Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Supporting/connective tissue is the term applied to tissues which provide general structure, mechanical strength, space filling (sculpting body shape), and physical and metabolic support for more specialised tissues.
Connective tissues usually have three structural properties with corresponding construction materials:
Tensile strength to resist pulling, stretching and tearing. This is provided by strong fibres of structural proteins from the collagen family.
Elasticity to facilitate return to original shape after mechanical distortion. This is provided mostly by specialised elastin fibrils which function like rubber.
Volume (i.e. bulk/substance). This is provided by glycoproteins and complex carbohydrates with profound water-binding ability, forming a wet gel known as ground substance .
The combined mix of fibres and ground substance is called extracellular matrix and this determines the physical properties of the tissue. Matrix is produced and assembled under the control of support cells , most commonly fibroblasts . The cells of supporting tissue are derived from precursor cells in primitive (fetal) supporting tissue called mesenchyme .
Supporting tissues occur with diverse physical properties. In most organs, loose connective tissue (also known as areolar tissue ) acts as a biological packing and wrapping material. Tissue with a greater density of fibres provides a structural framework. Dense forms of supporting tissue provide tough physical support in the dermis of the skin, comprise the robust capsules of organs such as the liver and spleen, and the specialised high–tensile strength ligaments and tendons. Cartilage and bone , both major skeletal components, are specialised forms of connective tissue that are considered separately in Ch. 10 .
Specialised fat storage is a further function, with adipose tissues having important metabolic roles. White adipose tissue also provides a structural fill and forms part of shock-absorbing padding. Highly metabolically active brown adipose tissue helps in the regulation of body temperature and body weight.
In addition, supporting tissues usually contain blood vessels, lymphatic vessels and associated nerves. Repair of tissue damage, especially wound closure and scar formation, is also largely a function of supporting tissues, involving both the support cells and blood and lymphatic vessels.
C capillary blood vessel F fibroblast
The fibrous components of connective tissues are of two main types: collagen (including reticulin , which was formerly considered a separate fibre type) and elastin .
Collagen is the main fibre type found in most supporting tissues and is the most abundant protein in the human body. Its notable function is the provision of tensile strength to resist pulling, stretching and tearing.
Collagen is secreted into the extracellular matrix by connective tissue cells (e.g. fibroblasts) in the form of a tropocollagen monomer . This consists of three polypeptide chains (each called an alpha chain and not necessarily all identical), bound together to form a helical protein structure 300 nm long and 1.5 nm in diameter. In the extracellular matrix, these tropocollagen molecules polymerise longitudinally and also side-to-side, forming collagen fibrils which are cross-linked by the enzyme lysyl oxidase.
At least 28 different types of collagen (designated by Roman numerals I to XXVIII) have now been delineated in the collagen super-family on the basis of morphology, amino acid composition and physical properties. Collagens can be fibre forming, mesh/network forming or cell membrane–associated proteins.
Type I collagen is the main structural collagen and is found in fibrous supporting tissue, skin (dermis), tendons, ligaments and bone. The tropocollagen molecules polymerise longitudinally and also side-to-side to form fibrils, and these are strengthened by numerous intermolecular bonds. Parallel collagen fibrils are further arranged into strong fibre bundles 2 to 10 μm in diameter, which confer great tensile strength to the tissue. These collagen fibres are visible with the light microscope, staining pink with H&E, with fibres in varying patterns of orientation, size and density according to the mechanical support required in the tissue.
Type II collagen is the main structural collagen of hyaline cartilage and consists of fibrils in the cartilage ground substance.
Type III collagen forms the delicate branched ‘reticular’ supporting meshwork which is prominent in highly cellular tissues such as the liver, bone marrow and lymphoid organs. This fibre was initially recognised by its affinity for silver salts and was (and often still is) called reticulin.
Type IV collagen is a network/mesh-forming collagen and is an important constituent of basement membranes .
Type VII collagen forms special anchoring fibrils that link extracellular matrix to basement membranes.
The remaining collagen types are present in various specialised situations.
Ap adipocytes BV blood vessels E elastin fibres F fibroblasts L longitudinal collagen fibres SM smooth muscle T transverse collagen fibres
Elastin is arranged as fibres and/or discontinuous sheets in the extracellular matrix where it confers the properties of stretching and elastic recoil. Elastin is a protein synthesised by fibroblasts in the form of a precursor monomer known as tropoelastin . The monomers are polymerised in the extracellular matrix by the enzyme lysyl oxidase, with extensive cross-linking of lysine amino acid side chains. Deposition of elastin in the form of fibres requires the presence of a template of microfibrils of the structural glycoprotein fibrillin and associated glycoproteins. These become incorporated around and within the ultimate elastic fibre.
Elastin is the name of both the fibre and the polymerised protein. There are also two named related fibres, oxytalan and elaunin , which have more fibrillin and less polymerised tropoelastin than generic elastin.
Elastin is found in varying proportions in most supporting tissues, conferring elasticity to enable recovery of tissue shape following normal physiological deformation. Elastin is present in large amounts in tissues such as lung, skin and urinary bladder. It is an important constituent of the wall of blood vessels; in arteries, elastin provides the stretch and recoil to smooth and transmit the pulse pressure generated by each heartbeat. In the lung, the stretch and recoil of the elastin is basic to that organ’s function.
Elastic fibres are eosinophilic and when large they are slightly refractile , meaning they bend light differently to other tissue components. This may enable their recognition; however, special elastin stains are usually needed.
Scurvy is a classical disease, historically of sailors, where defective collagen formation from lack of vitamin C results in loose teeth, skin haemorrhages and death. Small haemorrhages around skin hair follicles are an early sign.
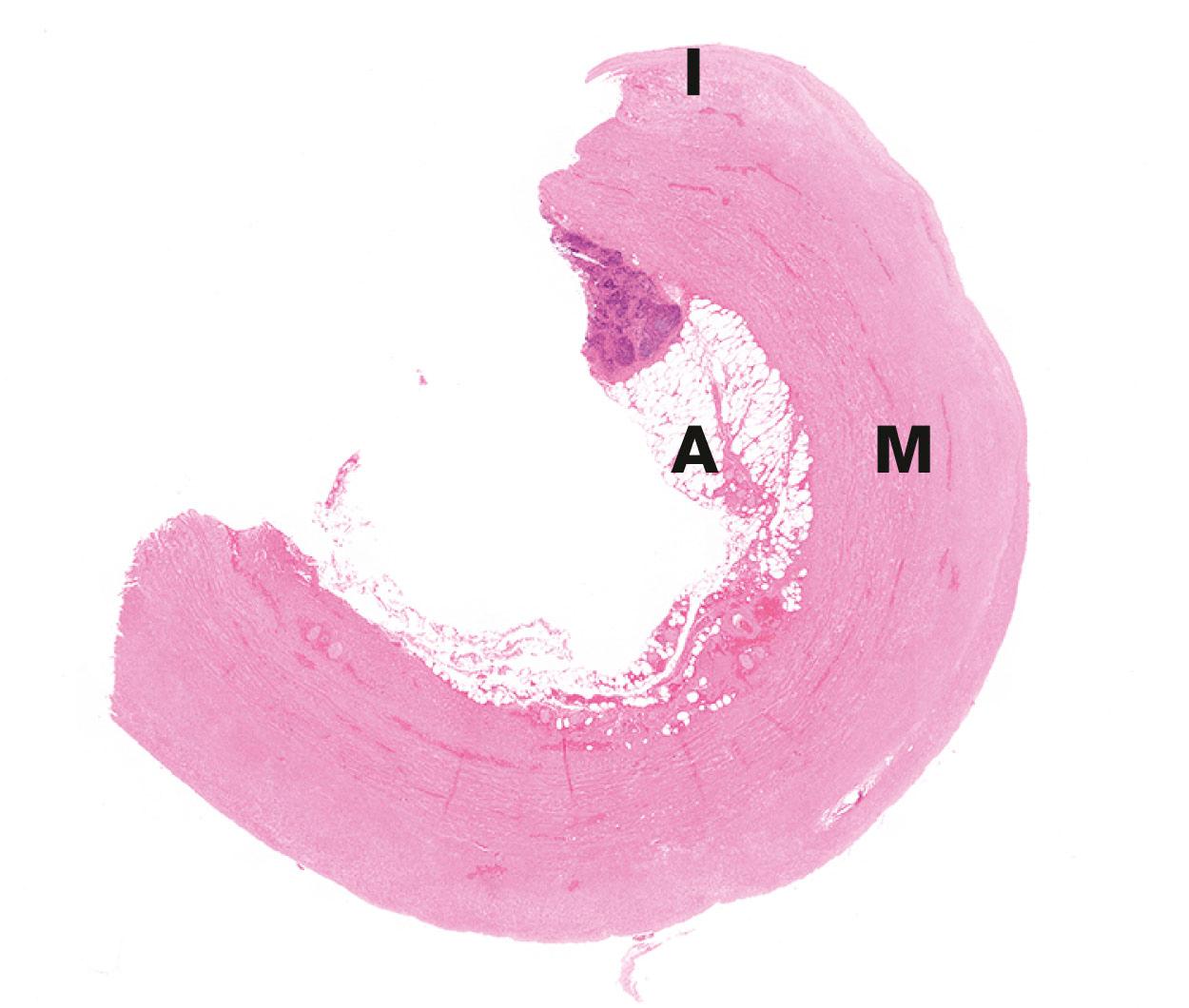
There are several inherited diseases caused by mutations in genes coding for types I and III collagen. Their main effects are in reduced tensile strength in supporting tissues, leading to abnormal tissue laxity or susceptibility to injury. Ehlers–Danlos syndromes, for example, are characterised by abnormal skin laxity and hypermobility of joints which can predispose to recurrent joint dislocations. Thirteen forms have been described, which have distinct clinical associations. The vascular subtype of Ehlers–Danlos syndrome can lead to bulges in the walls of arteries ( aneurysms ) . These can rupture, causing death.
Cap capillary E elastin fibres Ep epidermis F fibroblasts L longitudinal collagen fibres M microfibrils (fibrillin) Ma mast cells ML longitudinal microfibrils T transverse collagen fibres
Ground substance derived its name from being an amorphous transparent material with the physical character of semi-solid gel. It is a mixture of glycoproteins and complex carbohydrates with profound water-binding ability. Extra-cellular fluid, both water and salts (particularly sodium), are bound to these molecules, providing volume and compression resistance to the tissue and its tissue turgor (i.e. the internal pressure). They form the physical milieu and indirectly control the passage of both molecules and cells through the tissue and the exchange of metabolites with the circulatory system.
The carbohydrates are long, unbranched polysaccharide chains of seven different types, each composed of repeating units of two sugar derivatives, usually a uronic acid and an amino sugar such as N-acetyl glucosamine. This gives rise to the term glycosaminoglycan ( GAG ).
Hyaluronate , also known as hyaluronic acid , consists of repeating d-glucuronate (β1,3)-N-acetyl-d-glucosamine units and is the predominant GAG, forming huge unbranching linear molecules of 100,000 to 10,000,000 molecular weight.
The other GAGs include chondroitin-4-sulphate , chondroitin-6-sulphate , dermatan sulphate , keratan sulphate , heparan sulphate and heparin sulphate . Each of these molecules contains sulphated N-acetyl groups substituted onto galactosamine sugars in the repeating carbohydrate units, making them highly negatively charged (acidic). These charged groups prevent the carbohydrate chains from folding into globular aggregates, causing them to remain in an expanded linear form, thereby occupying a large volume for a small mass. The charged side groups also render them extremely hydrophilic, attracting a large volume of water and positive ions, particularly sodium.
These GAGs (other than hyaluronate) exist as carbohydrate chains covalently linked to various protein molecules, forming a range of molecular structures containing up to 90% to 95% carbohydrate. These are called proteoglycans . There are numerous specific proteins, including perlecan, syndecan, decorin, lumican and aggrecan . Proteoglycans have various specific functions. Some bind to hyaluronic acid producing massive quaternary structures, others interact with collagens or bind to various other matrix molecules including remodelling enzymes, enzyme inhibitors, growth factors, cytokines and cell surface receptors.
In addition to the proteoglycans, there are further glycoprotein molecules important in ground substance. These include two fibril-forming molecules, fibrillin (discussed in the section on elastin) and fibronectin .
Fibronectin has binding sites for many connective tissue components and plays a part in controlling the deposition and orientation of collagen in extracellular matrix. Fibronectin molecules bind to collagens, to heparan sulphate (a GAG) and to specific membrane receptors on cells.
Cell membranes incorporate a group of transmembrane protein complexes called integrins , which act as cell adhesion molecules. One of these acts as a fibronectin receptor; it binds internally within the cytoplasm to actin filaments of the cytoskeleton and externally binds with the fibronectin. This interaction forms part of a specialised layer of extracellular matrix called basement membrane where cells meet matrix. Here, other non-filamentous glycoproteins also play a structural role (see opposite).
Basement membranes are sheet-like arrangements of extracellular matrix proteins which act as an interface between the support tissues and epithelial or parenchymal cells. Basement membranes are also associated with blood vessels and muscle cells and form a limiting membrane around the central nervous system. The term derives from the initial recognition of membranes lying beneath the basal cells of epithelia. In the context of muscle and nervous tissue, the term external lamina is often applied.
Basement membranes have several functions:
Provide physical binding of the epithelium to the underlying tissue and physical support
Control of epithelial growth and differentiation, they form a barrier to downward epithelial growth; this is only breached if epithelia undergo malignant transformation (cancer).
Permit the flow of nutrients, metabolites and other molecules to and from an epithelium, as epithelium is devoid of blood vessels
Where a cell layer acts as a selective barrier to the passage of molecules from one compartment to another (e.g. between the lumen of blood vessels and adjacent tissues), the basement membrane assumes a critical role in regulating permeability. This role of forming a selective barrier reaches an extreme level of sophistication in the kidney, where the glomerular basement membrane is part of the highly selective filter for molecules passing from the bloodstream into the urine.
The main components of basement membranes and external laminae are the glycosaminoglycan heparan sulphate , type IV collagen , and the structural glycoproteins fibronectin , laminins and nidogen-1 . While fibronectin appears to be produced by fibroblasts of the supporting tissue, the rest are at least partly elaborated by the tissues being supported.
The structural framework is a fine meshwork of type IV collagen, a mesh/network-forming collagen found exclusively in basement membranes. The glycoprotein laminin, in concert with nidogen (also called entactin ), binds the type IV collagen and links to other basement membrane constituents and to laminin receptors on the basal plasma membranes of the epithelial cells.
Type III collagen (reticulin) is bound via the fibrillar glycoprotein fibronectin to integrins in the epithelial basal plasma membrane. Fibronectin also binds the GAG heparan sulphate. Basement membranes vary in their molecular details between sites and between types of epithelia.
BM basement membrane BV blood vessels E external laminae M mucin
Basement membranes are involved in several disease processes.
In the kidney (see Ch. 16 ) the fused basement membranes of endothelial cells and podocytes form the filtration barrier for the ultrafiltrate in the glomerulus. If the glomerular basement membrane becomes abnormal then renal function is impaired. In patients with diabetes mellitus, there is thickening of the glomerular basement membrane, which becomes abnormally permeable to proteins.
Epithelial tissues grow and regenerate and are anchored to, but separated from, support tissues by a basement membrane.
Mutations in epithelial cells lead to abnormal growth ( neoplasia ), forming cancers. A malignant tumour can grow from the site of origin and spread into local tissues (invasion). This is achieved by cancer cells secreting factors that facilitate destruction of basement membrane, allowing cells to grow into the extracellular matrix.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here