Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
As of 2007, 527,000 patients received treatment for end-stage renal disease in the United States. During this same year, approximately 1500 access interventions were performed per 1000 patients. With the exception of dialysis itself, the most commonly performed procedures on patients with end-stage renal disease are open or endovascular interventions directed at maintenance of arteriovenous access.
The modern era of hemodialysis began with the availability of reusable silicone elastomer (Silastic) external shunts between the artery and the vein, pioneered by Hegstrom and colleagues in 1960. For the first time repetitive arteriovenous access was available for maintenance hemodialysis in patients with renal failure. Although these shunts were cumbersome, prone to infection, and unsightly, they provided a simple means of allowing repetitive vascular access for dialysis of patients with renal failure.
In the mid-1960s Cimino and Brescia described the creation of a native arteriovenous fistula for chronic vascular access. In this technique needle cannulation of an enlarged segment of autogenous vein allowed repetitive hemodialysis access. The self-contained nature of this access allowed patients to function normally when not receiving hemodialysis. With the increased durability achieved by the Brescia-Cimino fistula, however, surgical strategies for effective access maintenance and for the treatment of access complications, including fistula degeneration, false aneurysm formation, and thrombosis, became imperative. In the 1990s percutaneous interventions were applied with increasing frequency for arteriovenous access maintenance. Although less invasive, the limited durability led to a dramatic increase in vascular access interventions.
Traditional management of arteriovenous graft thrombosis combined open surgical thrombectomy with surgical revision of the underlying cause of graft failure, often without the aid of direct intraoperative imaging techniques. However, the introduction of intraoperative venography has provided a means to define the cause of access failure as a routine component of the intervention.
Operative preparation. Many patients will present with an arteriovenous access thrombosis at a regularly scheduled hemodialysis session and often have recently eaten. Patients should be instructed to cease all oral intake.
Temporary dialysis. Preoperative discussion should include the possibility of placing a temporary hemodialysis catheter. Significant hyperkalemia or fluid overload warrants placement of a catheter for acute dialysis.
Prophylactic antibiotics. Administration of a preoperative intravenous antibiotic active against Staphylococcus species is routine, particularly in the presence of synthetic graft material. In addition, topical rifampin can be applied to exposed graft material during the procedure to minimize secondary biofilm infections.
Incomplete thrombectomy. Residual thrombus may compromise the durability of the access graft, if complete visualization is not afforded by intraoperative contrast venography.
Incomplete treatment. Anastomotic and other occult stenoses in the central venous system, as well as lesions in the graft and the arterial inflow, may be missed without thorough contrast imaging.
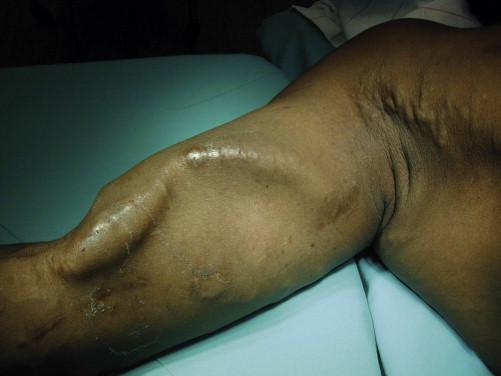
Treatment of arteriovenous access thrombosis that occurs 30 days or longer after the initial procedure has been traditionally approached by empirical operative revision of a stenotic venous anastomosis resulting from intimal hyperplasia, which remains the most common cause of access failure ( Fig. 68-1 ). In the absence of intraoperative imaging, revision of the distal anastomosis or distal portion of the access is often performed after open thrombectomy, where the presence of a pulse in the graft suggests a more distal obstruction or stenosis. Without contrast venography, physical examination becomes critical to the success of the procedure ( Fig. 68-2 ). Normal venous segments of an arteriovenous fistula typically do not thrombose.
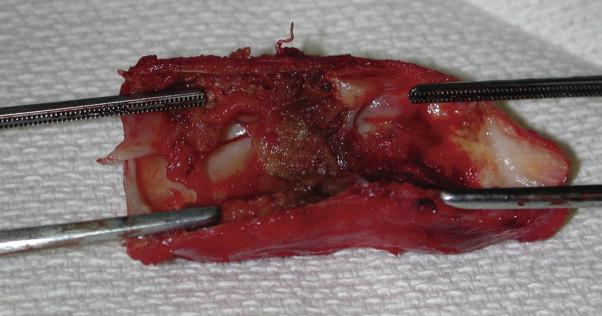
A segment of the synthetic arteriovenous graft distant from the previous skin incision chosen as the site to expose the prosthesis for planned thrombectomy. This segment is incorporated into the subsequent surgical plan for graft revision. By selecting a segment of graft underlying healthy skin, the risk of wound complications can be minimized. If intraoperative examination identifies the underlying cause of thrombosis, revision can be performed simultaneously with thrombectomy. Patch angioplasty or an interposition jump graft is often necessary to treat an underlying outflow stenosis. A limited dose of systemic intravenous heparin may be used, along with the administration of regional heparin using a heparin flush solution through the graft. Systemic doses of heparin range from 20 to 50 units per kilogram of body weight as an intravenous bolus, about one half the typical heparin dose used in patients without end-stage renal disease. Heparin flush solutions vary from 4 to 20 unit/mL, averaging about 10 unit/mL.
Graft thrombectomy and revision can often be performed under local anesthesia with intravenous sedation. The additional use of bupivacaine infiltration anesthesia can provide improved postoperative pain control. Typically, bupivacaine is used at a dose of 20 to 30 mL of 0.5% solution without epinephrine to a maximum dose of about 2 mg per kilogram of body weight. Systemic antibiotics should be given preoperatively. The use of a plastic antimicrobial skin barrier may also be helpful to prevent contact between skin and graft material. Generally, some form of barrier should be used to avoid skin flora contaminating the graft material. An incision is made over or near the distal anastomosis of the arteriovenous graft in a segment of healthy skin. In many cases this may incorporate the prior incision, but extension of the incision proximally or distally may be required to fully expose the involved segment. A sigmoid-shaped incision is useful near areas of flexion at the antecubital fossa.
Mechanical thrombectomy is performed using a Fogarty embolectomy catheter. Typically, venous thrombectomy is performed first by passing the catheter distally and slowly withdrawing the venous thrombus. Resistance to catheter withdrawal is often sensed if a stenosis is encountered. Most normal outflow veins can accommodate a fully inflated 4-Fr balloon catheter. If surgical revision of venous outflow is planned, this should be performed before completing the arterial thrombectomy. Otherwise, if a cause for graft thrombosis is not apparent, then arterial thrombectomy should be undertaken. Arterial thrombectomy is performed segmentally to remove the more liquid thrombus in the body of the graft, before withdrawal of the fibrous cap often found at the arterial end of the prosthesis. This minimizes the risk of embolization of the arterial cap retrograde into the arterial tree. Residual debris is removed by repetitive flushing once pulsatile inflow is achieved.
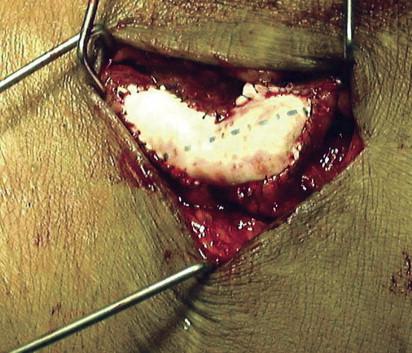
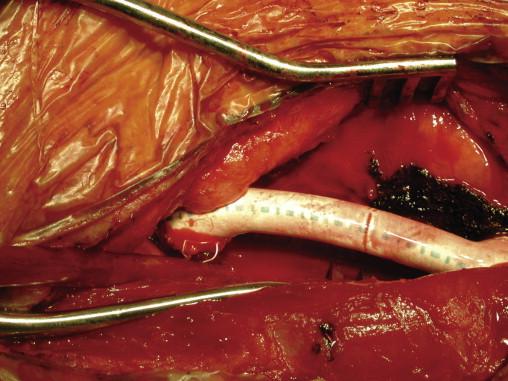
Revision of the distal venous anastomosis most often consists of either patch angioplasty ( Fig. 68-3 ) or extension interposition grafting ( Fig. 68-4 ). Although there is no consensus as to the best approach, patching is applied for short areas of stenosis and avoids loss of outflow vein. Interposition grafting is reserved for a longer-segment stenosis. Once the graft is free of thrombus and flow reestablished, a careful physical examination in the operating room should be performed. The graft should have an easily palpable pulse and thrill. Pulsatility in the absence of a thrill suggests significant residual outflow stenosis, and careful intraoperative imaging from the arterial anastomosis to the central veins is warranted.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here