Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
There are five special senses—smell, taste, feeling, vision, and hearing—each of which is perceived by receptors specific to that particular sense. The sense of smell and its receptors were described in Chapter 15 ; the sense of taste was described in Chapter 16 ; the sense of feeling (pressure, touch, pain, temperature, and proprioception) was partially described in Chapter 14 but will be elaborated on here; and the other two special senses, vision and hearing, will be described in detail to follow. Special sense receptors, when stimulated by environmental stimuli, transduce this sensory input into electrical signals and convey them to the central nervous system (CNS). These sensory receptors are classified into three types, depending on the source of the stimulus: exteroceptors, proprioceptors, and interoceptors.
Exteroceptors , located near the body surface, are specialized to perceive stimuli from the external environment. These receptors—sensitive to temperature, touch, pressure, and pain—are components of the general somatic afferent pathways and are described in the first part of this chapter. Other exteroceptors, specialized for perceiving light (sense of vision) and sound (sense of hearing), are components of the special somatic afferent pathways (discussed later).
Proprioceptors are specialized receptors located in joint capsules, tendons, and intrafusal fibers within muscles (see Chapter 8 ). These general somatic afferent receptors transmit sensory input to the CNS, which is translated into information that relates to an awareness of the body in space and movement. Certain receptors of the vestibular ( balance ) mechanism (see later discussion), located within the inner ear, are specialized for receiving stimuli related to motion vectors within the head. This input is transmitted to the brain for processing into awareness of motion for corrective balancing.
Interoceptors are specialized receptors that perceive sensory information from within organs of the body; therefore, the modality serving this function is general visceral afferent .
Certain peripheral receptors specialized to receive particular stimuli include mechanoreceptors, thermoreceptors, and nociceptors.
The dendritic endings of certain sensory receptors located in various regions of the body—including muscles, tendons, skin, fascia, and joint capsules—are specialized to receive particular stimuli. These adaptations help the dendrite respond to a particular stimulus. Thus, these receptors are classified into three types:
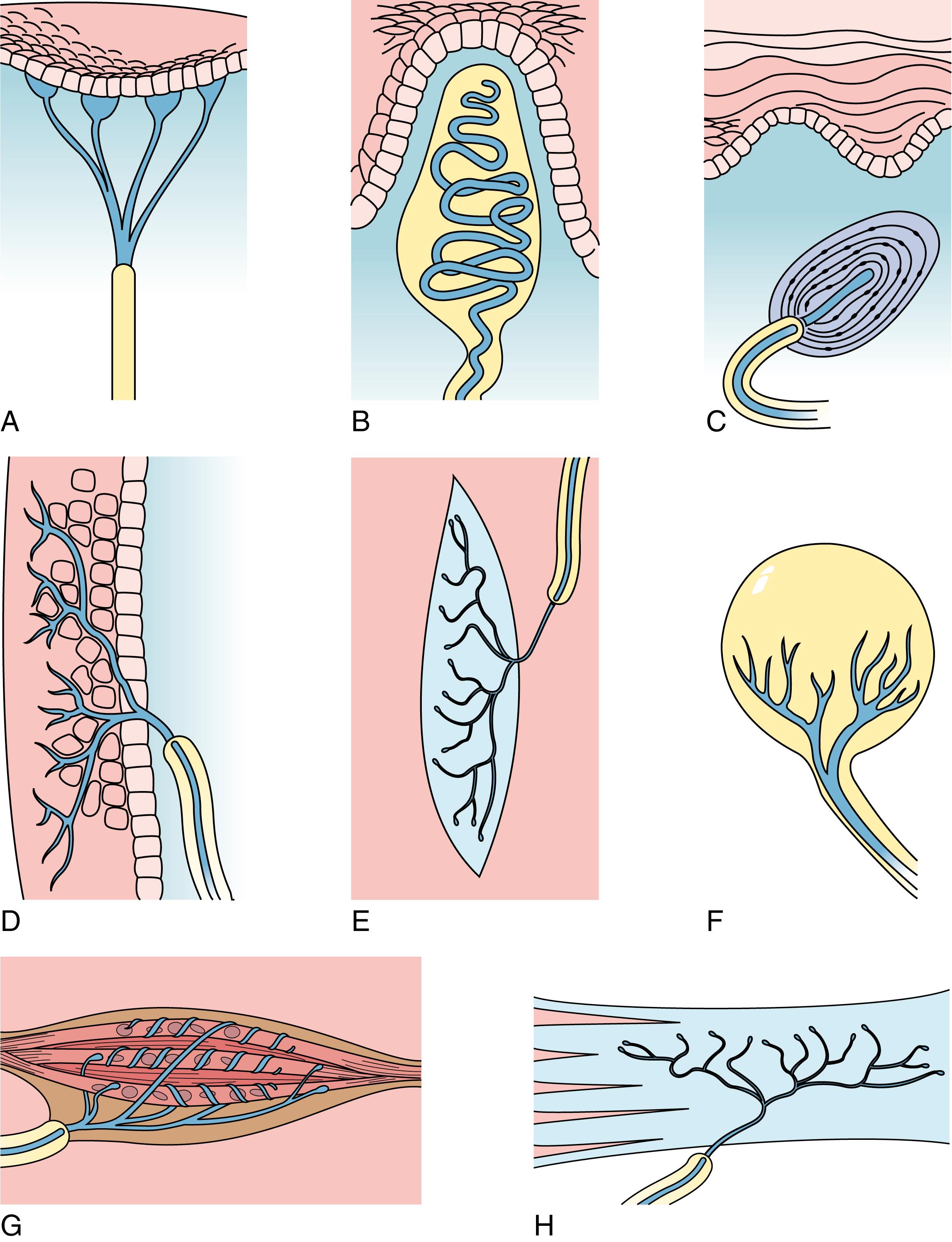
Mechanoreceptors, which respond to touch ( Figs. 22.1–22.4 )
Thermoreceptors, which respond to cold and warmth
Nociceptors (pain receptors) and pruriceptors (itch receptors)—the former respond to pain due to mechanical stress, extremes of temperature differences, and chemical substances; the latter respond to itch sensations
Although these specialized receptors generally are triggered only by a particular stimulus, any stimulus that is intense enough can trigger any receptor.
Mechanoreceptors respond to mechanical stimuli that may in some fashion alter the morphology either of the receptor or the tissues surrounding the receptor. Stimuli that trigger the mechanoreceptors are touch, stretch, vibrations, and pressure.
Nonencapsulated mechanoreceptors are simple unmyelinated receptors present in the skin, connective tissues, and surrounding hair follicles.
Peritrichial nerve endings , the simplest form of mechanoreceptors, are unmyelinated, lack Schwann cells, and are not covered by a connective tissue capsule. Such nerve endings are located in the epidermis of skin, especially in regions of great sensitivity, such as the face and the cornea, where they respond to stimuli related to touch and pressure (see Fig. 22.1D ). Additionally, peritrichial nerve endings are wrapped around the base and shaft of hair follicles and function in touch perception related to the deformation of the hairs. Moreover, some naked nerve endings function as nociceptors or as thermoreceptors.
Merkel disks are slightly more complex mechanoreceptors (see Fig. 22.1A ). Specialized for perceiving discriminatory touch, these receptors are composed of an expanded unmyelinated nerve terminal associated with Merkel cells , specialized epithelial cells interspersed with keratinocytes in the stratum basale of the skin (see Fig. 14.1 ). These receptors are located mostly in glabrous (nonhairy) skin and regions of the body more sensitive to touch.
Encapsulated mechanoreceptors exhibit characteristic structures and are present in specific locations.
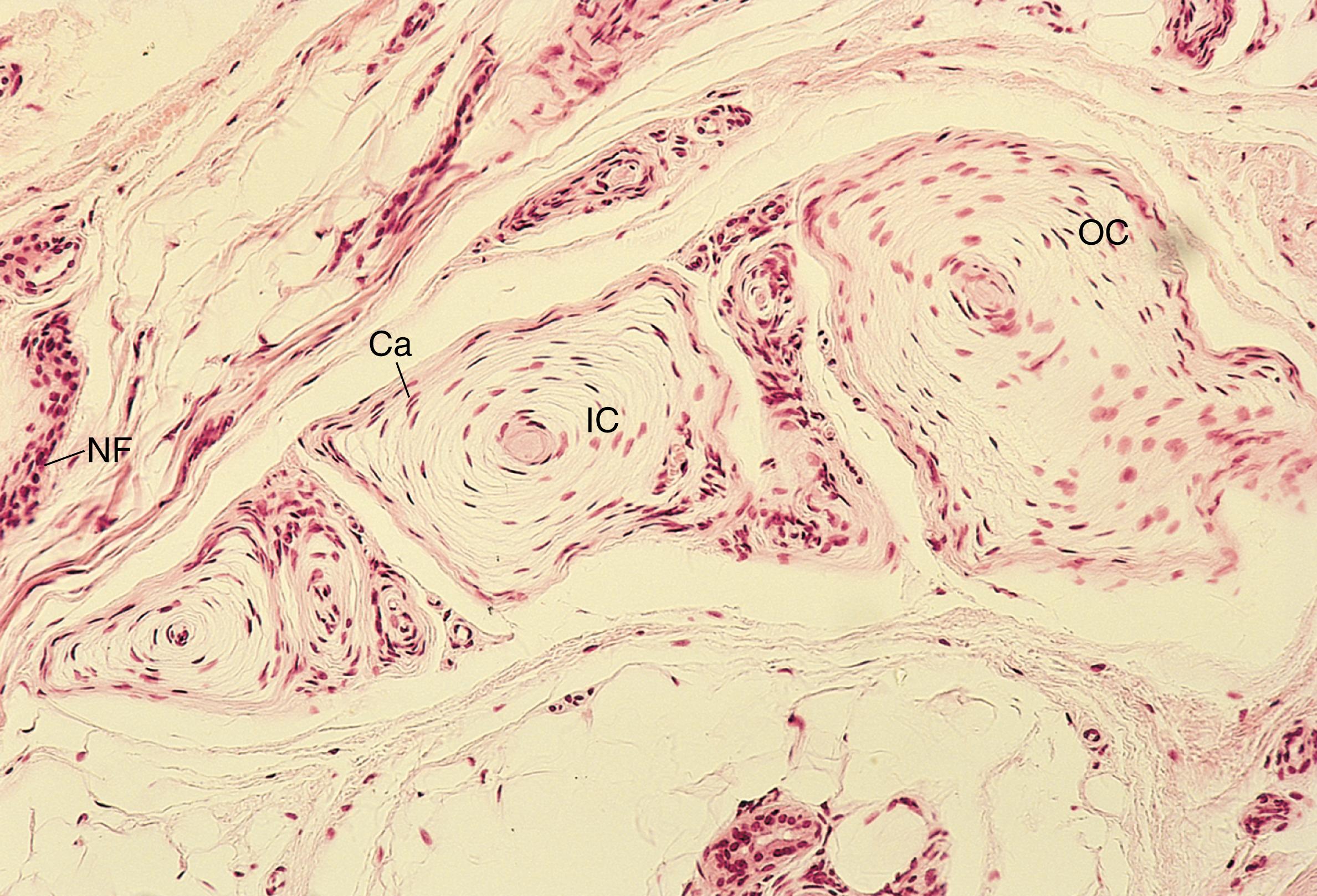
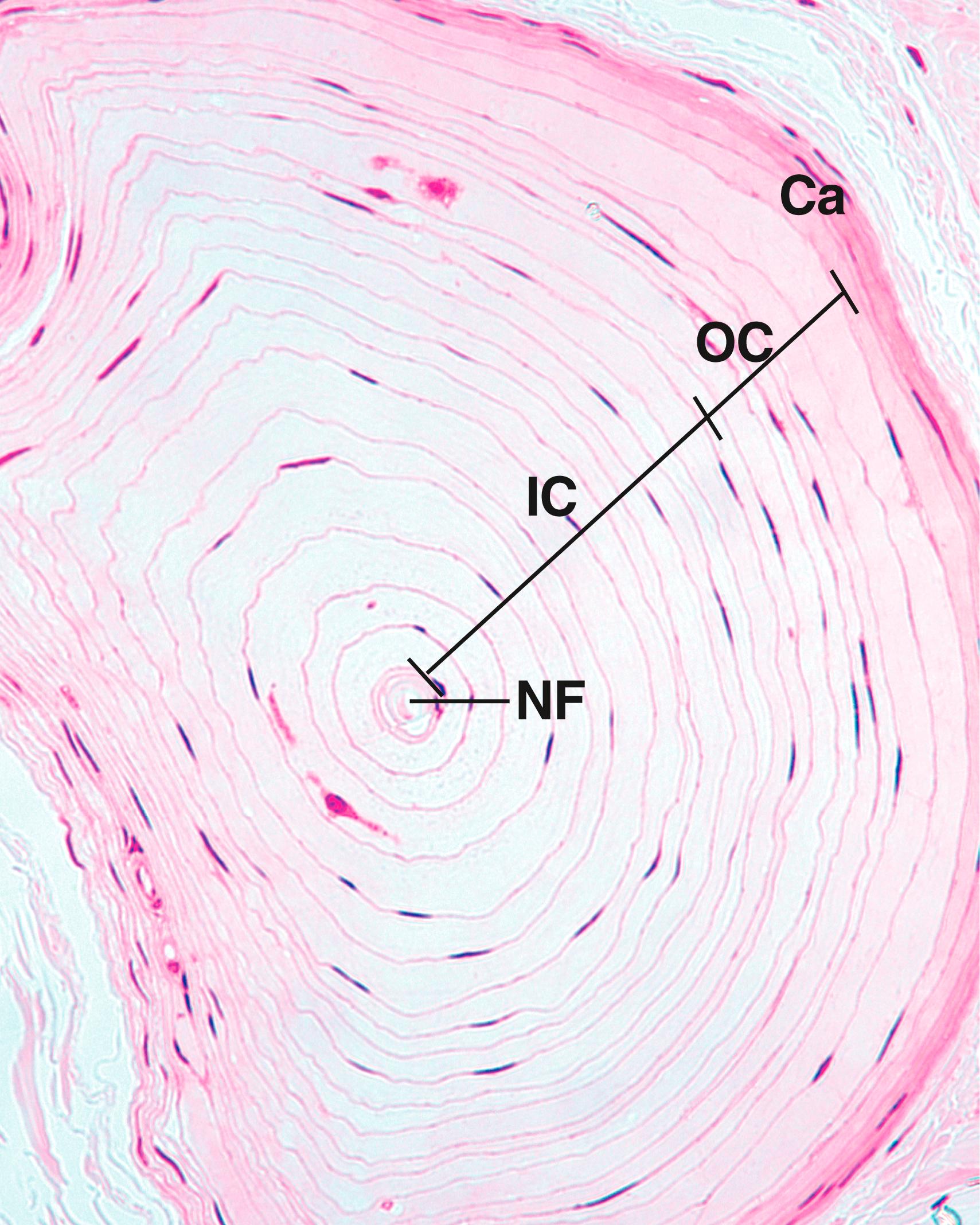
Pacinian corpuscles , another example of the encapsulated mechanoreceptors, are located in the dermis and hypodermis in the digits of the hands and in the breasts, as well as in connective tissue of the joints, periosteum, and mesentery. These mechanoreceptors are specialized to perceive pressure , touch , and vibration . Pacinian corpuscles are large, ovoid receptors 1 to 2 mm long by 0.1 to 0.7 mm in diameter (see Figs. 22.1C, 22.2, and 22.3 ). Each large Pacinian corpuscle is composed of a single myelinated fiber that, almost as soon as it enters the corpuscle, loses its myelin sheath and courses the entire length of the corpuscle as an unmyelinated nerve fiber. The inner core of the corpuscle contains the nonmyelinated nerve terminal and its Schwann cells, surrounded by approximately 60 layers of modified fibroblasts, each layer separated from the next by a small fluid-filled space. An additional group of 30 less dense modified fibroblasts, the outer core , surrounds the inner core. The entire structure has a collagenous connective tissue capsule that surrounds the outer core. The arrangement of the cells in the lamellae makes the histological section of a Pacinian corpuscle resemble an onion sliced in half.
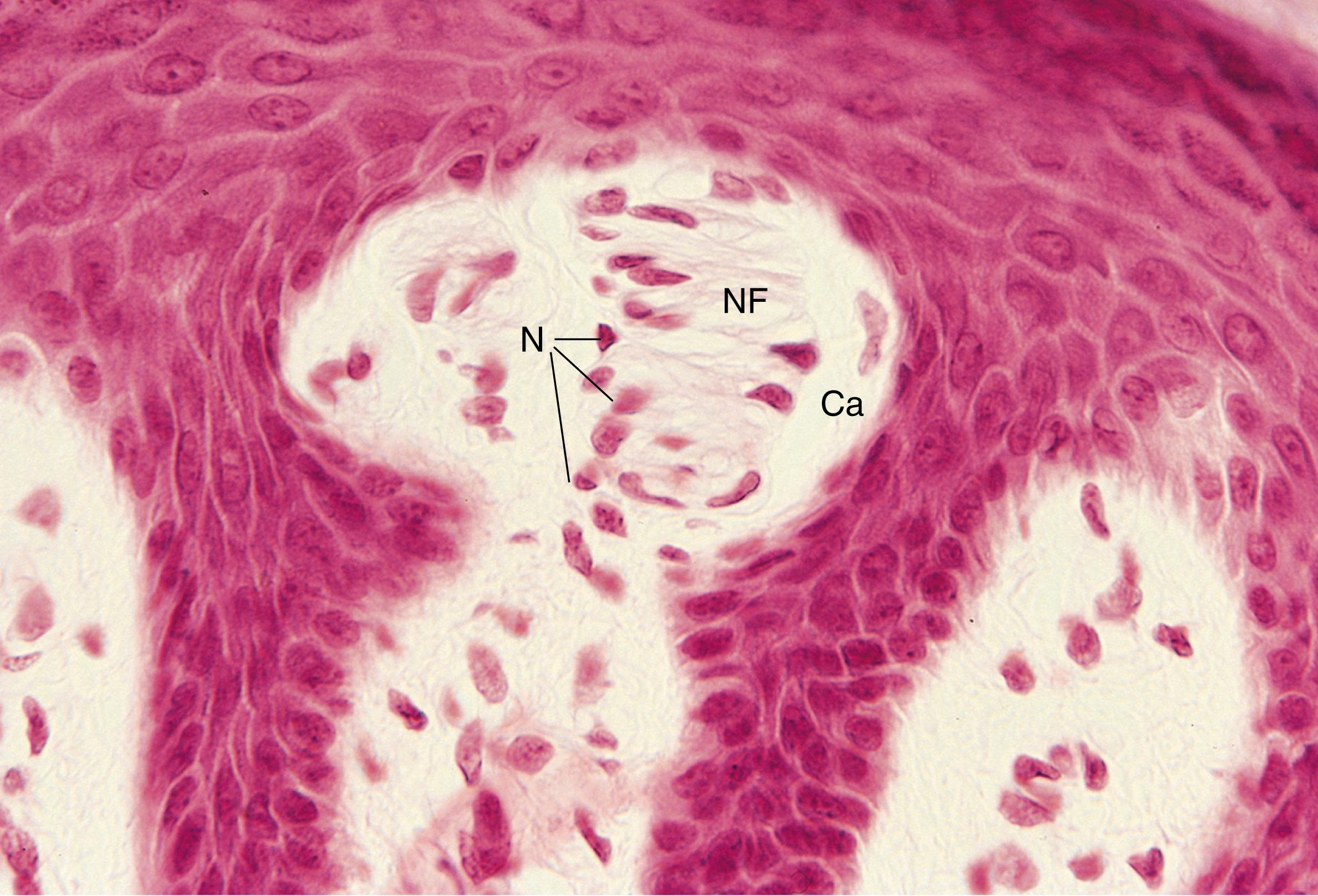
Meissner corpuscles (see Fig. 22.4 ) are encapsulated mechanoreceptors specialized for tactile discrimination . These receptors are located in the dermal papillae of the glabrous portion of the fingers and palms of the hands, where they account for about half of the tactile receptors. They also are located in the eyelids, lips, tongue, nipples, skin of the feet, and skin of the forearms. Meissner corpuscles, located in the dermal papillae, measure 80 to 150 μm × 20 to 40 μm with their long axes oriented perpendicular to the skin surface (see Fig. 22.1B ). Each Meissner corpuscle is formed by three or seven nerve terminals and their associated Schwann cells, all of which are encapsulated by connective tissue. A single myelinated nerve fiber branches to innervate several corpuscles; however, just after entering the corpuscle, the nerve fiber branch loses its myelin sheath. Contained within the capsule are stacks of epithelioid cells, possibly modified Schwann cells or fibroblasts, which serve to separate the branching nerve terminals. Meissner corpuscles respond to fine touch and are especially sensitive to edges and points and to movements of these objects, and they respond to slipping of the skin against an object so that the individuals can adjust the strength of their grip. These corpuscles have a receptive field of approximately 4 mm in diameter and respond to a pressure that depresses the skin of their receptive fields by less than 10 μm.
Ruffini endings ( corpuscles ) are encapsulated endings located in the dermis of the skin, nail beds, periodontal ligaments, and joint capsules. These large receptors, 1 mm long by 0.2 mm in diameter (see Fig. 22.1E ), are composed of branched nonmyelinated terminals interspersed with collagen fibers and surrounded by four to five layers of modified fibroblasts. The connective tissue capsule surrounding each of these receptors is anchored at each end, increasing their sensibility to stretching , touch , and pressure in the skin and joint capsules.
Krause end bulbs are spherical, encapsulated nerve endings located in the papillary region of the dermis, joints, conjunctiva, peritoneum, genital regions, and subendothelial connective tissues of the oral and nasal cavities (see Fig. 22.1F ). Originally, they were thought to be receptors sensitive to cold, but present evidence does not support this concept. Their function is unknown.
Both muscle spindles and Golgi tendon organs are encapsulated mechanoreceptors involved in proprioception. Muscle spindles (see Fig. 22.1G ) provide feedback concerning the changes in muscle length, as well as the rate of alteration in the length of the muscle; Golgi tendon organs (see Fig. 22.1H ) monitor the tension, as well as the rate at which the tension is being produced, during movement. Information from these two sensory structures is processed mostly at the unconscious levels within the spinal cord. However, the information also reaches the cerebellum, and even the cerebral cortex, so that the individual may sense muscle position. Golgi tendon organs and muscle spindles are discussed in Chapter 8 .
Thermoreceptors, which respond to temperature differences of about 2°C, are of three types: warmth receptors, cold receptors, and temperature-sensitive nociceptors.
Although specific receptors have not been identified for warmth, it is assumed that these receptors are naked endings of small nonmyelinated nerve fibers that respond to temperature increases (greater than 40°C to 42°C). It has been reported recently that ion channels, known as transient receptor V1 channels (also known as capsaicin receptors ) open when the temperature of the tissue in their immediate vicinity rises to higher than 43°C or when substances such as capsaicin or acids are encountered and Na + ions can enter the cell, depolarizing it. The information relayed to the CNS is interpreted as burning pain. Cold receptors are derived from naked nerve endings of myelinated fibers that branch and penetrate the epidermis and respond to temperatures lower than 25°C to 30°C. Because thermoreceptors are not activated by physical stimulation, they are believed to respond to differing rates of temperature-dependent biochemical reactions.
Nociceptors are receptors sensitive to pain; pruriceptors respond to itching sensations.
Nociceptors are responsible for pain perception. These receptors are naked endings of myelinated nerve fibers that branch freely in the dermis before entering the epidermis. Nociceptors are divided into three groups: (1) those that respond to mechanical stress or damage; (2) those that respond to extremes in heat or cold; and (3) those that respond to chemical compounds such as bradykinin, serotonin, and histamine.
Nociception is dependent on opening of voltage-gated Na + ion channels. There are nine types of known voltage-gated Na + ion channels; each type opens at slightly different voltages. Three of the nine channels react to painful stimuli. These are known as Na V 1.7 , Na V 1.8 , and Na V 1.9 , where Na V = voltage-gated sodium ion channel and 1.7 is the seventh, 1.8 is the eighth, and 1.9 is the ninth voltage-gated sodium ion channel.
Chronic neuropathic pain (usually in the hands and feet) is one of the most common illnesses in the world, affecting a large segment of the U.S. population. Many individuals with this condition present with mutations in the nociceptor ion channels (especially Na V 1.7) so that these ion channels are hyperactive and open even during conditions that would not be painful under normal circumstances. Normally, the resulting information is transmitted to the central nervous system (CNS), where interneurons intercept the information and inhibit its transmission (postsynaptic inhibition). The inhibition is due to the presence of a chloride transporter in the interneuron plasmalemma (also present in other neuron plasma membranes), known as potassium/chloride co-transporter 2 (KCC2) . In a normal individual, when pain is detected, microglia of the spinal cord release a signaling molecule that acts on interneurons to reduce their KCC2 transporters, and the interneuron can no longer inhibit the pain signal from being transmitted to higher levels in the CNS. In patients with neuropathic pain, what normally would be a nonpainful stimulus will cause the diminution of KCC2 levels in the interneurons and the stimulus, instead of being blocked by the interneuron, is permitted to be transmitted, erroneously, resulting in pain sensations.
Itching ( pruriception ) is a somatic sensation that may be due to local causes, such as a tiny insect walking on one’s arm or to generalized and systemic causes such as a form of dermatitis or even to cancer and organ failure. The mechanism of an itch is better known in mice than in humans, but it is assumed that there may be a close correlation in the two species. It has been demonstrated in mice that itching sensation is conveyed by C fibers to the murine spinal cord, where secondary neurons reside that are capable of expressing gastrin-releasing peptide receptors (GRPR). Apparently, there are a number of GRPR types of neurons and they are activated by various types of G protein–coupled receptors that respond to a particular cause of the itching sensation. Moreover, there seems to be a relationship between pain and itching sensations because they share similar but not identical pathways, but they both involve receptors in the skin, spinal cord, and brain.
The bulb of the eye is composed of three tunics: fibrous, vascular, and neural.
The eyes ( eyeballs ), the photosensory organs of the body, are approximately 24 mm in diameter and are located within the bony orbits of the skull. Light passing through the cornea and several refractory structures of the eyeballs is focused by the lens on the light-sensitive portion of the neural tunic of the eye, the retina , which contains the photosensitive rods and cones . Through a series of several layers of nerve cells and supporting cells, the partially assembled visual information is transmitted by the optic nerve to the brain for final processing.
About the fourth week of embryogenesis, the eyes begin to develop from three different sources. The future retina and optic nerve, the outgrowths of the forebrain, are the first to be observed. The continued growth of this outgrowth induces the surface ectoderm to develop into the lens and some of the accessory structures of the anterior portion of the eye. Later in development, adjacent mesenchyme condenses to form the tunics and associated structures of the eyeballs.
The eye is composed of three tunics (coats; Fig. 22.5 ): the tunica fibrosa ( fibrous tunic ), which forms the tough outer coat of the eye; the tunica vasculosa ( vascular tunic; uvea ), which is composed of the pigmented and vascular middle coats; and the retina ( neural tunic ), which constitutes the innermost coat of the eyeball.
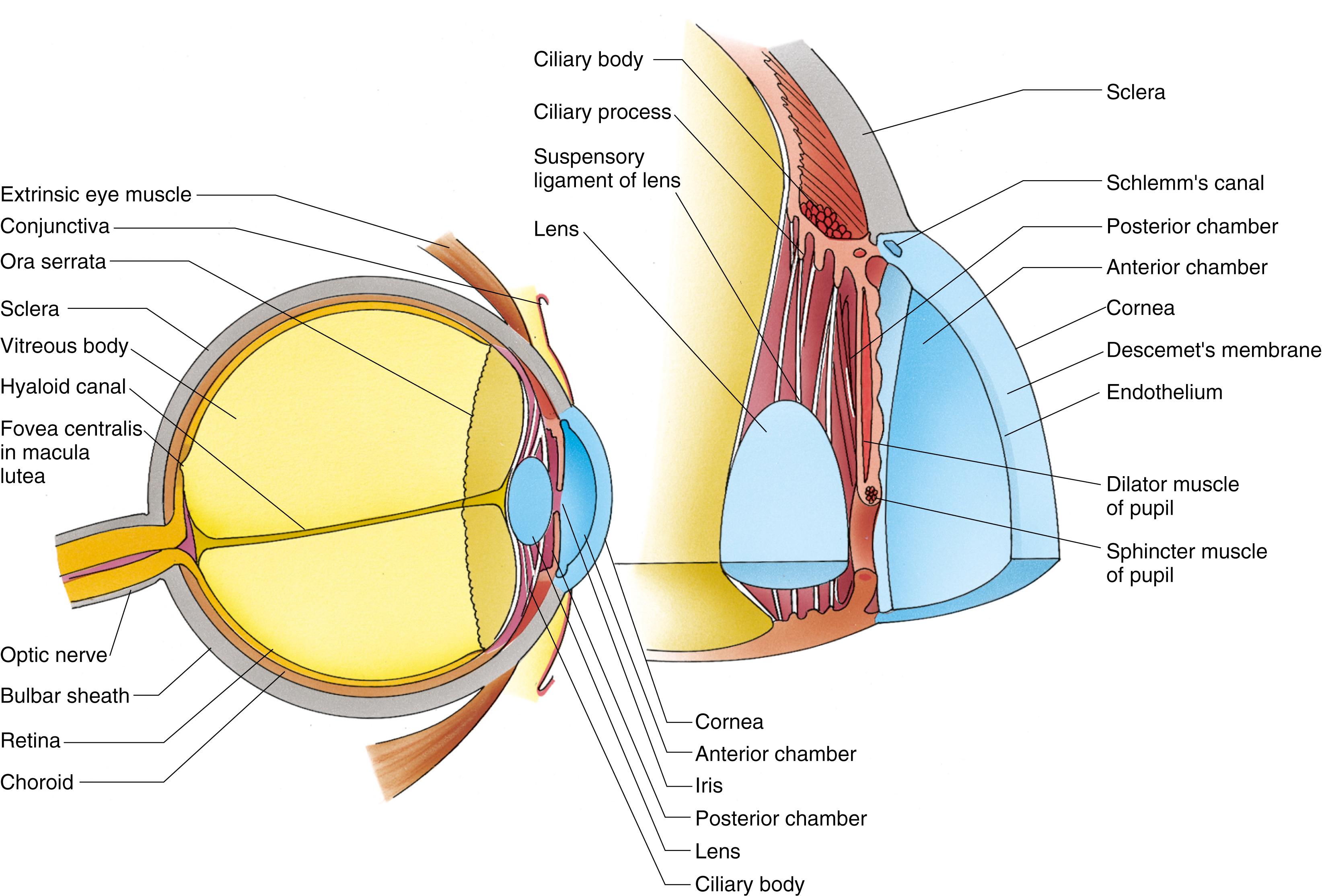
The extrinsic muscles of the eye, which are responsible for coordinated movements of the eyes to gain access to various visual fields, insert into the fibrous tunic. Smooth muscles located within the eyeball accommodate focusing of the lens and function to control the aperture of the pupil. Located outside the eyeball, but still within the orbit, is the lacrimal gland (tear gland), which secretes lacrimal fluid (tears) that moistens the anterior surface of the eye and the inner surface of the eyelids by passing through the conjunctiva , a transparent membrane that covers and protects the anterior surface of the eye.
The tunica fibrosa is composed of the sclera and cornea.
The external fibrous tunic of the eye, the tunica fibrosa , is divided into the sclera and cornea (see Fig. 22.5 ). The white, opaque sclera covers the posterior five-sixths of the eyeball, whereas the colorless, transparent cornea covers the anterior one-sixth of the eyeball.
The white opaque sclera is composed of type I collagen fibers interlaced with elastic fibers.
The sclera , a tough fibrous connective tissue layer that covers approximately 85% of the eyeball, is about 1 mm thick posteriorly, thinning at the equator and then thickening again near its junction with the cornea. It is covered by the conjunctiva, composed of a stratified squamous nonkeratinized to a stratified cuboidal epithelium with occasional goblet cells. Blood vessels visible on the surface of the sclera pierce the sclera to reach the tunica vasculosa, where they distribute to various components of the eye.
The sclera has three indistinctly recognizable layers. Just deep to the conjunctiva is the first layer, a thin, looser type of collagenous connective tissue layer, the episclera . The second and thickest layer, composed of a dense, collagenous connective tissue, is known as the stroma ( Tenon capsule, sclera proper ), whose interlacing type I collagen fiber bundles alternating with networks of elastic fibers provide the architectural support for the shape of the eyeball, which is maintained by intraocular pressure from the aqueous humor (located anterior to the lens) and the vitreous body (located posterior to the lens). The third layer, the suprachoroid lamina , is a thin, connective tissue layer that houses elongated, flat fibroblasts throughout its substance and melanocytes in its deeper aspect. At the sclera-corneal junction, the deep surface of the suprachoroid lamina is covered by a simple squamous epithelium, known as the scleral endothelium .
Tendons of the extraocular muscles insert into the stroma . The eyeball, along with its various parts and attached extraocular muscles, moves in unison within the periorbital fat–filled bony orbit ( Fig. 22.6 ).
The cornea is the transparent bulging anterior one-sixth of the eyeball.
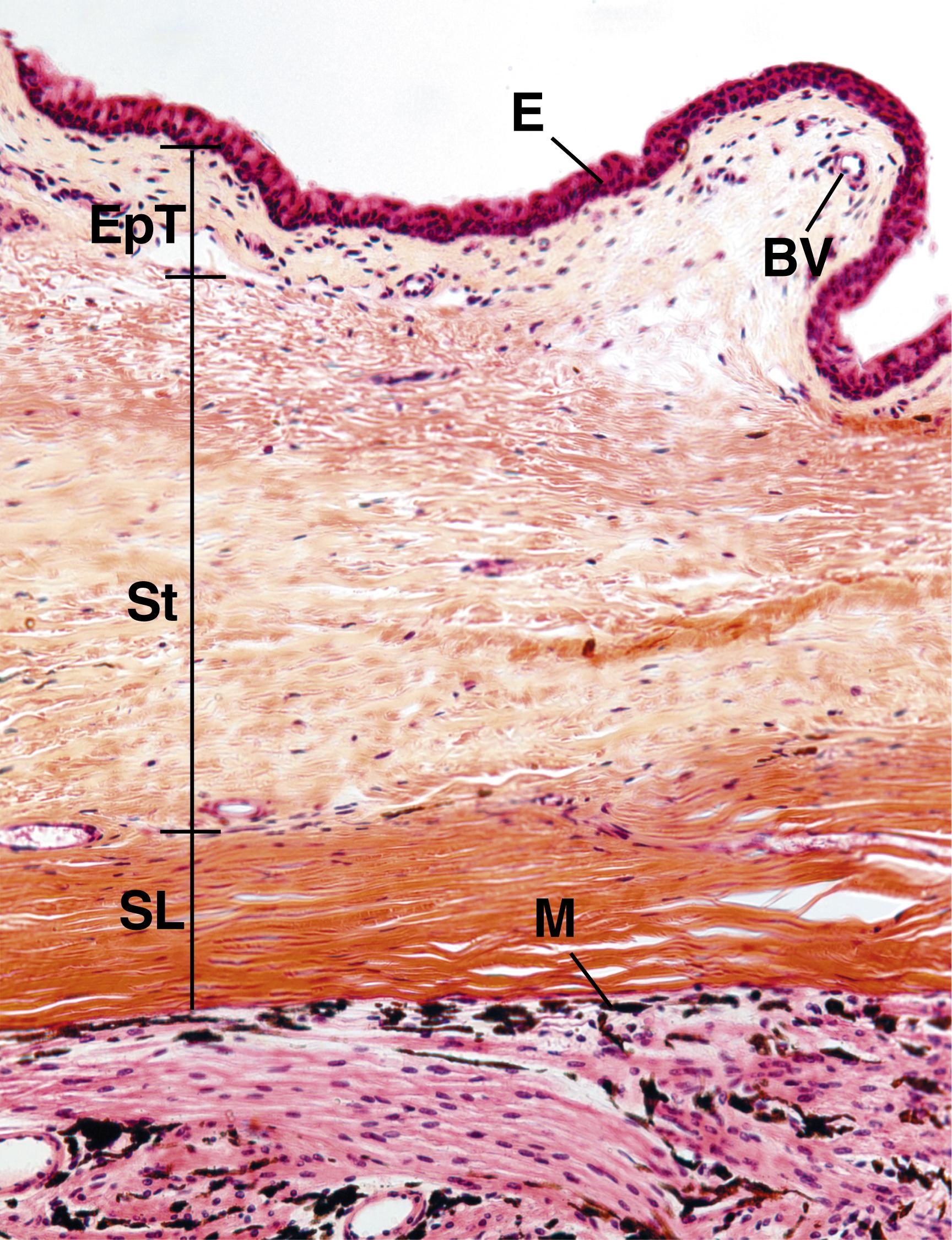
The cornea is the transparent, avascular, and highly innervated anterior portion of the fibrous tunic that bulges out anteriorly from the eyeball ( Figs. 22.7 and 22.8 ) It is slightly thicker than the sclera and is composed of six histologically distinct layers:
Corneal epithelium
Bowman membrane
Stroma
Pre-Descemet layer (Dua layer)
Descemet membrane
Corneal endothelium
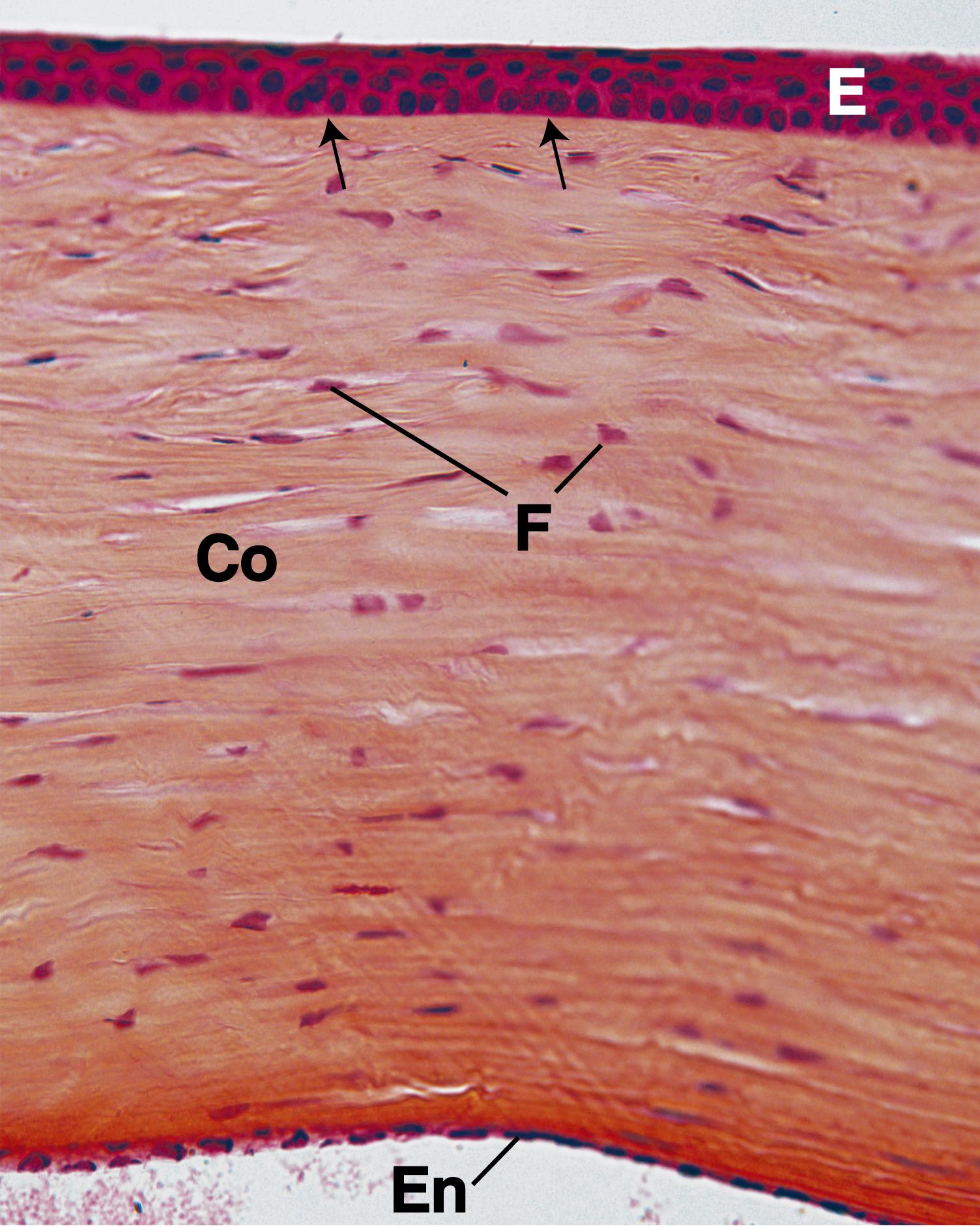
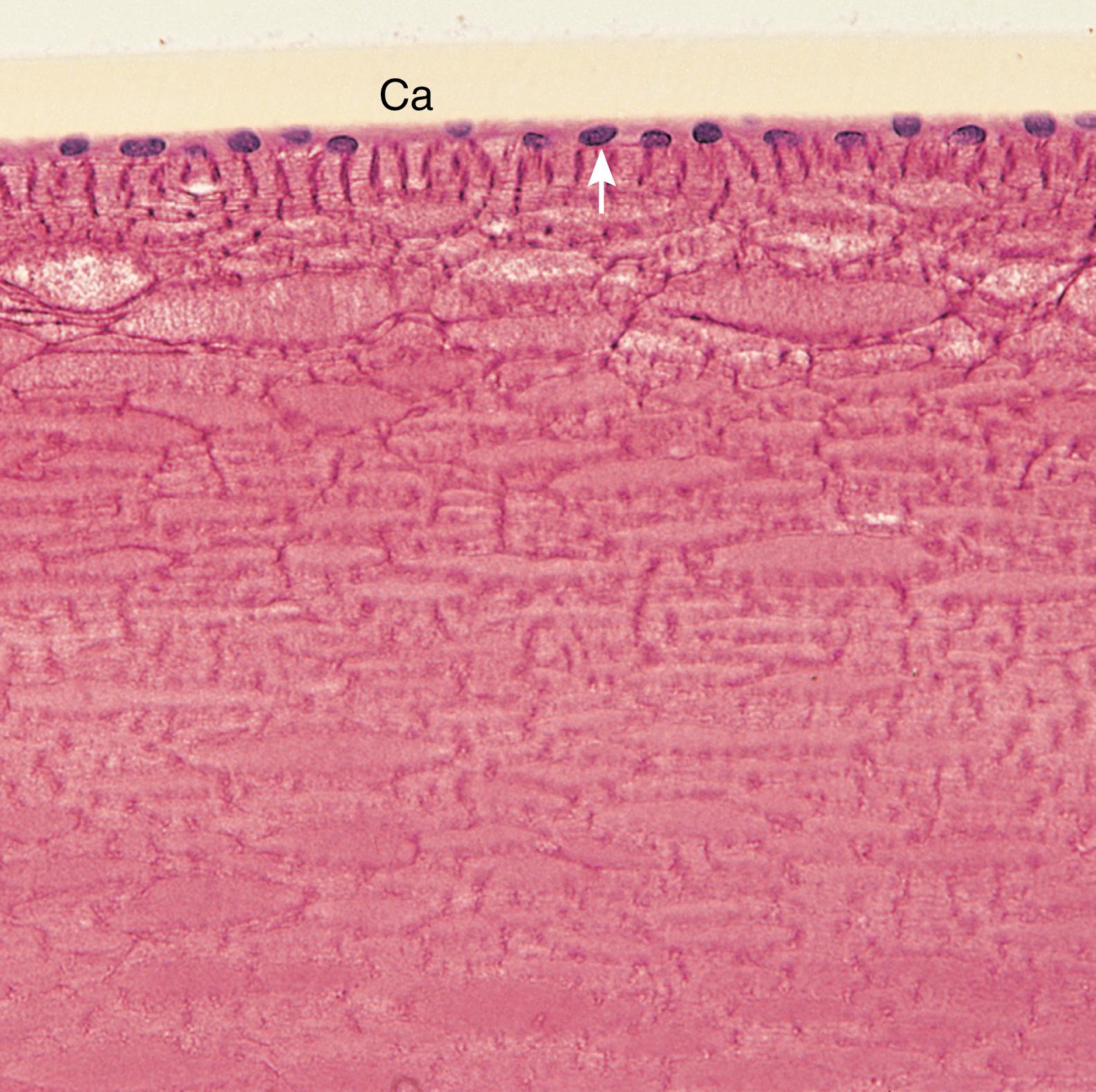
The corneal epithelium , the continuation of the conjunctiva (a mucous membrane covering the anterior sclera and lining the internal surface of the eyelids), is a stratified, squamous, nonkeratinized epithelium composed of five to seven layers of cells that covers the anterior surface of the cornea. The larger superficial cells have microvilli and exhibit zonulae occludentes, whereas the remaining cells of the corneal epithelium interdigitate with and form desmosomal contacts with one another, and their cytoplasm contains the usual array of organelles, along with intermediate filaments. The corneal epithelium is highly innervated by pain fibers with numerous free nerve endings. Mitotic figures are observed mostly near the periphery of the cornea, with a turnover rate of approximately 7 days, and damage to the cornea is repaired rapidly as cells migrate to the defect to cover the injured region. Subsequently, mitotic activity replaces the cells that migrated to the wound. The corneal epithelium also functions in transferring water and ions from the stroma into the conjunctival sac.
The Bowman membrane , a fibrillar lamina 6 to 30 μm thick composed of type I collagen fibers arranged in an apparently random fashion, lies immediately deep to the corneal epithelium. It is believed that this membrane is synthesized by both the corneal epithelium and cells of the underlying stroma. The sensory nerve fibers pass through this structure to enter and terminate in the epithelium.
The transparent stroma is composed of collagenous connective tissue consisting mostly of type I collagen fibers that are arranged in 200 to 250 lamellae, each about 2 μm thick. The collagen fibers within each lamella are arranged parallel to one another, but fiber orientation shifts in adjacent lamellae. The collagen fibers are interspersed with thin elastic fibers embedded in ground substance containing mostly chondroitin sulfate and keratan sulfate. Long, slender fibroblasts are also present among the collagen fiber bundles. The stroma constitutes approximately 90% of the cornea, making it the cornea’s thickest layer; during inflammation, lymphocytes and neutrophils infiltrate it. At the limbus (sclera-corneal junction) is a scleral sulcus whose inner aspect at the stroma is depressed and houses endothelium-lined spaces known as the trabecular meshwork that lead to the canal of Schlemm , the site of outflow of the aqueous humor from the anterior chamber of the eye into the venous system.
The sclera, the white of the eye, is nontransparent because of its high degree of hydration. However, if one dries a small area of the scleral surface by blowing dry air on it, that dried region becomes transparent. The cornea, however, is transparent because extracellular fluid is constantly being resorbed from its surfaces, partially dehydrating the substance of this transparent layer of the eyeball. Tears keep the modified conjunctival covering the cornea moist without adding water to the stroma of the cornea.
The pre-Descemet layer ( Dua layer ) is a 15-μm, thin, tough collagenous membrane that is probably manufactured by the fibroblasts of the stroma. It has been suggested that the durability of this recently discovered layer protects the cornea from damage, whereas physical injury that breaches the integrity of the pre-Descemet layer may result in corneal hydrops (infiltration of an aqueous fluid into the corneal stroma).
The Descemet membrane is a thick basement membrane interposed between the stroma and the underlying endothelium. Although this membrane is thin, only 5 μm at birth and homogeneous in younger persons, in older adults it becomes thicker (17 μm), presenting with cross-striations and hexagonal fiber patterns.
The corneal endothelium , which lines the internal (posterior) surface of the cornea, is a simple squamous to simple cuboidal epithelium , whose cells exhibit numerous pinocytotic vesicles, and their membranes have sodium pumps that transport sodium ions (Na + ) into the anterior chamber; these ions are passively followed by chloride ions (Cl – ) and water. Thus, excess fluid within the stroma is resorbed by the endothelium, keeping the stroma relatively dehydrated, a condition necessary for the maintenance of the refractive quality of the cornea. The corneal endothelium is responsible for synthesis of proteins that form the Descemet membrane.
H-Y antigens , products of genes located on the Y chromosome, are expressed on the cell membranes of almost every cell in the male body. If a female has not been exposed to these antigens prior to birth, then the unexposed female will react to the antigens as nonself and will mount an immune response against them. This is an important consideration in corneal transplants because in a major study, it was shown that approximately 22% of females receiving a male cornea rejected it, whereas in female-to-female corneal transplant the failure rate was 18%.
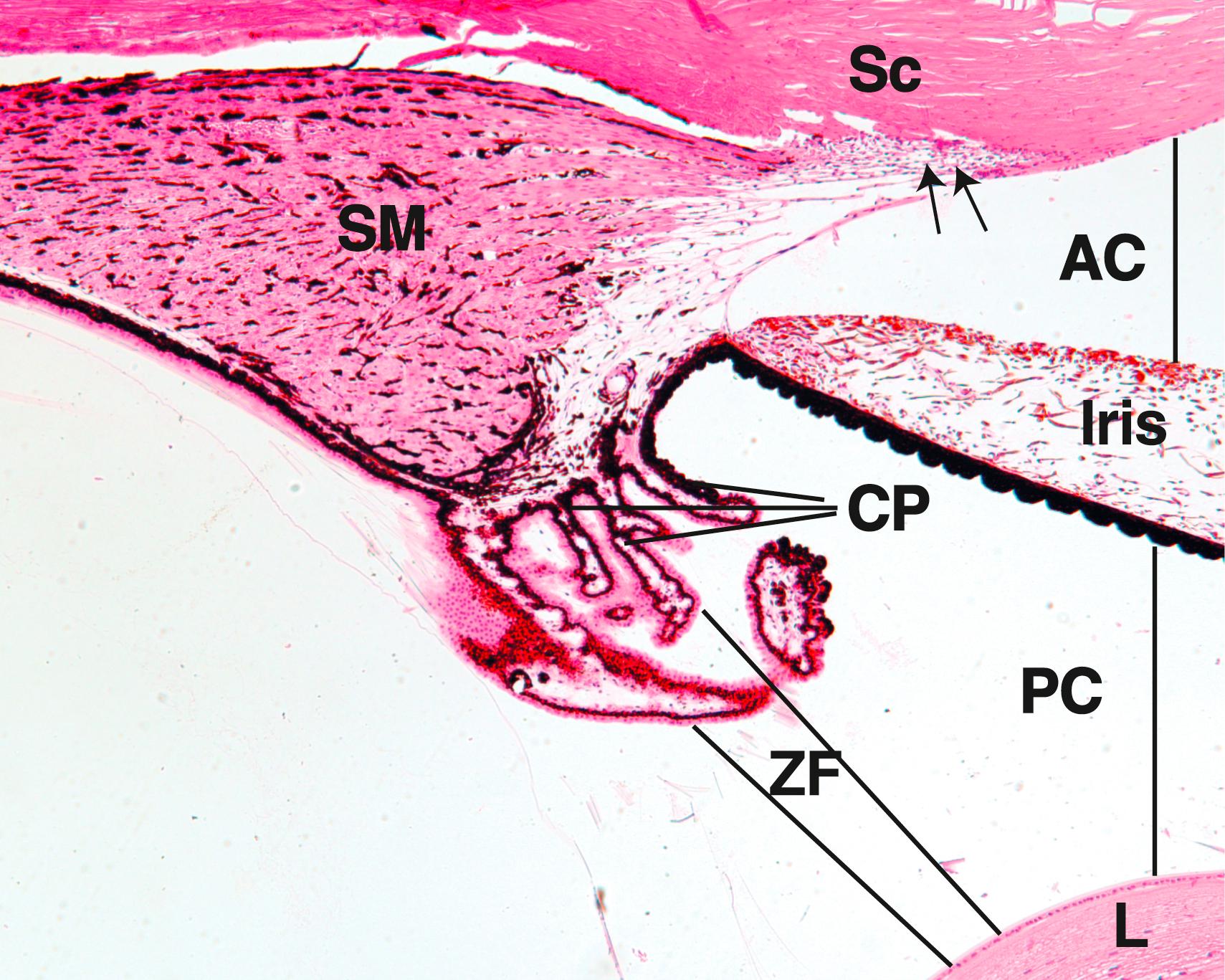
The vascular middle tunic of the eye, the tunica vasculosa (also known as the uvea), is composed of three parts: (1) the choroid, (2) ciliary body, and (3) iris ( Fig. 22.9 ; see Fig. 22.5 ).
The choroid, the pigmented posterior portion of the middle vascular tunic, is loosely attached to the sclera and separated from the retina by the Bruch membrane.
The choroid , the well-vascularized, pigmented layer of the posterior wall of the eyeball, is loosely attached to the tunica fibrosa. It is composed of loose connective tissue containing numerous fibroblasts and other connective tissue cells and has a rich vascular supply. The black color of the choroid is due to the myriad of melanocytes present in it. The inner surface of the choroid, because of its abundance of small blood vessels, is known as the choriocapillary layer and is responsible for providing nutrients to the retina. The choroid is separated from the retina by the 1- to 4-μm-thick Bruch membrane , which is composed of a network of elastic fibers located in the central region and sandwiched on both sides by collagen fiber layers. The outer aspect of each collagen fiber layer is covered by a basal lamina that belongs to capillaries on one side and the pigment epithelium of the retina on the other side.
The ciliary body is a wedge-shaped portion of the choroid located in the lumen of the orb between the iris and vitreous body and projecting toward the lens.
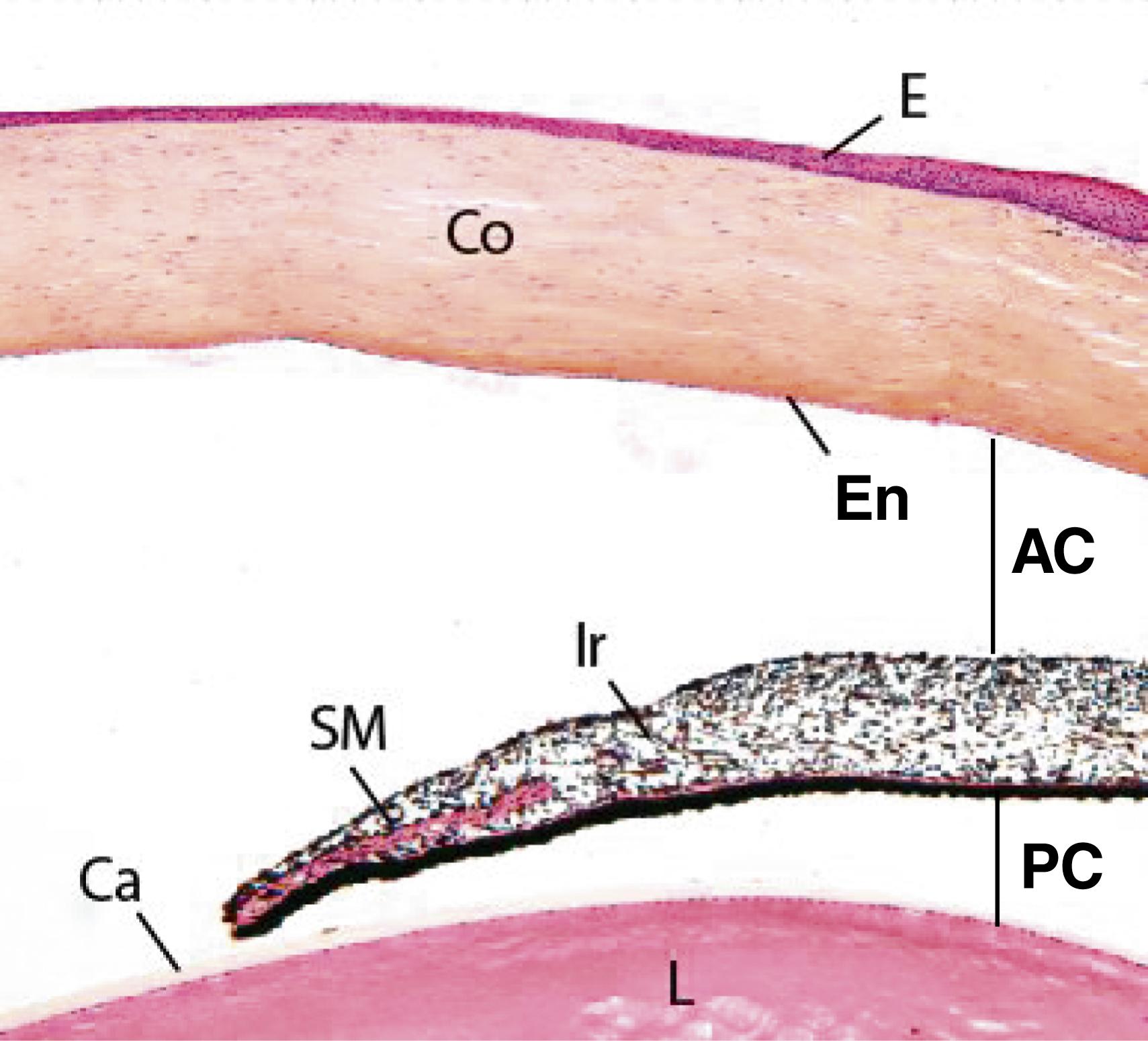
The ciliary body is the wedge-shaped extension of the choroid that rings the inner wall of the eye at the level of the lens, occupies the space between the ora serrata (the serrated junction where the retina ends) and the attached portion of the iris. One surface of the ciliary body abuts the sclera at the sclera–corneal junction; another surface abuts the vitreous body. The medial surface projects toward the lens, forming short, finger-like projections known as the ciliary processes (see Fig. 22.9 ).
The ciliary body is composed of loose connective tissue containing numerous elastic fibers, blood vessels, and melanocytes. Its inner surface is lined by the pars ciliaris of the retina , a pigmented layer of the retina that is composed of two cell layers. The outer cell layer, which faces the lumen of the eyeball, is a nonpigmented columnar epithelium ( nonpigmented ciliary epithelium ). The inner cell layer is composed of a pigmented simple columnar epithelium ( pigmented ciliary epithelium ), which is rich in melanin pigments.
The anterior third of the ciliary body has about 70 ciliary processes , which radiate out from a central core of connective tissue containing abundant fenestrated capillaries. Zonular fibers , composed of fibrillin, radiate from the ciliary processes to insert into the lens capsule, forming the suspensory ligaments of the lens , which suspend the lens in place.
The ciliary processes are covered by the same two layers of epithelia that cover the ciliary body. The inner nonpigmented layer has many interdigitations and infoldings; its cells transport a protein-poor plasma filtrate, the aqueous humor , into the posterior chamber of the eye. The aqueous humor flows from the posterior chamber into the anterior chamber by passing through the pupillary aperture between the iris and the lens. The aqueous humor exits the anterior chamber by passing into the trabecular meshwork near the limbus and, finally, as stated earlier, into the canal of Schlemm, which leads directly into the venous system. The aqueous humor provides nutrients and oxygen for the lens and cornea.
The bulk of the ciliary body is composed of three bundles of smooth muscle cells called the ciliary muscle . One bundle, because of its orientation, stretches the choroid, altering the opening of the canal of Schlemm for drainage of the aqueous humor. The remaining two muscle bundles, attached at the scleral spur (the junction point of the sclera, cornea, and ciliary body, just peripheral to the attachment of the iris), function in reducing tension on the zonulae. Contractions of this muscle, mediated by parasympathetic fibers of the oculomotor nerve (Cranial Nerve [CN] III), stretch the ciliary body, thereby releasing tension on the suspensory ligaments of the lens. As a result, the lens becomes thicker and more convex. This action permits focusing on nearby objects, a process called accommodation . Relaxation of all three muscle bundles increases tension on the zonule, which flattens the lens, enabling the eye to focus on distant objects. Constant adjustments between various degrees of contraction and relaxation are required to permit focusing on objects that are distant, intermediate, and close up.
Glaucoma is a constellation of conditions resulting from prolonged increased intraocular pressure caused by the failure of drainage of the aqueous humor from the anterior chamber of the eye. It is the world’s leading cause of blindness, second only to cataracts. In chronic glaucoma , the most common condition, the continued increase of intraocular pressure causes progressive damage to the eye, specifically, the optic nerve. The gradual loss of vision, if left untreated, results in blindness. Fortunately, a simple daily application of a variety of prescription eyedrops alleviates the condition. However, patient compliance (at least in the United States) is a major problem—approximately 50% to 75% of the patients fail to renew their prescription. Surgical treatments, such as canaloplasty, in which the canal of Schlemm is enlarged via microcatheterization, or trabeculectomy, in which a partial scleral flap is made and loosely sutured in position, provide a space for the fluid to drain from the eye. Additionally, in third-world countries, the access to ophthalmologists, as well as the availability and price of prescription drugs are major obstacles to proper glaucoma treatment. Currently, it is estimated that there are almost 60 million people affected worldwide and almost 3.5 million people with glaucoma in the United States.
The iris, the colored anterior extension of the choroid, is a contractile diaphragm that controls the diameter of the pupillary aperture.
The iris is a circular colored disc with a centrally located round hole known as the pupil ( pupillary aperture ). It is the anteriormost extension of the choroid, situated between the posterior and anterior chambers of the eye so that it completely covers the lens except at the pupil. The iris is thickest in the middle, becoming thinner both toward its attachment to the ciliary body and at the rim of the pupil (see Figs. 22.7 and 22.9 ).
The anterior surface of the iris displays two concentric rings: the pupillary zone , lying nearest the pupil, and the wider ciliary zone ; the two are separated by the collarette , the thickest part of the iris. This surface of the iris is irregular, with trenches extending into it; it also contains contraction furrows, which are easily distinguished when the pupil is dilated. An incomplete layer of pigmented cells and fibroblasts covers the anterior surface of the iris. Deep to this layer is the stroma , a poorly vascularized connective tissue containing numerous fibroblasts and melanocytes, which gives way to a well-vascularized, loose connective tissue zone.
The posterior surface of the iris is smooth and covered by the continuation of the two layers of retinal epithelium that cover the ciliary body. The surface facing the lens is composed of heavily pigmented cells, which block the light from passing through the iris except at the pupil. The epithelial cells facing the stroma of the iris have extensions that form the dilator pupillae muscle , consisting of myoepithelial cells. Another muscle, the sphincter pupillae muscle , is located in a concentric ring around the pupil. Contractions of these smooth muscles alter the diameter of the pupil, which changes inversely with the amount of light impinging on the iris. Thus, bright light causes constriction of the pupillary diameter, whereas dim light dilates it. As their names imply, the dilator pupillae muscle, innervated by the sympathetic nervous system , dilates the pupil; whereas the sphincter pupillae muscle, innervated by parasympathetic fibers of the oculomotor nerve (CN III), constricts the pupil.
The abundant population of melanocytes in the epithelium and stroma of the iris not only blocks the passage of light into the eye (except at the pupil) but also imparts color to the eyes.
The eyes are dark when the number of melanocytes is high, and they are blue when the number of melanocytes is low. Approximately 6000 to 10,000 years ago, every person had brown eyes, but a mutation in the HERC2 gene that appeared in a single female was transmitted down the generations. The mutation in this gene affects a neighboring gene, known as OCA2, which is responsible for the formation of melanin in the iris. The final result is that the pigment cells of the iris produce less melanin than in irises without the mutation and the stroma of the iris contains much less melanin than individuals with brown eyes. Consequently, the irises of these individuals are blue.
The lens, the transparent biconvex disk located directly behind the pupil, focuses light rays on the retina.
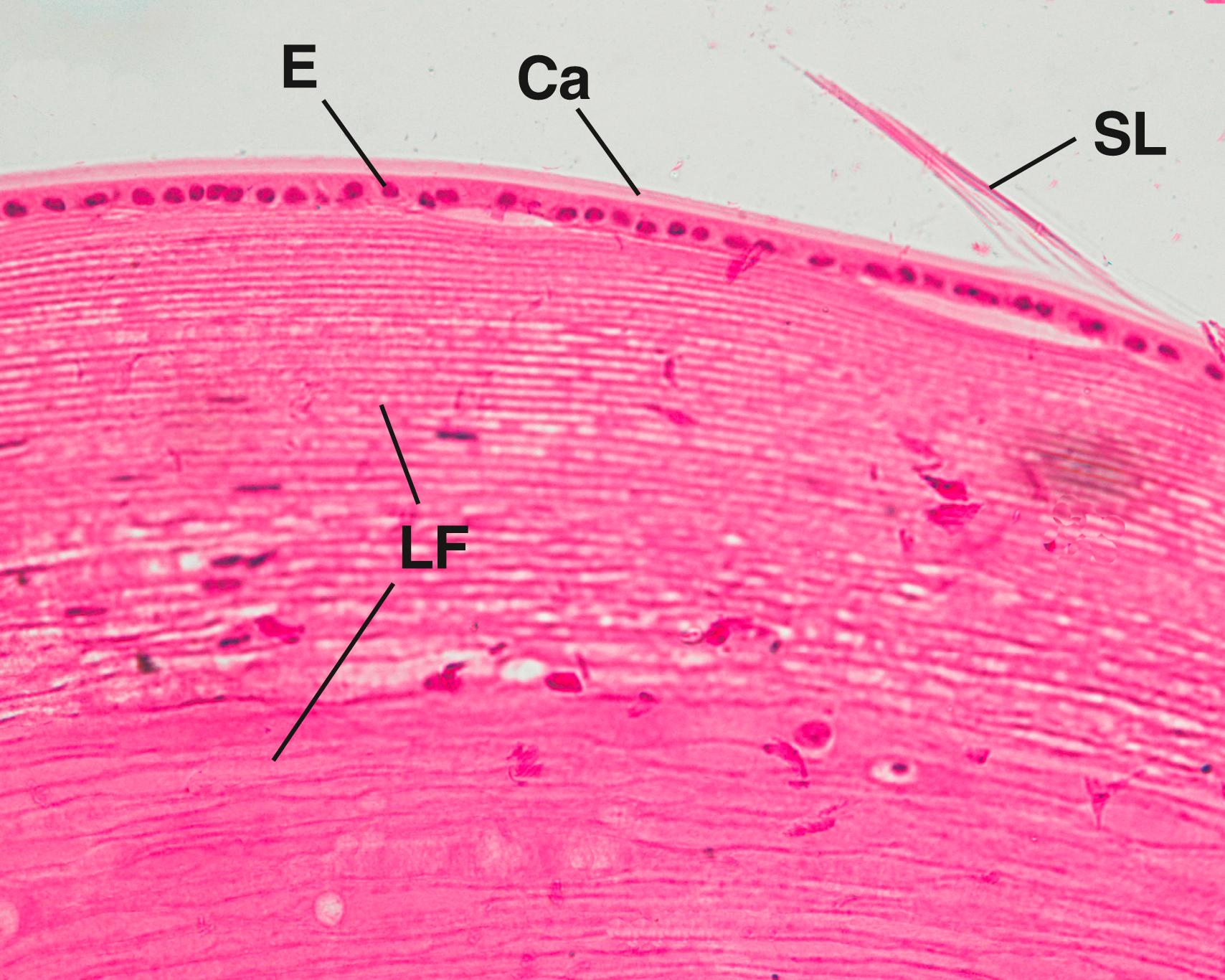
The lens of the eye is a flexible, biconvex, transparent disk composed of epithelial cells and their secretory products. The lens consists of three parts: lens capsule, subcapsular epithelium, and lens fibers (see Fig. 22.5 ).
The lens capsule is a basal lamina, 10 to 20 μm thick, containing mostly type IV collagen and glycoprotein that covers the epithelial cells and envelops the entire lens. This elastic, transparent, homogeneous structure, which refracts light, is thickest anteriorly.
The subcapsular epithelium is located only on the anterior and lateral surfaces of the lens, immediately deep to the lens capsule ( Figs. 22.10 and 22.11 ). It is composed of a single layer of cuboidal cells, which communicate with each other via gap junctions. The apices of these cells are directed toward the lens fibers and interdigitate with them, especially in the vicinity of the equator, where they are elongated and columnar in shape.
The substance of the lens is composed of approximately 2000 long cells, known as lens fibers , that lie immediately deep to the subcapsular epithelium (see Figs. 22.10–22.12 ). These highly differentiated cells in the shape of hexagonal solids arise from cells of the subcapsular epithelium, which lose their nuclei and organelles and continue elongating until they reach a length of 7 to 10 μm. This process of elongation, known as maturation , continues throughout the life of the individual. Eventually, these cells become filled with special lens proteins, known as crystallins , that increase the refractory index of the lens fibers.
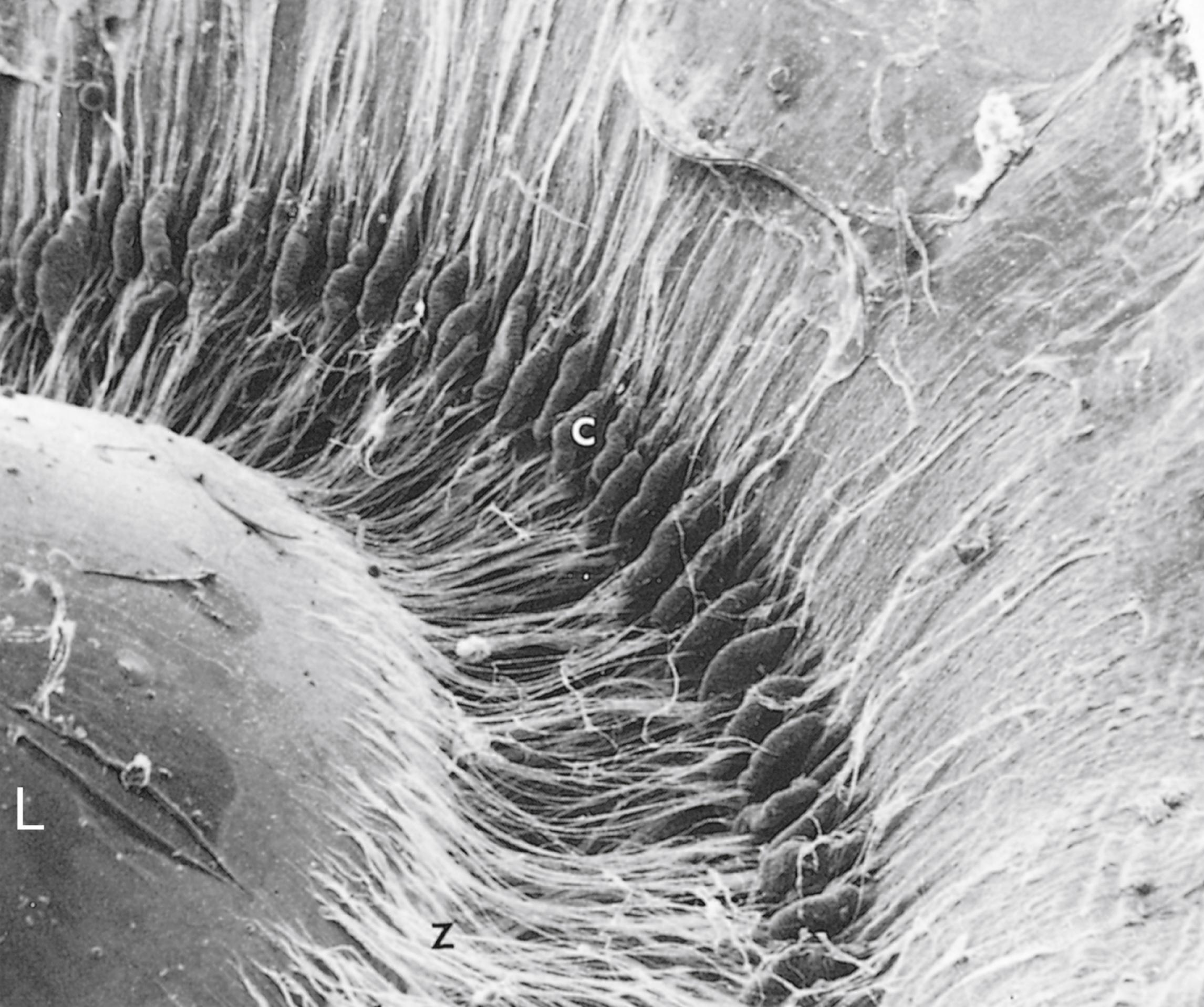
As indicated earlier, the lens is suspended in its location in the eyeball by the presence of zonular fibers (suspensory ligaments of the lens) that are attached to the ciliary processes. When the smooth muscles of the ciliary body are in the contracted state, there is little tension on the ligaments, and the lens is more convex so that near vision is sharper. During the relaxation of the ciliary muscles, the tension on the ligaments is increased, making the lens flatter and sharpening distant vision.
Presbyopia is the inability of the eye to focus on near objects (accommodation) and is caused by an age-related decrease in the elasticity of the lens. As a result, the lens cannot become spherical for exact focusing. This condition can be corrected with eyeglasses, contact lenses, or LASIK (laser-assisted in situ keratomileusis) eye surgery.
Cataract formation is usually also an age-related condition in which the lens becomes opaque, impairing vision. This condition may be due to an accumulation of pigment or other substances, as well as to excessive exposure to ultraviolet radiation. Although cataracts do not usually respond to medication and eventually lead to blindness, the opaque lens may be excised and replaced with a corrective artificial lens.
The vitreous body , a transparent refractile gel that fills the vitreous cavity (cavity of the eye behind the lens), is composed mostly (99%) of water containing a minute amount of electrolytes, collagen fibers, and hyaluronic acid. It adheres to the retina over its entire surface, especially at the ora serrata. Occasional macrophages and small cells called hyalocytes are observed at the periphery of the vitreous body; these are believed to synthesize the collagen and the hyaluronic acid. The fluid-filled hyaloid canal , a narrow channel that was occupied by the hyaloid artery in the fetus, extends through the entire vitreous body from the posterior aspect of the lens to the optic disk.
Eye floaters (vitreous opacities) specks, clouds, cobwebs and the like that individuals appear to see out in front of their eyes represent small debris that is floating in the vitreous body caused by its dehydration. These objects cast shadows on the retina that are translated by the brain as images in front of the eyes. Although most of the time these floaters will naturally resolve, some persons find that their lives are disrupted with the presence of floaters, especially when they are reading or driving. Specialized laser treatments can obliterate the floaters.
The retina, composed of 10 layers, possesses specialized receptor cells, called rods and cones, which are responsible for photoreception.
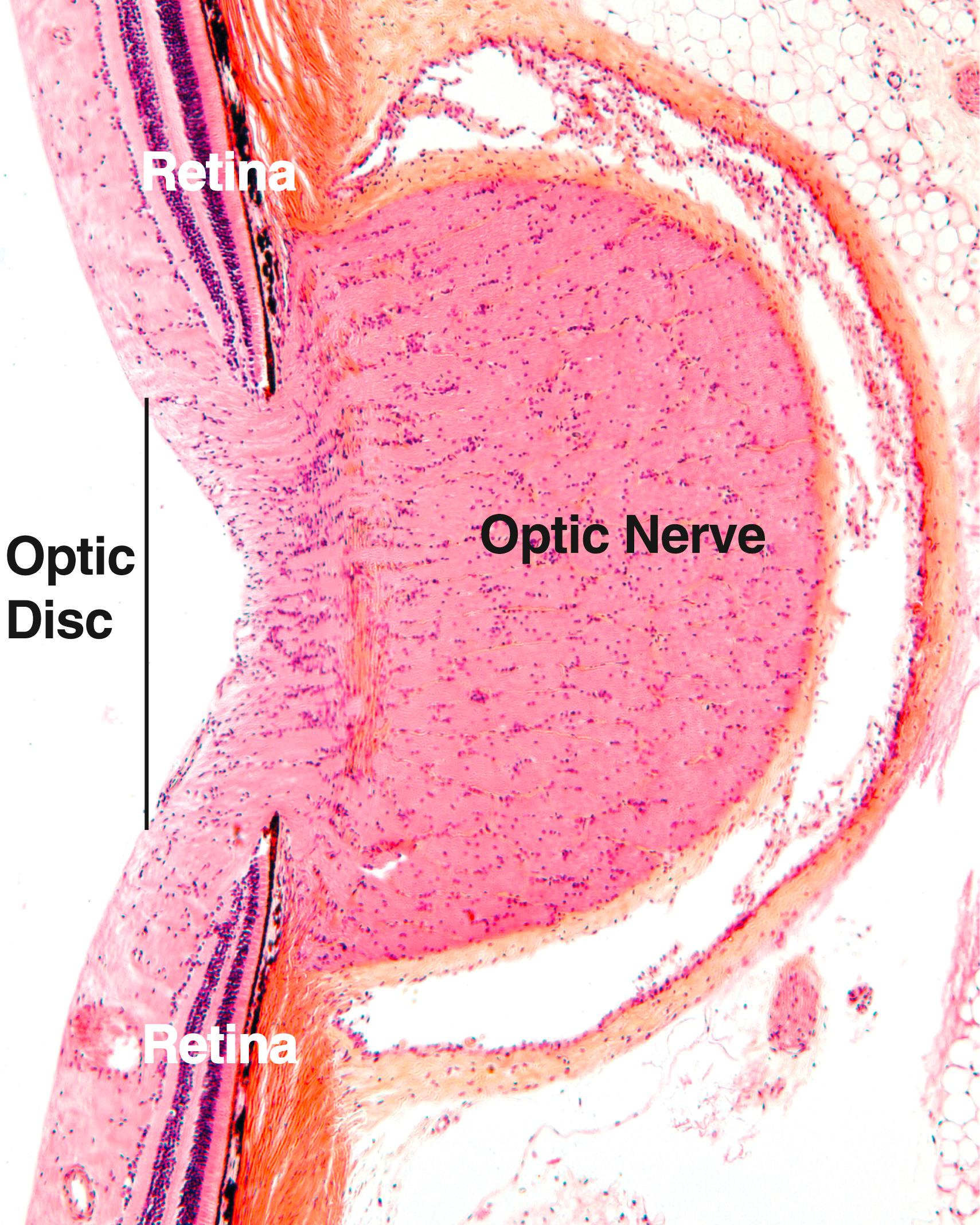
The retina , the third and innermost tunic of the eye, develops from the optic cup, an evagination of the diencephalon, which gives rise to the primary optic vesicle. Later in development, this structure invaginates to form a bilaminar secondary optic vesicle from which the retina develops, whereas the stalk of the optic cup becomes the optic nerve. The retina contains the photoreceptor cells, known as rods and cones .
The retina is formed of an outer pigmented layer that develops from the outer wall of the optic cup, whereas the neural portion of the retina develops from the inner layer of the optic cup and is called the retina proper . The pigmented layer of the retina covers the entire internal surface of the eyeball, reflecting over the ciliary body and posterior wall of the iris, whereas the retina proper stops at the ora serrata. The cells composing the retina proper constitute a highly differentiated extension of the brain.
The optic disk , located on the posterior wall of the eyeball, is approximately 1.75 mm in diameter and is the exit site of the optic nerve. Because it contains no photoreceptor cells, it is insensitive to light and is therefore called the blind spot of the retina ( Figs. 22.13 and 22.14 ). About 0.7 mm lateral to the optic disk is a yellow-pigmented zone, called the macula lutea ( yellow spot ), approximately 5.5 mm in diameter. At its center is a depression, 1.5 mm in diameter, known as the fovea ( fovea centralis ). Its central portion, the foveola , is where visual acuity is the greatest.
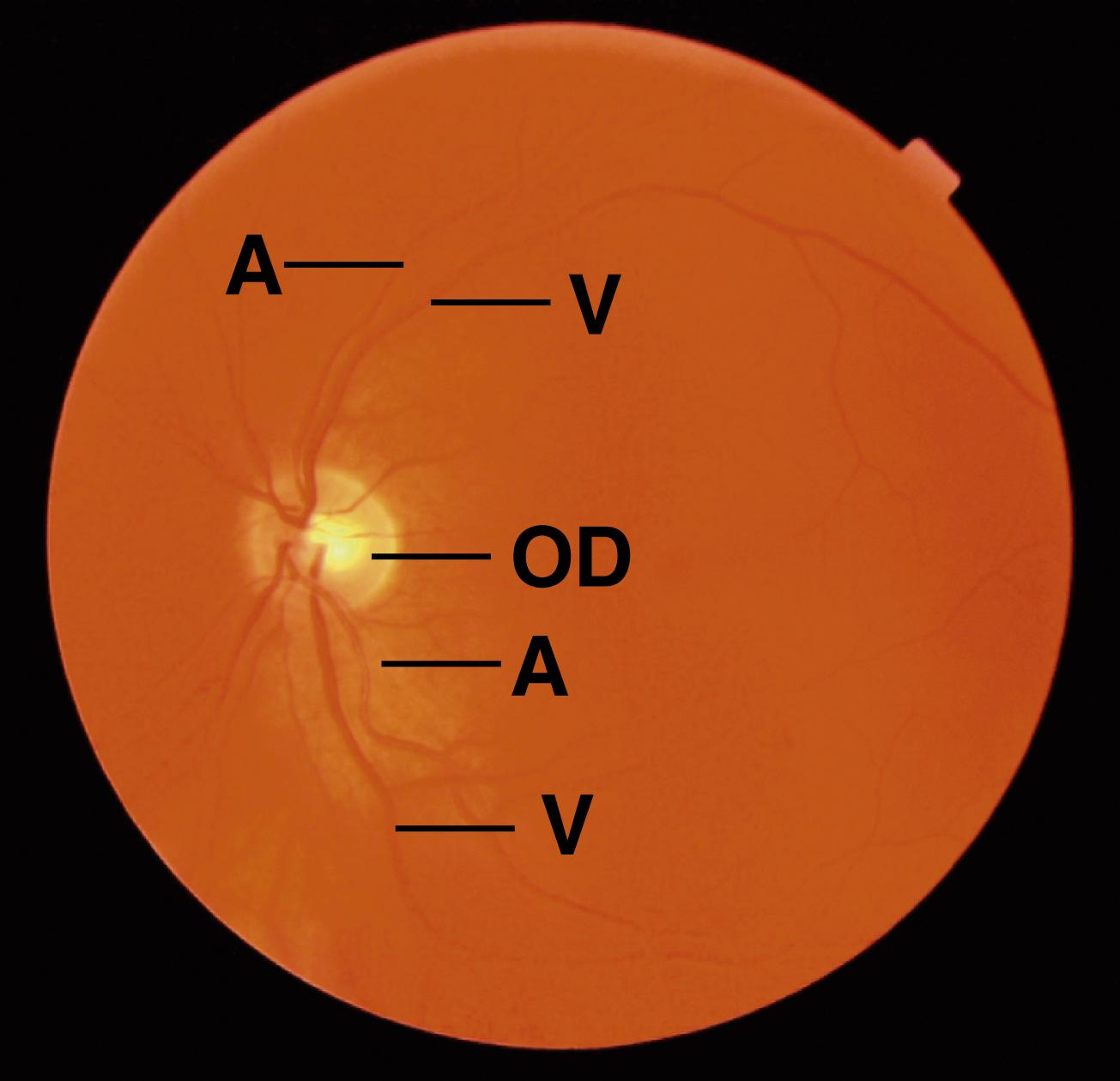
The retina has a dual blood supply. Much of the internal layers of the retina, from the internal segments of the rods and cones through the optic nerve fiber layer, is supplied by the central retinal artery , which gains access to the eyeball via the center of the optic nerve and forms numerous branches on the surface of the internal aspect of the retina. The external layers of the retina that constitute the outer segments of the rods and cones and the pigment epithelium are served by the vascular supply of the choroid .
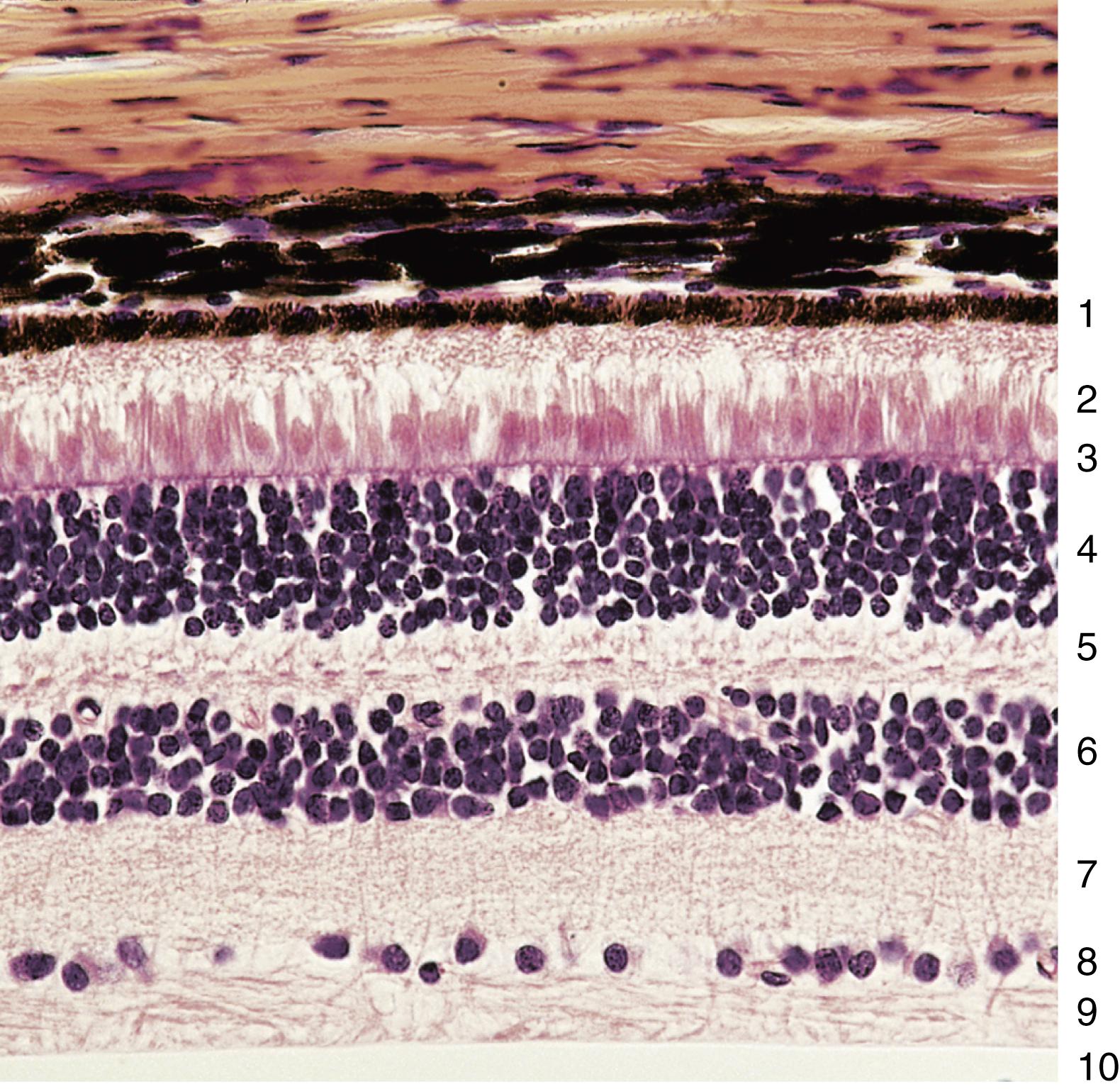
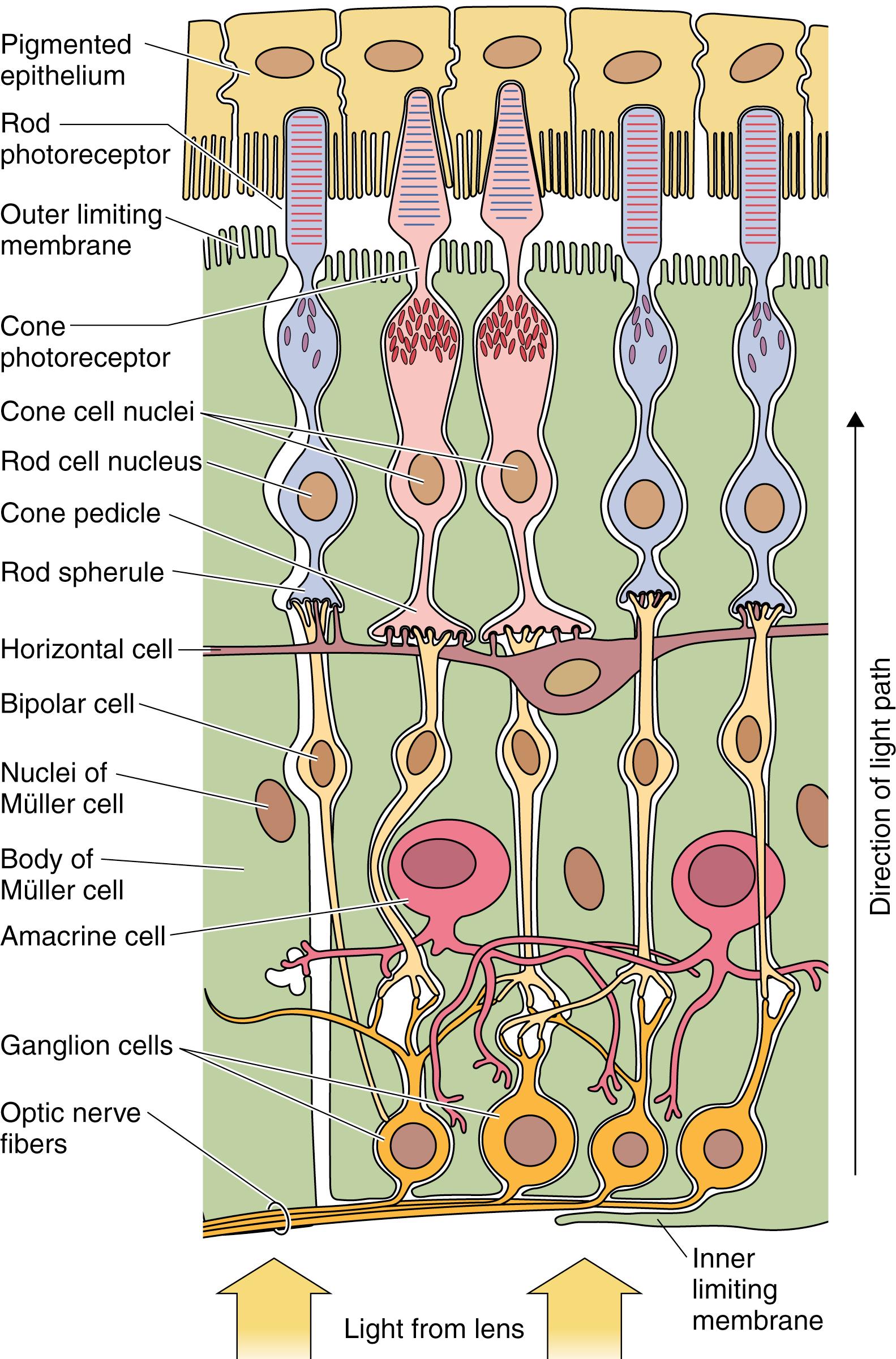
The portion of the retina that functions in photoreception faces the inner surface of the choroid layer from the optic disk to the ora serrata and is composed of 10 distinct layers ( Figs. 22.15 and 22.16 ). The retina has 10 named layers; by convention, the numbering starts from adjacent to the choroid to where the vitreous humor abuts the retina. It should be noted that the light entering the eyeball reaches the tenth layer (inner limiting membrane) first and the first layer (pigment epithelium) last. These 10 layers are as follows (see also Table 22.1 ):
Pigment epithelium
Layer of rods and cones
Outer limiting membrane
Outer nuclear layer
Outer plexiform layer
Inner nuclear layer
Inner plexiform layer
Ganglion cell layer
Optic nerve fiber layer
Inner limiting membrane
| Cell | Function |
|---|---|
| Pigment cells | Absorption of light, preventing light reflection; phagocytosis of spent membranous disks from rods and cones; esterifying and storing vitamin A to release it to rods and cones when needed. |
| Rods | Photosensitive cells responsible for monochromatic vision during low light conditions. |
| Cones | Photosensitive cells responsible for color vision during bright light conditions. |
| Bipolar neurons | Synapse with rods and cones and transmit information from these photoreceptor cells to dendrites of ganglion cells. |
| Horizontal cells | Transmit information from rods and cones to bipolar cells and regulate the transmission of this information by bipolar cells via lateral inhibition. |
| Amacrine cells | Act as interneurons controlling whether visual information should be transmitted to the visual center of the brain. |
| Müller cells | Support the cells of the retina physically and physiologically; act as if they were fiber-optic cables by guiding various wavelengths of light to the correct photoreceptor cells. |
| Ganglion cells | Relay information from rods and cones to the visual center of the brain; a small fraction of ganglion cells responsible for providing information concerning daylight and night to the regions of the brain responsible for establishing the body’s circadian rhythm; some of these cells are also control the pupillary reflex. |
Only three of the ten layers are composed of neurons that receive, integrate, and relay or transmit impulses to the brain for processing. These three layers are the photoreceptor cells (rods and cones), bipolar cells, and the ganglion cells; all of the other layers have supporting functions. The following sections describe the 10 layers of the retina starting with layer 1, which is adjacent to the choroid, and ending with layer 10, which contacts the vitreous humor.
The pigment epithelium , originating from the outer layer of the optic cup, is composed of cuboidal to columnar cells (14 μm wide and 10 to 14 μm tall) that are attached to the Bruch membrane, which separates these cells from the choroid. The nuclei of these cells are basally located, as are their mitochondria, which are especially abundant in the numerous cytoplasmic invaginations with the Bruch membrane. Desmosomes, zonulae occludentes, and zonulae adherentes are present on the lateral cell membranes, forming the blood–retina barrier . Moreover, gap junctions on the lateral cell membranes permit intercellular communication. The cell apices exhibit microvilli and sleeve-like structures that surround and isolate, but are not attached to, the tips of individual rods and cones.
The most distinctive feature of the pigment cells is their abundance of melanin granules, which these cells synthesize and store in their apical regions. Additionally, smooth endoplasmic reticulum, rough endoplasmic reticulum (RER), and Golgi apparatus are abundant in the cytoplasm.
Pigmented epithelial cells have a number of functions. They absorb light after it has passed through and stimulated the photoreceptors, preventing reflections from the tunics, which would impair focus; continually phagocytose spent membranous disks from the tips of the photoreceptor rods and cones; play an active role in vision by esterifying vitamin A derivatives in their smooth endoplasmic reticulum; and store vitamin A in their cytoplasm, which they release to rods and cones as needed.
Individuals with albinism are unable to produce melanin pigment; therefore, the light that enters the eyeball is not absorbed by the pigment epithelium. Instead, the light is reflected throughout the eyeball. Consequently, the impinging light excites many more rods and cones than in a nonalbino individual, reducing the ability of those with albinism to see clearly. Usually, these individuals, instead of having 20/20 vision, have 20/100 to 20/200 vision.
Because the sleeve-like extensions of the pigment epithelial cells merely surround the photoreceptor rod and cone tips, sudden hard jolts may disengage them, resulting in detachment of the retina , a common cause of partial blindness. The condition can be corrected surgically by “spot welding” the two structures back together. However, if this condition is left unattended, the rods and cones die because they will have lost the metabolic support normally provided by the pigment epithelium. Their death leaves a blind spot in the visual field corresponding to the area where the photoreceptors were lost.
Age-related macular degeneration ( AMD ) is a condition that appears usually in individuals who are at least 50 years old. The region of the macula of these patients begins to undergo degenerative changes resulting initially in blurred vision and eventually central blindness but peripheral vision is virtually unaffected. Unfortunately, the region of the macula provides the greatest visual acuity. Therefore, patients suffering from the late stages of macular degeneration are mostly blind. Early to intermediate AMD presents very little if any symptoms. However, ophthalmologic examination discloses the presence of medium (90–100 μm in diameter) to large (> 180 μm) drusen (yellow deposits of lipids and lipoproteins between the retina and the choroid) and even bundles of melanin pigments derived from cells of layer 1 (pigment epithelium). Late AMD has two distinct forms: neovascular AMD (wet AMD) and geographic atrophy (dry AMD). Of the 6 million people worldwide who have AMD, 80% have dry and 20% have wet AMD. Usually, neovascular AMD develops rapidly; blood vessels invade the region deep to the retina and, when they reach the region of the macula, foveal vision becomes compromised.Usually, these new blood vessels are fragile; as they break, they release blood that accumulates deep to the macula, further complicating the condition. If untreated, the damage to the retina will cause loss of retinal cells. Geographic atrophy is a much slower process that involves destruction of layers 1 and 2 (layer of pigment epithelium and layer of rods and cones, respectively) resulting in an irreversible loss of vision. There are treatment possibilities for neovascular AMD that involve the use of vascular endothelial growth factor (VEGF) inhibitors and lasers to coagulate the aberrant blood vessels (this procedure is not recommended in the region of the macula, however). At this writing, there were no treatment modalities for geographic atrophy; however, in 2017, two separate studies reported that injected stem cells programmed to differentiate into retinal pigment epithelial cells survived for more than 3 years and caused some improvement in patients’ vision. Further studies are needed to confirm these early results.
Rods and cones are polarized photoreceptor cells whose apical portions, known as the outer segments , are specialized dendrites. The outer segments of both rods and cones are surrounded by pigmented epithelial cells (see Fig. 22.16 ) and their basal regions form synapses with the underlying cells of the bipolar layer. There are approximately 100 to 120 million rods and only 3 million cones in each retina. Rods are specialized receptors for perceiving objects in dim light; cones are specialized receptors for perceiving objects in bright light . Cones are further adapted for color vision; rods perceive only light and cannot differentiate colors. Rods and cones are unevenly distributed in the retina in that cones are highly concentrated in the fovea; thus, this is the area of the retina where high-acuity vision occurs.
Rods are the photoreceptors of the retina specialized for perceiving dim light.
Rods are elongated cells (50 μm long by 2–5 μm in diameter) oriented parallel to one another but perpendicular to the retina. These cells are composed of an outer segment , inner segment , nuclear region , and synaptic region ( Fig. 22.17 ).
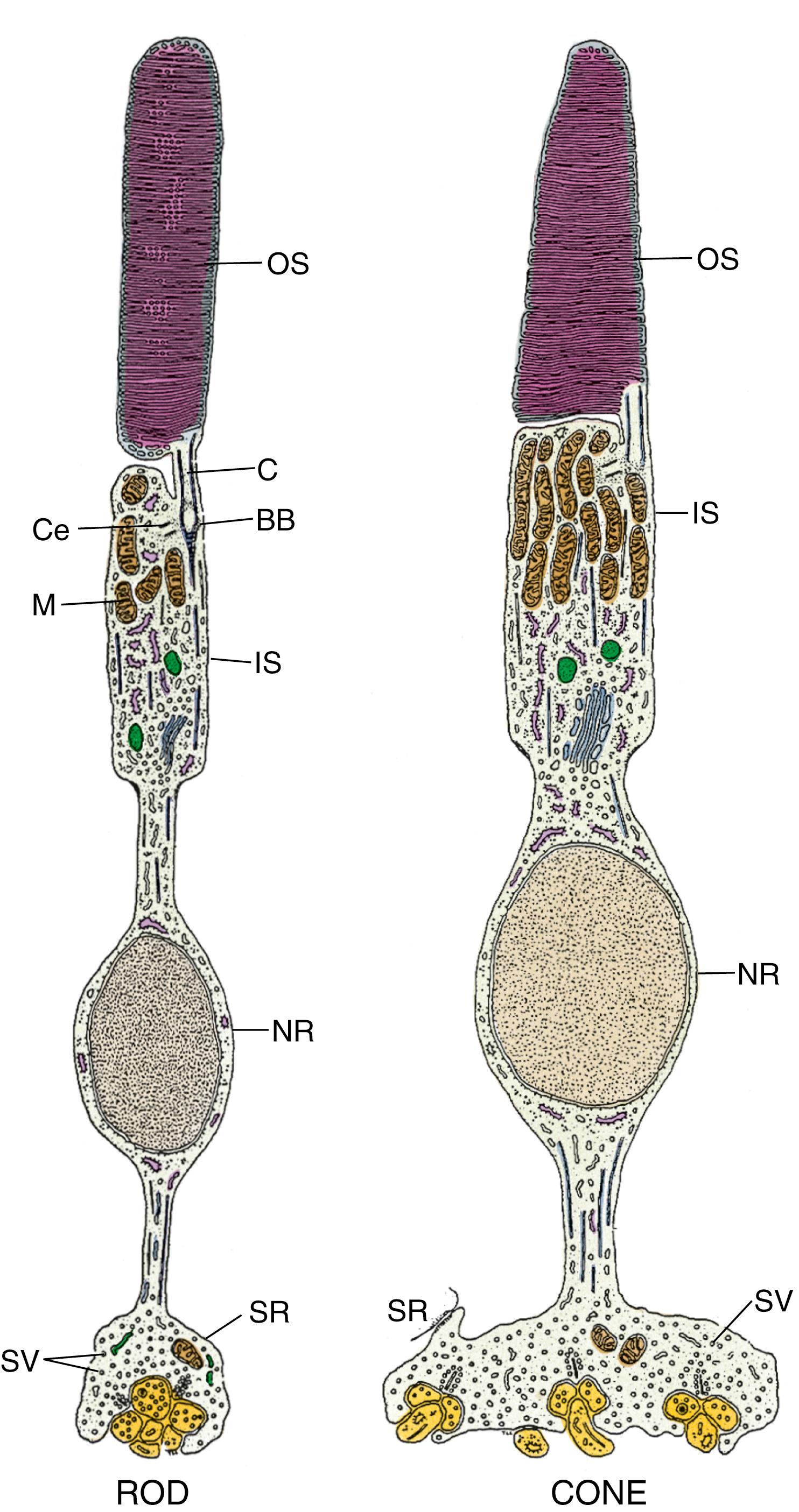
The outer segment of the rod , its dendritic end, presents close to 1000 flattened membranous lamellae oriented perpendicular to the rod’s long axis ( Fig. 22.18 ; see Fig. 22.17 ). Each lamella represents an invagination of the plasmalemma, which is detached from the cell surface, thus forming a disk. Each disk is composed of two membranes separated by an 8-nm space. The membranes contain a specific form of the light-sensitive pigments opsins , known as rhodopsin ( visual purple ). Because the outer segment is longer in rods than in cones, rods contain more rhodopsin, respond more slowly than cones, and have the capacity to collectively summate the reception. The cell membrane of the outer segment houses cyclic guanosine monophosphate (cGMP)–gated Na + ion channels that are kept open by the cGMP that are bound to the gates if there are no photons impinging on the rod, that is, if it is dark. Therefore, in the dark, Na + ions may enter the outer segment of the rod.
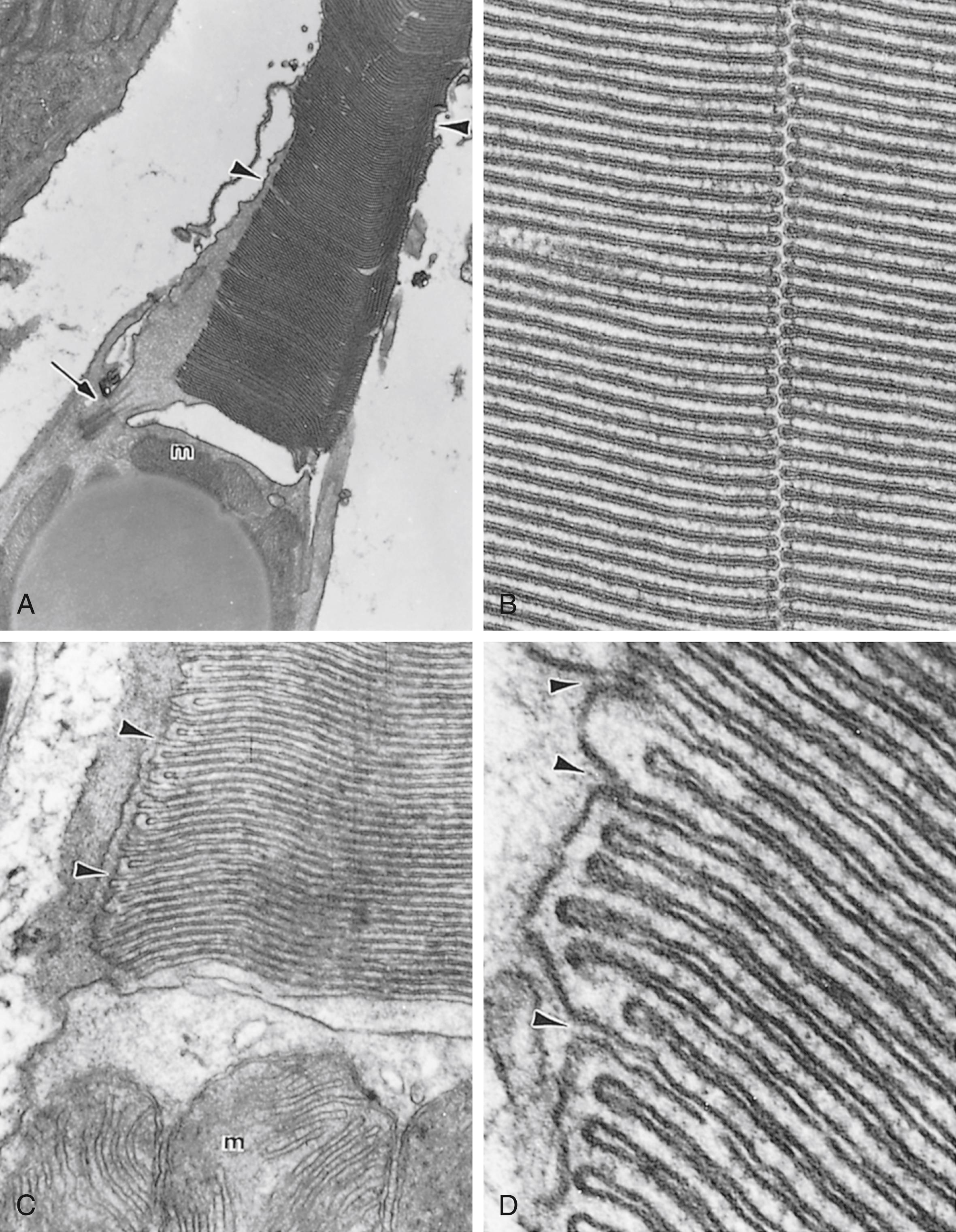
The inner segment of the rod is separated from the outer segment by a constriction called the connecting stalk . Passing through the connecting stalk and into the outer segment of the rod is a modified cilium (lacking the central singlet microtubules) that arises from a basal body located at the apical end of the inner segment. Congregated near the interface with the connecting stalk are abundant mitochondria and cytoplasmic glycogen granules, both necessary for the production of energy for the visual process. The cytoplasm basal to the mitochondria is rich in microtubules, polysomes, smooth endoplasmic reticulum, RER, and Golgi complexes. Proteins produced in the inner segment migrate to the outer segment, where they become incorporated into the disks. The disks gradually migrate to the apical end of the outer segment and are eventually shed into the sheaths of the pigment cells, where they will be phagocytosed. The length of time from protein incorporation through migration and finally to shedding is less than 2 weeks. The lateral cell membrane of the inner segment has adenosine triphosphate (ATP)–driven sodium-potassium pumps that deliver potassium into the rod and drive sodium out of the rod. The lateral cell membrane of the inner segment also houses nongated K + channels through which K + ions can leave the rod. These ion channels operate the same way whether light is impinging on the rod or not and maintain the cell membrane potential at −40 mV. When light impinges on a rod, the cGMP-gated Na + ion channels of the outer segment become closed. Thus, sodium cannot enter the rod and cause the cytoplasmic face of the rod cell membrane to become more negative (−70 to −80 mV) compared with its outer face, causing the rod to become hyperpolarized . Therefore, it is the hyperpolarization of the rod plasmalemma rather than depolarization that causes the rod to fire .
It was stated earlier that the rod membrane contains a light-sensitive pigment known as rhodopsin , a light-sensitive molecule composed of a carotene-like pigment 11- cis retinal and the protein scotopsin . When photons impinge on rhodopsin, within picoseconds the cis -retinal is transformed into all - trans retinal , changing its molecular conformation from a bent structure to a straight structure. This conformational alteration disrupts the bond between the retinal and scotopsin; within milliseconds, the rhodopsin molecule is transformed into activated rhodopsin , the molecule responsible for the electrical alterations within the rod. Activated rhodopsin is stable for 1 or 2 seconds and then is digested by the enzyme rhodopsin kinase into all- trans retinal and scotopsin. In order to replenish rhodopsin, the energy-requiring reaction occurs in which the enzyme retinal isomerase converts all- trans retinal to 11- cis retinal. Once converted, it immediately binds to scotopsin to form rhodopsin. Interestingly, the same enzyme can convert vitamin A (11- cis retinol) to 11- cis retinal, ensuring an abundant supply of rhodopsin.
Activated rhodopsin acts on transducin , a G protein, by inducing it to activate the enzyme cGMP phosphodiesterase to convert cGMP to 5′-cGMP. The decrease in cGMP levels causes the sodium channels of the outer segment to close because cGMP is no longer bound to the gates, preventing the entry of Na + ions into the rod, thus causing the rod cell membrane to become hyperpolarized. It should be noted that the impingement of even a single photon on a rod has a tremendous effect by being able to influence the movements of millions of sodium ions in that rod ( Fig. 22.19 ).
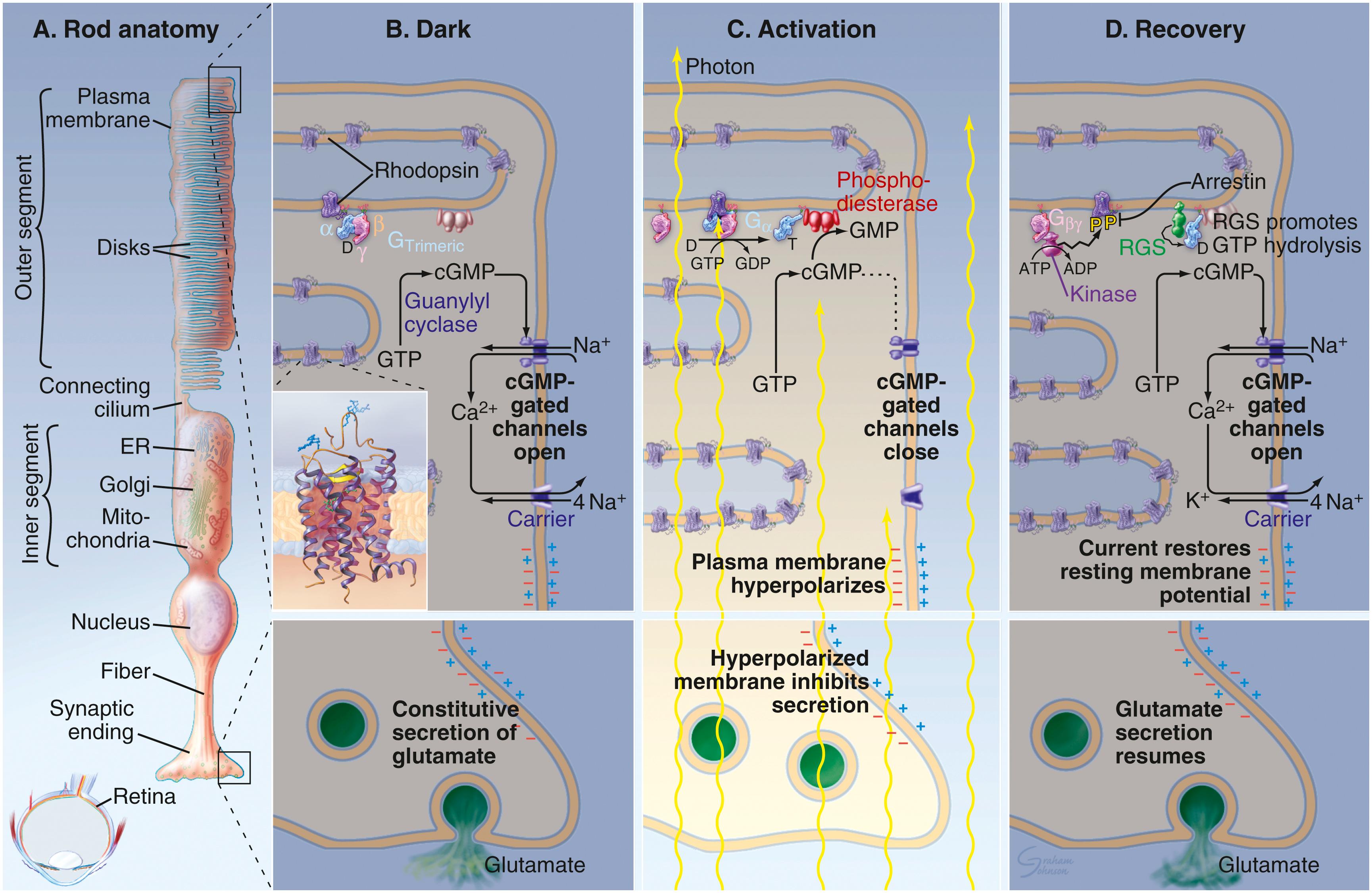
As indicated earlier, the signal from rods is not induced by depolarization, as it is in most cells; rather, light-induced hyperpolarization causes the signal to be transmitted through the various cell layers to the ganglion cells, where the signal generates an action potential along the axons to the brain.
The nuclear region of rods houses the nucleus and many of the organelles of the cell. The synaptic region displays short cytoplasmic processes that form synapses with bipolar and horizontal cells. The synaptic vesicles housed in the synaptic region contain the neurotransmitter substance glutamate , which they continuously release into the synaptic cleft when the rods are not hyperpolarized.
Rods are most sensitive to greenish-blue light whose wavelength is about 505 nm and completely insensitive to red light and beyond (640 nm).
A severe lack of vitamin A causes night blindness because the amount of available rhodopsin is greatly reduced. Because vitamin A is stored in great supply by the liver, it takes as long as 1 year of vitamin A deficiency in the individual’s diet before night blindness becomes evident. Fortunately, night blindness caused by a vitamin A deficiency can be reversed rapidly by a vitamin A injection.
Cones are specialized photoreceptors of the retina for perceiving bright light and color.
Although the morphology and mode of function of the cones is similar to that of rods, cones are activated in bright light and produce greater visual acuity compared with rods. There are three types of cones, L cones (long wavelength), M cones (medium wavelength), and S cones (short wavelength), each containing a different variety of the photopigment opsin , known as photopsin and responding to different wavelengths of light. Thus, each variety of photopsin has a maximum sensitivity to one of three colors of the spectrum—red, green, and blue. The difference resides in the opsins rather than in the 11- cis retinal (see Tables 22.1 and Table 22.2 ).
| Rod/Cones | Wavelength of Light (nm) |
|---|---|
| Rods | 505 |
| Blue-sensitive pigment in cones (S cones) | 445 |
| Green-sensitive pigment in cones (M cones) | 535 |
| Red-sensitive pigment in cones (L cones) | 570 |
Cones are elongated cells (60 μm long × 5 to 8 μm in diameter), being longer and narrower at the fovea centralis (70 μm long × 1.5 μm in diameter). Their structure is similar to that of rods, with the following few exceptions ( Fig. 22.20 ; see Figs. 22.17B and 22.18 ):
Their apical terminal (outer segment) is shaped more like a cone than a cylinder.
The disks of cones, although composed of lamellae of the plasmalemma, are attached to the plasma membrane, unlike the lamellae of the rods, which are separated from the plasma membrane.
Protein produced in the inner segment of cones is inserted into the disks throughout the entire outer segment. In the rods, it is concentrated in the most distal region of the outer segment.
Unlike rods, cones are sensitive to color and provide greater visual acuity.
Recycling of the cone photopigment does not require the retina pigment cells for the processing.
Cones are not nearly as sensitive (as much as 100 times less sensitive) to light as rods, but they are still able to discriminate color in relatively poorly lit conditions.
The synaptic vesicles housed in the synaptic region of cones, just as in rods, contain the neurotransmitter substance glutamate , which they stop releasing into the synaptic cleft when they become hyperpolarized.
The mechanism of photoreception in cones is very similar to that in rods, but cones react to different wavelengths than do rods (see Table 22.2 ).
Each type of cone can distinguish approximately 100 shades of color. Most mammals have two types of cones and are said to be dichromatic ; therefore, they distinguish 10,000 different colors. Because humans have three types of cones, people are said to be trichromatic and are able to distinguish 1 million different colors. It has been reported that some people possess four types of cones, tetrachromats, who can distinguish 100 million different colors.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here