Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The mature spermatozoon is an elaborate, specialized cell produced in massive quantity, up to 1200 per second. Spermatogenesis begins when Type B spermatogonia divide mitotically to produce diploid primary spermatocytes (2 n ), which then duplicate their DNA during interphase. After a meiotic division, each daughter cell contains one partner of the homologous chromosome pair, and they are called secondary spermatocytes (2 n ). These cells rapidly enter a second meiotic division in which the chromatids then separate at the centromere to yield haploid early-round spermatids ( n ). Thus, theoretically, each primary spermatocyte yields four spermatids, although fewer actually result, as the complexity of meiosis is associated with germ cell loss.
The process by which spermatids become mature spermatozoa within the Sertoli cell takes several weeks and consists of several events: the acrosome is formed from the Golgi apparatus; a flagellum is constructed from the centriole; mitochondria reorganize around the midpiece; the nucleus is compacted to about 10% of its former size; and residual cell cytoplasm is eliminated. With completion of spermatid elongation, the Sertoli cell cytoplasm retracts around the developing sperm, stripping it of unnecessary cytoplasm and extruding the sperm into the tubule lumen. The mature sperm has remarkably little cytoplasm.
The human spermatozoon is approximately 60 μm in length and is divided into three anatomic sections: head, neck, and tail. The oval sperm head, about 4.5 μm long and 3 μm wide, consists of a nucleus containing highly compacted chromatin, and an acrosome, a membrane-bound organelle harboring the enzymes required for penetration of the outer vestments of the egg before fertilization. The sperm neck maintains the connection between the sperm head and tail. It consists of the connecting piece and proximal centriole. The axonemal complex extends from the proximal centriole through the sperm tail. The tail harbors the midpiece, principal piece, and endpiece. The midpiece is 7 to 8 μm long and is the most proximal segment of the tail, terminating in the annulus. It contains the axoneme, which is the 9 + 2 microtubule arrangement, and surrounding outer dense fibers. It also contains the mitochondrial sheath helically arranged around the outer dense fibers. The outer dense fibers, rich in disulfide bonds, are not contractile proteins but are thought to provide the sperm tail with the elastic rigidity necessary for progressive motility. Similar in structure to the midpiece, the principal piece has several columns of outer dense fibers that are replaced by the fibrous sheath. The fibrous sheath consists of longitudinal columns and transverse ribs. The sperm terminates in the endpiece, the most distal segment of sperm tail, which contains axonemal structures and the fibrous sheath. Except for the end-piece region, the spermatozoon is enveloped by a highly specialized plasma membrane that regulates the transmembrane movement of ions and other molecules.
The spermatozoon is a remarkably complex metabolic and genetic machine. The 75 mitochondria that surround the axoneme contain enzymes required for oxidative metabolism and produce adenosine triphosphate (ATP), the primary energy molecule for the cell. Mitochondria are semiautonomous organelles that produce cellular energy and can also cause apoptotic cell death through the release of cytochrome c. Mitochondria are composed of double (outer and inner) membranes. Five distinct respiratory chain complexes span the width of the inner membrane and are necessary for oxidative phosphorylation: the NADPH dehydrogenase, succinate dehydrogenase, cytochrome bc 1, cytochrome c oxidase, and ATP synthase complexes. The sperm axoneme contains enzymes and structural proteins necessary for the chemical transduction of ATP into mechanical movement. The plasma membrane covering the sperm-head region harbors specialized proteins that participate in sperm–egg interaction.
The axoneme is the true motor assembly and requires 200 to 300 proteins to function. Among these, the microtubules are the best-understood components. Sperm microtubules are arranged in the classic “9+2” pattern of 9 outer doublets encircling an inner central doublet. The protein dynein extends from one microtubule doublet to the adjacent doublet and forms both the inner and outer “arms” of the axoneme. Sperm with outer arm mutants have reduced motility and those with inner arm mutants have no motility. Radial links or spokes connect a microtubule of each doublet to the central inner doublet and consist of a complex of proteins. Tektins are proteins associated with the outer microtubular doublets, and nexin links are proteins that connect the outer doublets to each other and maintain the cylindrical axonemal shape.
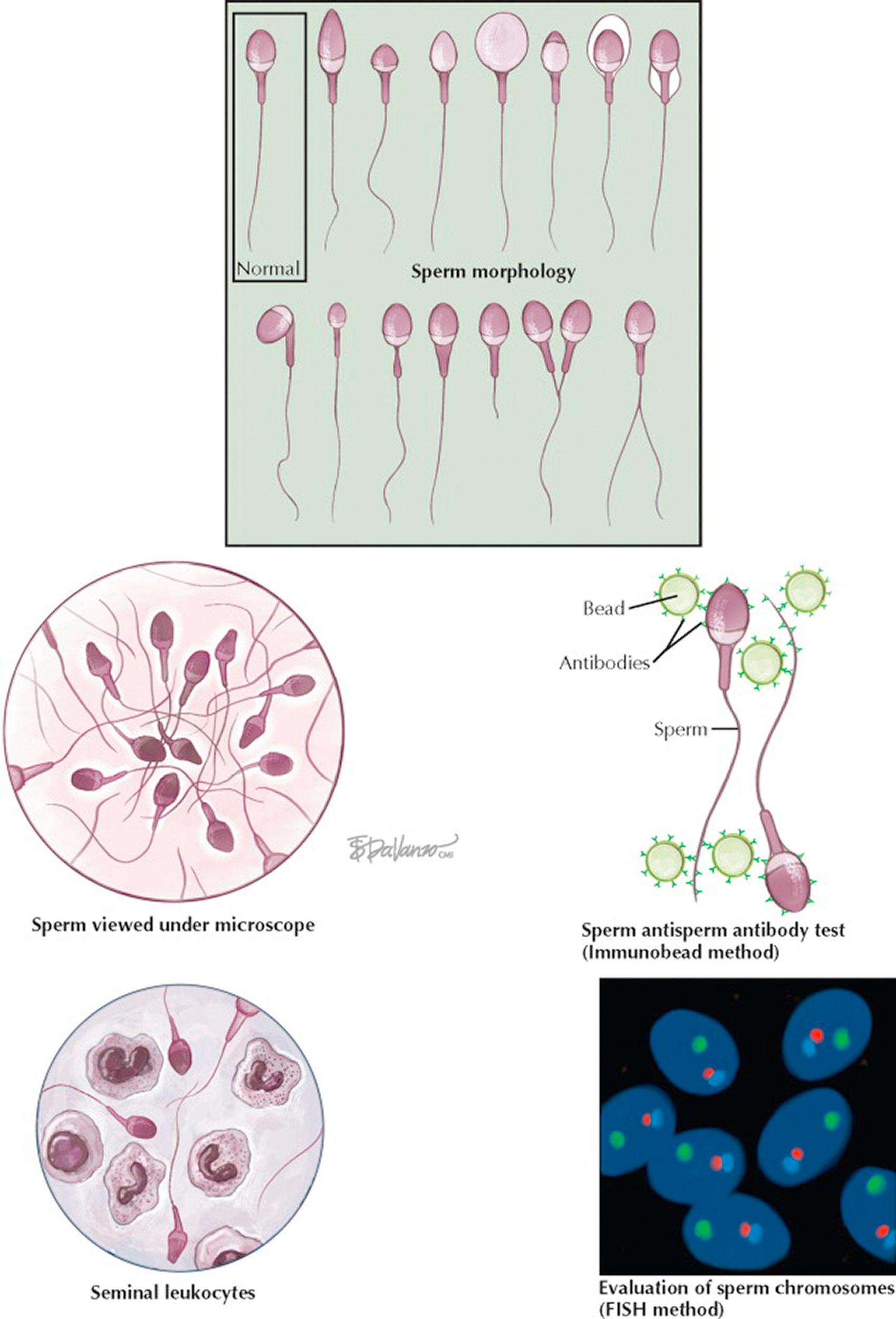
Although not a true measure of fertility, the semen analysis, if abnormal, suggests that the probability of achieving fertility is lower than normal. For a male infertility evaluation, two semen analyses, performed with 2 to 3 days of sexual abstinence, are sought due to the large biologic variability in semen quality. Lubricants should be avoided and the specimen kept at body temperature during transport.
Normal values have been defined for the human semen analysis by expert consensus (World Health Organization). Fresh semen is a coagulum that liquefies from 5 to 30 min after ejaculation. After liquefaction, semen viscosity is measured and should not show any stranding. Ejaculate volume should be at least 1.5 mL, as smaller volumes may not sufficiently buffer against vaginal acidity. Although most commonly a consequence of collection error, low ejaculate volume may also indicate retrograde ejaculation, ejaculatory duct obstruction, or androgen deficiency. Sperm concentration should be >20 million sperm/mL. Reasons for low sperm concentrations can include medications, exposures, systemic disease, hormonal disorders, varicocele, unilateral blockage, and genetic syndromes. Sperm motility is assessed in two ways: the proportion of all sperm that are moving and the quality of sperm movement. A normal value for sperm motility is 50% motile along with an average quality or progression score. The causes of low sperm motility, the most common semen analysis finding, are myriad and often reversible.
Recently, however, there has been debate concerning precisely which semen analysis values are to be considered “normal,” as controlled studies of fertile and infertile couples suggested other thresholds may be more appropriate, and sperm production is known to be susceptible to wide individual, geographic, and seasonal variation. When assessing semen quality, it is important to realize that spermatogenesis takes 60 to 80 days to complete, so that an individual semen analysis reflects biologic influences occurring 2 to 3 months prior. Likewise, medical or surgical therapy directed at improving semen quality will take several months to become manifest in improved semen quality.
Although seasonal variation exists, sperm production is a rapid and relatively constant process in humans. This is in part due to the anatomy of sperm production within the seminiferous tubules. A cycle of spermatogenesis involves the division of primitive germ cells into later germ cells. Many cycles of spermatogenesis coexist within the germinal epithelium at any one time, and they are described morphologically as stages. In addition, there is also a specific organization of spermatogenic cycles within the tubular space, termed spermatogenic waves. Although well described in other mammals, the exact configuration of spermatogenic waves in humans has been debated. The best evidence suggests that human spermatogenesis exists in a spiral or helical cellular arrangement that ensures that sperm production is a continuous and not a pulsatile process.
The formal evaluation of sperm shape is termed morphologic assessment. Several descriptive systems exist to evaluate morphology, and within each system, sperm are designated normal or abnormal based on specific size criteria. Although it is essentially judging a book by its cover, since the late 1980s it has been believed that sperm morphology may correlate with a man's fertility potential as reflected by in vitro fertilization (IVF). In general, the percentage of sperm with normal morphology has the greatest discriminatory power among all descriptors of semen quality in distinguishing fertile from infertile semen, although no particular value is diagnostic of fertility. Sperm morphology can be altered by toxic and occupational exposures, varicocele, fevers, medications, and systemic disease. It appears that sperm morphology is a sensitive indicator of overall testicular health, because the sperm morphologic characteristics are determined during spermatogenesis.
Other fertility assays can evaluate whether the seminal environment is abnormal, which may contribute to male infertility. Two such tests include an evaluation for excessive semen leukocytes (leukocytospermia) and testing for antisperm antibodies that can inhibit sperm transport through the female reproductive tract and impair sperm–egg interaction at fertilization. Sperm genetics can also be directly assessed for chromosomal normalcy with in situ hybridization techniques. These tests can complement the routine semen analysis in the male evaluation and better estimate the chances of fertility.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here