Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Introduction to Chapter 36, Surgical Anatomy and Monitoring of the Recurrent Laryngeal Nerve.
Recurrent Laryngeal Nerve Monitoring.
Continuous Vagal Nerve Monitoring.
Galen, in the second century, discovered and named the recurrent laryngeal nerve (RLN). He found that vagal sectioning in a pig resulted in aphonia. Until this time, speech was thought to be controlled by the heart. In the seventh century, Paulus Aegineta suggested that the RLN could be avoided during thyroid surgery. Vesalius, in the sixteenth century, provided anatomic drawings of the RLN and superior laryngeal nerve (SLN) distribution. In 1872, Kocher performed his first thyroidectomy. Kocher’s meticulous technique reduced operative mortality and was adopted by Halstead. At about this time, Billroth abandoned the procedure as a result of hemorrhage and postoperative sepsis. Wolfler noted a 29.5% RLN paralysis rate in 44 patients operated by Billroth during the course of 5 years. In 1909, Kocher was awarded the Nobel Prize for his work in physiology, pathology, and surgery of the thyroid gland. Safe thyroid surgery, introduced by Halsted in the United States after a period of study with Kocher, was the foundation on which the clinics of Crile, Lahey, and the Mayo brothers were built (see Chapter 1 , History of Thyroid and Parathyroid Surgery).
This chapter reviews the surgical anatomy of the recurrent laryngeal nerve (RLN), related surgical and management maneuvers, and monitoring techniques for the RLN. The superior laryngeal nerve (SLN) is discussed in a limited capacity, only in relation to complement RLN as it relates to thyroidectomy. For in-depth information on SLN, refer to Chapter 35 , Surgical Anatomy of the Superior Laryngeal Nerve.
RLN paralysis is one of the most feared postoperative morbidities after thyroid surgeries. Unilateral vocal cord paralysis (VCP) can lead to voice changes accompanied by dysphagia and aspiration; bilateral VCP may result in tracheostomy. Given the frequency and significance of RLN paralysis at thyroidectomy, the knowledge of the anatomy, surgical maneuvers, and monitoring techniques for the RLN is important. The reported incidence of RLN paralysis is reviewed in depth in Chapter 15 , Pre- and Postoperative Laryngeal Examination in Thyroid and Parathyroid Surgery.
Permanent RLN paralysis rates in expert hands have been reported in the 1% to 2% range. However, the rates of RLN paralysis after thyroidectomy in many studies are likely underestimates for several reasons. First, the thyroid units with unfavorable data are less likely to report findings. Second, most injuries are not detectable intraoperatively by surgeons. Lo found that surgeons had recognized intraoperative injury in only 1% of cases, although surgical injury had actually occurred in nearly 7% of patients. The third reason is the variability in practice of postoperative laryngeal examination in these studies; typically, only patients with significant and persistent symptoms undergo laryngoscopy. Several practitioners have shown the lack of reliability of clinical symptoms in VCP (see Chapter 15 , Pre- and Postoperative Laryngeal Examination in Thyroid and Parathyroid Surgery). Variability in symptoms results from variation in degree of injury, cord position, contralateral cord compensation, and the evolution over time in position of the paralyzed cord. The Scandinavian Quality Register has found that the rate of RLN paralysis doubles when all patients receive postoperative laryngeal examination. The true rate of RLN injury can only be appreciated if all patients uniformly receive preoperative and postoperative laryngeal examination.
Although the rate of RLN injury with thyroidectomy in expert hands may be low, Djohan recently wrote that “the estimated incidence of RLN injury in standard thyroidectomy is from 2% to 13%.” In patients undergoing surgery for thyroid cancer, 15% were found to have VCP when laryngoscopy was performed within 1 week. Foster, in reviewing 24,108 thyroidectomies, described a rate of 2.5% for tracheotomy. Recently, a large systematic review of the literature reviewing 25,000 patients found a postoperative rate of 9.8%.
Bilateral thyroidectomy is unique in head and neck surgery in that both left and right cranial nerves are subject to risk in one surgical procedure. Bilateral cord paralysis occurs frequently enough in patients undergoing bilateral thyroid surgery that indications for tracheotomy in patients undergoing bilateral thyroidectomy have been developed. Several practitioners have shown that rates of RLN paralysis during thyroid surgery are greater in cases associated with (1) lack of intraoperative RLN identification, (2) bilateral surgery, (3) surgery for cancer, (4) significant lymph node resection, (5) surgery for Graves’ disease or thyroiditis, (6) revision surgery, (7) surgery associated with substernal goiter, (8) surgery associated with longer operating room times or greater blood loss, and (9) patients brought back to surgery because of bleeding. Also, surgeon experience has been related to RLN paralysis rates with rates of < 1% being associated with surgeons performing greater than 45 nerve dissections per year. Kandil et al. specifically reported on post total thyroidectomy complications and found that low-volume surgeons were likely to have higher postoperative complications compared with high-volume surgeons (odds ratio 1.53, 95% confidence interval 1.12, 2.11, p = 0.0083). A large German multicenter study yielded the following risk factors, in descending order of their importance (odds ratio), for permanent paresis of RLN: carcinoma recurrence (6.66), goiter recurrence (4.67), carcinoma first operation (2.04) (comparison in each case with benign nodular goiter), hemithyroidectomy versus subtotal resection (1.76), no nerve identification versus nerve identification (1.41), hospital experience (1.34), and surgeon experience (1.23).
Surgical management of the RLN and RLN monitoring are necessarily wedded with knowledge of pre- and postoperative glottic function (see Chapter 15 , Pre- and Postoperative Laryngeal Examination in Thyroid and Parathyroid Surgery).
Preoperative laryngeal examination is necessary for the following reasons:
VCP may present preoperatively in the absence of voice complaints.
VCP present preoperatively suggests invasive malignancy. Its identification informs preoperative planning and radiographic evaluation.
VCP may be present preoperatively even in the setting of benign disease (discussed later).
Management of the invaded RLN found at surgery is, in part, affected by the knowledge of preoperative functional status.
Responsibility for postoperative VCP can be wrongly attributed if one fails to demonstrate its presence preoperatively.
Preoperative laryngeal examination provides a preoperative baseline for postoperative laryngeal assessment.
Postoperative laryngeal examination is necessary for the following reasons:
Glottic examination is the only existent accurate postoperative RLN outcome measure. Voice changes may occur without VCP, and VCP may occur without voice changes.
Postoperative functional glottic information allows for maximum interpretation of intraoperative electromyography (EMG) data from the neural monitoring.
Postoperative VCP has significant implications on swallowing and for the planning of future contralateral surgery. The presence of postoperative unilateral VCP is therefore important whether or not it is associated with symptoms. VCP has been found to have significant adverse effects to work-related outcome measures in up to 40% of patients affected.
Shonka and Terris discuss the latest American Thyroid Association (ATA) guidelines regarding voice assessment and laryngeal examination for patients in selective thyroid surgery; however, they note such a selective approach is insufficient, and preoperative and postoperative laryngeal examination should be performed routinely for all thyroid surgical patients. This is because these examinations provide important information to the surgeon, aid in patient counseling and medical decision making, and are imperative in assessing the quality outcomes of thyroid surgery.
This chapter assumes that all patients undergoing thyroid and parathyroid surgery have pre- and postoperative laryngeal examinations (see Chapter 15 , Pre- and Postoperative Laryngeal Examination in Thyroid and Parathyroid Surgery). Without this basic framing information, a discussion of intraoperative RLN management is not rational.
The presence of preoperative vocal cord paralysis (VCP) in the setting of benign thyroid surgical disease has been extensively reported. Overall, the rate of preoperative VCP in patients with benign thyroid disease is approximately 0.7%. However, Shin et al. reported that in their series of 198 patients, 2% patients presented with VCP in the setting of benign disease and in the absence of prior neck surgery.
The proposed mechanisms through which benign disease may result in VCP include distention-stretch, compression, edema, ischemia, perineural fibrosis, calcification, and toxic neuritis. Some cases of preoperative VCP are likely unrelated to the thyroid and may be related to left atrial enlargement, aortic aneurysm, and nonthyroid neoplastic disease of the head and neck; The esophagus, lung, or mediastinum or may be idiopathic. Certainly, the finding of preoperative paralysis warrants an aggressive radiographic evaluation, typically with axial contrast-enhanced computed tomography (CT) to evaluate the neck base fully.
The prognosis of VCP associated with benign thyroid disease is reasonably good, with reports ranging from 37% to 66% resolution after surgery. One may question whether some of these cases of motion recovery, which are not confirmed by laryngoscopy, are in fact cases of symptomatic improvement through contralateral vocal cord compensation or a gradual medialization of the affected cord. Our experience with preoperative VCP associated with benign disease shows that, if ipsilateral vagal stimulation cannot elicit electromyography (EMG), then postoperative recovery will not occur. (See the later section, “Preoperative Laryngeal Examination and Intraoperative Electrical Stimulability.”)
Although VCP is known to occur with benign disease, preoperative VCP is regarded as a strong predictor of invasive thyroid disease and mandates additional radiographic evaluation including CT scan or magnetic resonance imaging (MRI) to check for the extent of invasive disease. CT and MRI studies can also identify subtle laryngeal findings that are associated with VCP, including posterior cricoarytenoid (PCA) muscle atrophy, thyroarytenoid muscle atrophy, anteromedial deviation of the arytenoid cartilage, enlarged pyriform sinus, enlarged laryngeal ventricle, and paramedian cord position. In presence of known ipsilateral VCP, a mass judged as indeterminate for invasion by the radiologist should be considered invasive until proved otherwise. CT or MRI findings of VCP may not always be present and do require a fine-cut neck and airway CT scan as well. An experienced head and neck radiologist is necessary. Thus preoperative radiographic evaluation of the larynx does not replace the need for preoperative laryngeal examination.
The relationships among laryngeal examination, electrical stimulability, and nerve invasion are interesting. In our series of monitored patients, we have reviewed the laryngeal function and intraoperative EMG data in several interesting subsets of patients ( Figure 36.1, A and B ). Out of 22 cases with malignant nerve invasion, 45% of cases had normal preoperative laryngeal examination. RLN was routinely identified above and below the area of invasion as well as electrically stimulated, and 63% of invaded nerves were detected to retain electrical stimulability despite the intraoperative finding of nerve invasion. Among the invaded nerves that retained electrical stimulability, the average EMG amplitude in invaded nerves with normal preoperative laryngeal function was 180 μV, whereas in the invaded nerves with known preoperative VCP, it was only 63 μV. Thus invaded nerves are associated approximately one half of the time with preoperative clinical nerve paralysis, yet they retain electrical stimulability in two thirds of cases (with a low level of EMG response) (see Figure 36.1, A ). With maintenance of neural activity in the setting of invasion even when preoperative VCP is present, resection of such a nerve is expected to worsen glottic function; this affects the voice as well as swallowing. These patients should receive appropriate preoperative counseling. In fact, Chi et al. have demonstrated when invaded nerves with preoperative VCP and retained intraoperative stimulability are preserved, postoperative vocal cord atrophy and decline of vocal function are prevented.
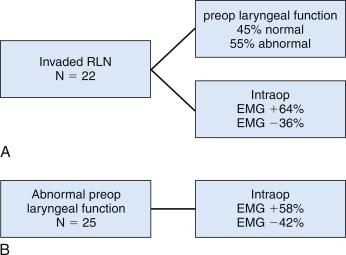
In the same study, among 25 patients presenting with abnormal preoperative laryngeal function, 58% patients demonstrated intraoperative stimulability. In these patients who retained electrical stimulability, if the laryngeal dysfunction was due to past surgery, the average amplitude was only 93 μV; if laryngeal dysfunction was due to malignant invasion, the average amplitude was only 63 μV. Notably, in patients with abnormal preoperative laryngeal function, the RLN failed to be electrically stimulable in 42% of patients. Chi et al. have presented a small series of such patients who retained some electrical stimulability despite preoperative paralysis. Thus in patients with abnormal preoperative laryngeal function, almost 60% maintain electrical stimulability with low-level EMG (see Figure 36.1, B ).
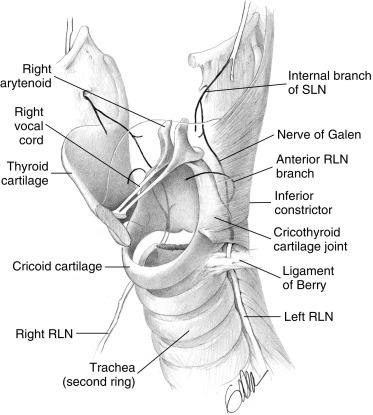
The RLN should be visualized in all cases. Lahey, in 1938, helped to introduce the routine dissection and demonstration of the RLN during thyroid surgery. Lahey believed that the identification of the RLN was so important that he routinely divided the inferior thyroid artery (ITA) laterally to facilitate dissection of the RLN to its laryngeal entry point. With nerve identification, Lahey’s paralysis rate reduced from 1.6% to 0.3% in more than 3000 nerve dissections over a 3-year period. Kocher’s technique of thyroidectomy involved medial mobilization of the thyroid after lateral ligation of the middle thyroid vein. He left a posterior capsule intact to protect the RLN and parathyroid glands. Lore has been an advocate for RLN preservation through operative identification, as has Lennquist. Crile believed that the RLN was extremely sensitive to surgical trauma, more so than other peripheral nerves, hence he proposed the “doctrine of vulnerability.” Wade and Perzik were also proponents of doctrine of vulnerability. These authors suggested a blind capsule technique for RLN management. We now understand that the RLN tolerates appropriate surgical dissection. Also, RLN identification and dissection does not seem to increase the rate of parathyroid devascularization.
Many studies prove that RLN identification during thyroidectomy is associated with lower rates of postoperative RLN paralysis. Jatzko reviewed 10 studies of 12,211 thyroid operations; in cases without RLN identification, he found temporary paralysis in 7.9% and permanent paralysis in 5.2%; with RLN identification, 2.7% of cases had temporary paralysis and 1.2% had permanent paralysis. Hvidegaard described a paralysis rate ranging from 3% to 9.4% without identification and a rate ranging from 0.3% to 2% with nerve identification. Wagner noted RLN paralysis in 7% of patients without and 3.8% of patients with RLN identification. Mountain noted a rate of paralysis three to four times higher without routine RLN exposure. Riddell found a 0.6% RLN paralysis rate with nerve identification and a 2% rate without identification. Interestingly, Jatzko noted that in cases with initial postoperative paralysis, intraoperative identification of the RLN was associated with better RLN recovery rates (57% recovered if the RLN had been identified versus only 34% if it had not). Several other researchers agree that RLN identification is essential during thyroidectomy and this is now embedded in guidelines of the American Academy of Otolaryngology Head and Neck Surgery as well as subsequently in the American Thyroid Association.
“People see what they are prepared to see.” Ralph Waldo Emerson, Journals, 1863
A thorough knowledge of vagal, RLN, and SLN anatomy, including branching patterns and anatomic variations, is vital knowledge for any thyroid or parathyroid surgeon.
The modern vagus nerve anatomy was first described by Willis in the 1600s. The vagus derives its blood supply from a discrete vagal artery, a branch of the inferior thyroid artery (ITA), and it is reinforced by branches from the internal carotid, common carotid, aortic arch, and bronchial and esophageal arteries. The cervical branches of the vagus nerve of concern during thyroid surgery include the SLN, both internal and external branches, and the RLN. The SLN’s internal branch brings general visceral afferents to the lower pharynx, supraglottic larynx, vocal cords, base of the tongue, and special visceral afferents to the epiglottic taste buds. The SLN’s external branch (external branch of the superior laryngeal nerve; EBSLN) brings branchial efferents to the cricothyroid muscle and inferior constrictor. As is described later, the internal branch of the SLN may also provide motor contributions to the posterior cricoarytenoid (PCA) muscle and intraarytenoid muscle, and the EBSLN may provide, at least in some patients, limited motor input to the thyroarytenoid muscle. The RLN contains branchial efferents to the inferior constrictor, cricopharyngeus, all laryngeal intrinsics except the cricothyroid muscle, general visceral afferents from the larynx (vocal cords and below), upper esophagus, and trachea. RLN branches also convey sympathetic and parasympathetic branches to the lower pharynx, larynx, trachea, and upper esophagus. Apart from the larynx and pharynx, the vagus nerve provides afferent and parasympathetic innervation to the heart, esophagus, stomach, intestines, liver, spleen, and kidneys.
Cortical areas (including Broca’s area, the motor cortex, and the anterior cingulum) that control laryngeal function project to brainstem nuclei bilaterally (primarily from the nucleus ambiguous). The nucleus ambiguous projects to the ipsilateral portion of the larynx. Gacek’s work in the cat has identified laryngeal motor supply arising in the ipsilateral nucleus ambiguous with adductor function, primarily in the dorsal division of the nucleus and abductor neurons primarily in the ventral division. He identified a second source of laryngeal innervation in the retrofacial nucleus, from which neurons extend to the cricothyroid and PCA muscles with abductor fibers arising centrally and adductor fibers more peripherally.
The vagus nerve forms through a gathering of a number of small vagal rootlets together with the bulbar roots of the accessory nerve on the lateral aspect of the medulla; these combined structures exit the skull through the pars nervosa of the jugular foramen. It descends the neck in the carotid sheath, initially on the medial aspect of the internal jugular, then becomes situated posteriorly between the internal jugular and carotid artery lower in the neck ( Figure 36.2 ). The initial branches of the vagus nerve include the meningeal filament extending to the dura, the auricular branch to the posterior external auditory canal (Arnold’s nerve), Jacobson’s nerve to the promontory of the middle ear, and the pharyngeal ramus. The superior ganglion of the vagus nerve lies within the jugular foramen, whereas the inferior or nodose ganglion is just beneath the jugular foramen. The pharyngeal ramus supplies motor fibers to the pharyngeal constrictors and sensory fibers to the underlying pharyngeal mucosa. The nodose ganglion contains cell bodies for sensory and parasympathetic fibers and is present just laterally to the superior cervical sympathetic ganglion. Immediately below the nodose ganglion, the SLN is given off.

Dionigi et al. have shown the vagus nerve is located directly posterior to the carotid artery and jugular vein in 73% of cases, lies directly posterior to the carotid artery in 15% of cases, lies directly posterior to the jugular vein in 8% of cases, and lies anterior to the carotid and jugular vein in the carotid sheath in 4% of cases.
As the heart and great vessels descend during embryologic life, the RLN is dragged down by the lowest persisting aortic arch (see Chapter 2 , Applied Embryology of the Thyroid and Parathyroid Glands). The right vagus runs from the posterior aspect of the jugular vein in the neck base to cross anterior to the first part of the subclavian artery. The RLN branches and courses up and behind the subclavian artery (fourth branchial arch) running medially along the pleura and cranially behind the common carotid artery into the right thoracic inlet in the base of the neck. The left vagus courses from the carotid sheath in the left neck base anterior to the aortic arch (sixth arch, ligamentum arteriosus). The RLN branch curves up under the aortic arch just lateral to the obliterated ductus arteriosus ( Figure 36.3, A ).
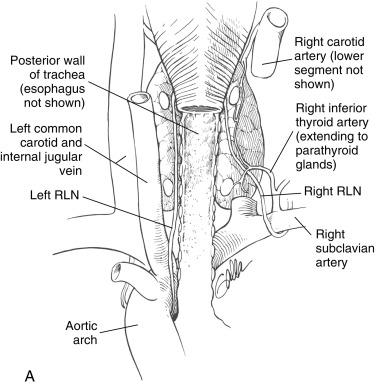
Because of its course around the right subclavian artery, the right RLN enters the neck base at the thoracic inlet more laterally compared with the left RLN (see Figure 36.3, A ). The right RLN then ascends the neck, enters the thoracic inlet, emerges from under the common carotid artery, tracks laterally to medially as it travels superiorly, and ultimately crosses the ITA. It assumes a paratracheal position in the last centimeter of its course as it approaches the lowest edge of the inferior constrictor. Shindo et al. found in their distal course both right and left RLNs typically form an angle of between 15 and 30 degrees relative to the trachea; they showed the right RLN as having a more oblique course in paratracheal region as compared with the more vertical/strictly tracheoesophageal groove course of the left RLN.
At the lower edge of the cricoid cartilage posterolaterally, the RLN travels under the inferior-most fibers of the inferior constrictor (i.e., the cricopharyngeus muscle), extending deep to the inferior constrictor and up behind the cricothyroid articulation to enter the larynx. In approximately 30% of cases, the RLN actually penetrates the lowest fibers of the inferior constrictor on its way to the larynx (see Figure 36.3, B ). Incorrect descriptions of this anatomy exist, in which the RLN is described to pass deep to the cricothyroid, rather than the inferior constrictor muscle. In this chapter, the point at which the RLN disappears under the lowest fibers of the inferior constrictor will be termed the laryngeal entry point; it marks the distal-most exposure of the RLN in the thyroid surgical field. For the last centimeter or so before laryngeal entry, the RLN travels close to the lateral border of the trachea.
The vagus diameter in its cervical course is approximately 4 mm with the epineurium and 3 mm without. The diameter decreases to approximately 2 mm after the RLN takeoff point. The RLN diameter averages approximately 2 mm and ranges between 1 and 3 mm. The average length of the vagus from the second cervical vertebra to the separation of the RLN (based on 30 dissections in 15 adult humans) is 11.5 cm on the right and 13.5 cm on the left. The length of the RLN from vagal takeoff to laryngeal entry point is 8.5 cm on the right and 10 cm on the left. The different lengths result in discretely different latencies in the evoked EMG, and the specific latencies allow recognition of the corresponding nerve when electrically stimulated (discussed later).
The SLN arises immediately beneath the nodose ganglion of the upper vagus and descends medial to the carotid sheath. It divides into its internal and external branches about 2 or 3 cm above the superior pole of the thyroid, although this point may vary (see Chapter 35 , Surgical Anatomy of the Superior Laryngeal Nerve). The internal branch travels medial to the carotid system and enters the posterior aspect of the thyrohyoid membrane, which provides sensation to the ipsilateral supraglottis and base of the tongue. The EBSLN descends to the region of the superior pole and extends medially along the inferior constrictor fascia to enter the cricothyroid muscle. As the EBSLN slopes downward on the inferior constrictor musculature, it has a close association with the superior thyroid pedicle. Several practitioners have shown that in approximately 20% of cases, the EBSLN is closely associated with the superior thyroid vascular pedicle at the level of the capsule of the superior pole; this places it at risk during ligation of these superior pole vessels. In approximately 20% of cases, the EBSLN runs subfascially on the inferior constrictor muscle and may not be directly visualized, yet it can be stimulated electrically. Depending on the degree of superior pole development, the sternothyroid muscle may “hood” the superior pole region. Isolated sternothyroid division can help with exposure in this region. With EBSLN injury, there is a loss of vocal cord tensing, which is manifested by increased vocal tiredness and a loss of higher registers. Postoperative examinations after unilateral external branch injury are subtle and controversial, but they are generally believed to include a bowed and somewhat lower cord and a larynx rotated to the affected side. Such an injury ended the operatic career of Amelita Galli-Curci; however, there is intrigue regarding the veracity of this claim (see the “SLN Monitoring” section presented later in the chapter and also Chapter 35 , Surgical Anatomy of the Superior Laryngeal Nerve).
Gacek has shown in cats, that the RLN is 2 cm below the cricoid and is composed of approximately 55% motor fibers and 45% sensory fibers. The percentage of motor fibers rises to 80% more distally at the laryngeal entry point, with sensory fibers having branched from the RLN to adjacent tracheal and esophageal locations. Nonmyelinated fibers within the RLN represent sympathetic (carotid plexus) and parasympathetic (postganglionic) fibers, the cell bodies of which are within the nodose and plexiform ganglia. Gacek and others found a higher number of fibers in the right versus the left RLN.
Murtagh described between 2000 and 3000 myelinated fibers in the human RLN. The number of motor axons in the distal-most branches of the intralaryngeal portion of the RLN in the dog ranges from 143 to 370. Malmgren described three fiber types within the human RLN. The first group comprised 4- to 12-μm fibers staining for acetylcholinesterase; the second group consisted of small fibers from 1 to 5 μm, also staining for acetylcholinesterase; and a third group consisted of small fibers from 4 to 12 μm in size, but without acetylcholinesterase staining. The last group was believed to be sensory and autonomic fibers. We now recognize in the human RLN the following fiber types:
Efferent Type A fibers which are larger myelinated fibers comprising ~ 30%,
Afferent unmyelinated fibers of medium size,
Autonomic fibers with varing sizes including both:
large Type B myelinated fibers,
thinner Type C unmyelinated fibers. It is these thinner Type C autonomic fibers that mediate the bulk of the vasovagal symptoms of central, pulmonary and gastrointestinal autonomic effects.
Malmgren and Gacek noted that the RLN’s epineural covering was notably thicker than for most other human peripheral nerves and that the RLN was organized into between 1 and 11 fascicles.
Within the vagus, adductor and abductor fibers are not spatially segregated, although all laryngeal motor fibers tend to be in the anterior half of the vagus of the upper neck and the medial half of the vagus in the lower neck.
At the laryngeal entry point in the RLN, the adductor and abductor fibers lack spatial segregation; they are diffusely distributed throughout the entire nerve. The RLN contains two to four times as many adductor fibers as abductor fibers.
The RLN is white and approximately 2 mm wide. Although it generally follows a linear course, it can have a somewhat curved profile and be similar in appearance to the spinal accessory nerve in surgery of the lateral portion of the neck. Virtually always, the normal RLN has a vessel running on its surface (vasa nervosum), which can be seen as a ventral “red strip.” This may be less apparent if the nerve has been attenuated over time, as in massive goiter or if the nerve is placed on stretch. All that visually appears to be the RLN may not be. Electrical confirmation complements the visual impression and avoids visual false-positives. Raffaelli and colleagues noted that sympathetic chain branches to the distal RLN branch can be large enough to mimic a nonrecurrent nerve. They also describe rare, medially directed branches of the sympathetic system that can mimic the normal RLN. They have found at least one such case, in which the nerve that “perfectly mimicked the RLN, when dissected fully, originated from the sympathetic stellate ganglion, not the vagus.” Sympathetic nerve branches, when stimulated, should not yield laryngeal EMG activity; thus functional information provided by RLN monitoring aids in distinguishing such nerves. The RLN is amendable to surgical dissection without injury. Chiang et al. have shown that RLNs that require extensive dissection of > 5 cm in the setting of goiter did not have higher rates of paralysis than nerves requiring less dissection.
The right nonrecurrent RLN (NRLN) occurs in 0.5% to 1% of cases and is associated with a right subclavian artery takeoff from the distal aortic arch. The right subclavian artery in these cases follows a retroesophageal course to the right or, less commonly, between the esophagus and the trachea. The left NRLN is extremely rare, with only 0.04% cases reported in the literature; it is associated with situs invertus. Henry found that the symptoms of dysphagia secondary to subclavian interaction with the esophagus (dysphagia lusoria) were not consistently present in cases of right NRLN. When dysphagia is present, dysphagia lusoria is difficult to separate from dysphagia referable to a pathologic condition of the thyroid.
Epstein suggests nonrecurrent RLN (NRLN) is associated with compressive symptoms in 10% of patients (typically patients with dysphasia). The retroesophageal subclavian artery is associated with Kommerell’s diverticulum (dilation at its aortic takeoff) in 60% and with other aortic, cardiac, vertebral artery, and thoracic duct abnormalities including right-sided duct location.
Several preoperative imaging such as barium swallow, ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), and angiography are employed and shown to provide successful identification of associated vascular anomaly and hence the NRLN. However, preoperative identification of NRLN may be difficult, especially when limited preoperative imaging is performed before thyroid surgery. NRLN has no functional implications, but it is prone to intraoperative injury due to abnormal anatomy. A study of 31 patients at-risk of NRLN demonstrated a 12.9% paralysis rate. Recently, an intraoperative electrophysiological and anatomic algorithm has been shown to reliably identify NRLN before dissection in the related area (discussed later under RLN monitoring section). The NRLN derives from the vagus as a direct medial branch in the neck and extends generally with a downward looping course from behind the carotid artery to the laryngeal entry point; however, it can follow more horizontal or ascending paths.
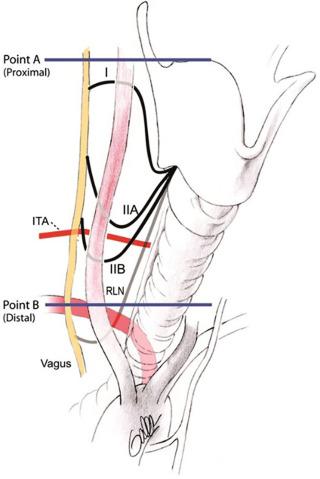
Avisse’s classification scheme includes three types of NRLN. Type I is characterized by a high takeoff from the vagus traveling from the vagus in association with the superior pole vessels. Type IIA is derived from the vagus at the level of the laryngotracheal junction traveling along the path of the ITA, and type IIB is derived from the vagus at the level of the laryngotracheal junction; it then travels in a downward looping course below the level of the ITA before it extends to its laryngeal entry point ( Figure 36.4 ). The NRLN may be very closely related to the ITA. It may be bifid or have multiple branches in 18% to 40% of cases ( Figure 36.5 ). According to some researchers, NRLN may be associated with a second, smaller right RLN in the normal RLN position. Katz found this in 5 of 11 NRLNs, and Karlin found it in 2 of 5 NRLNs. Not all agree that NRLN may coexist with a small, normally positioned right RLN; however, the finding of a small narrow right RLN should alert the surgeon to search for a larger NRLN trunk. Communicating branches between the cervical sympathetic system and RLN have an unclear function; they are perhaps involved in vasomotor laryngeal control. These fibers typically originate from the middle cervical sympathetic ganglion and have a diameter slightly less than a normal RLN. Large cervical sympathetic RLN anastomotic branches have been found in 1.5% of cases (more frequently than the rate of right NRLN). Maranillo’s cadaveric dissections suggest a rate as high as 17% of specimens. When present, such large sympathetic RLN anastomotic branches connect to the RLN within 2 cm of the RLN’s laryngeal entry point. The large sympathetic RLN anastomotic branches may be confused with true NRLN. One should be able to differentiate between these through neural stimulation.
RLN position may be significantly abnormal in the setting of goitrous change, especially when substernal or retrotracheal extension exists. RLN position and identification may also be more difficult if significant paratracheal RLN chain nodal disease exists. The nerve in the setting of goitrous change can be displaced in any direction and may even come to lie ventral to the inferior pole. Importantly, goitrous enlargement can be associated with fixation and splaying of the RLN to the undersurface of the enlarged thyroid lobe. In such cases, routine identification of the RLN in the thoracic inlet is prohibited because of the size of the goiter. Recommendations have been made that blunt dissection will allow delivery of the goiter from the wound or the substernal goiter into the neck without RLN identification. In our study of large cervical and substernal goiters, 16% were associated with abnormally positioned RLNs, which were either fixed to the undersurface of the goiter or splayed significantly over the surface of the goiter. Left and right lobes were equally affected. In 184 cases not involving goiter, we did not find any nerves that were fixed or splayed except in the presence of invasive malignancy. We noted that fixation and splaying of the RLN occurred more frequently in larger goiters and goiters with substernal extension, with tracheal compression evident on preoperative CT scan, and in goiters with intubation difficulties. Sinclair reported a 17.5% rate of postoperative RLN paralysis in patients with retrosternal goiter in whom the RLN was not specifically identified during blind digital goiter delivery. Sinclair reported several cases in which the nerve was associated with the thyroid gland and was thus at serious risk when the retrosternal mass was mobilized into the neck (a maneuver usually achieved by dislocating the mass with a finger from below and behind). We believe that this hazard must be recognized by all thyroid surgeons and that every strand of tissue stretched over the retrosternal component of the goiter should be presumed to be nerve until anatomically proven otherwise.
Lahey, in 1938, recommended RLN identification “even in deep intrathoracic goiter extending nearly to the diaphragm.” We believe that because of the possibility of nerve fixation and splaying on the undersurface of a goiter, blunt dissection without nerve identification risks stretch injury. Identification of the RLN in such cases is a necessary initial step. The nerve that is fixed to or splayed on the undersurface of the goiter should be dissected off before the gland is delivered. The nerve can be identified through a superior approach (discussed later) and can be dissected retrograde off the goiter before digital delivery of the goiter. After goiter resection, the nerve so dissected can appear to be significantly redundant, but it will stimulate normally and function postoperatively despite the intraoperative appearance of laxity. We have noted that goiters associated with retrotracheal extension as identified by preoperative CT scanning may be associated with RLN displacement to the ventral surface of the goiter. This is a disorienting position and places the nerve at extreme risk, even in experienced hands. Therefore analysis of preoperative CT scanning in patients with retrotracheal goiter may empower the surgeon to have low suspicion for such ventral RLN displacement (see Chapter 6 , Surgery of Cervical and Substernal Goiter).
Aside from thyroid tissue that may actually infuse the ligament of Berry (LOB; see the discussion of the LOB presented later in the chapter), surface nodules and lobulation of the thyroid gland near the ligament may make distal RLN dissection more difficult. The tubercle of Zuckerkandl (TOZ) is a lobule of thyroid tissue, which, if present, typically occurs just caudal to the LOB at the posterolateral margin of the thyroid lobe. The tubercle was described by Zuckerkandl in 1904 as the “processus posterior glandulae” and by Madelung in 1867 as the “posterior horn of the thyroid” (see Chapter 2 , Applied Embryology of the Thyroid and Parathyroid Glands, Figures 2.2 and 2.3, D ).
The adult orthotopic thyroid is derived from the fusion of medial and lateral elements (see Chapter 2 , Applied Embryology of the Thyroid and Parathyroid Glands).
The lateral thyroid anlage derives from the fourth pharyngeal pouch and includes the ultimobranchial body (from the ventral part of the fourth pharyngeal pouch and possibly a portion of the fifth pouch) and parafollicular C cells of neural crest origin. This lateral element fuses with the medial anlage (which derives from the tuberculum impar of the embryonic tongue base) as the thyroid descends before the seventh week of gestation. This point of fusion is closely juxtaposed to several regional, important structures: the upper parathyroid glands (PIV), which arise from the adjacent dorsal wing of the fourth pharyngeal pouch); the RLN (the vagus and RLN arise between the fourth and fifth pharyngeal pouches); and the ITA, which derives from the fourth aortic arch.
When thyroid tissue is present as a posterior lateral projection of the lateral thyroid lobe, it can be termed the TOZ, and it is believed to represent the point of fusion between the lateral anlage and the medial thyroid elements; its association with the RLN and upper parathyroid glands (PIV) are of special surgical importance.
When present in its typical position, the tubercle is caudal to the LOB. The tubercle points to the nerve as the nerve interacts with the LOB and laryngeal entry point. The nerve is typically deep to the tubercle and may be entrapped in a cleft between the tubercle’s deep surface and the adjacent deep surface of the thyroid lobe ( Chapter 2 , Applied Embryology of the Thyroid and Parathyroid Glands, Figures 2.2 and 2.3, D ). The nerve is said to be in this deep position relative to the TOZ in 93% of cases, but in the remaining 7% of cases the nerve may ride ventral to the tubercle and thus be in an extremely vulnerable position at surgery. Hisham reported that the RLN was located anterior to the tubercle in 6% of patients and felt this variation was more common in revision patients (see Chapter 2 , Applied Embryology of the Thyroid and Parathyroid Glands, Figures 2.2 and 2.3, D ). When the tubercle is extending dorsally and the nerve is brought ventrally, the TOZ is felt to be the initial manifestation of what would be in the neck: a retrotracheal goiter and a posterior mediastinal goiter (in the mediastinum); both of these are associated with such high-risk “ventral” RLNs (see Figure 6.7 , B in Chapter 6 , Surgery of Cervical and Substernal Goiter). Even if the nerve is in its more typical position (deep to the TOZ), it may be relatively entrapped in the cleft between the TOZ and adjacent thyroid; it may be stretched, as the TOZ is serially dissected and retracted to expose the RLN.
This lobule of thyroid tissue may have a varying relationship to the RLN and, in general, tends to obscure its distal course and make more difficult its distal dissection. The exact position of the tubercle of Zuckerkandl (TOZ; when it is present) varies along a cranial caudal axis. Most typically, the TOZ is present at the posterolateral margin of the thyroid midpole region and is below the ligament of Berry (LOB); in this position, the TOZ points superiorly to the RLN as the nerve extends to the LOB and laryngeal entry point. However, the TOZ may occur higher on the thyroid lobe and so may overlie the LOB region, which may obscure the region of the nerve-LOB and nerve entry site. The TOZ may occur even more superiorly from the posterolateral margin of the superior pole above the RLN entry site. Additionally, the TOZ is felt to have a relatively constant relationship to the ITA—typically dorsal relative to the TOZ—and will typically have the PIV on its lateral or superolateral margin (see Chapter 2 , Applied Embryology of the Thyroid and Parathyroid Glands, Figures 2.2 and 2.3, D ).
A TOZ grading scheme has been offered: grade 1 (less than 5 mm), grade 2 (between 5 and 10 mm), and grade 3 (greater than 1 cm). Significant TOZ (i.e., grade 3) is felt to be present in 14% to 61% of patients and seems to vary in its prevalence in relation to country of origin. One study has shown that significant TOZ may be more common on the right side than on the left side (69.6% versus 53.2% respectively). Of course, as thyroid tissue, the TOZ is subject to any benign or malignant process. This variability in existence of the tubercle from patient to patient has hindered this as being a reliable landmark for nerve identification (see Chapter 2 , Applied Embryology of the Thyroid and Parathyroid Glands, Figures 2.2 and 2.3, D ).
Various surgical and cadaver dissection series show the RLN branches before the laryngeal entry point in from 30% to 78% of cases. These studies do not usually distinguish between the small branches arising from the RLN and extending to the adjacent trachea (sensory), esophagus (sensory and motor), inferior constrictor (sensory and motor), and sympathetic chain, and the typically larger branches arising as a terminal division of the nerve destined to innervate laryngeal musculature. Of course, branches that contain laryngeal intrinsic motor fibers must together enter the larynx at the laryngeal entry point and not extend more ventrally or posteriorly, which implies their sensory or nonlaryngeal motor nature. Obviously, only the branches extending to the laryngeal entry point will affect laryngeal motor function. Both the research of Serpell et al. and our own research has noted that 50% to 60% of patients have some small branches of the RLN to the trachea, esophagus, or inferior constrictor; however, only 20% to 30% have true RLN extralaryngeal branches that enter the larynx and with stimulation resulting in laryngeal EMG activity. This is in agreement with the older work of Morrison (who noted true extralaryngeal branches in approximately one third of patients on at least one side) as well as more recent studies. Endotracheally based monitoring systems identify only thyroarytenoid depolarization, whereas assessment of laryngeal twitch or posterior laryngeal electrodes will inform regarding PCA depolarization. In our experience, the small RLN branches to the esophagus and inferior constrictor, when stimulated, usually result in local contraction of the associated esophageal or inferior constrictor musculature. With careful dissection, Brok found four or five branches of the RLN to the cricopharyngeus in nine of nine patients.
Any true extralaryngeal RLN branches not recognized during surgery are at risk for injury. The total diameter of the normal RLN in the neck is only approximately 1 to 2 mm, so these branches are often less than 1 mm. Most laryngeal branches of the RLN arise from the distal RLN segment, with 90% of branching occurring above the intersection of the RLN and the ITA. Reed found that only 5.4% of 506 dissected nerves branched at the level of the RLN and ITA crossing. It is known that the distal-most RLN intralaryngeal branches are always given off by the time the RLN is above the cricothyroid joint. Serpell’s group has shown that the most common branch point occurred in the distal 2 cm course of the RLN measured from the bottom of the inferior constrictor and averaged approximately 18 mm with a range of 5 to 34. True “extralaryngeal RLN branches” (i.e., major branches destined to enter the larynx) can be assumed to be analogous to intralaryngeal branches, except that they are premature and occur more proximally below the lower edge of the inferior constrictor. The lowest segment of the inferior constrictor is termed the cricopharyngeus.
Thus extralaryngeal nerve branches, when present, usually exist at the level of the LOB and are usually not present below the ITA. The lack of branches below the ITA is part of the rationale for the inferior approach to the RLN in the thoracic inlet (as described by Lore). We have seen that RLN branching occurrence and pattern vary from side to side within the same patient; this observation is in agreement with the work of Morrison and Katz. Katz noted, in a study of 1771 nerves, that if extralaryngeal branches occur on one side, 39% of patients will have branching on the opposite side.
The most important message regarding patients with extra laryngeal branching, because of this increased anatomic complexity and narrow diameter of the branched RLN, is that they are at increased risk for both transient and permanent VCP. Sancho et al. suggested that branched nerves are twice as likely to have transient postoperative VCP (15.8%) than nonbranched nerves (8%). Cassella’s work also suggested an increased rate of paralysis in patients with branched RLN: about 7 to 12 times that of patients with unbranched RLNs.
See Figure 36.6 .
Some believe that extralaryngeal branches, when present, represent two functionally discrete fibers: posterior extralaryngeal branches that innervate abductor muscle (primarily PCA) and anterior extralaryngeal branches that represent adductor fibers that innervate the TA, interarytenoid, lateral cricoarytenoid, and thyroepiglottic muscles. Most agree there is limited cross innervation from the bilateral RLNs to the interarytenoid muscles ( Figure 36.6 ). Based on this assumption of functional segregation of fibers, some have speculated that injury to a particular branch at thyroidectomy would result in discrete and predictable laryngeal motor abnormality. A reliable functional segregation of fibers in extralaryngeal RLN branches is not accepted by all. Many researchers (i.e., Sunderland and Swaney, Sepulveda, Biller, Pichler, and Gisel) who work in humans as well as researchers (i.e., Gacek, Sihler, and Wu) who work in animals have confirmed that there is no consistent segregation of adductor and abductor fibers associated with the extralaryngeal branches. Sunderland and Swaney, who examined 65 human patients, showed no consistent pattern of segregation of abductor and adductor fibers into discrete bundles within the RLN trunk. Similarly, Gacek’s work in the cat does not show a segregation of fibers into discrete abductor and adductor groups in the vagus or RLN. Although Sepulveda dissected the RLN into discrete bundles that could be traced back to the vagus, he in no way identified these bundles as abductor and adductor fibers. Pichler and Gisel, in 100 dissections, failed to identify discrete abductor and adductor branches of the RLN. In five whole canine larynges stained with Sihler’s technique, Wu et al. found that posterior RLN branches joined with the IBSLN and that the anterior RLN gave off small posterior fibers to the PCA. Biller has written that, “variations have been reported in which the anterior branch innervates all the laryngeal musculature or where the abductor muscles were supplied by both branches.”
Sunderland and Swaney found the following variations:
Anterior RLN branch
May innervate the lateral cricoarytenoid, TA, and thyroepiglottis
May innervate all the laryngeal intrinsics
May innervate all the laryngeal intrinsic muscles and also provide an anastomotic branch to the SLN’s internal branch (Galen’s anastomosis to be described hereafter)
Posterior RLN branch
May innervate the PCA and interarytenoid muscles and give an anastomotic branch to the SLN’s internal branch (Galen’s anastomosis)
May not innervate any laryngeal muscles and continue to anastomose with the SLN’s internal branch
Sunderland and Swaney’s work suggests that the RLN anterior branch contains adductor fibers or adductor fibers and abductor fibers; it also suggests the RLN posterior branch contains abductor or no motor fibers; Sunderland and Swaney’s research is in agreement with recent work of Sanudo and Sato and with our current understanding of intralaryngeal RLN branching and Galen’s anastomosis. Recently, Maranillo et al. found PCA fibers arose from the anterior division of the intralaryngeal portion of the RLN, but that the PCA also in 4% of patients received a fiber from the posterior division (i.e., ramus anastomoticus), which may or may not contain motor fibers. True extralaryngeal RLN branching, we believe, is best understood as a variant form of normal intralaryngeal RLN branching where the branching occurs more proximally; the branching may be seen below the lowest edge of the inferior constrictor and is thus visible to the thyroid surgeon.
See Figure 36.7 .
Sanudo, Sato, and others have shown that the most posterior (dorsal) RLN branch fibers in virtually all patients join the posterior most branch of the SLN’s internal branch to form Galen’s anastomosis (i.e., ramus anastomaticus ). Galen ( ad 160–220), Martin (1734), Habershon (1876), and Portman (1951) described this RLN/SLN posterior anastomosis as Galen’s anastomosis or Galen’s ansa. Galen’s anastomosis has been described in humans and other species by Bowden. Lamere found that Galen’s anastomosis in both humans and dogs can be a single trunk, several branches, or it may exist as a plexus of fibers; some of these fibers can pass into the substance of the PCA muscle. Galen’s anastomosis is traditionally believed to be primarily an anastomosis of sensory fibers; however, new evidence suggests that it may have some motor function. Thus the first posterior or dorsal branch of the RLN should be considered the primary sensory contribution of the RLN to Galen’s anastomosis. It may or may not contain abductor motor fibers ( Figure 36.7 ). As Sunderland and Swaney noted, the degree to which the RLN’s posterior branch contains abductor fibers varies. Certainly, studies that involve sectioning of various RLN branches designated as abductor or adductor in cats, dogs, and humans do suggest that at least some abductor function resides in the posterior branch of the RLN in at least some individuals. Maranillo et al. recently showed that in nearly 90% of dissections, the nerve innervating the PCA muscle also contained fibers to one or more adductors. Some studies of RLN branch function have involved detailed intralaryngeal dissection of branches with the identification of Galen’s nerve; these studies are able to identify discrete PCA branches and anterior adductor branches. Although distal arborization within the larynx ultimately results in functional segregation of fibers, this is not anatomic information that is available at thyroidectomy when one is viewing the branches below the inferior constrictor.
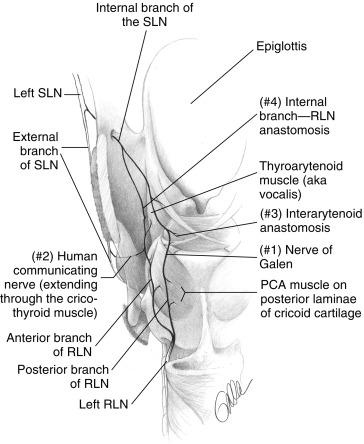
Serpell et al. recently applied vocalis EMG and laryngeal twitch assessment of the PCA in 64 patients with branching nerves and reported that all patients had both adductor and abductor motor fibers in the anterior branch. This may not imply that the posterior branch never has PCA fibers given the anatomic body of work presented previously. Need of larger studies and more definitive PCA assessment are necessary to conclusively make this statement. Experienced practitioners agree that damage to any of the RLN branches in the thyroid surgical field may engender VCP. Selective division of anterior or posterior branches at thyroidectomy does not result in a predictable discrete adductor or abductor dysfunction (Joseph Attie, personal communication, 1996). This agrees with our unpublished EMG data from intraoperative stimulation of anterior and posterior branches of the RLN during thyroidectomy. Therefore all extralaryngeal nerve branches of the RLN must be identified and preserved during thyroidectomy. However, Serpell’s work is important because it makes clear that the most common pattern of branching is an anterior branch that contains both adductor and abductor fibers and is certainly at risk if a posterior branch is first identified and felt to be the only RLN trunk; this could lead to injudicious and injurious dissection more anteriorly with disruption of the important anterior branch. Intraoperative nerve monitoring (IONM) can clearly help inform such a situation.
The relationship between stimulation frequency and glottic function informs us about the interrelationship of the adductor and abductor muscle systems within the larynx. RLN stimulation during intraoperative RLN monitoring involves stimulation at 1 to 2 mA, with pulses of 100 ms duration at 4 pulses per second. This results in a transient sphincteric-like twitch of the vocal cord in humans and dogs (Randolph unpublished observation). It is frankly difficult to characterize this brief movement, but it involves predominantly adduction and is a manifestation of the greater adductor muscle bulk (compared with abductor muscles) with transient muscle stimulation. Saunders found in dogs with transcutaneous stimulation at 10 mA of 1 ms duration that evoked vocal cord motion varied with stimulation frequency. Stimulation at 30 Hz resulted in maximal abduction, whereas stimulation ≥ 40 Hz resulted in adduction. Nakamura and others also found that 20 Hz stimulation resulted in abduction, whereas greater than 40 Hz stimulation resulted in adduction. Sanders speculated that this frequency-dependent vocal cord motion was related to different contraction times for abductor and adductor musculature. All laryngeal muscles have fast contraction times, compared with other somatic muscles, but abductor musculature is slower than adductor musculature. The PCA (the primary abductor) is described as being composed primarily of slow-twitch type 1 muscle with a longer contraction time of about 30 ms. The lateral cricoarytenoid (a primary adductor) is described as a fast-twitch type 2 muscle with a shorter contraction time of about 15 ms. With a suprathreshold electrical stimulation of the RLN, all axons are stimulated and both abductor and adductor muscles simultaneously contract. The movement from a single transient stimulation then results from interaction between the greater contraction time of the PCA and the greater bulk of the laryngeal adductors. With repetitive stimulation at lower frequencies of approximately 30 Hz, the slow-twitch PCA is in tetanic contraction, whereas the faster-twitch adductor muscles are going through cycles of repetitive contraction and relaxation. Thus abductor movement predominates. As the stimulation frequency increases, the adductor muscles approach tetanic contraction; with their greater bulk, the adductor muscles begin to predominate in terms of overall vocal cord motion.
To appreciate fully the functional significance of extralaryngeal branching and to interpret intraoperative RLN monitoring information, the thyroid surgeon needs to understand not only the anatomy of the RLN and SLN systems but also their interconnections (see Figure 36.7 available on expertconsult.com ). The SLN internal branch chiefly relates to the afferent innervation of the hypopharynx, base of tongue, supraglottis, and vocal cords. The SLN’s external branch provides motor innervation to the cricothyroid muscle and sensory innervation to the anterior subglottis. It is believed that the afferent activity, important in regulating laryngeal protective mechanisms, resides primarily in the IBSLN.
Connections between the RLN and SLN systems have been documented in a variety of dissection studies in 15% to 83% of cases. Sato, in 201 dissections of 113 cadavers, found direct connection between the RLN and the IBSLN in 53.7% of cases. Most have regarded these connections as primarily anastomotic connections between distal sensory branches of SLN and RLN, although some have speculated that these anastomotic links are associated with the possible motor complement within SLN branches. Dilworth noted that anastomotic interconnections between vagal branches innervating a given organ are a pattern seen throughout the body. Galen, and later Martin, believed that return of function (i.e., voice) that sometimes occurs after RLN transection resulted from regrowth from branches of the SLN. More recently, practitioners have again suggested that one method of vocal cord recovery after RLN injury involves reinnervation through supplemental motor branches of the SLN. Functionally important connections between the SLN and RLN systems can be divided into four groups (see Figure 36.7 available on expertconsult com): I. Galen’s anastomosis, II. SLN external branch/distal RLN anastomosis (human communicating nerve), III. interarytenoid anastomosis, and IV. SLN internal branch—RLN TA region anastomosis.
Galen’s anastomosis has been described earlier. Sañudo found this connection in 100% of 90 human microdissected larynges. The posterior branch of the RLN, just before or at the laryngeal entry point, reliably forms a robust anastomotic connection on the posterior surface of the PCA; controversy exists as to whether the IBSLN has motor fibers to the PCA or interarytenoid muscles; Berlin and Lahey, Vogel, and Sanders favor this position, but Lamere and King and Gregg argue against it. Sanudo found two cases in which Galen’s nerve sent branches to the PCA muscle. Sanders showed that, in five human dissections, axons of the IBSLN form connections with RLN branches in the interarytenoid region. Sanders found that some of these axons, ultimately innervating PCA muscle fibers, extend to these fibers from a superior direction, which suggests IBSLN origin. As discussed in the extralaryngeal RLN branch section, the posterior-most branch of the RLN may contain PCA fibers, or it may be mainly sensory with PCA RLN fibers deriving as small, twiglike, posteriorly oriented branches from the anterior branch of the RLN.
Sanudo et al. found that in 68% of 90 human microdissected specimens, the EBSLN, after innervating the cricothyroid muscle, continues on, extending through the cricothyroid membrane to innervate the anterior TA muscle region. Sanders found an anastomotic connection between the external branch and the RLN in this region in 4 of 10 human larynxes with Sihler staining technique. Maranillo recently confirmed this finding with external branch to RLN connections found in 83% of humans. Wu et al. found that of 27 human hemilarynxes treated with Sihler’s stain, 44% had external branches that extended to the anterior TA muscle; they termed these fibers the human communicating nerve. 100 Others, including Dilworth, Durham, and Meng, have found such a communicating nerve in 10% to 62% of human larynxes. In the work of Wu et al., four of these distal external branch fibers stained positively for acetylcholinesterase, which is a marker for motor neurons. Thirty-one percent of the fibers were judged to be motor neurons. Wu et al. described two parts to the nerve: an intramuscular set of fibers that usually combined with distal RLN branches terminating in the anterior portion of the TA muscle, and an extramuscular branch that passed through the TA muscle and terminated in subglottic mucosa (likely sensory). In a study of canine larynxes, Nasri found evidence of motor connection from the EBSLN to the TA muscle in three of seven dogs, confirmed by TA EMG data. The EBSLN penetrated the cricothyroid membrane between the medial and lateral cricothyroid muscle insertions. In two such animals, the innervation was present bilaterally. Stimulation of the ipsilateral RLN in these dogs gave a TA EMG amplitude of 2050 μV, whereas stimulation of the ipsilateral EBSLN gave TA EMG activity of only 437 μV. Interestingly, they noted the greatest EMG activity in the anterior one third of cord.
SLN motor contribution to the TA may, in part, explain differences in cord position seen with given RLN or vagal injuries. It may also explain Dedo and Venker-van Haagen’s finding of ongoing TA EMG activity, which could be seen immediately after RLN transection and persisted after thyrotomy (making contralateral RLN activity unlikely), but it stopped after SLN section. SLN motor contribution to the TA may also explain in part the poor, long-term control of adductor spasmodic dysphonia after RLN transection. Notably, cordal EMG with SLN stimulation may relate to recording electrode configuration and is controversial.
For the first 3 months after RLN section, laryngeal musculature atrophies. After this period, from 3 to 9 months, there is evidence of ipsilateral laryngeal reinnervation by laryngeal EMG. Controversy exists as to where these reinnervating fibers arise. Some suggest that spontaneous reinnervation of the deinnervated larynx arises from the existing proximal RLN stump. It may also be that motor fibers from the SLN system provide reinnervation through SLN/distal RLN connections. Wu et al. speculated that the human communicating nerve may be (intermediate between the fourth branchial arch SLN and the sixth branchial arch RLN) the fifth branchial arch nerve.
I have found, in more than 400 patients undergoing thyroidectomy, that SLN external branch can be found in virtually all cases either visually or through electrical stimulation, on or under the inferior constrictor fascia. In cases in which the nerve cannot be seen existing under the inferior constrictor fascia, the nerve stimulator can be swept over the lateral surface of the inferior constrictor. In more recent work with a novel endotracheal tube we were able to idenitify measureable evoked EMG on the vocal cord in 100% of patients. Nerve is indicated by the narrow patch of inferior constrictor, which when stimulated generates a discrete cricothyroid twitch. We have found that when we stimulate the EBSLN, we obtain significant TA EMG activity (via vocal cord surface endotracheal tube-based electrodes) in approximately 70% to 80% of cases (see the SLN Monitoring section at the end of this chapter). In agreement with Nasri’s canine data, we found the amplitude to be substantially less than for ipsilateral RLN stimulation. In cases in which external branch stimulation did not give TA EMG activity, we assume that either the human communicating nerve branch did not exist or that subtle electrode rotation issues precluded recording of activity that is likely centered on the anterior third of the cord. TA activity with external branch stimulation is not, in our patient series, mediated through an afferent vagal reflex arc because the latency of response is too short. We have found that the waveform amplitude with external branch stimulation is approximately one third of that obtained with ipsilateral RLN stimulation. Using vocalis muscle surface electrodes, Mermelstein also found EMG activity during SLN stimulation. The SLN waveform was complex (biphasic or triphasic); it had an average latency of 0.5 ms. Lipton also noted TA/lateral cricoarytenoid EMG activity as measured by hook-wire during external branch stimulation.
Both Sañudo and Sanders found RLN-SLN anastomotic plexus in the interarytenoid region. Nordland, Vogel, Mu, and Dilworth claimed that some of the SLN’s internal branch fibers in this area might contribute to motor function to the interarytenoid muscle.
Sanudo described an additional anastomotic connection between the TA branches of the RLN and the IBSLN as the “TA anastomosis.” He found this present in only 14% of cases; it has also been infrequently described by other practitioners. A cricoid anastomosis occurs in the posterior subglottis and is undoubtedly a sensory anastomosis between SLN and RLN systems.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here