast majority of intraventricular tumors. This chapter will address proven and reliable methods to maximize tumor removal with minimal morbidity.
The lateral ventricles are divided into five areas: frontal horns, bodies, atria, occipital horns, and temporal horns ( Fig. 19.1 ).
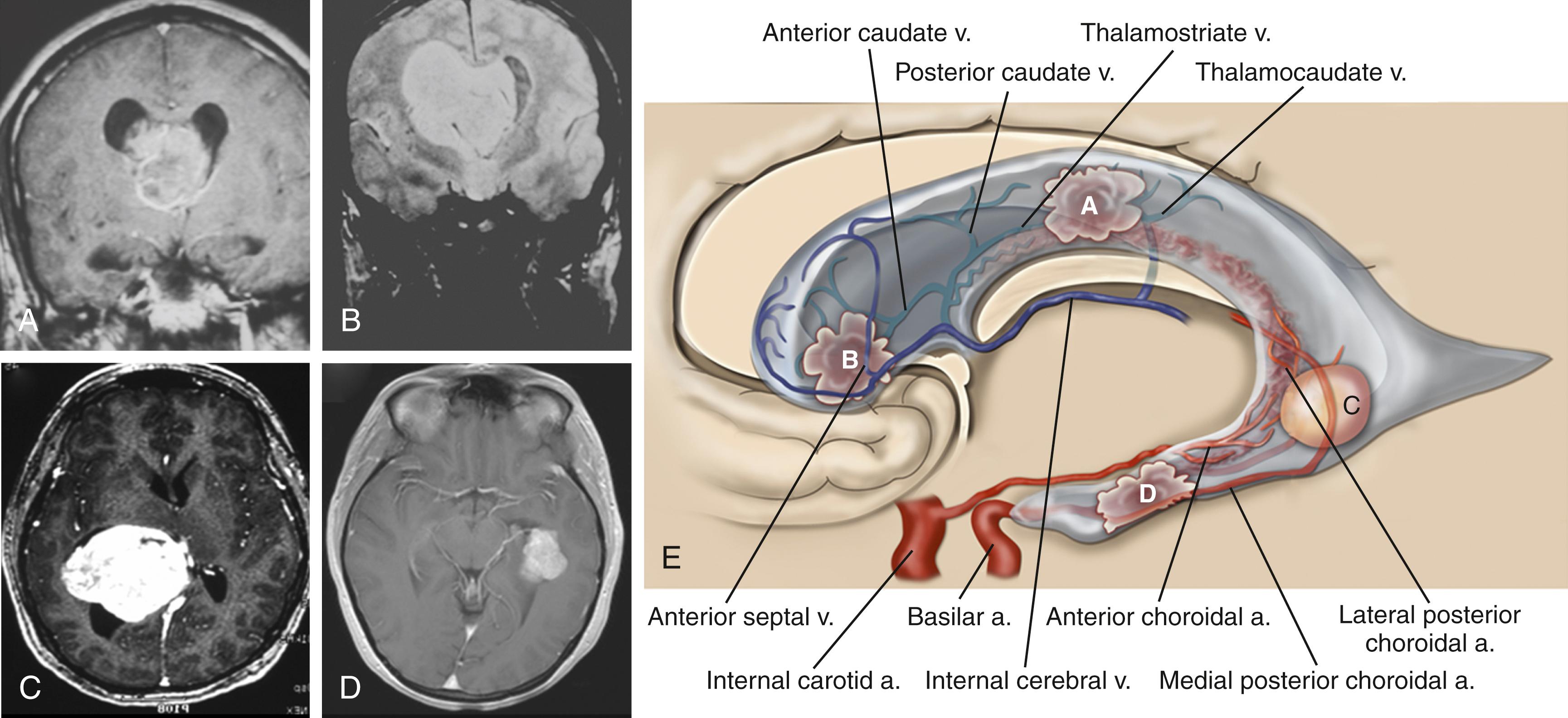
The frontal horns are triangular extensions of the lateral ventricles anterior to the foramen of Monro and are bounded laterally by the head of the caudate, anteriorly and superiorly by the corpus callosum, and medially by the septum pellucidum. The foramen of Monro marks the posterior extent of the frontal horns, and the boundary consists of the forniceal columns that run just anterior to the foramen of Monro as they bend inferiorly to start their descent toward the mammillary bodies. The frontal horn contains no choroid plexus but has on its wall two important veins that help with surgical orientation. On its medial border, the septal vein leads into the medial foramen of Monro, where it enters the velum interpositum in the roof of the third ventricle to join the internal cerebral vein (ICV). Laterally, the anterior caudate vein runs medially to join the thalamostriate vein near the foramen of Monro.
The body of the lateral ventricles begins at the posterior edge of the foramen of Monro and extends posteriorly to the anterior border of the splenium of the corpus callosum. The body of the ventricle is covered superiorly by the corpus callosum and laterally by the body of the caudate. There are two sets of veins that travel on the lateral wall of the body: the more anterior thalamocaudate (the size of which is inversely proportional to the thalamostriate vein) and the posterior caudate vein, which drains either into the thalamostriate or directly into the ICV through the velum interpositum. Inferiorly, the junction of the lateral wall and floor of the body of the ventricle is demarcated by the striothalamic sulcus, which separates the caudate from the thalamus. In this sulcus, the thalamostriate vein courses anteriorly to the foramen of Monro to drain into the ICV. Separating the thalamus from the body of the fornix is the choroidal fissure. The medial posterior choroidal artery, which enters the ventricular system just lateral to the pineal and travels anteriorly in the roof of the third ventricle in the velum interpositum, can be seen in the lateral ventricle as it ascends through the foramen of Monro and bends posteriorly to run in the direction of the choroid plexus. Medially, the two leaves of the septum pellucidum separate the two ventricles.
The trigone or atrium of the lateral ventricles is a confluence of the body and temporal and occipital horns. The atrium begins as a continuation of the body at the posterior edge of the thalamus and ends further posteriorly as the corpus callosum blends into the occipital lobe. The splenium (superiorly) and the tapetum of the corpus callosum (more posteriorly) make up the roof of the atrium. Because the roof bends into the lateral wall posteriorly, the tapetum covers this lateral wall segment. More anteriorly, the caudate tail covers the lateral wall as it curves downward, on its way toward the temporal lobe. The anterior boundary of the atrium starts just medial to the caudate tail with the pulvinar eminence of the thalamus. Medial to the pulvinar, covered by choroid, is the crus of the fornix. At the atrial level of the choroid plexus, two choroidal arteries can often be seen, one curving with the choroid medially, and the anterior choroidal artery, which can course into the body of the ventricle. More laterally, the lateral posterior choroidal artery, which may have several branches, runs to supply the atrium and body of the choroid. The triangular enlargement of the choroid plexus at the trigone is called the glomus. The medial wall of the atrium has two prominences. The upper prominence consists of the forceps major fibers and is called the bulb of the corpus callosum. The lower prominence is called the calcar avis and is simply the ventricular protrusion of the calcarine sulcus. The floor similarly consists of the upward protrusion of the collateral sulcus forming the collateral trigone.
The occipital horn is a posterior extension of the atrium and can vary in size. Medially, the wall consists of the same structures that make up the atrial medial wall, namely, the forceps major superiorly and, inferiorly, the calcar avis. Likewise, the collateral trigone forms the floor of the occipital horn. The roof and lateral wall blend into one and are both covered by the tapetum. There is no choroid in the occipital horn.
The temporal horn is an extension of the lateral ventricles into the medial temporal lobe. The floor displays two prominences: (1) laterally, the collateral eminence formed by the underlying deep collateral sulcus and (2) medial to that, the hippocampus, which protrudes prominently into the floor. The lateral wall, which angles into the roof of the temporal horn, is lined by the tapetum. In the medial part of the roof, the tail of the caudate projects anteriorly toward the amygdaloid nucleus. Medial to the caudate tail, forming the medial wall of the temporal horn, is the thalamus and, inferior to it, the fimbria of the fornix. The choroid fissure separates the thalamus from the fornix. The choroid plexus is attached as it continues anteriorly to end just posterior to the amygdaloid nucleus at the inferior choroidal point. The anterior choroidal artery enters the choroidal fissure at approximately this point and courses posteriorly in the plexus. More posteriorly, the lateral posterior choroidal artery enters the fissure and is seen more laterally in the choroid plexus. The temporal horn ends in the amygdaloid nucleus.
The anterior transcallosal route is useful for lesions arising within the body of the lateral ventricle (see Fig. 19.1A ). Cortical veins draining into the sagittal sinus can be a significant obstacle to interhemispheric access, and a preoperative cerebral angiogram or magnetic resonance venogram can be important for surgical planning. Furthermore, the ventricular venous and arterial structures can be distorted by the tumor and should be noted preoperatively. The patient is placed in a supine position, with the head slightly flexed. Alternatively, the patient’s head is fixed in a lateral position with the affected hemisphere toward the floor to use gravity to assist with retraction. A bicoronal incision is made anterior to the coronal suture. The craniotomy is also centered anterior to the coronal suture. Midline exposure should extend up to the superior sagittal sinus. The dura is opened and reflected medially up to the sagittal sinus. Often, cortical draining veins enter the dura before reaching the midline. These veins may be preserved by opening the dura on all sides around the veins, leaving the dura covering the venous access to the sagittal sinus intact. If exuberant arachnoid granulations are encountered, they can be divided with sharp dissection and bipolar cautery. Once the midline is reached, the falx is followed to its depth. At this point, the operating microscope is used. The arachnoid below the falx may be adherent, and this must be carefully divided to avoid injury to the cingulate gyrus on either side. Once the corpus callosum is reached, the two pericallosal arteries are visualized ( Fig. 19.2 ). Ventricular access between the two arteries helps to prevent vascular injury. The midline of the corpus callosum is often demarcated by a very small callosal artery. The callosotomy can be started just posterior to the genu and developed 2 to 3 cm posteriorly to gain access to the lateral ventricle. By performing the callosotomy off midline and toward the ventricle of interest, opening the contralateral ventricle can be avoided. Proper orientation is confirmed by locating the choroid plexus and the thalamostriate vein entering into the foramen of Monro. If the vein is to the right of the choroid plexus, the surgeon is in the right ventricle, if the vein is to the left of the choroid, the surgeon is in the left ventricle ( Fig. 19.3 ). Throughout the course of tumor resection, the interface between the tumor and the ependymal surface ( Fig. 19.4 ) should be identified and preserved because this prevents losing the plane of dissection. Because many lateral ventricular tumors can reach a very large size, an internal decompression may be required prior to isolating the tumor capsule away from surrounding ventricular structures. ,
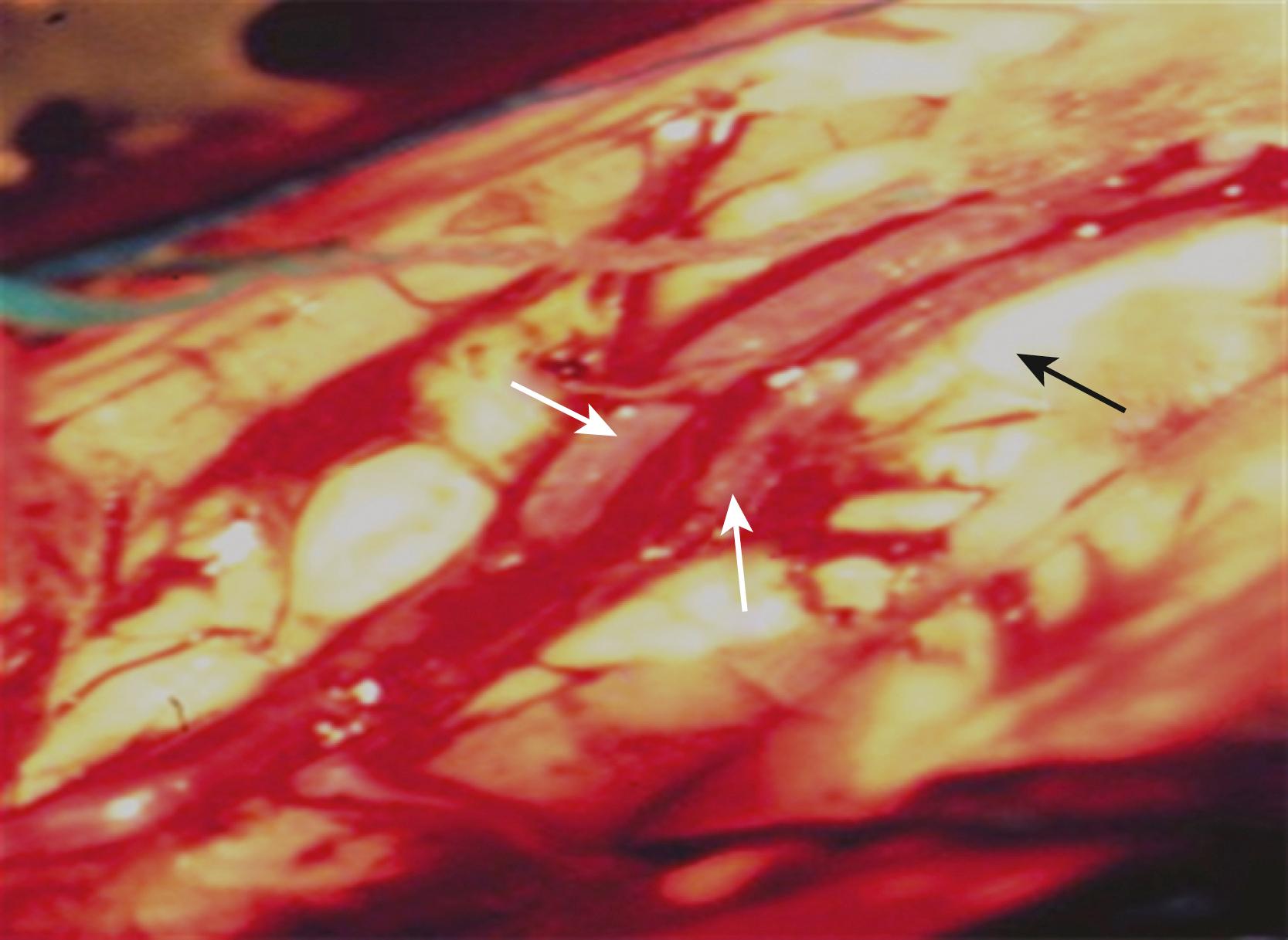
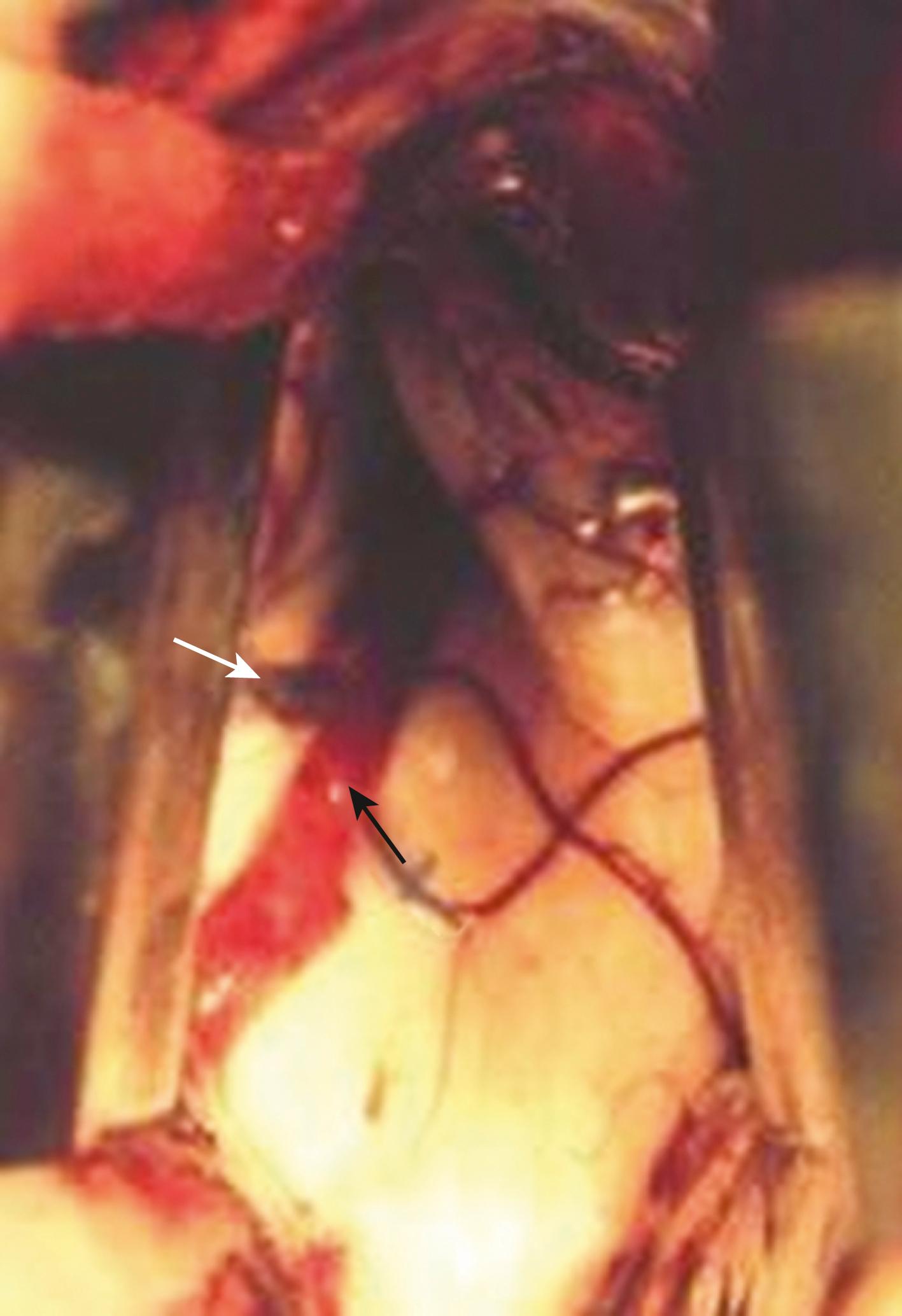
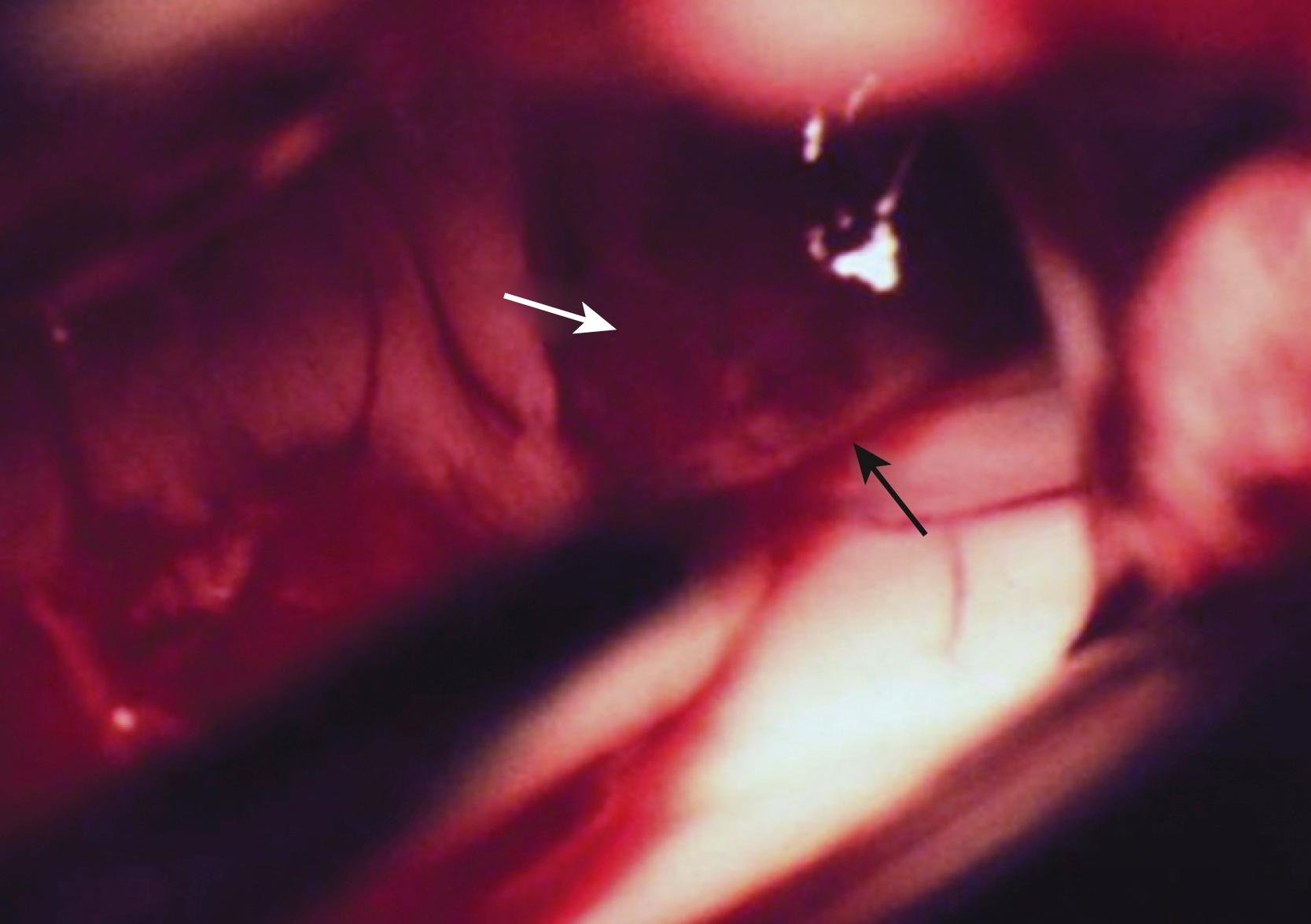
The use of the operative microscope at this stage merits consideration. High magnification is helpful in identifying tumor anatomy and vascularity but can diminish an appreciation of the volume of the lesion outside the field of view and its interface with regional anatomy. Expanding the operative field by zooming out to lower magnification can be helpful in reorienting the surgeon and in maintaining the interface with the ependymal surface.
The anterior transsulcal approach (superior frontal sulcus) is used commonly for lesions in the anterior lateral ventricles, especially when associated with hydrocephalus. In addition, this approach is preferred over the transcallosal route to avoid large, midline-draining cortical veins. The craniotomy is similar to the one used for the transcallosal approach and uses a bicoronal incision lateral to the midline, measuring 4 to 6 cm in length. The superior and middle frontal gyri need to be exposed. Intraoperative ultrasound or stereotaxis can provide guidance for direct ventricular access. The sulcus is dissected open to its depths (or a 2- to 3-cm gyral incision is made) and developed down into the ventricle. The operative microscope is used after the ventricular chamber is opened. Tumor removal is achieved by delivering the lesion to the area of exposure while maintaining the tumor interface with the ependymal surface.
Very large tumors that extend into the frontal horn and body of the ventricle may require both a transsulcal and transcallosal approach (see Fig. 19.1B ). This is necessitated by the limitations of each operative exposure. Entering the ventricle through the corpus callosum cannot provide access to the frontal horn without excessive retraction. Similarly, an anterior transsulcal exposure limits access to the posterior body of the ventricle. In those tumors that fill the lateral ventricle, an initial decompression through a transsulcal corridor can generate adequate relaxation of the hemisphere to permit interhemispheric dissection. Once this is achieved, the transcallosal corridor can be opened to complete the tumor resection without excessive retraction to either region.
The interparietal sulcus approach (superior parietal lobule) is the preferred route to the atrium of the lateral ventricle and allows access to both medial and lateral segments of this part of the ventricle (see Fig. 19.1C ). The patient is positioned in the three-quarter prone position with the parietal region at the highest point in the field. The craniotomy extends over the superior parietal lobule. A preoperative magnetic resonance venogram or cerebral angiogram is helpful in determining the position of major draining veins. The craniotomy does not cross midline. Once the cortex is exposed (or the cortical incision is made), dissection proceeds along the interparietal sulcus. The atrium is more lateral at this location, and the dissection can be guided by ultrasound or stereotaxis. Once inside the atrium, the surgeon can visualize the thalamus anteriorly, the choroid plexus more medially, and the crus of the fornix. It should be remembered that the optic radiations define the lateral wall of the atrium, and the surgeon should avoid manipulation of that area. When tumors compress the lateral wall of the atrium, the tumor should be decompressed before separating it from this lateral ependymal surface. The vascular pedicle of the tumor should be identified and coagulated at the earliest possible time to avoid excessive bleeding because bleeding from the ependymal surface can be particularly challenging to control. ,
The posterior transcallosal approach gains access to the roof and medial part of the atrium of the lateral ventricles. This is achieved by splitting the splenium of the corpus callosum and is contraindicated for patients with a preoperative homonymous hemianopia contralateral to the dominant hemisphere due to a risk of alexia. Because the lateral ventricle extends laterally in this region, the lateral part of the atrium is not well visualized by this route. Preoperatively, as in the anterior approaches, a magnetic resonance venogram or cerebral angiogram helps to guide the placement of the craniotomy by visualizing the dominant cortical draining vessels. The patient is positioned in the three-quarter prone position, with the parietal area of the operated side in the dependent position. The craniotomy begins at the posterior edge of the postcentral gyrus and extends approximately 4 cm posteriorly. The craniotomy exposes the superior sagittal sinus and extends laterally 3 to 4 cm. The dura is reflected medially, and care is taken to maintain the large draining veins. The parietal lobe is gently retracted (approximately 2 cm) away from the falx. Once the arachnoid adhesions are opened, the distal pericallosal arteries and the splenium are identified. Below the splenium, the ICVs join the vein of Galen, and these can be seen once the splenium is cut. The splenium is incised with a bipolar cautery, and this incision must be made lateral to the midline because the atrium of the lateral ventricle deviates laterally. Access into the atrium is now achieved; however, tumors not found in the medial part of the atrium will be hard to resect by this route, and the surgeon should consider the posterior transcortical route for lateral atrial tumors. , ,
The posterior temporal approach can be a useful route for lesions that are located laterally in the atrium. The patient is positioned either supine with the head tilted at least 60 degrees away from the craniotomy side or in the lateral position. The posterior temporal region is exposed just above the plane with the transverse sinus. Extreme care should be taken not to injure the vein of Labbe as it courses to the junction of the transverse and sigmoid sinus. The vein of Labbe can be further protected by placing a pledget of thrombin-soaked Gelfoam over it. Once the dura is exposed, on the nondominant side, an incision into the posterior middle or inferior temporal gyrus (middle temporal sulcus) will gain access to the atrium. The incision should be along the axis of the gyrus. Once the ventricle is accessed, the tumor is removed piecemeal and separated away from surrounding ependyma. In the dominant hemisphere, the approach can be varied to avoid impairment in language abilities. Specifically, the inferior temporal bone and mastoid air cells can be removed to gain access to the subtemporal area. The cortical incision is then made near the occipitotemporal gyrus. Although this avoids more of the optic radiations and is further removed from the speech cortex, this route requires more temporal lobe retraction. Care should be taken to avoid stretching or kinking the vein of Labbe. Furthermore, on closure of the subtemporal craniotomy, the mastoid air cells must be closed, and closure must be watertight to avoid postoperative cerebrospinal fluid (CSF) leakage. Lateral transcortical approaches to the atrium place the optic radiations at risk of injury, minimizing the usefulness of this approach.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here