Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Stroke broadly describes the sudden onset of neurologic dysfunction due to an abnormality of blood supply to the brain, retina, or spinal cord. Ischemic stroke makes up the majority of all strokes and is often considered synonymous with stroke although the definition extends to intracerebral hemorrhage, cerebral venous sinus thrombosis, subarachnoid hemorrhage, and retinal and spinal ischemia. In a 2013 update from the American Heart Association and the American Stroke Association, cerebral ischemia and cerebral hemorrhage that were present on brain imaging without an overt neurologic symptom (i.e., were “silent”) were included in the definition of stroke to underscore the significance of the pathology regardless of clinical manifestation. This definition of stroke is not widely accepted outside of the United States and will have implications for comparing outcomes and disease prevalence internationally. Cerebrovascular accident (CVA) is a related term that has fallen out of favor in part because it implies the outcome as unanticipated. On the contrary, ischemic and hemorrhagic strokes are usually not “accidents,” but rather manifestations of chronic conditions.
Ischemic stroke has an association with many general medical conditions. It is a heterogeneous disorder caused by any combination of thrombosis, embolism, or hypoperfusion. Ischemic stroke etiology is commonly subtyped into broad categories including small artery (lacunar), large vessel atherosclerotic, or cardioembolic, and there is overlap in the medical conditions that lead to these stroke subtypes. This chapter will review the most common medical conditions that underlie stroke.
Hypertension is the most common risk factor for ischemic stroke and is responsible for the greatest proportion of preventable strokes. The relationship between stroke and blood pressure seems to be maintained well under the traditional threshold of 140/90 mmHg, and even modest decreases in blood pressure reduce stroke risk. Elevated blood pressure exerts injury throughout the cerebrovascular system and is a risk factor for multiple types of stroke including intraparenchymal hemorrhage, aneurysmal subarachnoid hemorrhage, and ischemic stroke of multiple subtypes, including small vessel, large vessel, and cardioembolic. The mechanisms underlying each stroke type are distinct but result from a combination of mechanical and inflammatory injury to both small and large vessel artery walls. Elevated blood pressure also increases the risk of cardiac structural changes, atrial fibrillation, and myocardial infarction and therefore is an indirect but important cause of cardiac embolus formation leading to stroke.
Treatment of blood pressure is effective for both primary and secondary stroke prevention, and higher intensity strategies of blood pressure reduction are thought to have played a major role in the reduction of the population stroke risk in the United States over the past half century (see Chapter 7 ).
Elevated total cholesterol and low-density lipoprotein (LDL) are correlated with atherogenesis of the carotid arteries and with heart disease, while high-density lipoprotein seems to be protective. Although the correlation between atherosclerotic disease and heart disease is well known, there is little correlation between the absolute levels of serum cholesterol and LDL and ischemic stroke risk.
HMG-CoA reductase inhibitors (statins) reduce LDL by inhibiting their synthesis and are an important component of stroke prevention therapy. Although their effect can be measurable in terms of LDL reduction, it is currently believed that statin medications exert their stroke reduction via other antiatherogenic mechanisms. Ezetimibe and PCSK9 antibodies are mechanistically distinct from statins and are used as add-on therapy for further LDL reduction when required; currently, these medications are recommended for use in patients who are considered to be at high risk for atherosclerotic cardiovascular disease.
While often present with other metabolic risk factors, the presence of diabetes independently increases stroke risk (see Chapter 19 ). The pathogenesis may be mediated by an enhanced atherogenesis in diabetics, microvascular disease of the arterial walls, and the promotion of coagulation by way of platelet activation and changes in coagulation factors. As with hypertension, diabetes mellitus is associated with several ischemic stroke subtypes.
Hyperglycemia is associated with an increased risk of mortality following stroke, and prevention of severe hyperglycemia during this period confers an improved outcome. However, intensive blood glucose control in the immediate period following acute stroke (i.e., maintaining blood glucose less than 130 mg/dL) does not seem to render any benefit in stroke recovery compared to strategies that aim to prevent hyperglycemia (blood glucose greater than 180 mg/dL).
Homocysteine is an intermediate in methionine metabolism, and hyperhomocysteinemia may result from an acquired or genetic deficiency in the enzymes or co-factors involved. Elevated plasma homocysteine is associated with all-cause vascular disease, mortality, and an increased risk of ischemic stroke. High levels of homocysteine are also linked to vascular injury and atherosclerotic plaque formation.
Severe hyperhomocysteinemia results in homocystinuria and is usually caused by inborn errors of metabolism. Individuals with homocystinuria experience premature atherosclerosis, thromboembolic disease including stroke, developmental delay, osteoporosis, marfanoid appearance, and ectopia lentis. This condition is usually diagnosed prior to young adulthood due to the overt manifestations.
In contrast, mild to moderate hyperhomocysteinemia may be clinically asymptomatic and accompany vitamin deficiencies (e.g., B 12 , B 6 , folate), and genetic variants including mutations in the methylenetetrahydrofolate reductase gene ( MTHFR ). Mild to moderate elevations of homocysteine are also associated with vascular disease and ischemic stroke; however, whether mild to moderate elevations of homocysteine directly contribute to vascular injury or are merely a marker of vascular disease is debated.
Folate, B 12 , and B 6 vitamin supplementation reduce levels of plasma homocysteine, even in the absence of overt vitamin deficiency, and have been studied for their effect on stroke reduction without much promise to date. In a 2017 Cochrane review including 15 randomized trials, 71,422 participants, and up to 7.3 years follow-up concluded that homocysteine-lowering interventions had a possible but small reduction in stroke risk. In combination with antihypertensive medications, homocysteine-lowering treatments may prevent one stroke in 143 people treated for 5.4 years. At this time, routine screening and treatment for hyperhomocysteinemia in stroke patients is not recommended unless there is clinical suspicion for homocystinuria.
Cardiogenic thromboemboli are a common source of ischemic stroke. Thrombi may originate from the chambers of the heart, the cardiac valves, or from systemic veins gaining access to the arterial system through a right-to-left shunt in the heart (e.g., a patent foramen ovale (PFO) or atrial septal defect). The latter, termed paradoxical embolism , is still referred to as a cause of cardiogenic stroke though this may be a misnomer since the embolus comes from a noncardiac source, accessing the cerebral circulation via a structural heart defect. Paradoxical emboli can also occur in the setting of noncardiac shunts such as a large arteriovenous malformation (AVM) located elsewhere in the body.
Atrial fibrillation (AF) and atrial flutter combined are the most frequently associated conditions with cardiogenic stroke. Traditionally, AF was thought to lead to intracardiac clot formation from dysrhythmic contractility leading to stasis and subsequent thrombus formation in the left atrium or atrial appendage. However, given the dependence on comorbid vascular risk factors and advanced age to substantiate a high stroke risk in AF, this mechanism is probably not entirely explanatory.
In elderly patients with vascular risk factors, AF leads to structural remodeling of the left atrium. Whether AF is directly causal to stroke or whether stroke results from an atrial cardiopathy remains a matter of debate. Nevertheless, the discovery of persistent or episodic atrial fibrillation in patients with cardiogenic stroke is important as these patients benefit from anticoagulation more than antiplatelet therapy in the secondary prevention of stroke; long-term rhythm monitoring for the detection of atrial fibrillation is recommended following embolic stroke. The benefit of anticoagulation in patients without known atrial fibrillation but with atrial cardiopathy is not yet known.
Valvular atrial fibrillation, commonly from mitral valve stenosis, is also associated with an increased risk of stroke. Anticoagulation with warfarin is recommended for stroke prevention in patients with valvular AF, whereas direct oral anticoagulants have emerged as a preferred therapy for nonvalvular AF. See Chapter 5 for further discussion.
A PFO occurs when the foramen ovale fails to close after birth. This is a common cardiac finding, estimated to occur in one out of every four individuals and generally thought to be of no consequence in otherwise healthy people. However, young patients with embolic-appearing stroke tend to have a higher proportion of PFO, raising the question of causality in certain patients with stroke. Previously, surgical closure of PFO for secondary stroke prevention was not known to be of benefit but recent studies have suggested a long-term benefit for closure in select young patients with high-risk PFO and cryptogenic embolic stroke.
In the United States, women are disproportionally impacted by stroke. Women experience a higher life-time risk of stroke, and as a result of stroke, women are more likely to be institutionalized and have poorer outcomes including a higher mortality rate. Some of these sex-specific differences may be due to longer life expectancies in women and their older age at stroke onset, but certain vascular risk factors have higher prevalence or risk attribution in women including hypertension, diabetes mellitus, migraine with aura, and atrial fibrillation.
There are also sex-specific stroke risk factors unique to women including pregnancy, pre-eclampsia, gestational diabetes, menopause, and exposure to exogenous hormones from oral contraceptives and hormone replacement therapy ( Table 11-1 ). Because women tend to be underrepresented in major stroke trials, increasing inclusion and awareness in order to understand the unique aspects of stroke in women is critical.
| Risk Factor | Sex-Specific Risk Factor | General Risk Factor Stronger or More Prevalent in Women |
|---|---|---|
| Pregnancy | X | |
| Pre-eclampsia | X | |
| Gestational diabetes | X | |
| Oral contraceptive use | X | |
| Postmenopausal hormone replacement therapy | X | |
| Menopause | X | |
| Migraine with aura | X | |
| Atrial fibrillation | X | |
| Diabetes mellitus | X | |
| Hypertension | X | |
| Depression | X | |
| Psychosocial stress | X |
Stroke in pregnancy is a major cause of long-term morbidity and an important cause of mortality for these women. Recent pooled estimates suggest that stroke may complicate as many as 30 out of 100,000 pregnancies, which is a threefold higher risk compared to young adults overall. Stroke in pregnant women includes ischemic, hemorrhagic, and cerebral venous sinus thrombosis occurring in roughly equal proportions, which diverges from the general population where stroke incidence is predominantly ischemic.
The peripartum and postpartum periods tend to be the highest risk periods for stroke, with pregnancy-related hypertension and the acquired thrombophilia of pregnancy factoring most prominently (see Chapter 31 ). Other conditions that predispose to stroke can also manifest during pregnancy such as vascular dissection, congenital cardiac complications, moyamoya disease, and hemorrhage from aneurysm or vascular malformation.
Changes in the coagulation system that accompany pregnancy tend to manifest in the third trimester and are implicated in the increased risk of ischemic stroke and cerebral venous sinus thrombosis during pregnancy. These changes include a general increase in procoagulant factors (e.g., I, VII, VIII, IX, X, XII, XIII, and functional APC resistance), and a decrease in anticoagulants (e.g., antithrombin III and protein S).
Pre-eclampsia manifests in the latter half of pregnancy and is common among pregnant women with ischemic stroke, hemorrhagic stroke, and subarachnoid hemorrhage. The mechanism of stroke related to pre-eclampsia and eclampsia is likely complex related to both abnormal vascular tone and prothrombotic effects. Pregnancy cardiomyopathy and amniotic fluid embolization are other causes of ischemic stroke in pregnancy.
Successful use of tissue plasminogen activator (tPA) as a treatment for acute ischemic stroke in pregnant women is understood at the case report level, and has not been studied empirically. Since tPA does not cross the placenta, it would not be expected to cause direct harm to the fetus; however, the major concern is the risk of placental hemorrhage and abruption prompting preterm delivery. Based on these limited case reports, tPA should be considered in the treatment of disabling ischemic strokes in pregnant women. For large-vessel occlusions, thrombectomy without intravenous thrombolysis is also a reasonable approach.
Women who experience stroke related to pregnancy may also have a higher risk of lifetime recurrent stroke. This may be due to a persistence of vascular risk factors that were simply unmasked in pregnancy (e.g., women with gestational diabetes have a higher risk of developing diabetes mellitus later in life). Women with stroke related to pregnancy should therefore be monitored closely to ensure proper lifetime risk reduction and optimal prenatal planning for future pregnancies.
The physiologic transition into menopause is associated with an increased risk of ischemic stroke, and the preponderance of current data suggests that earlier menopause may be associated with a greater risk of stroke compared to those with typical age of onset. Endogenous estrogen in the premenopausal state has been hypothesized as providing a protective effect against stroke risk; however, as reviewed in the section below, exogenous hormone replacement is now thought to increase the risk of stroke. Thus, hormone replacement therapy should be used cautiously in women with vascular risk factors, and only with the intent to control symptoms related to menopause.
Postmenopausal women experience an increase in all subtypes of ischemic stroke. While premenopausal women tend to have lower rates of hypertension when compared to their age-matched male counterparts, this reverses in postmenopausal women; hypertension tends to be more common in women with stroke than men with stroke, and women are also less likely to achieve adequate blood pressure control pre- and post-stroke. Whether there is a physiologic basis for medication resistance or higher rates of nonadherence is unknown. There is an increased incidence of comorbid vascular risk factors in elderly women including central obesity and elevated total cholesterol and LDL.
While many conditions that predispose to stroke are broadly prothrombotic, there are a variety of major hematologic disorders that result in pathologic activation of hemostatic pathways and the coagulation cascade. These can arise from inherited, acquired, or mixed causes. Procoagulant perturbations of the coagulation cascade result from loss of function of a natural anticoagulant, such as protein C, protein S, or antithrombin III inherited or acquired deficiencies, or a gain of function of a procoagulant such as factor V Leiden, activated protein C (APC) resistance, or prothrombin gene mutations. Other conditions can bolster the hemostatic pathway including the antiphospholipid antibody syndrome, heparin-induced thrombocytopenia (HIT), and certain malignancies and their treatments (see below).
Current clinical guidelines from the American Heart Association and American Stroke Association do not specify for whom testing for hypercoagulable states should be performed post-stroke and this continues to be an area of variability in practice. The yield of thrombophilia testing is probably greatest in young patients with a cryptogenic stroke, or those with a personal or family history of thrombosis or unexplained pregnancy loss. See Table 11-2 for a suggested work-up for thrombophilia.
| Basic coagulation screen: INR, aPTT |
| Antithrombin III functional assay |
| Protein C functional assay |
| Protein S functional assay |
| APC resistance assay, including genetic testing for factor V Leiden if APC abnormal |
| Prothrombin (factor II) gene mutation 20210A genetic testing |
| Antiphospholipid antibodies: anticardiolipin IgG and IgM, β2-glycoprotein-1 IgG and IgM, lupus anticoagulant assay |
Antiphospholipid antibodies, a misnomer given their indirect action on anionic phospholipids, function in various ways to enhance hemostasis through their interaction with vascular endothelial cells and subsequent activation of the coagulation cascade. Antiphospholipid syndrome (APS) is defined by a venous or arterial thrombosis or pregnancy loss in the presence of persistent positivity of one or more antiphospholipid antibodies (aPL). APS can be a primary disorder or secondary to a rheumatologic condition such as systemic lupus erythematosus (SLE). The mechanism of stroke in APS may be a result of in situ arterial thrombosis or cardioembolism secondary to APS-related nonbacterial endocarditis ( Fig. 11-1 ).
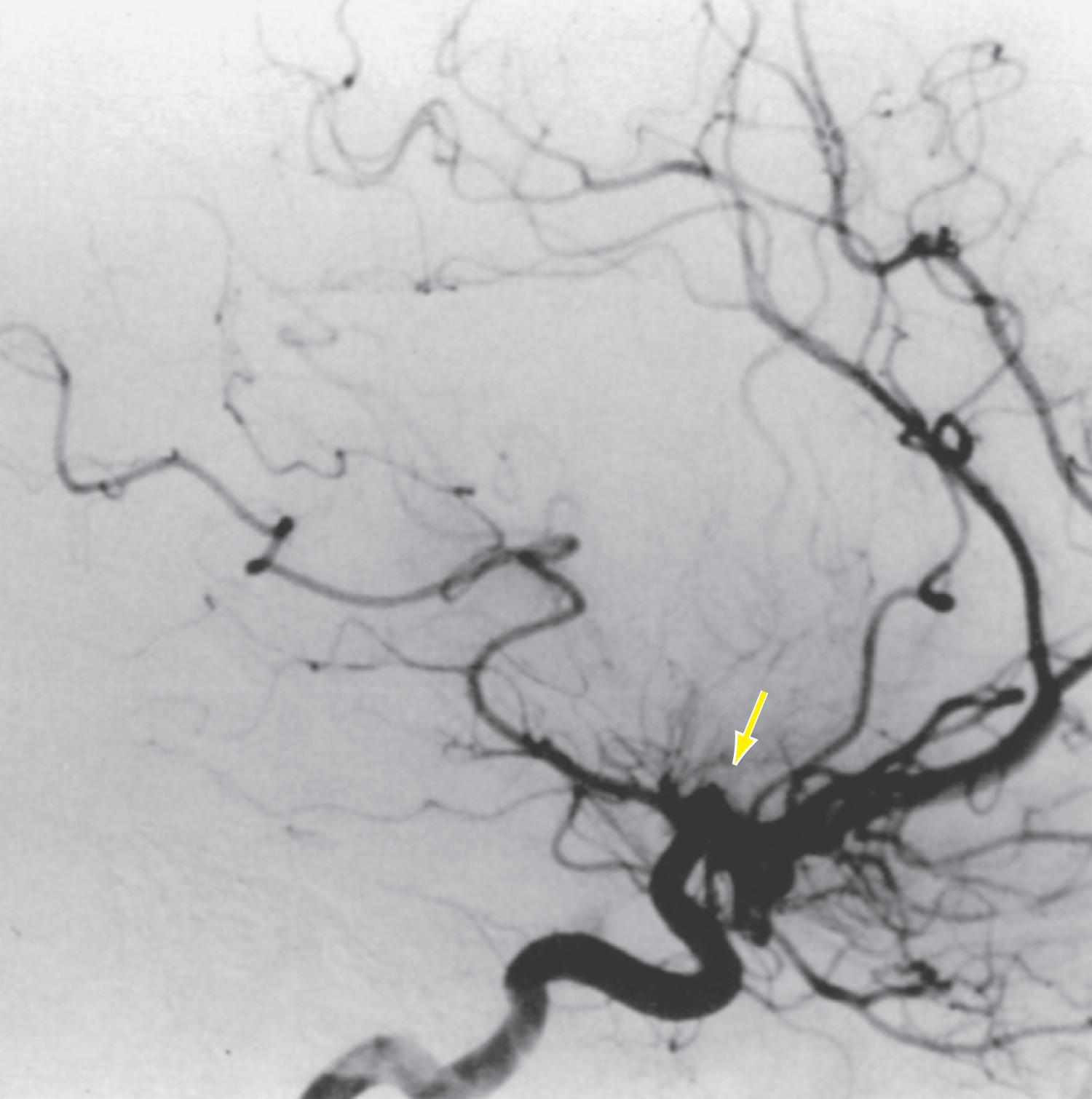
Antiphospholipid antibodies that fulfill diagnostic criteria for APS include anti-β2 glycoprotein-I antibodies (aβ2GPI), anticardiolipin antibodies (aCL), or a positive functional lupus anticoagulant (LA) assay. There is emerging evidence that other antiphospholipid antibodies may be relevant to APS and stroke, although they are not yet formally part of the diagnostic criteria. These other antibodies include antiphosphatidylserine (aPS), antiphosphatidylserine-prothrombin antibodies (aPS/PT), antiannexin A5, and antiphosphatidylethanolamine (aPE). In terms of serologic risk prediction, the highest risk is often attributed to (1) triple positivity (aβ2GPI, aCL, and LA), (2) moderate to high titers, and (3) IgG isotype (versus IgM). The strongest association with non-criteria antibodies and risk of stroke and death is with aPS/PT.
There are currently no disease-modifying drugs used in the management of APS, and the mainstay of therapy consists of antithrombotic medications to reduce the risk of future thrombotic events. The data regarding an optimal regimen do not address the safety and efficacy of newer oral anticoagulants for APS. Current expert consensus is that standard intensity warfarin (INR 2–3) should be used for secondary stroke prevention in APS unless there are significant bleeding concerns or a perceived low risk of recurrent thromboembolic events. If warfarin is not initiated for any reason, patients should be treated with antiplatelet therapy for secondary prevention. Patients with stroke and aPL who do not meet criteria for APS are not recommended for treatment with anticoagulation for secondary stroke prevention.
Given the increasing awareness that aPL may be an independent risk factor for thromboembolism, primary stroke prevention is an important clinical question with insufficient data. At this time, many consider antiplatelet therapy in asymptomatic aPL carriers if there are co-morbid vascular risk factors; however it is unknown whether this exerts a significant stroke risk reduction in otherwise healthy, young patients.
Sneddon syndrome is a neurocutaneous disorder associated with antiphospholipid antibodies that primarily affects middle-aged women. Histopathologically, Sneddon syndrome is a noninflammatory thrombotic arteriopathy of medium and small vessels in the dermis and in the brain. Skin biopsy reveals dermal inflammation without vasculitis. Clinically, it is characterized by recurrent strokes and livedo reticularis. Affected patients may also have Raynaud phenomenon or acrocyanosis of the digits. Antiphospholipid antibodies are often prominent and progressive cognitive decline from the arteriopathy may occur even in young persons. Accordingly, any young patient presenting with progressive cerebrovascular disease or cognitive decline and livedo reticularis should be evaluated for the presence of antiphospholipid antibodies. Optimal treatment is unknown and both antiplatelets and anticoagulants have been used. Immunosuppressive therapy is not routinely indicated.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here