Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() For videos accompanying this chapter see ExpertConsult.com. See inside cover for access details.
For videos accompanying this chapter see ExpertConsult.com. See inside cover for access details.
Abnormal eye movements in the infant or young child can be congenital or acquired. They may be associated with abnormal early visual development, or a sign of neurologic, neuromuscular or orbital disease. Abnormal eye movements in an apparently well child should never be labeled as congenital or benign without careful investigation. This should include a careful history and clinical examination. Neuroimaging, laboratory testing, and electrophysiologic investigation of the visual sensory system may also be indicated. Ocular motility analysis can indicate the type of eye movement disturbance and whether this is associated with an underlying ocular or neurologic condition.
The neural integrator is an engineering term applied to an ocular motor “function” carried out by groups of cells in the cerebellum (flocculus and paraflocculus), the prepositus hypoglossus and the medial vestibular nucleus (MVN). The neural integrator is needed for all conjugate eye movements. To move the eyes at a constant speed or hold them in an eccentric gaze position, two neural signals must overcome the elastic tendency of the eyes to go back to their “resting” position. These signals are the desired speed (phasic component) and a tonic component that counterbalances the elastic restoring forces. A changing tonic component can be precisely generated by a premotor neural signal that mathematically computes the “integration” of the velocity signal, thus the term “neural integrator.” The role of the neural integrator in generation of saccades and maintenance of eccentric gaze positions occurs after inhibitory burst neurons (IBNs) are inhibited and excitatory burst neurons (EBNs) fire rapidly, yielding an intense phasic signal that is transmitted to the appropriate yoked pair of extraocular muscles via the appropriate cranial nerves. The “burst” signal is also transmitted to the neural integrator, which “integrates” that burst signal (counts the number of discharge spikes) and generates a neural signal (again transmitted via the cranial nerves) appropriate to hold the eye steady at the new position (tonic discharge).
| Type of eye movement | Function | Stimulus | Clinical tests |
|---|---|---|---|
| Vestibular | Maintain steady fixation during head rotation | Head rotation | Fixate on object while moving head; calorics |
| Saccades | Rapid re-fixation to eccentric stimuli | Eccentric retinal image | Voluntary movement between two objects; fast phases of OKN or of vestibular nystagmus |
| Smooth pursuit | Keep moving object on fovea | Retinal image slip | Voluntarily follow a moving target; OKN slow phases |
| Vergence | Disconjugate, slow movement to maintain binocular vision | Binasal or bitemporal disparity; retinal blur motion | Fusional amplitudes; near point of convergence |
The neural integrator is not perfect and the “tonic” signal slowly decays or “leaks” over time. This decay is normally not seen in lighted conditions because visual feedback, with the use of the smooth-pursuit/fixation system, helps to hold the eye steady. At birth, the neural integrator is leaky, but by about 1 month of age it functions well.
A saccade is a rapid eye movement, which may occur volitionally (voluntary saccade), reflexively, or as part of the fast phases of nystagmus. It purposefully redirects the fovea to a specific target. Voluntary saccades may be predictive command generated (i.e. look right), memory guided, and antisaccades. Involuntary saccades include the fast phase of nystagmus, spontaneous saccades, and reflexive saccades. The pathway of saccades proceeds through the anterior limb of the internal capsule and then through the diencephalon. It then divides into dorsal and ventral pathways, the dorsal limb going to the superior colliculi and the ventral limb (which contains the ocular motor pathways for horizontal and vertical eye movements) to the pons and midbrain. The superior colliculus acts as an important relay for some of these projections. In the brainstem, the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF) and pontine paramedian reticular formation (PPRF) provide the saccadic velocity commands, by generating the “pulse of innervation” immediately before the eye movement, to cranial nerves III, IV, and VI. Horizontal saccades are generated by EBNs in the PPRF, which are found just ventral and lateral to the MLF in the pons, and by IBNs in the nucleus paragigantocellularis dorsalis, caudal to the abducens nucleus in the dorsomedial portion of the rostral medulla ( Fig. 91.1 ). Vertical and torsional components of saccades are generated by EBNs and IBNs in the riMLF, located in the midbrain ( Fig. 91.2 ).
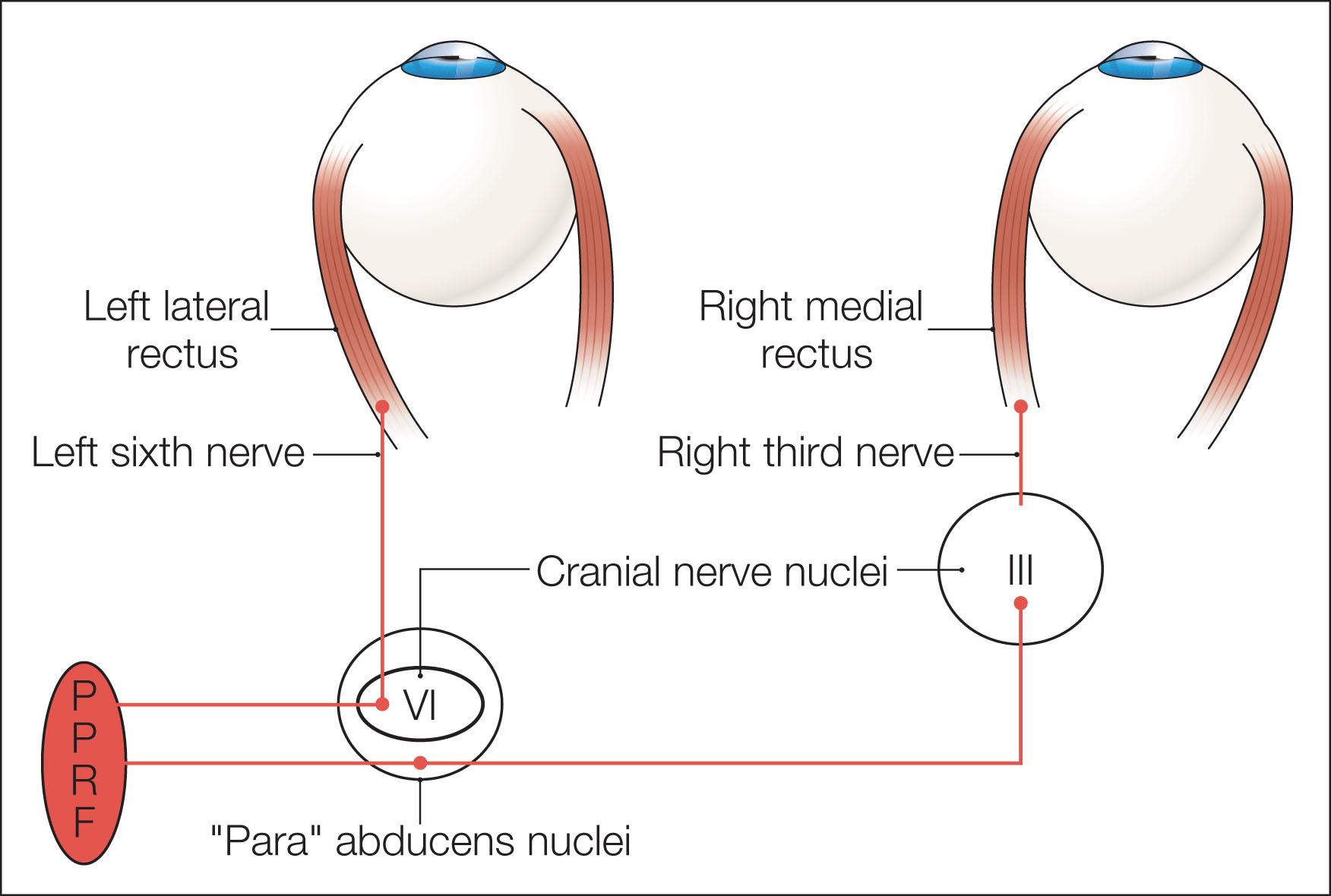
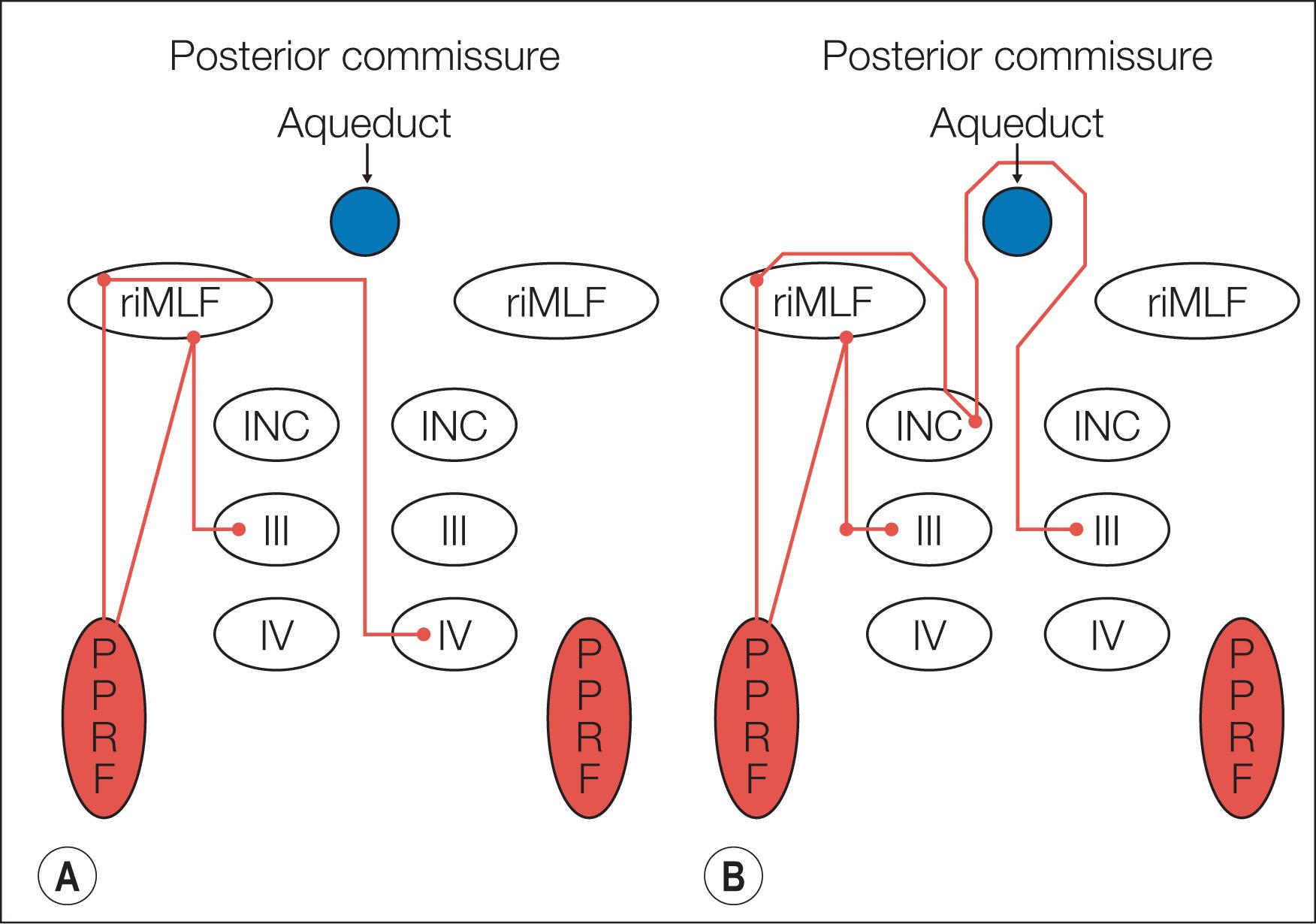
Following a saccade, a “step of innervation” occurs, during which a higher level of tonic innervation to ocular motor neurons keeps the eye in its new position against orbital elastic forces that would restore the eye to an anatomically “neutral” position in the orbit. For horizontal saccades, the step of innervation comes from the neural integrator (NI), most importantly from the nucleus prepositus–MVN complex. The eye is held steady at the end of vertical and torsional saccades by the step of innervation provided from the interstitial nucleus of Cajal in the midbrain. Other burst neurons termed long-lead burst neurons (LLBNs) discharge 40 ms prior to saccades, whereas excitatory burst neurons (EBNs) discharge 12 ms prior to saccades. Some LLBNs lie in the midbrain, receiving projections from the superior colliculus and projecting to the pontine EBNs, medullary intermediate burst neurons (IBNs), and OPNs. Other LLBNs lie in the nucleus reticularis tegmenti pontis (NRTP), projecting mainly to the cerebellum but also to the paramedian pontine reticular formation (PPRF). It appears that LLBNs receiving input from the superior colliculus may play a crucial role in transforming spatially coded to temporally coded commands, whereas other LLBNs may synchronize the onset and end of saccades.
The saccadic system is not fully developed until about 1 year of age; infants make multiple hypometric saccades to reach the target. If the head is held still, this hypometria can be seen clinically in infants less than 3 months of age, especially for large saccades. There is a progression toward “normometria” during the first 7 months of life. In healthy adults and children older than 1 year, saccades are typically hypometric, reaching about 90% to 100% of the target distance, followed by secondary saccades – normometria. In optokinetic nystagmus and vertical nystagmus, quick phases occur less frequently in children than in adults.
The function of smooth pursuit (SP) is to hold fixation on a moving target, and both eye and head movements are required. This requires a prediction to overcome the time delays of the visuomotor system and the ability to suppress the vestibulo-ocular reflex (VOR). VOR suppression is required because, when the head moves, there is a reflexive VOR that moves the eyes equally in the opposite direction. SP and VOR suppression are probably the same ocular motor functions. The frontal and extrastriate visual cortexes transmit information about the motion of both the target and the eyes to the dorsolateral pontine nuclei, thence to the paraflocculus, flocculus, and dorsal vermis, and then via the vestibular and fastigial nuclei to the ocular motor nerve nuclei III, IV, and VI. Unilateral lesions in the cortex and cerebellum affect ipsilateral SP. Brainstem lesions are less well defined clinically.
SP is present in the first week of life but is immature in the young infant. Horizontal gaze probably develops before vertical gaze. SP gain increases with age, and at 5 months the SP is more apparent. It is not known at what point in time pursuit matures to the adult form. It does not appear to happen before 6 months of age, and may not be mature until late adolescence.
The vestibular apparatus drives reflex eye movements to keep images steady on the retinas as we move our heads. The eyes move in the opposite direction to the head so that they remain in a steady position in space. The three-neuron arc – vestibular ganglion, vestibular nuclei, and ocular motor nuclei – are the principal connections. The direct neuronal pathways include both excitatory and inhibitory contributions. Each semicircular canal influences a pair of extraocular muscles that move the eyes in the plane of that canal. The anatomy of the vestibular nuclei has been well characterized ; they receive projections from the 14,000 to 18,000 axons of the vestibular nerve.
There are four major vestibular nuclei and accessory subgroups, including the interstitial nucleus, with its cells distributed among the vestibular rootlets as they enter the brainstem, and the y-group, near the superior cerebellar peduncle. The MVN has the greatest volume and is the longest vestibular nucleus. The lateral vestibular nucleus (LVN) also has projections to the spinal cord, mainly via the ipsilateral lateral vestibulospinal tract but also through the contralateral medial vestibulospinal tract. In its most rostral aspect, the dorsal vestibular nucleus (DVN) also projects to the ocular motor nuclei.
The primary vestibular afferents enter the medulla at the level of the LVN. Almost all bifurcate, giving a descending branch to terminate in the MVN and DVN and an ascending branch to the superior vestibular nucleus (SVN), with a final destination in the cerebellum, especially the anterior vermis and the nodulus and uvula. All canals and otoliths project to the borders of ventromedial LVN, medial MVN, and dorsomedial DVN. All canals also converge on a small patch in the ventromedial SVN. Utricular afferents project to the rostral MVN and saccular afferents project to the y-group. For both the horizontal and vertical VOR, many neurons in the vestibular nuclei that receive inputs from primary vestibular afferents encode head velocity, eye position, and varying amounts of SP and saccadic signals. Vestibular nucleus neurons do not project just to motor neurons; they also send collaterals to the nucleus prepositus hypoglossi, the nucleus of Roller, and the cell groups of the paramedian tracts.
When testing the VOR in the infant, it is not unusual to find a slightly high VOR gain, which gradually decreases during preschool years, and a short VOR time constant. In the premature infant and in some healthy full-term infants, rotation induces a tonic deviation or “locking up.” The eyes deviate in the direction opposite to rotation when the doll's head maneuver or the Barany chair rotation test is used. They deviate in the same direction as the rotation when the infant is rotated at arm's length. A “corrective” fast phase develops at approximately 45 weeks post-conceptual age. This lock up may be prolonged in the infant with delayed visual maturation. Usually there are no more than a couple of beats of post-rotational nystagmus when the child stops spinning; if there are more than a few beats of nystagmus, a severe visual deficit or abnormality of the SP pathway should be suspected. This test can be influenced by behavioral state and wakefulness.
Vergences are eye movements that turn the eyes in opposite directions so that images of objects will fall on corresponding retinal points. In other words, it is the ability to change the angle between the two visual axes to permit near (convergence), far (divergence), and torsional (cyclovergence) foveation, for binocular vision. Three major stimuli are known to elicit vergences: (1) retinal disparity that leads to fusional vergences; (2) retinal blur that evokes accommodative vergences; and (3) motion induces both disparity and accommodative vergence. The neural substrate for vergence lies in the mesencephalic reticular formation, dorsolateral to the oculomotor nucleus where neurons discharge in relation to vergence angle (vergence tonic cells), velocity (vergence burst cells), or both angle and velocity (vergence burst–tonic cells). Although most of these neurons also discharge with accommodation, some remain predominantly related to vergence. Like versional movements, a velocity-to-position integration of vergence signals is necessary: the NRTP is important in this integration. The cells in the NRTP that mediate the near response are separate from cells that mediate the far response. Lesions of the NRTP cause inability to hold a steady vergence angle. The NRTP has reciprocal connection with the cerebellum (nucleus interpositus) and receives descending projections from several cortical and subcortical structures.
The eyes of neonates, particularly if premature, often appear divergent, and there is little voluntary convergence until 2–3 months. By 3–6 months of age, 75% of premature and 97% of full-term infants are orthophoric. Fusion is not completely established until 6 months. Accommodation-driven vergence can be detected at 2 months of age and disparity-driven vergence at about 4 months, which is when stereopsis and fusion develop.
The optokinetic system is responsible for conjugate slow following of the eyes to movement of large areas of the visual field. Optokinetic nystagmus (OKN) is a reflex conjugate physiologic nystagmus in which a slow-phase pursuit response to movement of the visual surround (optokinesis) is followed by a corrective saccade or quick phase. OKN is elicited naturally during head and eye movements, or unnaturally by looking out of the window of a moving vehicle. Together with the vestibular system, the optokinetic system holds images steadily on the retina during sustained motion of the head or world or both. The neural substrate for optokinesis includes the nucleus of the optic tract and accessory optic pathways. Optokinesis can be elicited by either full-field (natural head or eye movements) or small-field (foveal) motion (a moving drum or tape). Both SP and optokinetic systems contribute to the stabilization of images of stationary objects during head rotations. In primates, OKN has two components, each of which has a separate but parallel neural pathway. Delayed (indirect, slow) OKN (OKNd) has a slow buildup (tens of seconds) and gives rise to optokinetic after-nystagmus (OKAN), which is a gradual decay of the nystagmus after the lights have been extinguished. OKNd is closely related to VOR and is driven by visual motion signals in the visual cortex via the nucleus of the optic tract in the pretectum and the vestibular nuclei. Early (direct, fast) OKN (OKNe) has a rapid buildup (<1 s) and does not give rise to OKAN; it ceases promptly in the dark. The OKNe pathway is similar to the SP pathway, which is mediated by a corticopontocerebellar route, and it is doubtful that the pretectum has a direct role in OKNe/SP, although it may be involved in its adaptive control.
Finally, the cerebellum plays an important role in eye movements. Together with several brainstem structures, including the nucleus prepositus and the medial vestibular MVN, it appears to convert velocity signals to position signals for all conjugate eye movements through mathematical integration. Figure 91.3 shows the anatomical areas of the brain responsible for the “localizing” forms of acquired nystagmus.
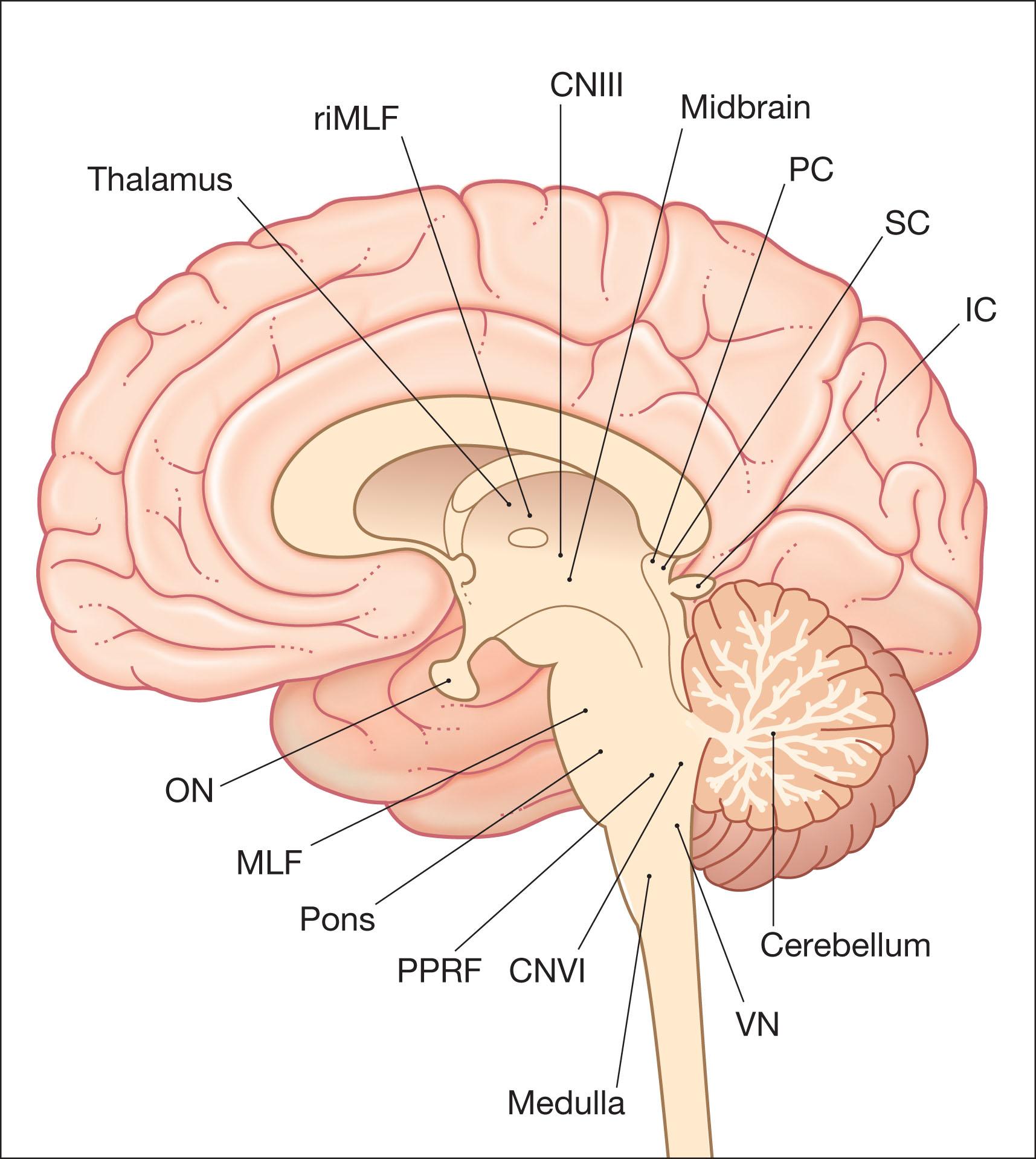
The workup of supranuclear eye movement disorders and nystagmus is directed toward identifying ophthalmic and neurologic features which point to the underlying etiology. This is usually apparent by history, examination, or neuroimaging.
The history should determine the age of onset of the nystagmus.. A family history of eye disease, the pregnancy, labor, delivery, growth and development since birth should be sought. Nystagmus onset dating back to the neonatal period is generally benign, while acquired forms require further investigation. Since anxiety can affect nystagmus, the eye movements should be observed from a comfortable distance, in a non-threatening manner, while talking to the child or the parent. The most important features of nystagmus can usually be ascertained while “playing” with the child. Head turns or tilts while the child is viewing distant or near objects should be noted. An adequate fundus examination is a necessary part of the evaluation of eye movement disorders and involuntary ocular oscillations in infants and children ( Figs. 91.4 and 91.5 ).
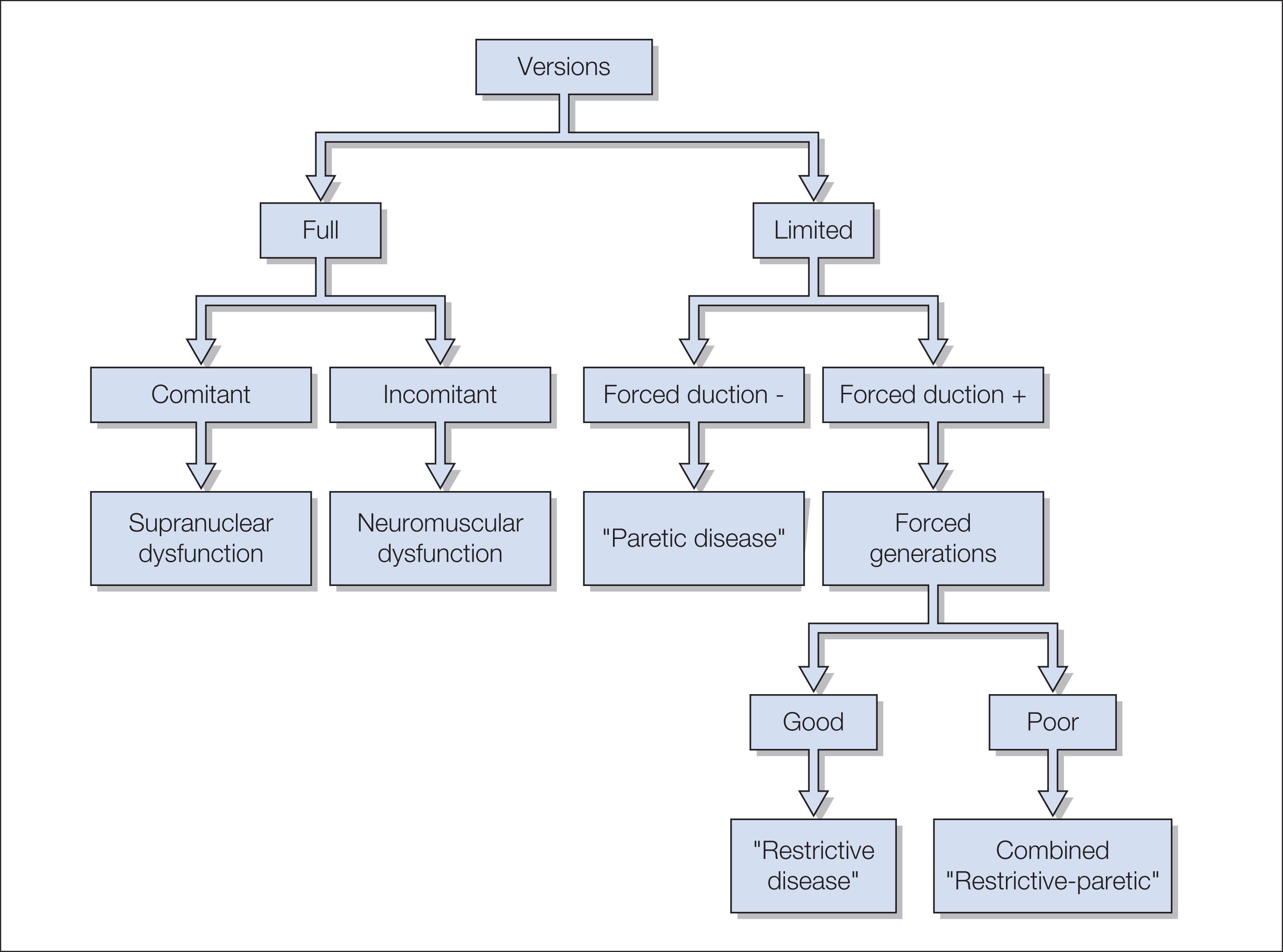
Vision testing depends on the patient's age and their neurologic status. The “binocular acuity” should be tested first, allowing the patient to assume any anomalous head posture (AHP) they wish. During the examination of visual acuity in nystagmus patients with an AHP, the direction of the posture must be observed over a 5–7 minute period. Up to 17% of patients with infantile nystagmus (and also some with acquired nystagmus) manifest a periodic change in the direction of their nystagmus fast phase and, consequently, their head posture.
Binocular acuity is the “person's” acuity and monocular acuity is the “eye's” acuity; they are often very different in patients with nystagmus. In a non-verbal child or adult, various tests can be used to help determine both binocular and monocular acuity. These include fixation behavior, the 10 prism diopter base-down test, Teller acuity cards, and matching of single, crowded, HOTV optotypes or Lea symbols. Simple testing of binocular function, e.g. Worth 4-dot, and near stereopsis should be attempted. The results are important if convergence is to be stimulated or fusion is to be aided by refractive therapy.
In older children, a subjective refraction is the foundation for any type of refractive therapy. All refractionists develop their own idiosyncrasies that assist them with rapid and accurate refraction. Try to ignore the oscillation and start with distance retinoscopy in a phoropter for patients without an AHP or trial frame or loose lenses (for those with a significant AHP). The next step is to do binocular refraction: this is the most important step in these patients, because some patients have significant changes in their nystagmus under monocular conditions. The best way to do this is to fog the eye not being refracted with enough plus to decrease the vision by 1–3 lines.
It is important in infants and young children to perform an objective refraction procedure; a cycloplegic refraction provides additional and important data for treatment decisions. In those patients in whom there is a different refraction under cycloplegia, record both subjective and objective refraction for decision-making regarding spectacle prescription.
Clinical evaluation of the ocular oscillation includes fast phase direction, movement intensity, conjugacy, gaze effects, convergence effects, and effect of monocular cover. The amplitude, frequency, and direction of the nystagmus in all directions of gaze can be documented with a simple diagram. The clinician can also observe the nystagmus while moving the patient's head. Associated motility systems (e.g. strabismus, pursuit, saccades, and VOR) can be clinically evaluated and recorded. Changes in the nystagmus with convergence or monocular viewing should be noted. If the nystagmus increases beneath closed eyelids, vestibular or brainstem pathology should be suspected since visual fixation may suppress nystagmus from lesions in these regions. Conjugacy of eye movements should be observed.
Eye movement recordings provide a basis for eye movement abnormality classification, etiology, and treatment, and have impacted eye movement systems research ( Figs. 91.5 and 91.6 ). There are four commonly used methods:
electro-oculography
infrared reflectance oculography
scleral contact lens/magnetic search coils: this is the most sensitive technique and is minimally invasive, but its use in children between 6 months and about 10 years is difficult (see Figs. 91.5 and 91.6 )
video oculography.

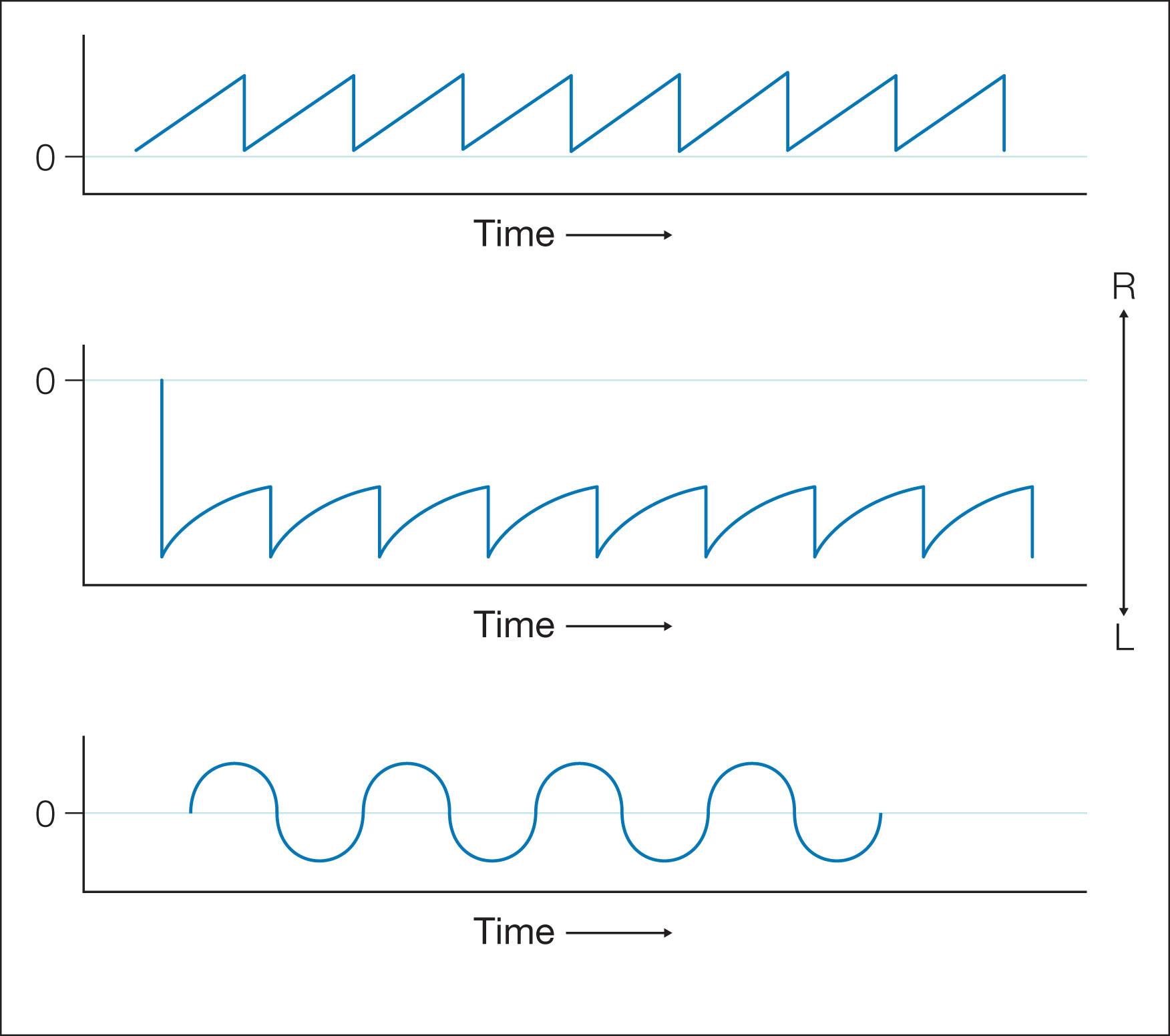
Practical applications of eye movement recording technology in clinical medicine include diagnosis/differentiation of eye movement disorders and utility as an “outcome measure” in clinical research. Eye movement recordings display the data during continuous periods of time. Position and velocity traces are clearly marked, with up being rightward or upward eye movements and down being leftward or downward eye movements. The basic types of nystagmus patterns observed after eye movement recordings are shown in Fig. 91.7 .
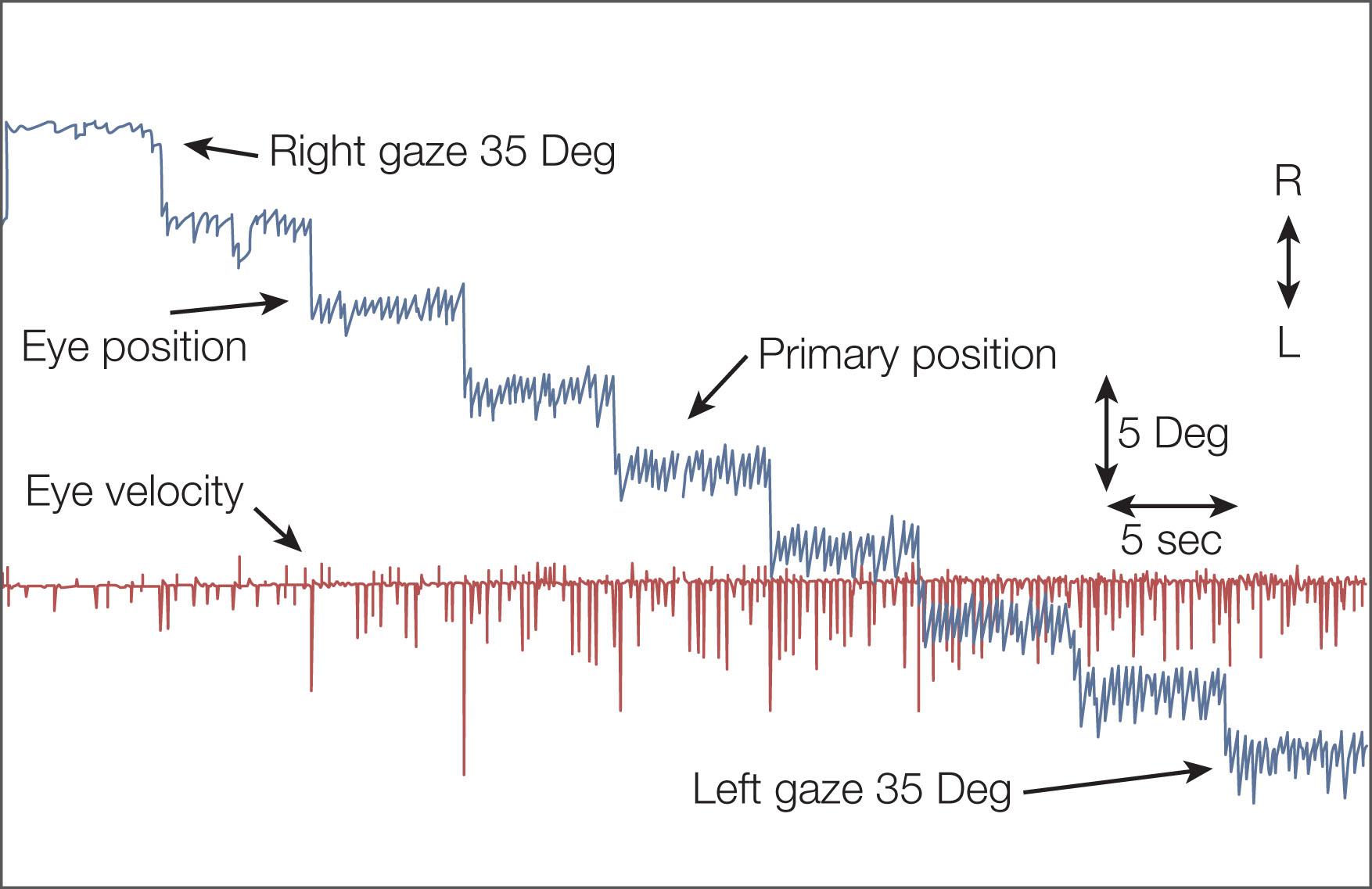
To test the neural integrator clinically, observe primary position fixation, fixation in eccentric gaze, saccades, pursuit, and OKN, and also test for rebound nystagmus and VOR cancellation. To examine for rebound nystagmus, first ask the patient to fixate on a target from the primary position, then to re-fixate on an eccentric target for 30 seconds, and then return to the primary position target. A patient with rebound nystagmus shows transient nystagmus with the slow phases toward the previous gaze position. To evaluate a child's VOR cancellation, it is easiest to place your hand on top of the patient's head to control both the head and a fixation target that will extend in front of the child's visual axis. If the child is unable to cancel the VOR, you will observe nystagmus instead of the steady fixation expected in normal subjects.
“Gaze-evoked” nystagmus is a sign of a leaky neural integrator and occurs with attempts to maintain eccentric gaze. These “beats” of nystagmus toward the eccentric position persist as long as the child attempts to view a peripherally placed target. This is different from physiologic endpoint nystagmus, where only a few beats of nystagmus, gradually decreasing in amplitude, are present while viewing eccentrically placed targets.
If an abnormality of saccadic eye movements is suspected, the quick phases of vestibular and OKN can be easily evaluated in infants and young children. To produce and observe vestibular nystagmus, hold the infant at arm's length, maintain eye contact, and spin first in one direction and then in the other ( Fig. 91.8A,B ). An OKN response can be elicited by passing a repetitive stimulus, such as stripes or an OKN drum, in front of the baby, first in one direction and then in the other ( Fig. 91.8C ). In addition, reflex saccades will be induced in many young patients when toys or other interesting stimuli are introduced into the visual field. Older children are asked to fixate alternately upon two targets so that the examiner can closely observe the saccades for promptness of initiation, speed, and accuracy ( Fig. 91.9 ). However, do not confuse a saccade toward sound as a visually guided saccade in a child with poor vision; it should first be established that the child can see. In children with strabismus, it is necessary to test saccades monocularly. The OKN drum/tape or the VOR response can also be used to assess saccadic function. It is sometimes easier to observe the eyelashes moving vertically while testing vertical saccades using a vertically rotating OKN drum (see Fig. 91.9C ).

SP is tested by having the child follow a slow-moving, easily seen target (e.g. a brightly colored toy or mirror), both horizontally and vertically. The child's head should initially be kept still. If the child is uncooperative or is thought to have hysterical blindness, a mirror should be slowly rotated before his/her eyes, or an OKN drum or tape can be used. When examining the pursuit system, one should always include an assessment of VOR suppression, which is an essential component of the SP task during motion. In the older child, VOR suppression can be examined by asking the child to hold his/her finger about 6–8 cm in front of their face. The child is then asked to slowly rotate his/her head with their finger from left to right while maintaining fixation on their finger. If the VOR response is suppressed, there is no movement of the eyes relative to the head. Failure of VOR suppression results in a jerk nystagmus beating in the direction of the rotation. In an infant or young child, you can test VOR suppression by holding the child in outstretched arms and spinning in both clockwise and counterclockwise directions, while encouraging the child to fix on your face.
Vergence is usually tested by objective tests such as cover/uncover testing, alternate cover testing, prisms in front of the eye, and manual tests of near point of accommodation and vergence. Subjective aspects of vergence are tested by measuring the child's ability to perceive stereopsis. Many of these tests are dependent on the child's ability to cooperate, but alternate cover testing can be performed as soon as the child is able to fix on a near object.
Both slow and fast phases of OKN can be elicited by using OKN drum, tape, or full-field OKN stimuli (see Fig. 91.8C ). OKN responses are evident with both eyes open in the full-term infant from the first day of life. In healthy neonates, a monocular OKN response can be obtained when the stimulus is moved in the temporal-to-nasal direction, but not in the nasal-to-temporal direction (physiologic/developmental, monocular OKN asymmetry). After 3 months, this monocular OKN asymmetry declines for moderate stimulus speeds, but persists beyond 6 months of age at high stimulus speeds.
Neural integrator dysfunction manifests clinically as gaze-evoked nystagmus and is often associated with low pursuit gain (gaze-holding deficiency nystagmus, eccentric gaze nystagmus) and rebound nystagmus. Quick phases are away from the central position (see Fig. 91.7 ). Neurologic signs and symptoms include vertigo, nausea, dizziness, and oscillopsia. Gaze-evoked nystagmus is induced by moving the eye into lateral or vertical gaze, with sustained attempts to look eccentrically ( Fig. 91.10 ). After the eyes are then returned to the central position, a short-lived nystagmus with quick phases opposite to the direction of the prior eccentric gaze occurs (rebound nystagmus). Abnormal suppression of the VOR and low-gain OKN responses are also associated with clinical findings and, again, probably reflect cerebellar disease (see Fig. 91.10 ).
Most types of pathologic nystagmus increase their intensity as the eyes move in the direction of the fast phase (Alexander's Law); this is believed to be due to a physiologically adapted “leaky” neural integrator responding to the pathologic vestibular imbalance that is part of the nystagmus.
Most frequently, gaze-holding deficiency nystagmus is seen in conjunction with use of anticonvulsants and sedatives, cerebellar and brainstem disease, or other drug intoxication. Magnetic resonance imaging (MRI)/computed tomography (CT) of the brain reflects underlying disease and ocular motility recordings show slow phases that have decelerating velocity characteristics. Acquired eye movement abnormalities suggesting defective neural integration, whether isolated or associated with other neurologic deficits, alert the examiner to investigate for a serious central nervous system (CNS) abnormality. Structural anomalies affecting the brainstem and cerebellum, e.g. Arnold–Chiari malformation, as well as metabolic, vascular, and neurodegenerative disorders, may also produce abnormalities of the neural integrator.
Normal and abnormal saccades are often dysmetric (inaccurate). Saccadic inaccuracy can be a result of gain dysmetria or pulse-step dysmetria. In saccadic dysmetria, the saccade misses the target and corrective saccades are needed for foveation ( Fig. 91.11 ). If the saccades fall short of the target, they are known as hypometric; if they overshoot the target, they are hypermetric. In both cases, one or more secondary saccades are needed to eventually fixate the target. Hypometria is often seen in cerebellar disease, including autosomal dominant spinocerebellar ataxia type 3, and ocular motor apraxia (OMA). It is far more common than hypermetria. Consistent marked hypometria below 90% that persists beyond 7 months of age suggests neurologic disease. Hypometric saccades can be secondary to changes in visual magnification; for example, the removal of aphakic spectacles may lead to a temporary hypometria until adaptation takes place.
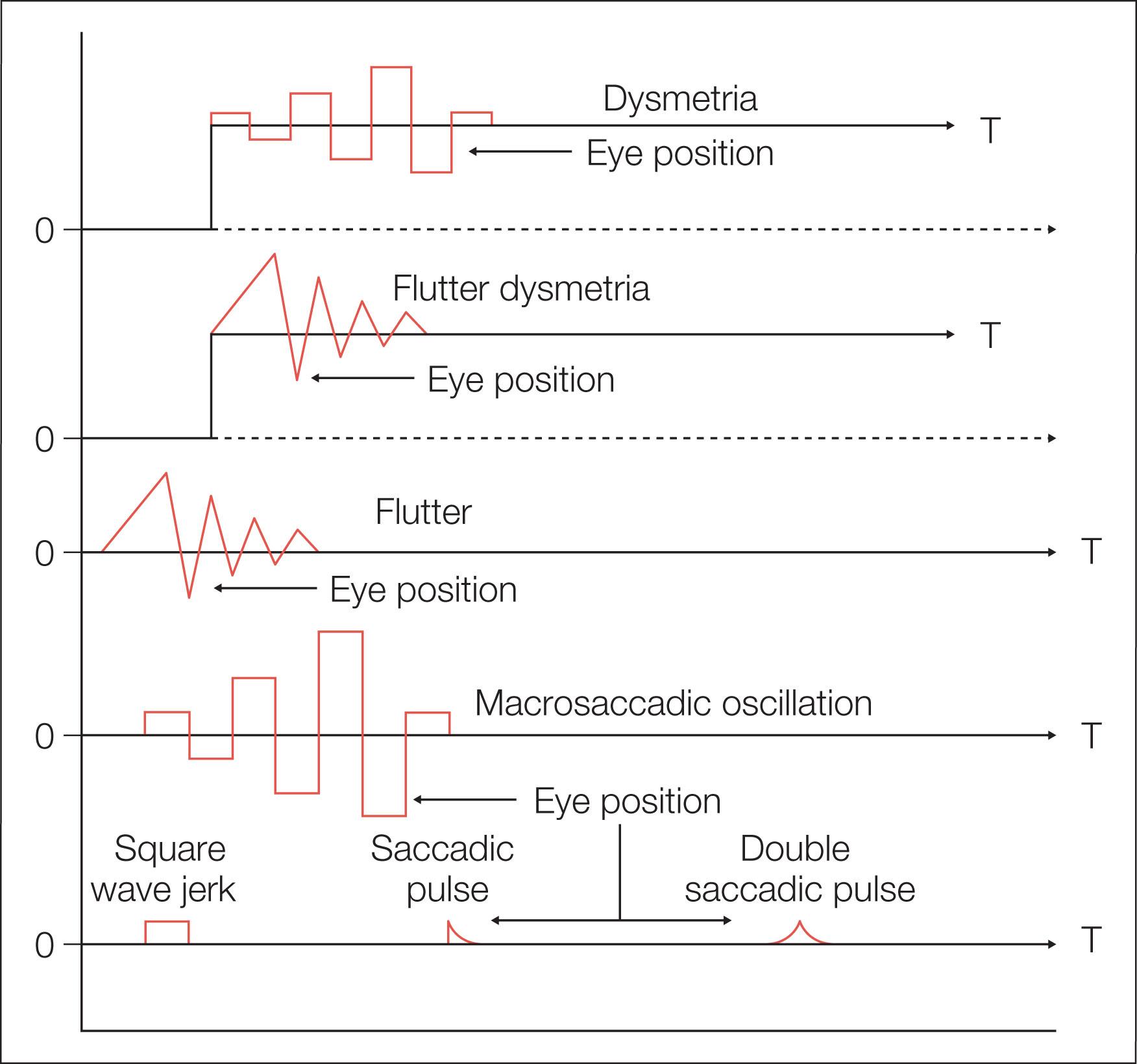
Hypermetria is much less common and appears flutter-like (but should not be mistaken for ocular flutter). With the exclusion of centripetal saccades, hypermetria is abnormal at any age, and, when conjugate, it is almost always associated with cerebellar disease, including spinocerebellar ataxia type 1. If hypermetria is severe, the corrective saccade may be as large as the primary saccade, thus causing the eyes to oscillate back and forth with saccades. This phenomenon has been termed “macrosaccadic oscillations.” After recovery of opsoclonus, there may be persistent hypermetric saccades (see Fig. 91.11 ).
Slow saccades may not be obvious clinically, especially in children. They may result from an abnormality involving the paramedian pontine reticular formation and have been thought to be pathognomonic of burst cell dysfunction. Slow saccades may be seen in mitochondrial disorders, progressive external ophthalmoplegia, myasthenia gravis, basal ganglia disease, Duane syndrome, and families with spinocerebellar ataxia type 2. Slow horizontal, and sometimes vertical, saccades may occur in patients with Gaucher type 3 disease.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here