Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
On completion of this chapter, you should be able to:
Understand the complexity of and criteria for receiving a transplant
Gain knowledge of surgical techniques of liver, renal, and pancreatic transplants
Identify the necessary imaging protocol needed for imaging the transplanted organ
Recognize and define normal sonographic features of the transplant patient
Recognize and define common and complex pathologies
Know the importance of imaging in the immediate postoperative patient
Identify and describe immediate complications after transplantation and those that occur within the months and years to follow
Understand your role in donating life
According to the Organ Procurement and Transplantation Network (OPTN) , as of January 2021, there are more than 120,000 people in need of an organ transplant. Of these, 108,464 are actively waiting, and every 9 minutes a new candidate is added to the national transplant waiting list. The average wait time is 5 to 7 years. With this severe shortage of donor organs, 17 people die each day on average while waiting for their transplant. In 2020, 39,034 patients received a transplant, which was the 10th consecutive annual record, with 70% of these transplants coming from deceased donors. The number of living donor transplants were reduced due to the COVID-19 pandemic. Many transplant programs deferred living donor transplantation to reduce unnecessary exposure to the COVID-19 virus. The majority of transplant recipients were age 50 and older during the past 10 years, with the most common age range between 50 and 64 years.
To ensure a successful transplant, each patient needs to receive the best of care from the entire transplant team. The team consists of clinical transplant coordinators, transplant physicians and surgeons, financial coordinators, and social workers. The United Network for Organ Sharing (UNOS) celebrated 35 years in 2019. UNOS maintains a centralized computer networking system for all organ procurement organizations and transplant centers while seeking to be fair and effective in selecting transplant candidates. The UNOS database can be accessed 24 hours a day. Once a patient is referred to a transplant center, the patient will have a series of tests, and their mental and physical health will be evaluated by a physician. The social support available to the patient is also a consideration. If the center accepts the candidate, the medical information will be entered into the national organ transplantation waiting list. Once a deceased donor is located, a transplant coordinator from an organ procurement organization will access the UNOS database. Each patient on the waiting list is matched with the donor characteristics using the computer. The computer will then display the ranked list of the candidates according to the OPTN organ allocation process. Factors in receiving a transplant include tissue match, blood type, immune status, length of time on the waiting list, and the distance between the donor and recipient. The organ is offered to the transplant team and candidate that are on top of the list. The person at the top does not always receive the organ because they may be too unhealthy or laboratory results indicate an incompatible match and rejection would likely occur. Once the patient is selected, they would be contacted for the transplant surgery to be scheduled and performed.
Patients who have severe liver disease require a liver transplant for survival. Acute liver failure is sudden and most commonly caused from a drug-induced injury such as an acetaminophen overdose. Chronic liver failure or end-stage liver disease is progressive over a period of months and years. Scar tissue is formed within the liver and normal functioning liver tissue is reduced, creating cirrhosis. The most common reason for transplantation in the United States is cirrhosis due to chronic hepatitis C, followed by alcohol abuse ( Fig. 20.1 ). Many other liver diseases can also cause the liver to fail resulting in the need for transplantation, including the following:
Chronic hepatitis B and autoimmune hepatitis
Nonalcoholic steatohepatitis, caused by fat and inflammation of the liver
Hemochromatosis, due to iron overload within the liver
Wilson disease, due to copper overload within the liver
Budd-Chiari syndrome, caused by occlusion of the hepatic veins draining the liver
Biliary diseases, including the following:
Biliary atresia, absent or blocked bile ducts and occurs only in infants
Alagille syndrome, blocked or malformed bile ducts and ductal paucity
Primary biliary cirrhosis, destruction of intrahepatic ducts
Primary sclerosing cholangitis (PSC), destruction of intrahepatic and extrahepatic ducts
Cancer originating in the liver such as hepatocellular carcinoma (HCC), hepatoblastoma, and cholangiocarcinoma
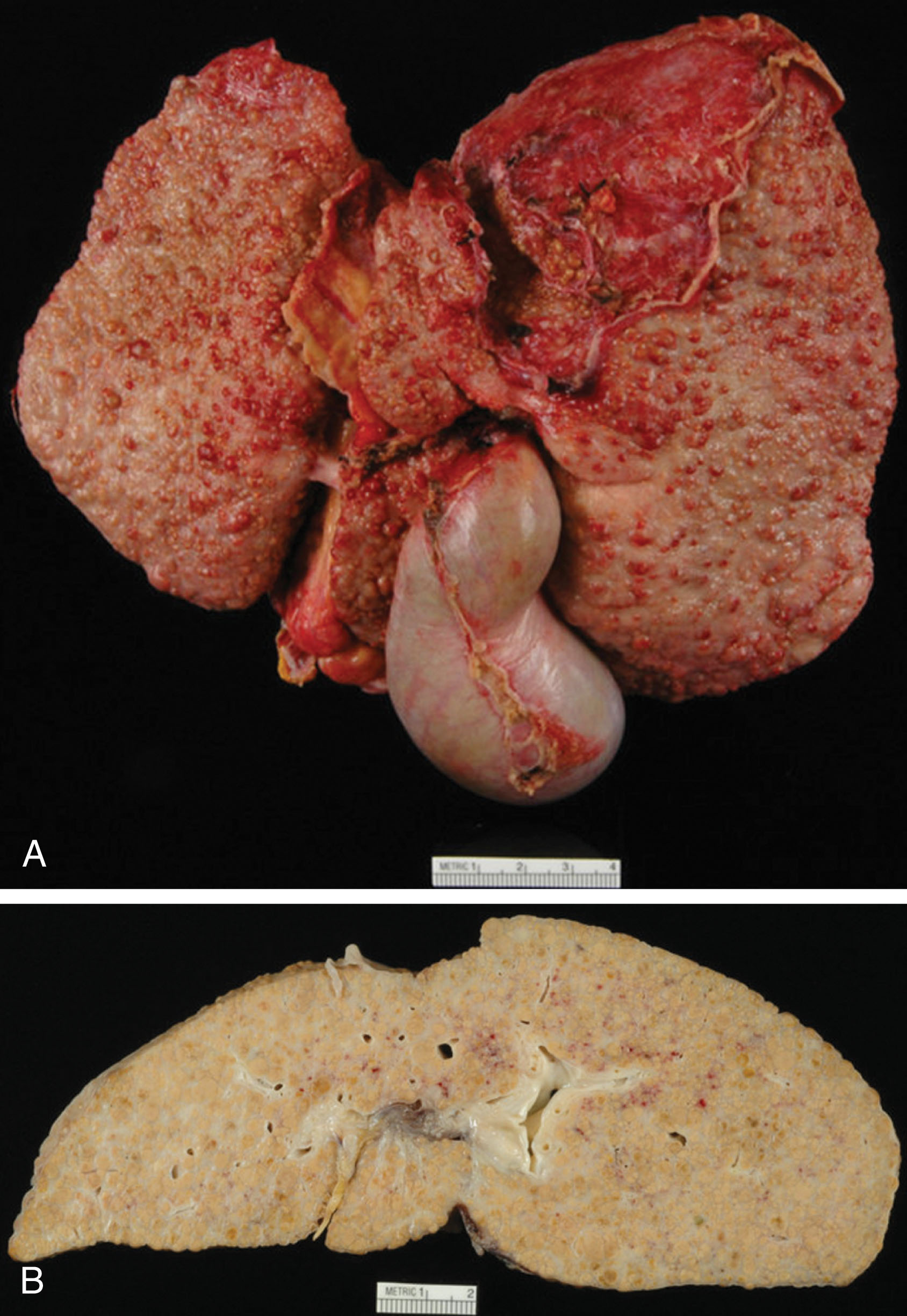
Symptoms of liver disease include jaundice, fatigue, weight loss, itching, abdominal ascites, bleeding in the stomach from varices, confusion, black stools, nausea, and loss of appetite. A patient may not be a liver transplant candidate if they present with severe irreversible illness, widespread cancer, human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), severe pulmonary hypertension, active infection, alcohol or drug abuse, or history of noncompliance.
Patients who are placed on the transplant waiting list receive a score that determines how urgently they need a new liver. Dr. Patrick Kamath originally developed the Mayo End-Stage Liver Disease Score at Mayo Clinic in 2001. With modification from UNOS, the Model for End-Stage Liver Disease (MELD) scale for adults and Pediatric End-Stage Liver Disease (PELD) for younger than 12 years are still actively used today. MELD was initially used to predict death within 3 months in patients who had a transjugular intrahepatic portosystemic shunt (TIPS) performed. This scale was also found to be beneficial in predicting prognosis and prioritizing liver transplant recipients. MELD scores range from 6 to 40 and PELD scores range from negative values to 99. The scores identify the likelihood of death within 3 months without receiving a transplant. Higher scores denote an increased need for a transplant. For example, a patient with a MELD score of 22 would have a 10% mortality risk at 3 months, whereas a patient with a score of 38 would have an 80% mortality risk. The MELD score is a mathematical calculation based on laboratory values of bilirubin (measurement of bile pigment), creatinine (kidney function), and international nationalized ratio (INR; blood clotting ability).
In 2002 there was an immediate decrease by 12% of the time spent on the waiting list, which also led to a reduction of almost 15% mortality. The mean waiting time in the MELD era went from 656 to 416 days. The reduction in mortality went from 2046 deaths in 2001 to 1364 by 2005. The average wait time in the United States based on the MELD scores is 21 months for a MELD of 11 to 18, 4 months for a MELD of 19 to 24, and 20 days for a MELD greater than 25.
The Milan criteria also play a role in liver transplantation. If a cirrhotic patient has HCC, the patient may be considered for a transplant if they have one HCC smaller than 5 cm, or up to three lesions smaller than 3 cm without vascular invasion or extrahepatic involvement. HCC is the most common primary malignant tumor in the liver and is the fifth most common cancer. There is a 10% to 50% chance of recurrence at 2 years and 70% at 5 years with liver resection. Transplantation and radiofrequency ablation (RFA) are potentially curative options.
Dr. Thomas Starzl performed the first liver transplant on March 1, 1963. The 3-year-old patient with biliary atresia died due to uncontrolled bleeding. The first five transplant recipients died within 23 days. In 1967 the first successful liver transplant performed by Dr. Starzl in Denver survived 1 year. The patient died of recurrent HCC. With the help of Sir Roy Caine, cyclosporine was introduced in 1979, improving survival rates significantly. In 1989 Starzl and colleagues reported that 1179 patients survived between 1 and 5 years after transplantation. Since the first transplant, more than 10,000,000 liver transplants have been performed worldwide with an 80% to 90% survival rate within the first year. There are currently more than 125 transplant programs performing approximately 5000 to 6000 transplants each year. As of June 15, 2012, 16,773 patients were awaiting a deceased liver transplant.
In 2020 the average cost of receiving a liver transplant was $878,400. If a patient received a combined liver and kidney transplant, the cost was $1,355,100. These costs include 30 days pretransplant, procurement, hospital transplant admission, transplant physician, 180 days posttransplant discharge, and immunosuppressants.
The most common cause for needing a renal transplant is chronic end-stage renal disease or renal failure. Acute renal failure may also occur, requiring transplantation, but this is significantly uncommon. Symptoms of renal failure begin when the kidney only has 10% functioning tissue. Some signs and symptoms are decreased urine output, edema in the legs or feet, shortness of breath, fatigue, confusion, nausea, chest pain, heart arrhythmias, difficulty sleeping, itching, hypertension, loss of appetite, muscle cramps, and seizures or coma in severe cases. Depending on the cause of renal impairment, it may be treatable, yet some may show irreversible damage. Renal function is evaluated using blood laboratory values of blood urea nitrogen, creatinine, and glomerular filtration rate. With progressive loss of kidney function, patients will ultimately require dialysis to filter waste and remove excess fluid from the blood. Dialysis is the only means of survival until a renal transplant becomes available. Patients may not receive a transplant if they have widespread cancer, active infection, HIV/AIDS, liver or heart disease, history of noncompliance, or alcohol or drug abuse. The following are causes that can lead to renal failure and a need for transplantation:
Hypertension and renal artery stenosis or renal vein thrombosis
Diabetes mellitus: high blood glucose can damage the filtering system (diabetic nephropathy)
Urinary tract obstruction such as renal stones or certain cancers
Inherited kidney disease such as polycystic kidney disease
Glomerulonephritis: inflammation of small filters, glomeruli
Interstitial nephritis: inflammation of kidney tubules
Hemolytic uremic syndrome: premature destruction of red blood cells
Systemic lupus erythematosus: immune system attacks kidney as foreign object
Scleroderma: hardening and tightening of the skin and connective tissue
Vasculitis: inflammation of blood vessels
Toxins such as alcohol, cocaine, or heavy metals
Recurrent kidney infection
Medication therapy used for other diseases
Severe dehydration
On December 23, 1954, Dr. Joseph Murray performed the first successful kidney transplant at Peter Bent Brigham Hospital in Boston. The surgery involved identical twins, Richard and Ronald Herrick, eliminating possible rejection. In 1990 Dr. Murray won the Nobel Prize in Physiology of Medicine. Between 1954 and 1973, about 10,000 renal transplants were performed. More than 99,000 people were on the deceased kidney donor list as of June 15, 2012. Due to shortage of kidneys, the system of expanded criteria donors (ECDs) was introduced in 2002. This includes the kidneys that once were considered not suitable. ECD kidneys are any donors older than 60 years or ages 50 to 59 years with two of the following conditions: hypertension, serum creatinine greater than 1.5 mg/dL, or cause of death from a cerebrovascular accident.
In 2020 the average cost of receiving a renal transplant was $442,500. If a patient received a combined kidney and liver transplant, the cost was $1,355,100. The cost of a combined kidney and pancreas transplant was $713,800. These costs include 30 days pretransplant, procurement, hospital transplant admission, transplant physician, 180 days posttransplant discharge, and immunosuppressants.
More than 15 million people in the United States have diabetes mellitus, with 798,000 new patients diagnosed each year. Patients receive a pancreas transplant in hopes to cure type 1 diabetes. In these patients, the pancreatic islet cells cannot produce enough insulin, resulting in dangerously elevated blood glucose. Insulin provides movement of the glucose in the blood into the muscles and fat to provide energy for the body. Initially, patients routinely receive insulin injections or take medications to help control blood glucose. Transplantation may be a consideration if glucose levels are poorly managed, if glucose levels show frequent insulin reactions, or if severe kidney damage occurs. It is common for a patient to receive a combined pancreas and renal transplant to ensure healthy organs are unlikely to be affected by the damage that comes from diabetes. High blood glucose can lead to many complications such as amputations, heart disease, stroke, vascular disease, blindness, nerve damage, or kidney damage.
Type 2 diabetes patients are not a consideration for pancreas transplant. In these patients, insulin is produced from the pancreas, but the body develops insulin resistance and a new pancreas will not be a cure in this case. Transplantation usually is not performed on a patient with cancer, active infection, HIV/AIDS, lung disease, obesity, severe heart disease, or an ongoing history of alcohol or drug abuse.
Dr. William Kelly and Dr. Richard Lillehei performed the first successful pancreas transplant combined with a kidney transplant on December 17, 1966, at the University of Minnesota. The surgery was considered experimental until about 1990. There are about 1200 pancreas transplants performed each year in the United States, with 75% of cases being a simultaneous pancreas-kidney transplant (SPK) . There have been more than 23,000 pancreas transplants reported to the International Transplant Registry.
In the United States there were 3202 patients that were listed for a pancreas transplant as of February 2014. During the years of 2012 and 2013, there were 16,410 deceased donors with only 2826 pancreas allografts procured. This shortage of organs is due to lack of procurement or the pancreas being deemed unsuitable. The ideal donor is between 10 and 40 years of age with a body mass index (BMI) less than 27.5 kg/m2, and with a cause of death not from cerebrovascular disease. Recent studies have suggested organs from extended criteria donors can receive similar graft survival as the “ideal” donor. The accumulation of risk factors and extended cold ischemic time should be avoided when possible to ensure a healthy allograft.
In 2020 the average cost of receiving a pancreas transplant was $408,800. The cost of a combined pancreas and kidney transplant was $713,800. These costs include 30 days pretransplant, procurement, hospital transplant admission, transplant physician, 180 days posttransplant discharge, and immunosuppressants.
The liver can be preserved between 8 and 12 hours on ice and using a special preservation solution. The shorter the ischemic time, the better the allograft function once transplanted. For every liver transplant, the donor and recipient are rechecked to verify a match. The blood type and compatibility need to be confirmed and the UNOS database per protocol as well. Before coming into the operating room, the team will discuss the risks, benefits, and alternatives with the patient and obtain consent. All institutions have certain protocols and techniques that they follow. The following will discuss the basic current practices at my institution.
The patient is brought into the operating room confirming patient identification and procedure to be performed. Following general anesthesia and line placement, a Foley catheter is placed into the bladder. The abdomen is prepped and draped sterilely. Bilateral subcostal skin incisions are made and extended to the midline up to the xiphoid process. The scar resembles a “Mercedes sign.” The diseased liver is mobilized, vessels clamped and ligated, and dissected free from the body cavity becoming “anhepatic.” This diseased liver is sent for pathologic examination.
The procured liver is prepared on the “back table” for any reconstruction to occur and submerged into a saline slush solution. Patients present with variant anatomy and that needs to be taken into consideration. Some may have normal anatomic variants such as additional veins or arteries to anastomose or vessels need to be trimmed down to size. Some may have short veins or arteries that are not long enough and require an interposition graft in which a deceased donor iliac artery or vein may be used. The iliac vein can act as a conduit between the recipient superior mesenteric vein and the donor portal vein. The iliac artery can be used as a conduit between the recipient infrarenal aorta and the donor hepatic artery.
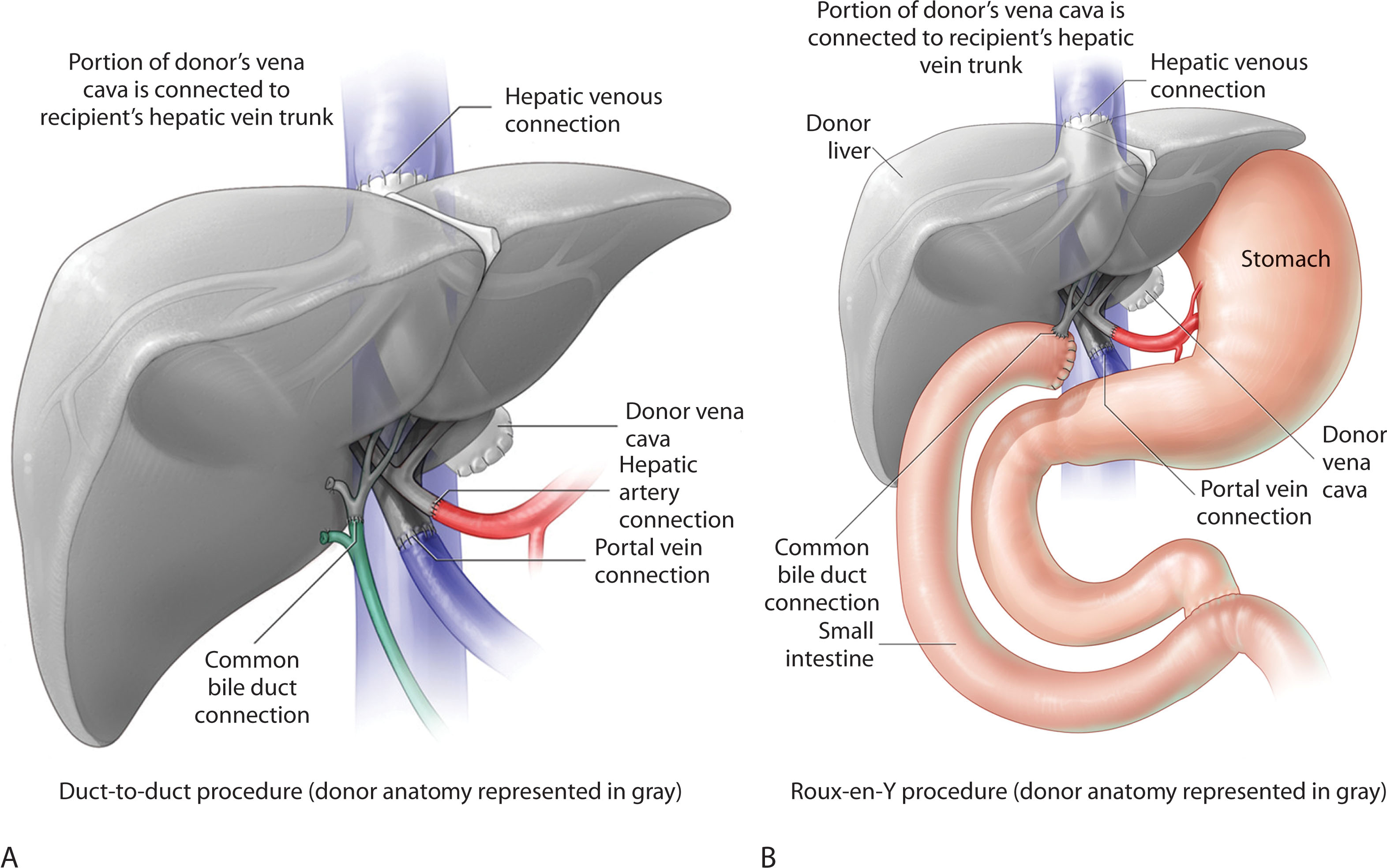
Once the donor liver has been prepared, it is placed within the recipient’s abdominal cavity and covered with laparotomy pads containing saline slush. Vent tubing is placed in the infrahepatic cava to prepare the suprahepatic cava for anastomosis. The upper caval anastomosis is sewn end to side with running sutures. The recipient portal vein is occluded with a clamp and flushed with glycine irrigant, and then an end-to-end portal vein anastomosis is sewn with sutures. The recipient common hepatic artery is flushed with heparinized saline, and occluded with a clamp. The donor and recipient common hepatic arteries are then sewn end-to-end using running sutures to create the arterial anastomosis. The arterial and portal venous clamps are removed and the liver flushed with blood to begin reperfusion. The surgeon examines all the anastomoses to ensure they are dry and do not have a leak and makes sure the patient is hemodynamically stable. At this point, a cholecystectomy is performed on the donor liver. A choledochodochostomy is sewn between donor and recipient common bile ducts using running sutures. The anastomosis is sewn over a polyurethane biliary stent or tube, which is brought out through the cystic duct stump. The catheter is then secured to the stump with a suture. Another option would be an end-to-side choledochojejunostomy using a suture over the jejunal biliary tube. In patients with PSC or retransplantation, a roux-en-Y limb is created for reconstruction ( Fig. 20.2 ). The patient is rechecked for any significant bleeding. Drains are placed within the right and left subhepatic spaces and secured to the skin with stitches. Sponge, needle, and instrument counts are conducted before closure. If indicated, the patient will receive units of blood, platelets, or fresh frozen plasma. The incision is closed in two layers with a running layer suture on the midline and posterior fascia and then anterior fascia is closed. The skin is closed with staples. The wound is dressed with a dry sterile dressing and the patient is transported to the intensive care unit (ICU) when stable.
There is a shortage of organs available for donation. Living donor organ donation offers the alternative for ones waiting for a transplant and also increases the existing organ supply. A friend or family member may consider being a donor for their loved one. If the donor is not a match, there is a paired exchange program. The donor’s organ would go to someone else for a better match rather than directly going to their friend or family member. The one needing the transplant will receive their organ in the exchange that meets their match.
The first adult living liver donor donation was performed in 1989; more than 4000 have been performed in the United States. The 1-, 3-, and 5-year survival rates are 90%, 83%, and 78%, respectively. Ultimately, a donor liver will regenerate to more than 85% of its original volume. Living donor transplantation has many ethical issues and has been a source of some controversy. Donors may experience a psychological syndrome due to the stress of surgery. Gokce and colleagues showed that 21% of donors feel anxious about the complications and quality of life after surgery. The donor complication rate is 40% with incision infection, biliary leak, or stricture being the most common. Some donors develop complications that lead them to suicide or drug overdose. Living donor liver transplants are optimal in urgent situations; however, 27.1% of living donors demonstrated one or more complications in the postoperative period.
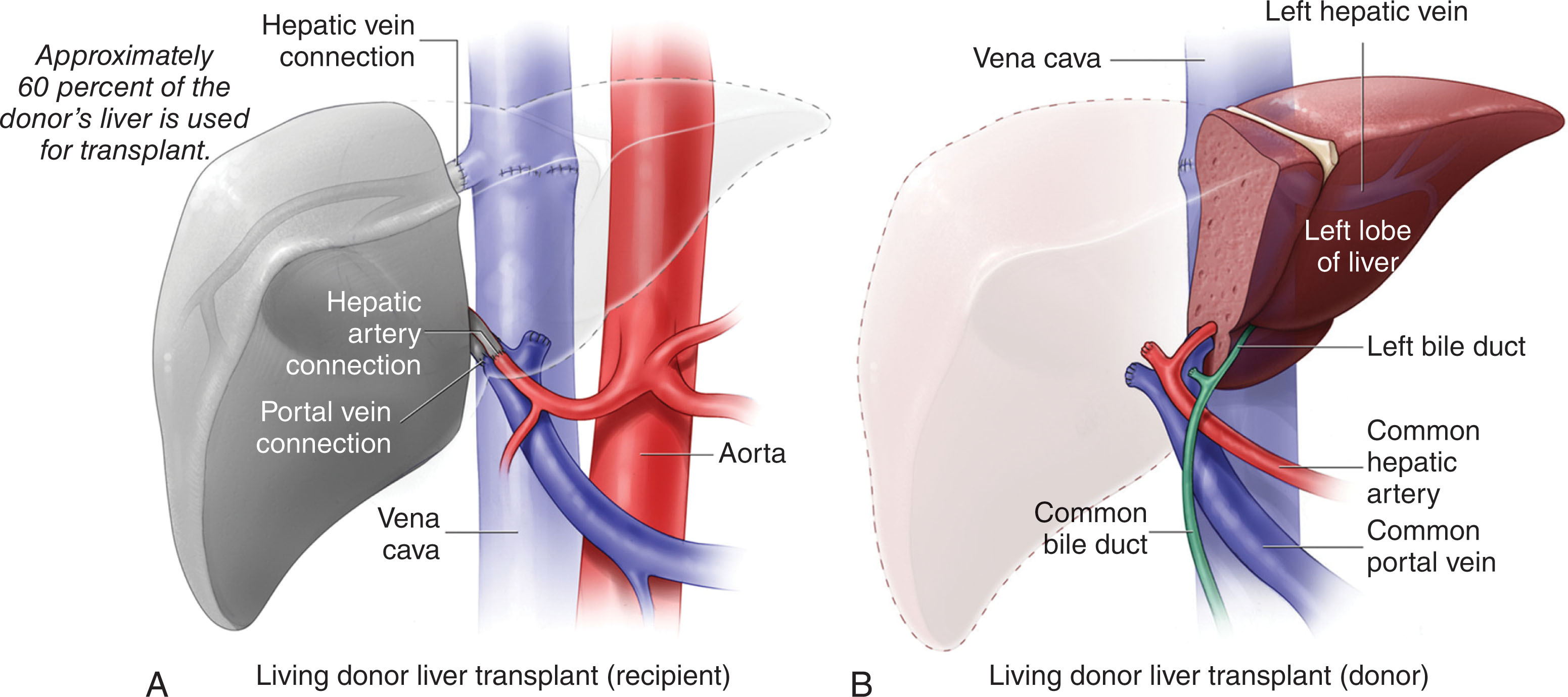
The surgical technique is very similar compared with a cadaveric liver. The allograft may demonstrate better function more quickly due to the decreased preservation time in a living donor. The recipient will receive part of a liver rather than a full organ, usually the right hepatic lobe. The patient is brought into the operating room confirming patient identification and procedure to be performed. Following general anesthesia and line placement, a Foley catheter is placed into the bladder. The abdomen is prepped and draped sterilely. Bilateral subcostal skin incisions are made and extended to the midline up to the xiphoid process. The scar resembles a Mercedes sign. The diseased liver is mobilized, vessels clamped and ligated, and dissected free from the body cavity becoming anhepatic. This liver is sent off for pathologic examination.
The procured liver is prepared on the back table for any reconstruction to occur and submerged into a saline slush solution. Patients present with variant anatomy and that needs to be taken into consideration. Some may have additional veins or arteries to anastomose or vessels need to be trimmed down to size, or some may have short vessels and require an interposition graft. Once the donor liver is prepared, it is placed within the recipient’s abdominal cavity and covered with laparotomy pads containing saline slush. The recipient’s left middle hepatic vein trunk is stapled and the right hepatic vein is occluded using a clamp. The donor right hepatic vein opening is extended inferiorly on the vena cava, and two sutures are placed on the superior and inferior venotomy. The sutures are then placed through the donor right hepatic vein. An end-to-end anastomosis is performed between the donor and recipient right hepatic vein and vena cava using running sutures. The recipient portal vein is flushed free of clot, and an end-to-end portal vein anastomosis is created using running sutures. Reperfusion of the liver is accomplished by releasing the venous and portal vein clamps. Following reperfusion, the perihepatic area is carefully inspected for hemostasis. If bleeding is noticed, the surgeon will control using additional sutures. The donor right hepatic artery is flushed with heparinized saline and occluded with a clamp. The recipient gastroduodenal artery is ligated and divided. The common hepatic artery is occluded with a clamp and divided in its distal portion. An end-to-end anastomosis is performed between the donor right hepatic artery and the recipient common hepatic artery using running sutures. Once arterial reperfusion is achieved, the perihepatic area is again carefully inspected for hemostasis. An end-to-end duct anastomosis is created. In patients with PSC or retransplantation, a roux-en-Y limb is created for reconstruction. An end-to-side hepaticojejunostomy is performed using a running suture on the posterior layer and an interrupted suture on the anterior layer ( Fig. 20.3 ). Hemostasis is again ensured. Two drains are placed posterior to the liver and inferior to the incision. Sponge, needle, and instrument counts are conducted before closure. If indicated, the patient will receive units of blood, platelets, or fresh frozen plasma. The incision is closed with a running suture in the anterior and posterior layers and in the midline. Staples are used for skin closure. The wound is dressed with a dry sterile dressing and the patient is transported to the ICU when stable.
As a sonographer, evaluate the liver transplant as a native liver. Assess the size, echotexture, contour, biliary tree, and vasculature, and look for any masses, fluid collections, or ascites. Institutions instill their own routine protocols on timing of examinations and specific images that are required. The following is the basis of what my institution practices. Once the patient has arrived at the ICU from the surgical suite, the sonographer will go remotely to perform the “day 0” examination. Typically the patient will receive an ultrasound examination on postoperative day 1 and day 7 as well. The patient’s liver function is closely monitored and if any issues arise, the transplant physicians will tailor their imaging requests. The patient usually has a follow-up ultrasound every 6 months to 1 year if there are no complications.
It is best to image using a 2.5- to 5.0-MHz curvilinear transducer and have the patient fasting 4 to 6 hours. Obese patients and livers with fatty infiltration will likely require lower frequencies. It is helpful to ask the patient to take a breath in and hold it if they are able to do so. This will give better visualization of the liver and reduce respiratory motion. Rolling the patient left lateral decubitus will also help aid visualizing the right lobe more clearly.
The sonographer should scan completely through the liver before taking any images using subcostal and intercostal approaches while adjusting the frequency, depth, gain, time-gain compensation (TGC), and focal zones as they go. Once the liver is fully accessed, the examination needs to include gray-scale longitudinal and transverse images of the left and right hepatic lobes including the caudate lobe and vascular landmarks of the inferior vena cava (IVC), hepatic veins (HVs), and portal veins (PVs). The right lobe of the liver should be compared with the right kidney to evaluate the echogenicity. Document any pathology in two planes with and without measurements and include color Doppler flow. Knowing the type of transplant the patient received (i.e., living or cadaveric) is helpful before scanning because in living donor transplanted patients only the right lobe will be present. The donor liver receives a cholecystectomy during the surgical process and, as a result, no gallbladder images will be acquired. The biliary tree needs to be evaluated for intrahepatic and extrahepatic ductal dilation, wall thickening, biliary leaks, and stones or sludge. It is common for biliary stents to be present. Quadrant images need to be obtained checking for free fluid or signs of bleeding.
The Doppler portion is crucial for the transplanted liver. Venous and arterial color and spectral Doppler needs to be obtained looking for any signs of thrombus or stenosis. Ultrasound is commonly the first method of imaging in these patients, and the sonographer can help the physicians diagnose the patient and treatment will be administered quickly. The hepatic arteries (HAs) are not routinely included in the native liver examination, but they are very important to image in your transplanted patient. It is critical to angle correct in the left, right, and main HAs with pulsed-wave Doppler to accurately assess for stenosis. The sonographer will need to measure the peak systolic and end diastolic velocities to obtain the resistive index (RI) measurement. This is a common indirect Doppler technique to evaluate the HA. A low RI can be a strong indicator of a proximal stenosis the sonographer may not be able to otherwise identify or a presence of an arteriovenous fistula (AVF). A high RI may indicate rejection or hepatic venous congestion. These vessels are small in size, and the sonographer may choose to use the zoom feature on the ultrasound machine for better visualization. Although sometimes it may be difficult to identify the anastomosis, attempt to evaluate the HA as proximally as possible. If a stenosis is present, the anastomotic site is the most common location. Color flow aliasing in the PVs, HVs, or IVC, should be further evaluated with spectral Doppler to help identify a narrowing. For all spectral Doppler, the angle should be parallel with the vessel that is being sampled and less than 60 degrees to avoid falsely elevated velocities. To increase diagnostic accuracy, all of the spectral waveforms should fill the spectral window while eliminating spectral aliasing.
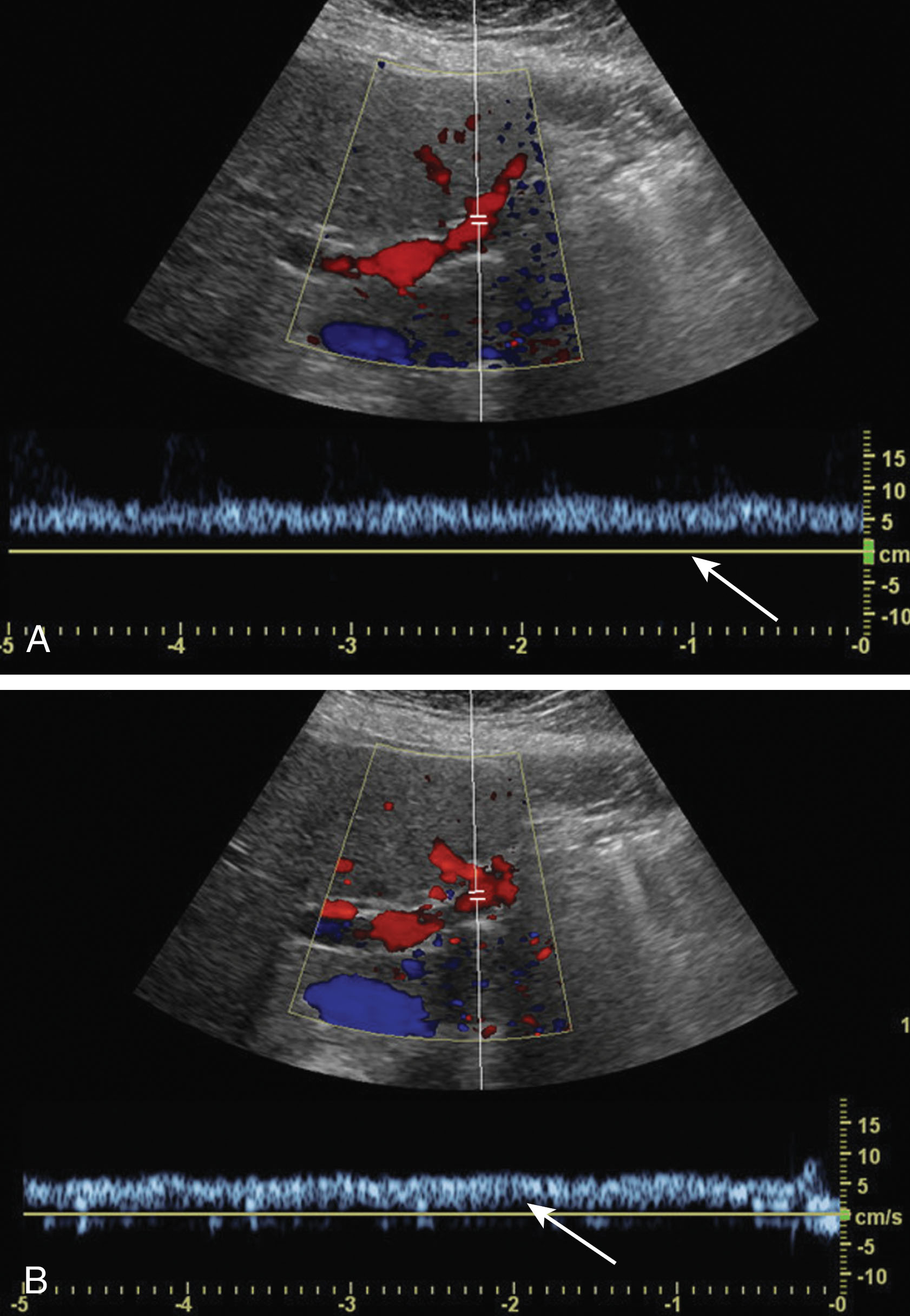
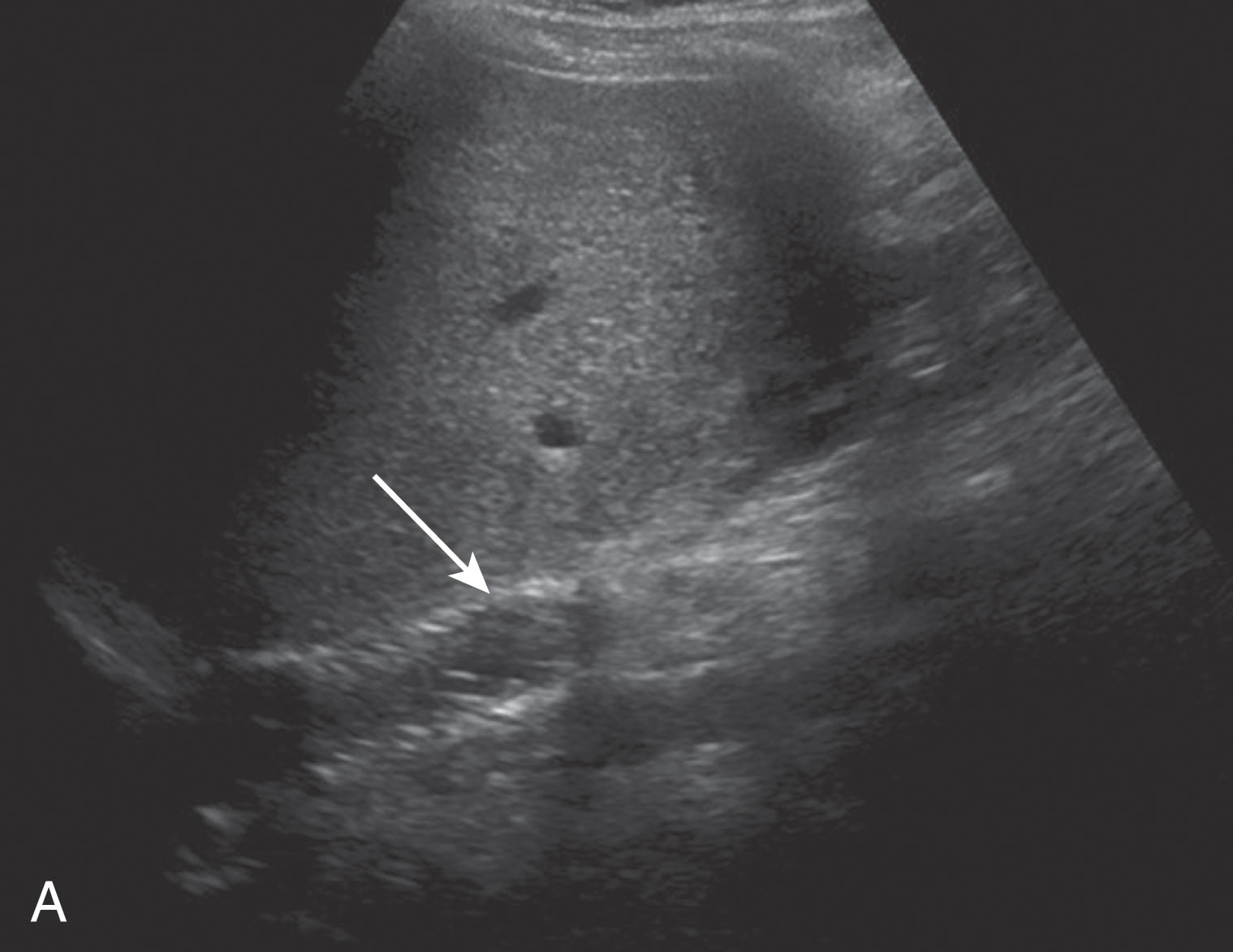
When very slow flow is present in a vessel, a Doppler signal can be difficult to obtain and either the Doppler scale will need to be decreased or the color gain will need to be increased. Some ultrasound machines may have special settings to detect slow flow. These can often be found in other settings besides the abdominal settings such as those for detecting low flow in a lower extremity vein or the iliac vein. If these are not available, the renal artery setting can be helpful filling in the vessels as well. Sometimes the wall filter needs to be decreased if the very slow frequencies are rejected as “cluttered noise” ( Fig. 20.4 ). For obese patients, decreasing the gray scale, color, and spectral Doppler frequencies will enable deeper penetration.
On gray-scale imaging, the liver parenchyma should appear smooth and homogeneous being isoechoic relative to the right renal cortex. All vessels and biliary ducts should be anechoic. The HVs and IVC lack a distinct wall whereas the PVs, HAs, and ducts demonstrate echogenic walls running parallel from the liver hilum and branching within the liver. A normal common hepatic duct (CHD) ranges in diameter from 3 to 7 mm, and the common bile duct (CBD) can range from 5 to 9 mm. See Chapter 9 for a more detailed explanation of normal liver anatomy.
The Doppler portion of the transplanted liver examination is crucial to perform. Some vascular complications can only be diagnosed using color and spectral Doppler. The HA will be red on color imaging and the spectral waveform demonstrates a rapid systolic upstroke with continuous diastolic flow above baseline demonstrating a low-resistance waveform. The velocities should be below 200 cm/sec. A normal RI in the HA is in the range of 0.50 to 0.70. The PV flow should be toward the liver showing flow above the baseline with a red color. The spectral waveform of the PV has continuous flow with minimal respiratory changes. The IVC and HVs should be primarily blue in color with a phasic bidirectional spectral Doppler waveform representing changes in the cardiac cycle. The LHV can appear more pulsatile because you are in closer proximity to the heart ( Fig. 20.5 ). When measuring velocities using spectral Doppler imaging, turbulence or any velocity doubling or tripling in the vessel from one location to the next should not be seen.
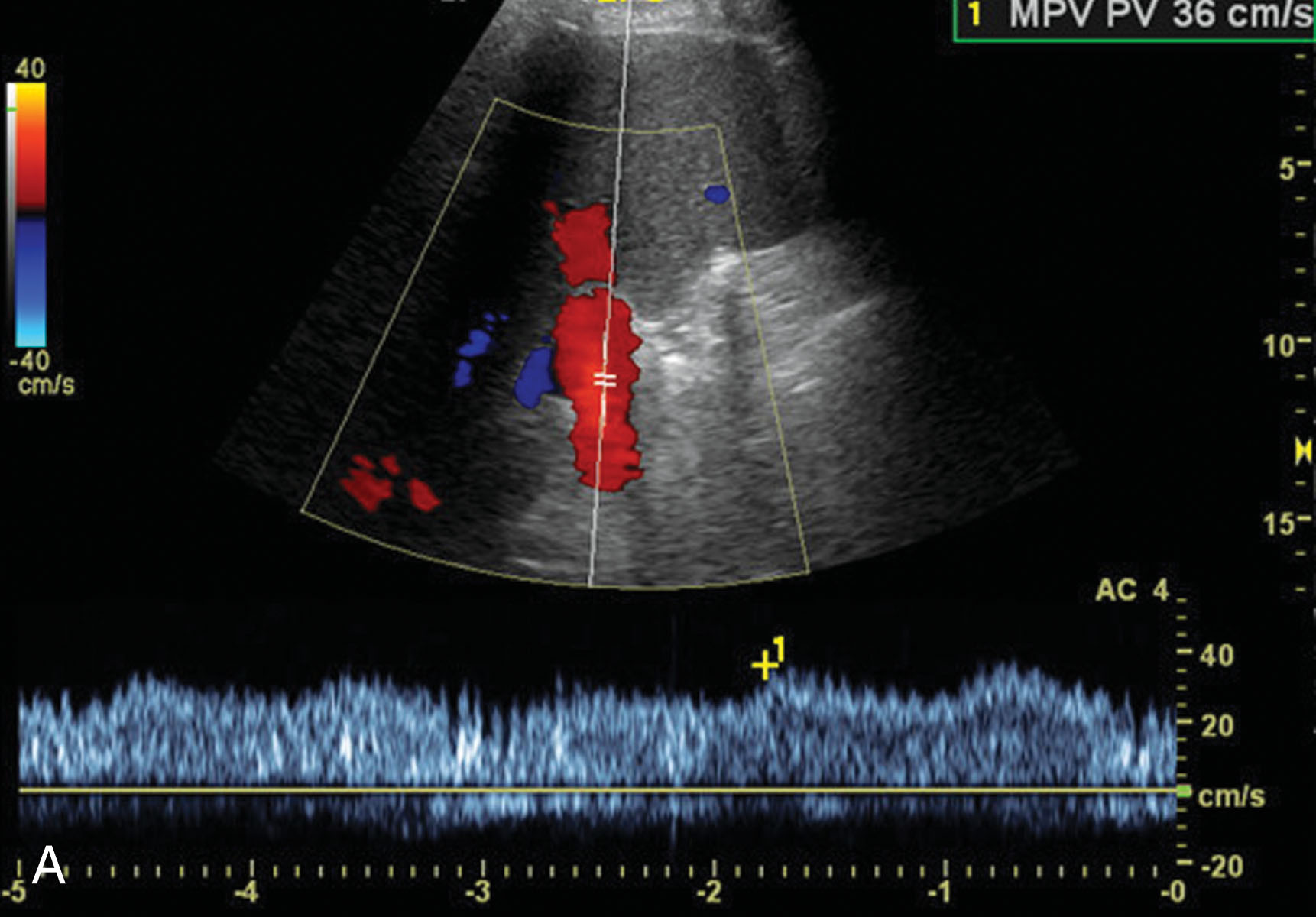
It is imperative to image the newly transplanted liver in the immediate postoperative period. Ultrasound is the initial modality of choice. There is no ionizing radiation, and the cost is relatively inexpensive compared with computed tomography (CT) or magnetic resonance imaging (MRI). A skilled sonographer provides early detection of transplant complications and prevents misdiagnosis.
On day 0 of ultrasound examination, the patient will be in the ICU and intubated. As a result, these patients will not be able to roll onto their side or help assist with their breathing. It is very helpful to image the patient without bandages on, if possible. This helps attain the best imaging windows possible and prevents missing pathology. Although this is not a sterile scanning environment, using sterile gel while the patient’s incisions are healing can potentially reduce the risk of infection. It is common and helpful to have a member of the transplant team or surgeon present for this examination. They want to ensure that the allograft has adequate arterial and venous flow and look for signs of hemorrhage. If there is an urgent complication noted, the surgeon can transfer the patient back to the operating room to correct the issue immediately. Not all complications are considered urgent, and a follow-up ultrasound will often be recommended within 24 hours to ensure stability or determine whether the issue has resolved or is getting worse. Urgent findings include, but are not limited to, HA thrombosis, PV, HV or IVC thrombus or occlusion, or active bleeding. It is very common to see postoperative fluid collections, and these usually resolve on their own. Fluid collections will be monitored on serial ultrasound examinations making sure they are not enlarging and causing liver function problems. Another common finding involves the vasculature. Postoperative edema can create elevated blood flow velocities within the HAs or venous system. In this situation, the HAs can have an elevated RI and may demonstrate little or no diastolic flow. It may be common to see reversal of flow within the LPV due to the high flow volume state. Once the edema decreases, the vessels should return to their normal state ( Fig. 20.6 ). Once the ultrasound examination has been completed, the incision should be redressed to help prevent infection. If no complications arise during recovery in the transplant unit, the liver transplant patient is typically discharged from the hospital around postoperative day 5.
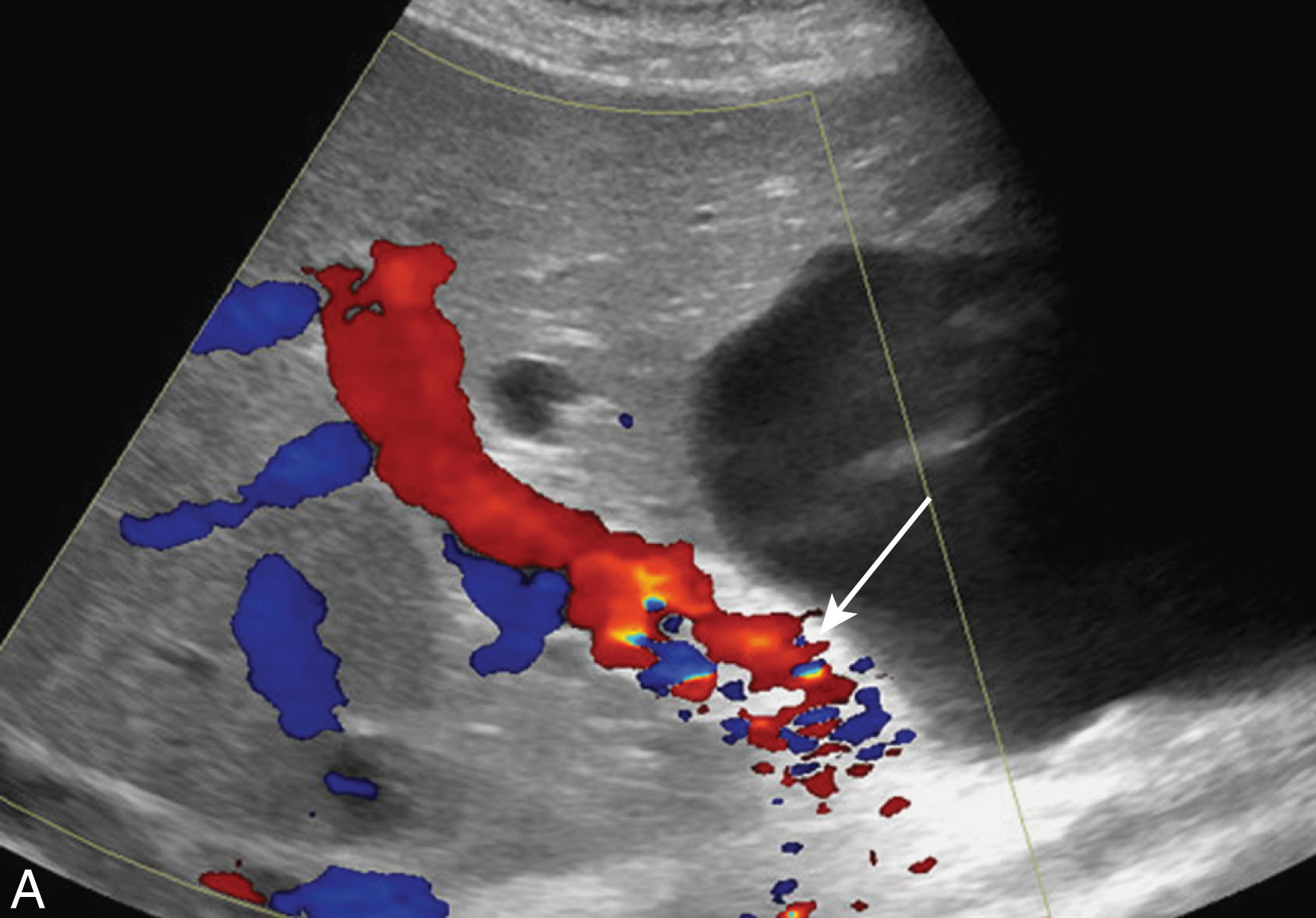
Once patients receive their liver transplant, they are closely monitored with routine follow-up examinations and procedures. Blood levels to monitor hepatic function and coagulation studies are checked frequently: daily for 1 week, then 3 times a week, then 2 times a week, then once weekly for 4 months, then monthly. Ultrasound examinations are routinely performed on days 0, 1, and 7. If the transplant team is concerned with complications, the patient will often have more frequent follow-up ultrasounds. Long term, the liver transplant patient typically is followed with a yearly ultrasound.
It is common to perform a routine parenchymal biopsy around day 7 after transplantation, and some patients may require a biopsy yearly to ensure no disease recurrence and rule out rejection. If the patient is symptomatic with pain, fever, or elevated liver function tests (LFTs), a biopsy may be ordered if the ultrasound does not help provide a diagnosis ( Fig. 20.7 ).
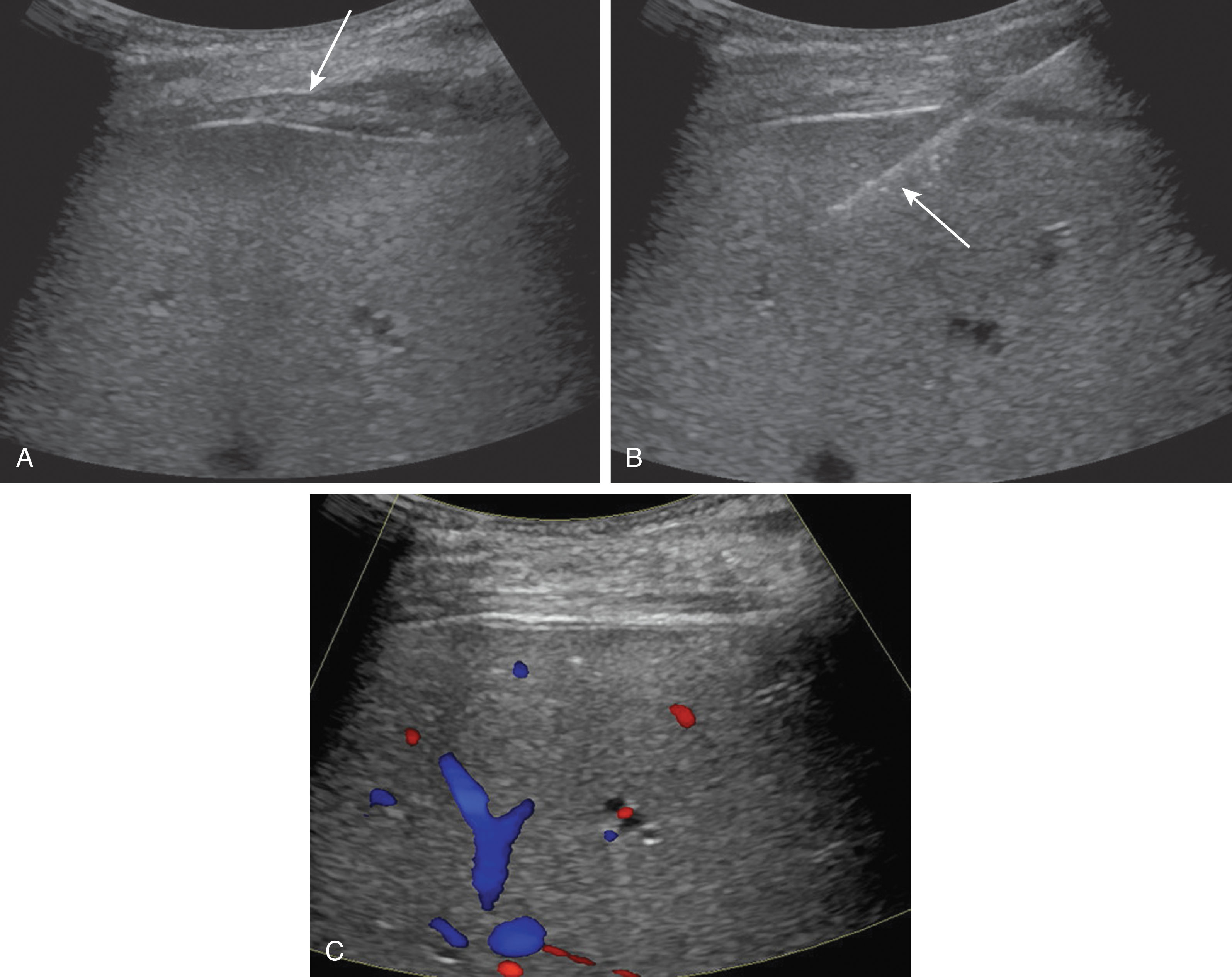
Complications may arise while performing these biopsies. The most common complication is bleeding. The patient’s INR and platelet counts are checked before the procedure. To reduce the risk of bleeding, patients are asked to stop taking anticoagulants 3 to 5 days before the procedure. Another risk of bleeding is elevated blood pressure or hypertension. Some patients may have known hypertension and be placed on an antihypertensive medication, and this should be taken as usual. Anxiety can significantly worsen hypertension as well in the acute setting. Blood pressures should not exceed 160/90 mm Hg. The radiologist or physician performing the biopsy may administer mild to moderate sedation to help calm the patient, reducing these pressures. Even after preventive measures, bleeding may still occur. Following the biopsy, the physician should image the site assessing for a bleed. Arterial flow along the biopsy tract outside of the liver parenchyma may be visualized on ultrasound. Color and spectral Doppler should be used to document the bleed. At times, a large collection of lidocaine may collect mimicking a subcapsular bleed. This area should be noted before the procedure so it is not confused with a postprocedural hematoma. The bleeding may be painful if it is associated with the liver capsule or painless. Most bleeds will stop on their own after applying moderate pressure over the biopsy tract. If the bleeding is severe, the patient may become vasovagal and blood pressure may drop. Fluids should be given, and the head should be lowered. If there is active bleeding that cannot be controlled, the patient may be transferred to the interventional radiology department for further imaging and possible embolization.
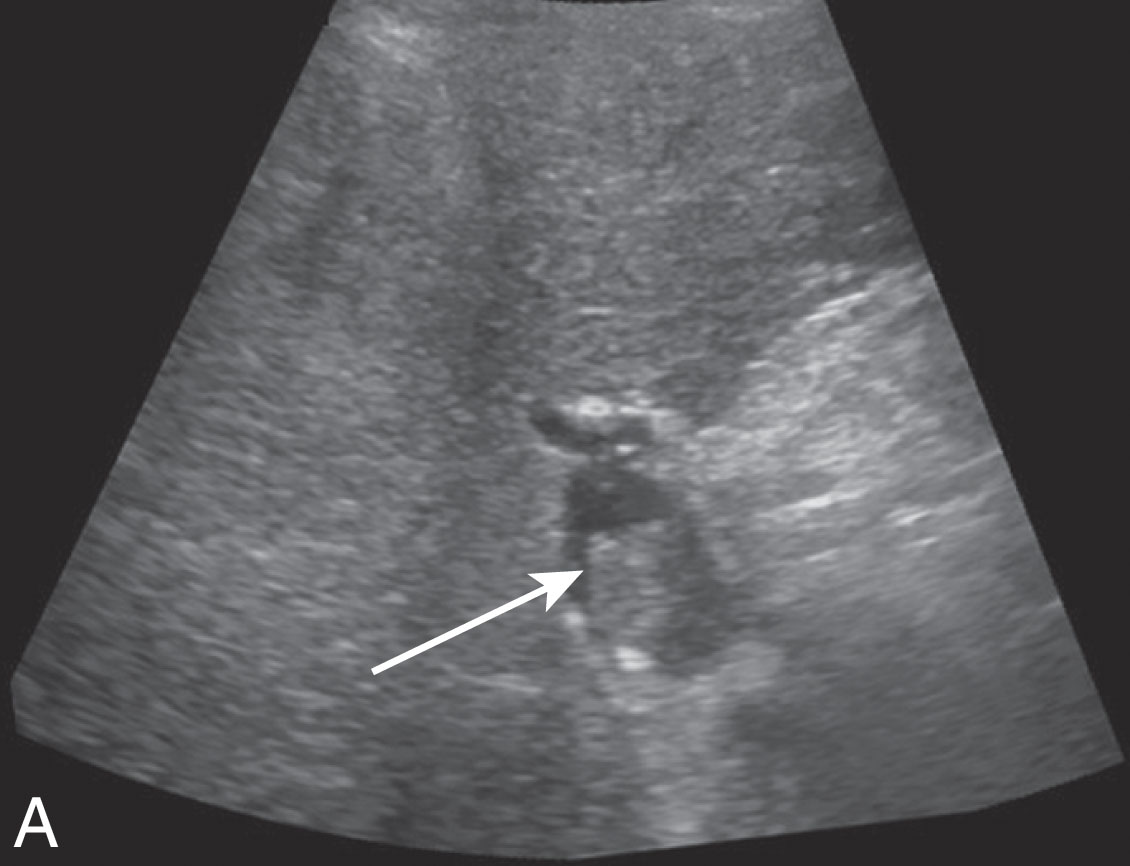
The biopsy needle is ultrasound guided to avoid major blood vessels and biliary ducts. At times, the needle path may cross small vessels for a potential risk creating an AVF or small intrahepatic ducts causing a bile leak forming a biloma ( Fig. 20.8 ). Another rare complication after biopsy is creating a hepatic artery pseudoaneurysm. You can visualize the to-and-fro waveform on spectral Doppler imaging. These findings are usually clinically insignificant and will likely just be followed on serial ultrasound examinations. The radiologist can reduce these risks by avoiding the liver hilum.
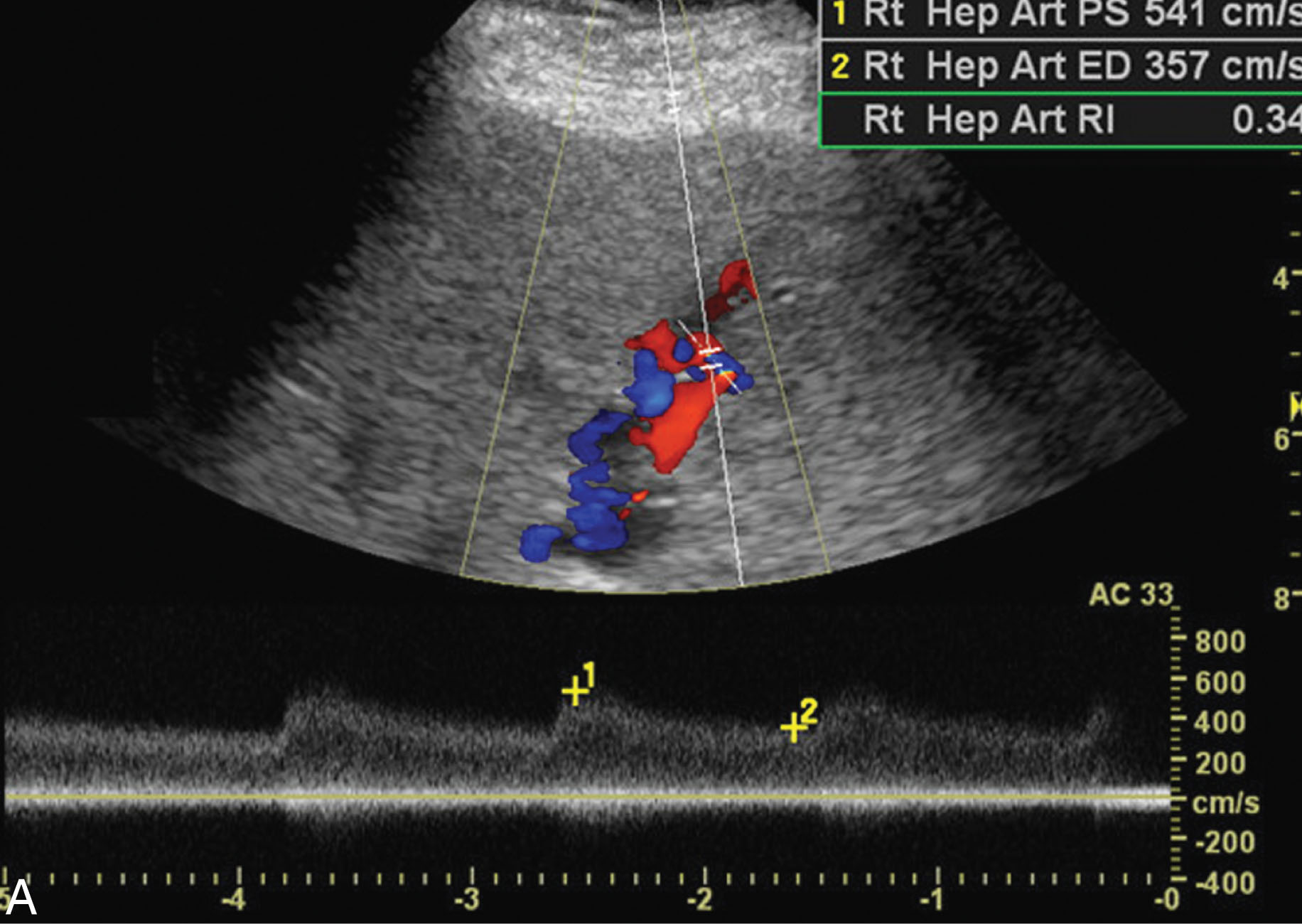
The most common cause for liver transplant failure is rejection. Acute rejection occurs within the first 10 days of transplantation. Some signs and symptoms include right upper quadrant pain, fever, tachycardia, hepatomegaly, and ascites. Liver function tests may be elevated and the patient may develop encephalopathy. Chronic rejection evolves over an extended period of time slowly deteriorating the liver graft, causing fibrosis. The ultrasound findings of rejection are often nonspecific and can include an elevated RI or periportal edema, most often requiring a liver biopsy for diagnosis.
Intrahepatic abscesses are localized fluid collections of necrotic inflammatory tissue that contains purulent material and an infectious organism. Common signs and symptoms are fever, right upper quadrant pain, and jaundice. These abscesses are often seen secondary to a liver infarction. Patients who are on immunosuppressive medications who present with biliary stricture or arterial insufficiency are at increased risk for developing an infection. Infection is likely to spread to the chest and lungs in the immunocompromised patient. On ultrasound imaging, an abscess will typically have thick walls and be hypoechoic and complex in appearance with an air-fluid level with poorly defined borders. Gas bubbles may be present within the abscess and will appear as a “dirty” shadow. Abscesses can have varied appearances though and may have internal septations or some may even appear solid mimicking a mass. The treatment of choice is usually a percutaneous drainage catheter using CT or ultrasound guidance and antibiotics ( Fig. 20.9 ).
There is less than a 1% complication rate involving the IVC and HVs after transplantation. Thrombosis and stenosis most commonly occur at the anastomosis sites due to size discrepancy between the native and transplant vessels or suprahepatic caval kinking. Chronic IVC stenosis is more common in retransplanted or pediatric patients. The IVC can be narrowed from extrinsic compression from postoperative edema or a large hematoma creating a stenosis. Color Doppler aliasing and tripling of the spectral Doppler waveform velocities demonstrating turbulence may be visualized. Hepatic vein dilation with a dampened monophasic waveform may also be visualized. Angioplasty with stent placement is the treatment of choice ( Fig. 20.10 ). Stenosis of the HV mostly occurs in living donor transplants. A focal narrowing along with aliasing on color Doppler and a monophasic, turbulent waveform demonstrating elevated velocities on spectral Doppler imaging will be seen. IVC and HV thrombosis is caused from a hypercoagulable state and surgical factors. Intraluminal thrombus is identified filling the vessel with no color Doppler flow. There may be imaging features of Budd-Chiari syndrome ( Fig. 20.11 ). If the ultrasound examination is inconclusive, CT or MRI may be considered to confirm diagnosis. These patients are typically treated with anticoagulants.
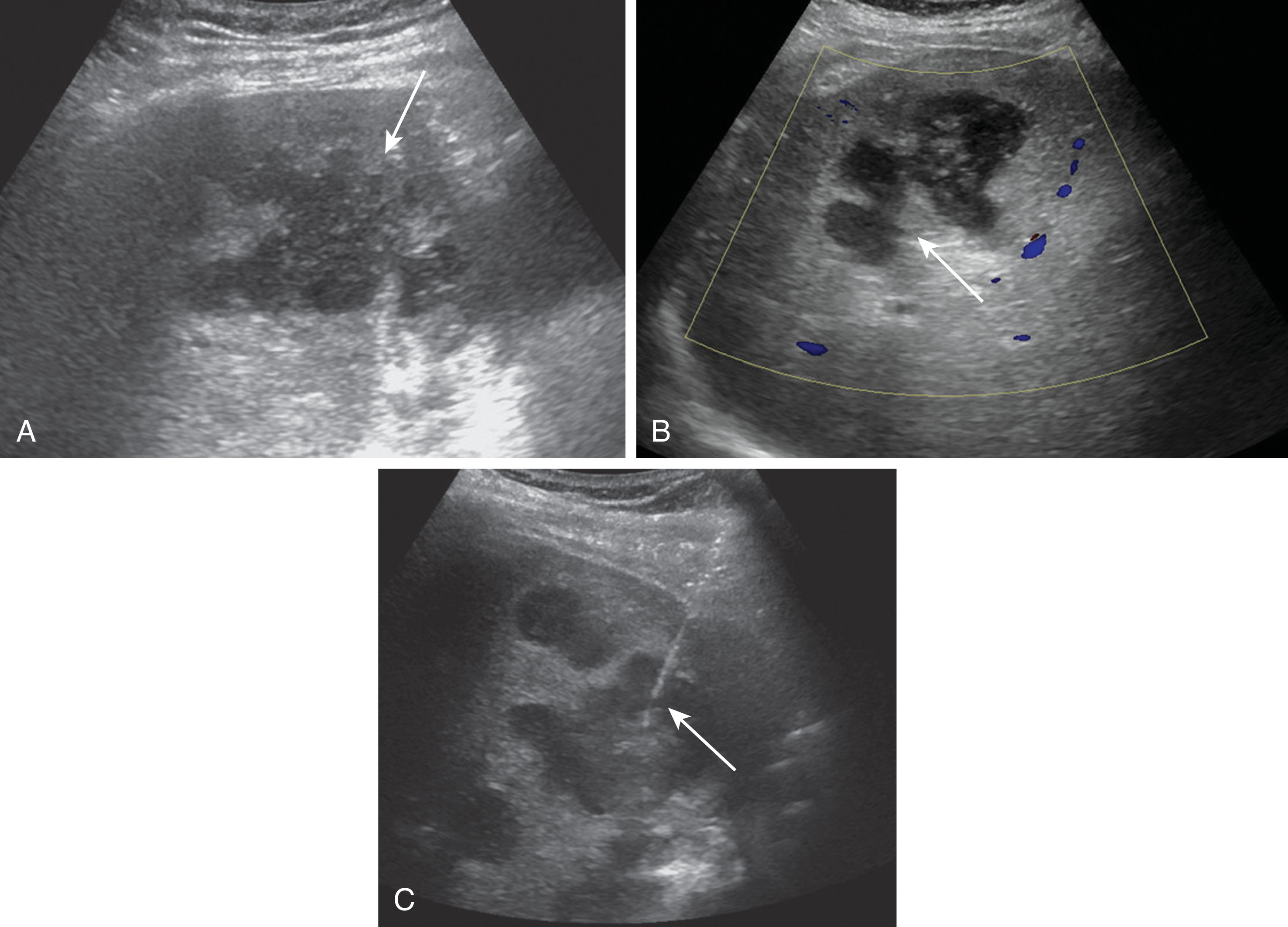
Portal vein stenosis occurs in about 1% of patients after transplantation. Stenosis is usually found at the anastomosis site and may occur when there is a size discrepancy of the vessels. On ultrasound imaging, the PV will demonstrate color and spectral Doppler aliasing, typically with a 3:1 ratio. Patients may undergo balloon angioplasty, stent placement, or resection. Thrombosis of the PV occurs in about 3% of liver transplants. This commonly results from mismatched caliber differences in the vessels, stretching of the PV near the anastomotic site, slow portal inflow, or hypercoagulable states. Fresh thrombus appears echogenic and can be either occlusive or nonocclusive. Occlusive thrombus will demonstrate no flow with color or spectral Doppler ( Fig. 20.12 ). With nonocclusive thrombus, some flow will be visualized around the thrombus. As a thrombus ages, it typically becomes more anechoic. In the symptomatic patient, thrombectomy, thrombolysis, stent placement, or a venous graft may be necessary ( Fig. 20.13 ). With chronic PV thrombosis, cavernous transformation can occur with the hepatic artery.
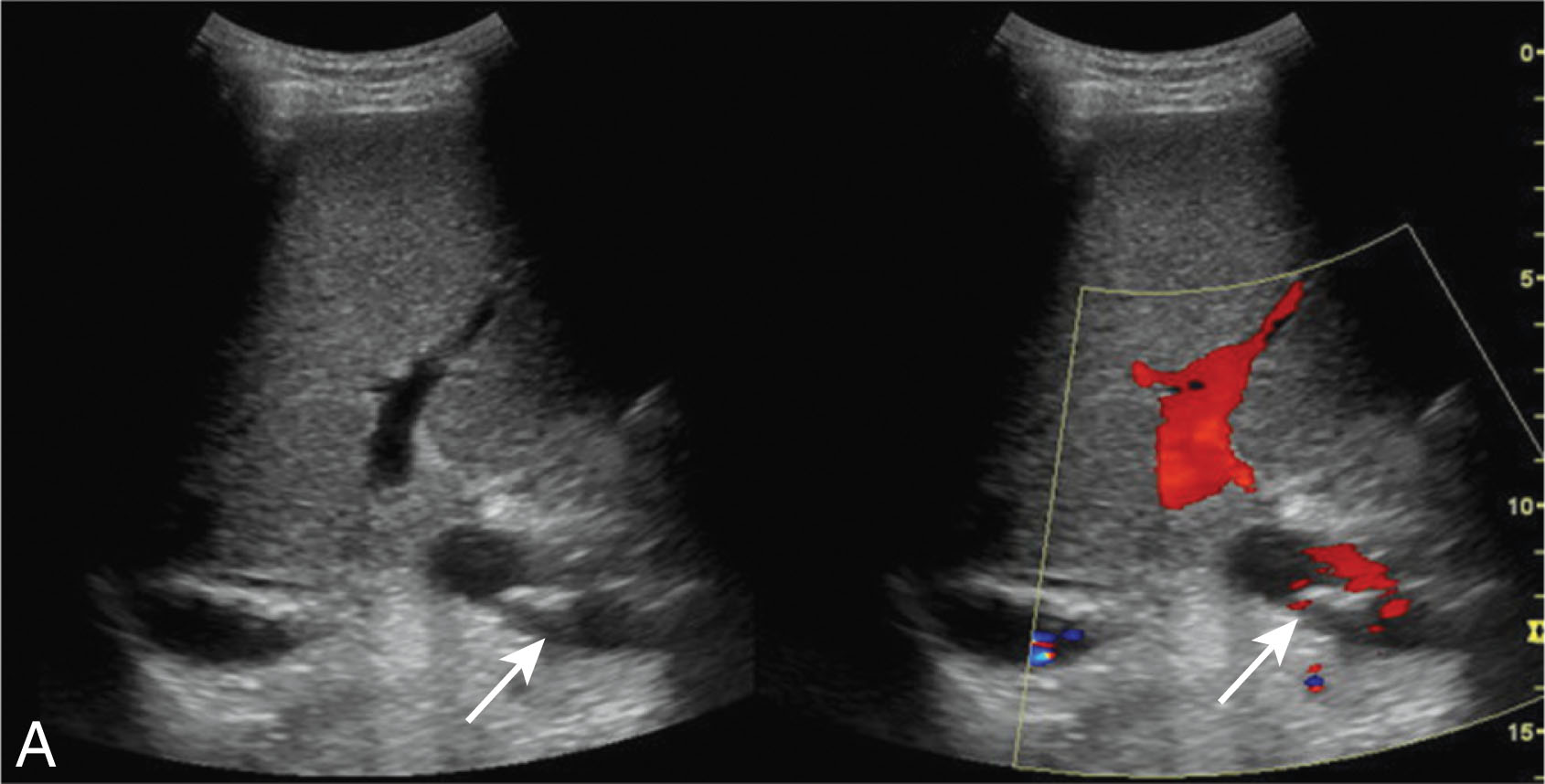
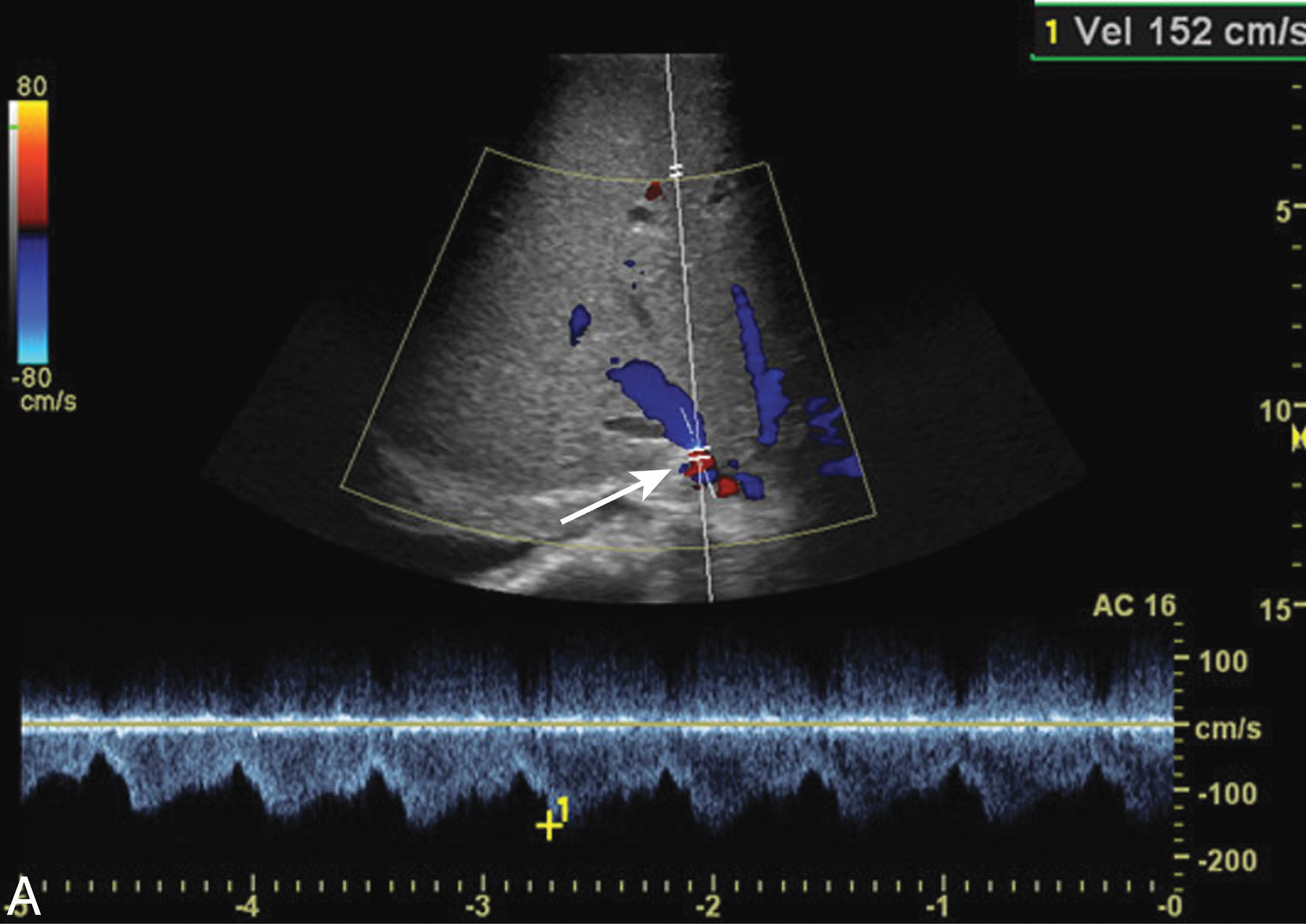
Hepatic artery stenosis occurs in 2% to 11% of liver transplant patients with a median time to diagnosis of 100 days. Risk factors include rejection, poor surgical technique, or clamp injury. The sonographer will notice color and spectral Doppler aliasing with turbulent flow. Pulsed-wave Doppler will demonstrate a low RI (less than 0.50) with a velocity greater than 200 cm/sec and a tardus parvus waveform. In the early postoperative period, less than 72 hours of transplantation, the RI can be elevated greater than 0.80, but usually will return to normal within a few days. The most common cause for this is postoperative edema. The increased hepatic artery resistance can be associated with an older donor age and an extended period of ischemic time. It is common to get an elevated false velocity measurement when the hepatic artery is tortuous. If the ultrasound examination is inconclusive, a CT angiography (CTA) or MR angiography (MRA) should be considered. Treatment may include balloon angioplasty with stent placement or surgical revision ( Fig. 20.14 ). If the stenosis is left untreated, it may lead to HA thrombosis, liver ischemia, biliary stricture, sepsis, and allograft loss. Early detection is critical to avoid the need for retransplantation.
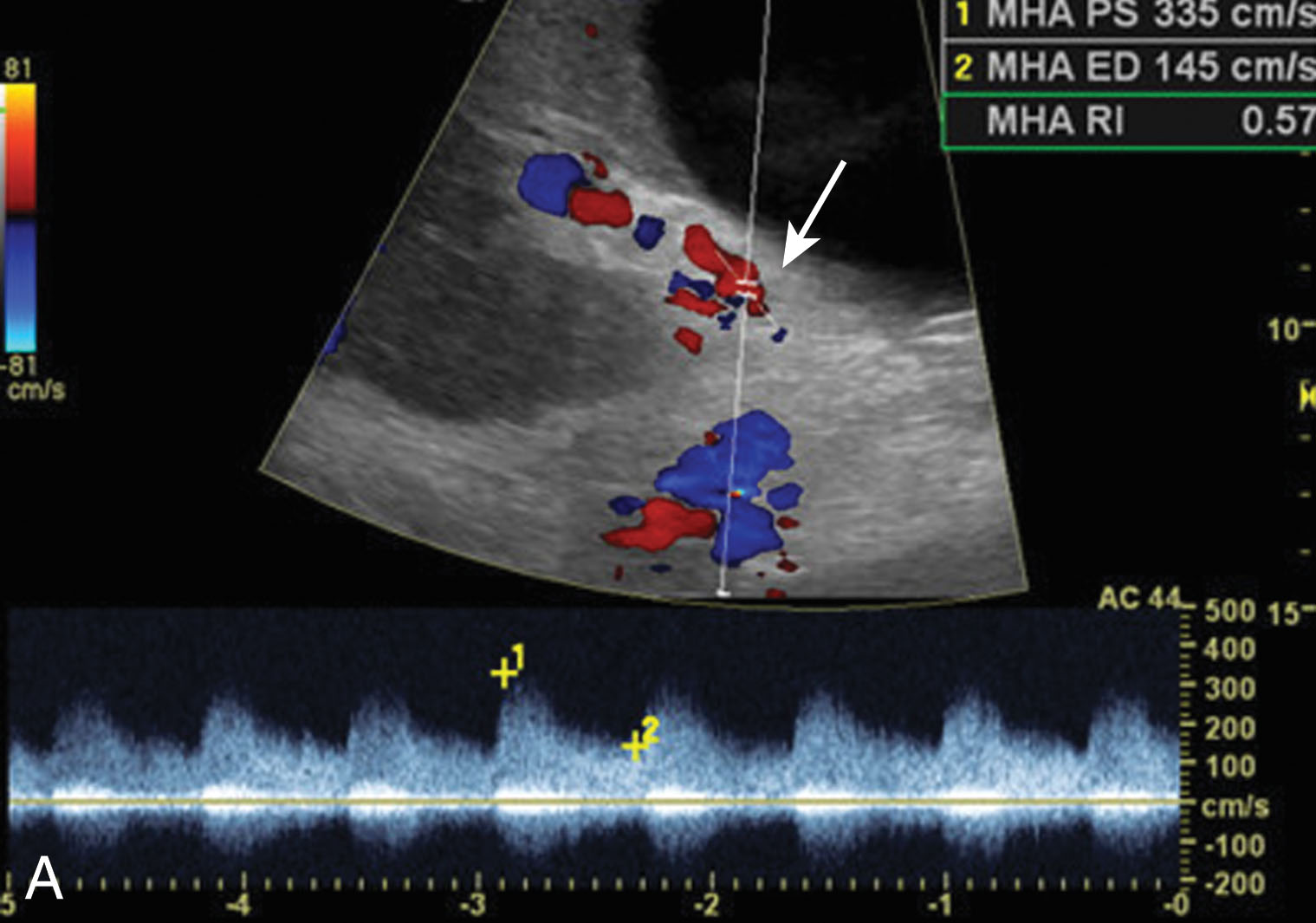
The most common vascular complication of liver transplantation is HA thrombosis. Thrombosis occurs in 4% to 12% of adults and 42% of children between postoperative days 15 and 132. Risk factors include rejection, short warm ischemic time, end-to-end anastomosis, and pediatric transplantation. HA thrombosis and stenosis can lead to biliary ischemia because the HA is the only vascular supply to the biliary ducts. There will be absence of color and no flow on spectral Doppler imaging. On gray scale, echogenic thrombus filling the HA lumen may be visualized. When there is slow flow present due to low cardiac output or arterial spasm, ultrasound may not detect flow leading to a false-positive diagnosis when the hepatic artery is patent. If the ultrasound is inconclusive, a CTA may ultimately be required. CT may demonstrate areas of infarction as well. Treatment includes an emergent thrombectomy, but many cases ultimately require retransplantation ( Fig. 20.15 ).
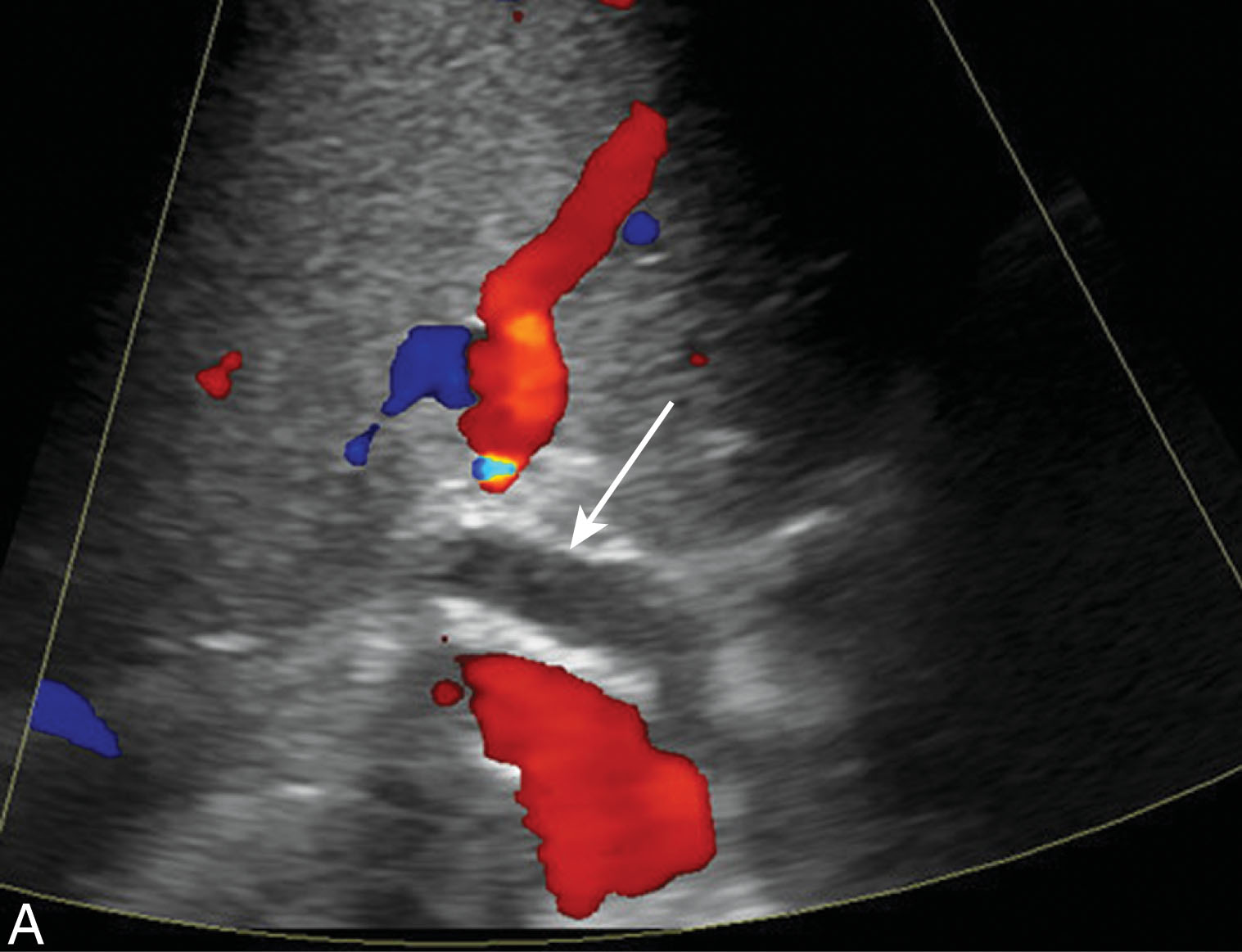
In a native liver, it is rare for an infarction to take place. If the HA were to occlude, collateral vessels would take over to give collateral flow to the liver. Within a transplant, development of collateral vessels is not usually possible as most of the collateral vessels are ligated during the surgical process. In 85% of cases, hepatic infarction is associated with HA complications with portal vein occlusion being less likely. Ischemic areas within the liver can liquefy over time and may become infected and calcifications may be present. Infarction usually appears hypoechoic and wedge shaped located along the periphery. Infarcts can be difficult to distinguish from focal abscesses and an aspiration or biopsy may be needed for confirmation ( Fig. 20.16 ).
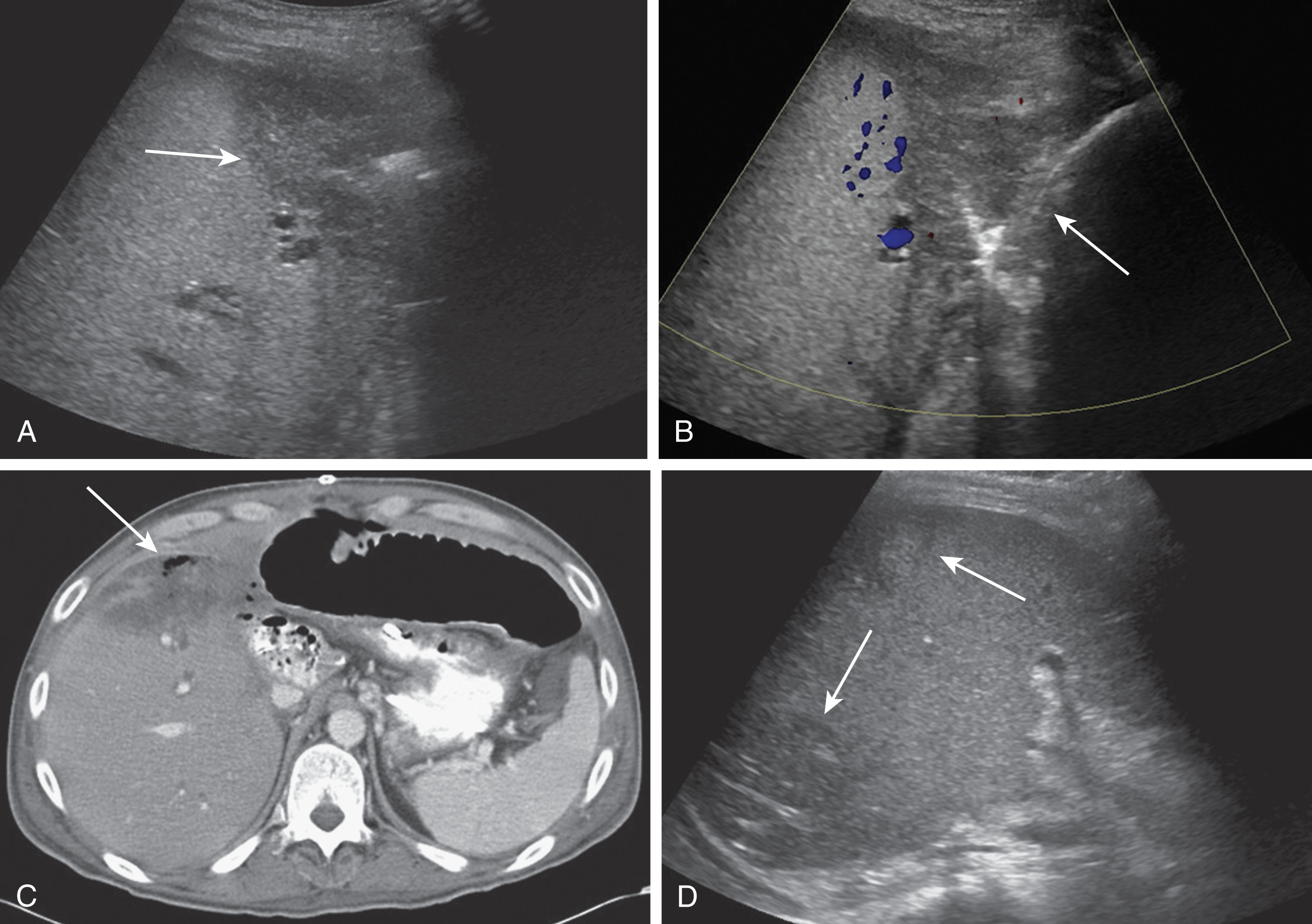
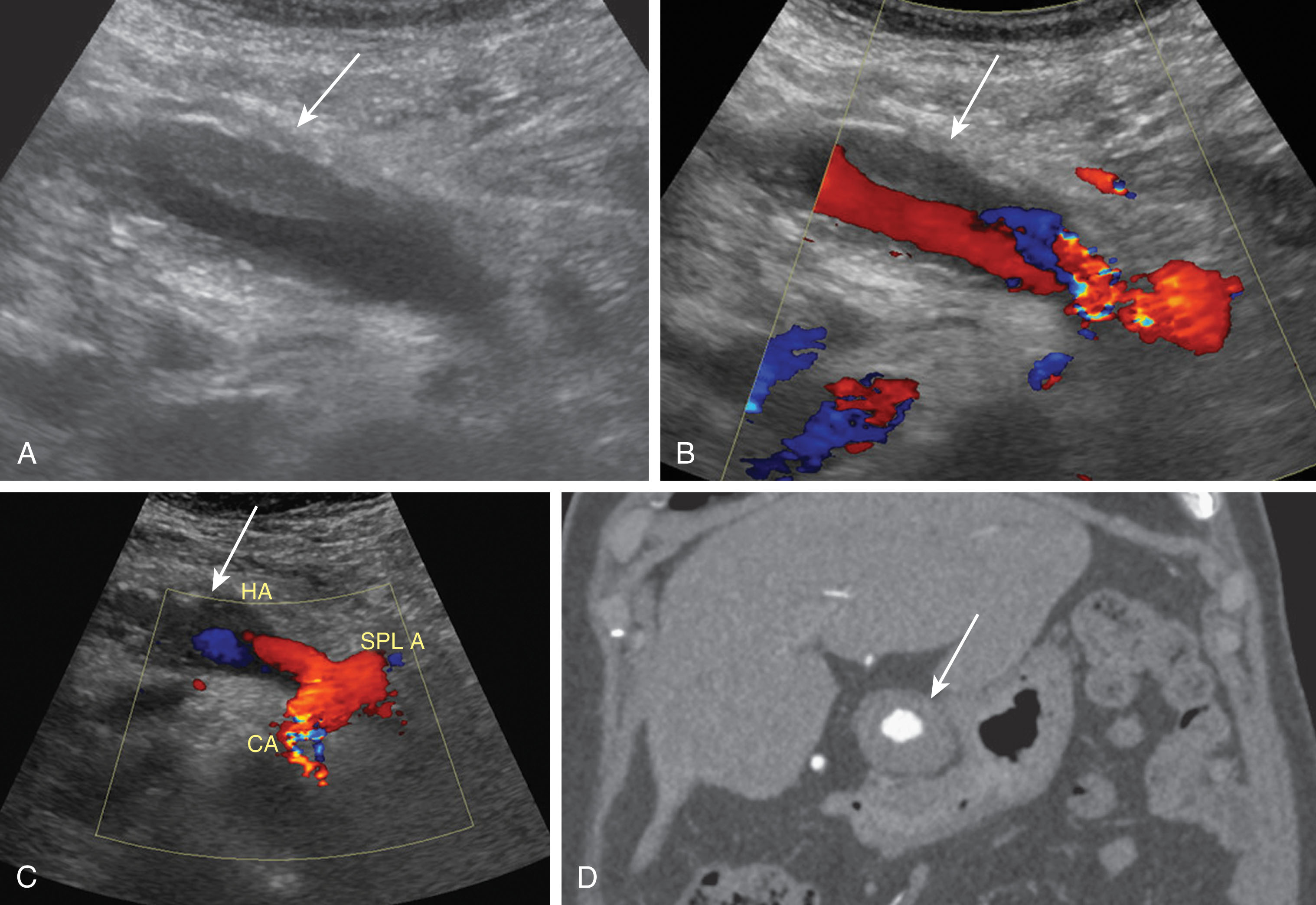
Hepatic artery pseudoaneurysms are relatively uncommon and usually located at the anastomosis site or occur due to angioplasty complications. These may also be intrahepatic secondary to a liver biopsy, biliary procedures, or infection. Patients are most often asymptomatic. If the pseudoaneurysm ruptures, however, the patient may go into acute shock and a portal or biliary fistula can develop. Treatments for extrahepatic pseudoaneurysms include resection, coil embolization, or stent placement. The treatment option of choice for an intrahepatic pseudoaneurysm is coil embolization. On ultrasound imaging, a pseudoaneurysm appears as an anechoic structure often containing nonocclusive thrombus along the walls, and located along the course of the HA. On Doppler imaging, it fills with color and there is a disorganized, bidirectional flow pattern. A characteristic “yin-yang” pattern of flow on Doppler imaging has been described. Pseudoaneurysms can be visualized using CTA or MRA. These appear as round masses at the anastomotic site and enhance avidly during the arterial phase. If slow flow is present, enhancement may only be noted during the portal or delayed venous phases ( Fig. 20.17 ).
In about 5% to 15% of liver transplant patients, biliary complications occur. These typically occur within the first 3 months after transplantation. Complications include biliary ductal obstruction, stenosis or stricture at the anastomosis, stone formation, biliary necrosis, sphincter of Oddi dysfunction, and recurrent biliary disease. Bile leaks and bilomas are also complications that are discussed later in this chapter. Biliary complications taken as a whole are the second most common cause of allograft dysfunction following rejection.
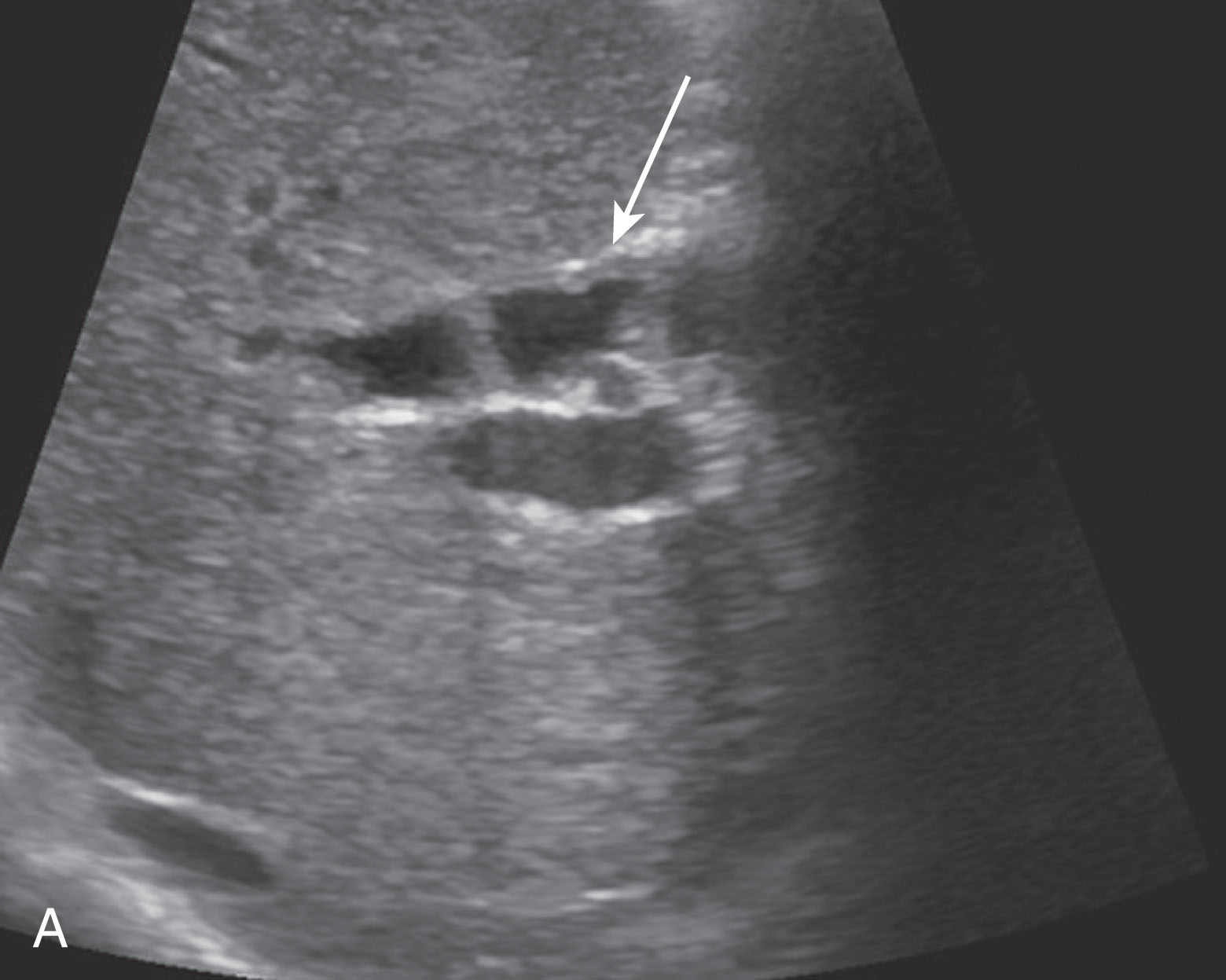
Obstruction is the most common biliary complication usually caused from a stricture at the anastomosis but may also be secondary to choledocholithiasis. The donor CBD will demonstrate postobstructive dilation in patients with choledocholithiasis. It is important to note that some patients may present with nonobstructive ductal dilation secondary to papillary dyskinesia and may be clinically insignificant. Imaging findings should be correlated with clinical findings and symptoms. Ultrasound is less reliable when mild ductal obstruction is present. As a result, MRI cholangiopancreatography is useful in detecting biliary obstruction. Most strictures are extrahepatic and near the anastomosis caused from fibrotic tissue scarring. These are usually treated with angioplasty with or without stent placement. As opposed to extrahepatic strictures, intrahepatic strictures are due to ischemia or cholangitis and treatment includes percutaneous biliary drain placement ( Fig. 20.18 ). Stones, sludge, and debris can be found in 5.7% of liver transplants. Altering the bile composition could be a predisposing factor for stone and sludge formation. Choledocholithiasis is usually treated with an endoscopic sphincterotomy and stone retrieval ( Figs. 20.19 and 20.20 ). Biliary ductal ischemia is secondary to HA thrombosis or stenosis as the ducts are dependent on the HA for their only blood supply. Biliary necrosis occurs and biliary stenosis, bile leaks, and bilomas may follow. If angioplasty of the stenotic duct is not successful, retransplantation is often necessary.
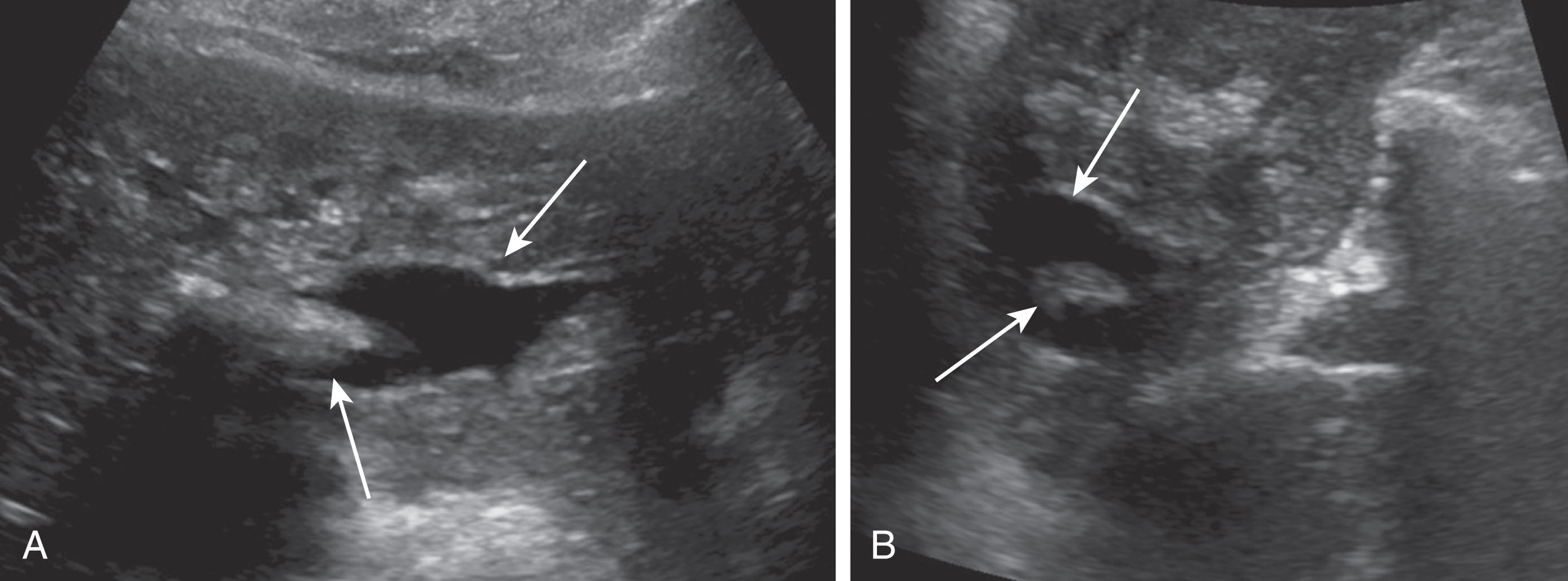
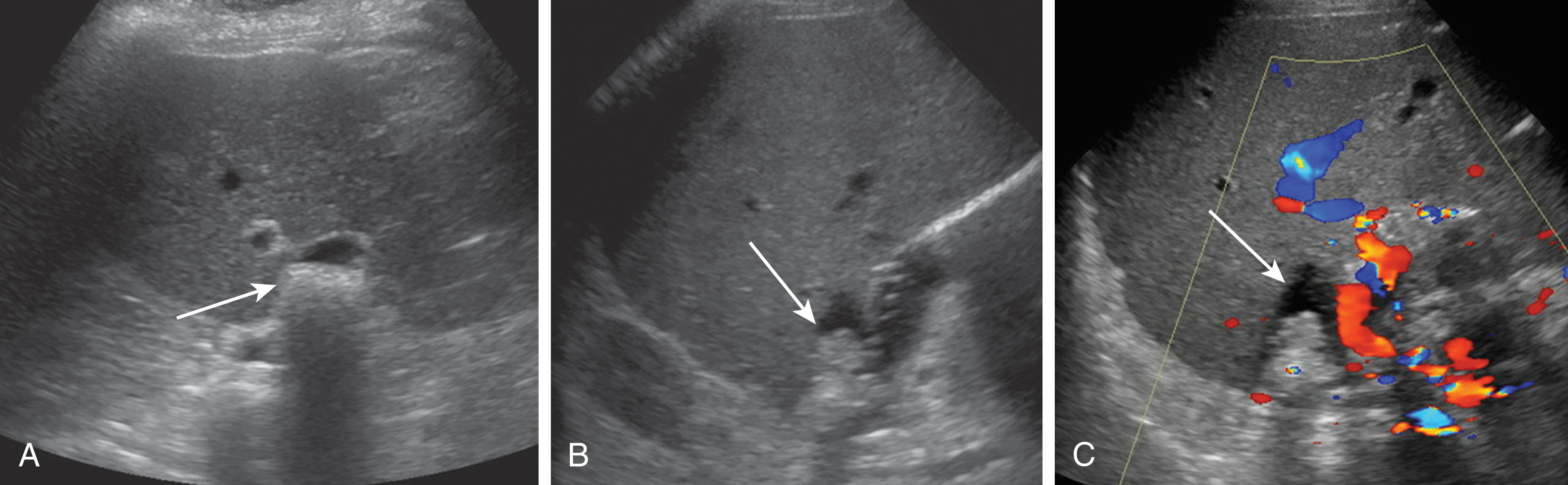
Hematomas are collections of blood and usually occur within the first 2 weeks postoperatively, and most are present and can be visualized on day 0 ultrasound examination. Hematomas are most common along the perihepatic spaces and near the vascular and biliary anastomoses sites. Most hematomas will resolve spontaneously with no intervention needed. At rare times, there may be an underlying infection within a hematoma and a percutaneous drainage catheter may need to be placed. Hematomas can have variable appearances as they age. They typically have irregular walls, are ovoid in shape, and decrease in size over time. Fresh hematomas have more internal echoes and can appear echogenic. As they age, they liquefy and become more anechoic and may contain septations ( Fig. 20.21 ).
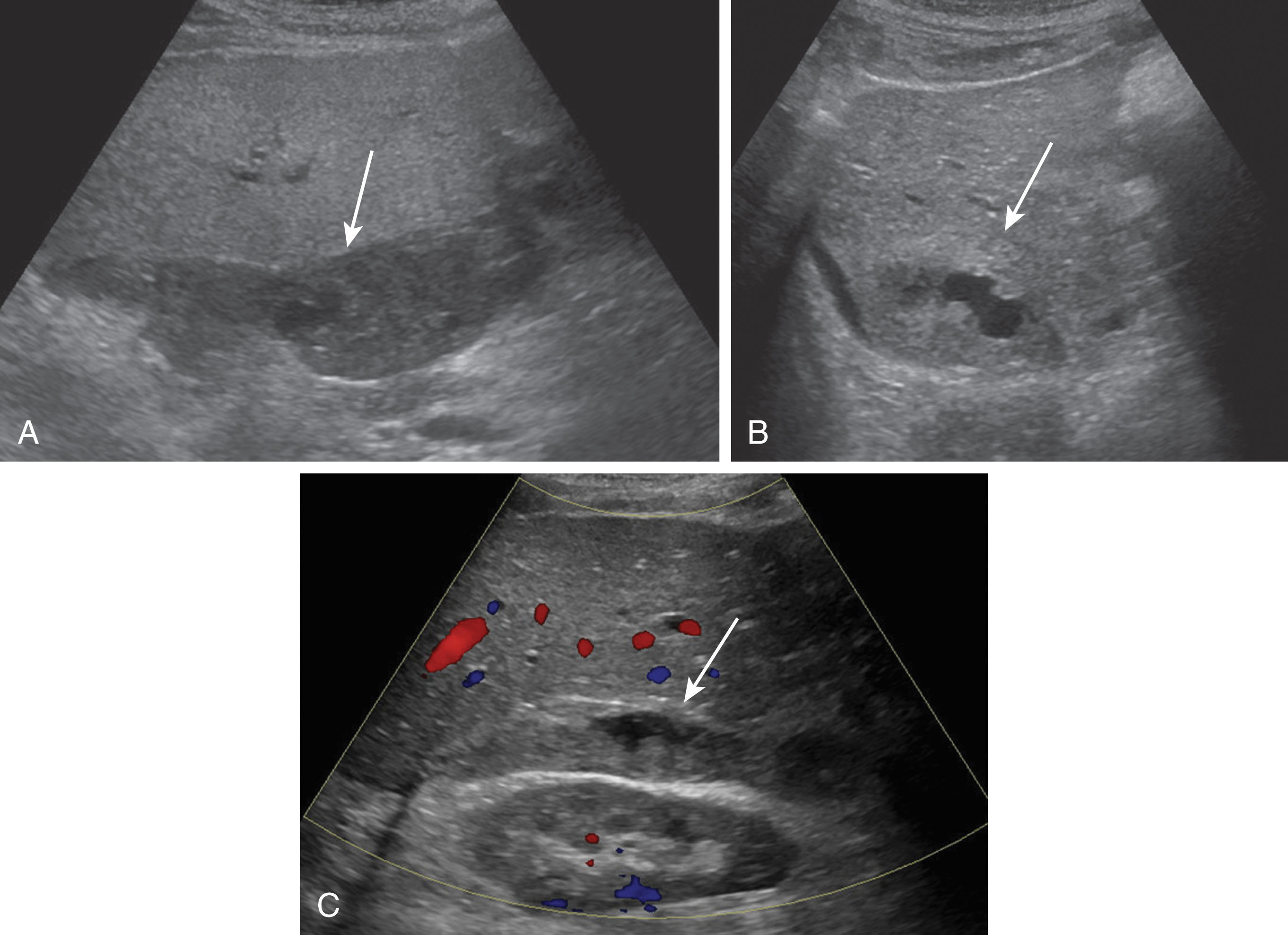
Seromas are clear, serous fluid collections that are usually found within the first few days after transplantation. They are most common along the perihepatic spaces and near the vascular and biliary anastomoses sites. Most seromas will resolve spontaneously within a few weeks. On ultrasound, a seroma is a round, localized, anechoic, thin-walled fluid collection, which may contain debris or septations. Ultrasound is very sensitive to diagnose a fluid collection but not specific of contents. Unfortunately, blood, pus, lymph, bile, and serosanguineous fluid can have similar appearances on ultrasound, and ultimately CT or MRI is helpful in differentiating the collections. If there is concern the fluid collection is infected or creating mass effect on the vasculature, an aspiration may be performed ( Fig. 20.22 ).
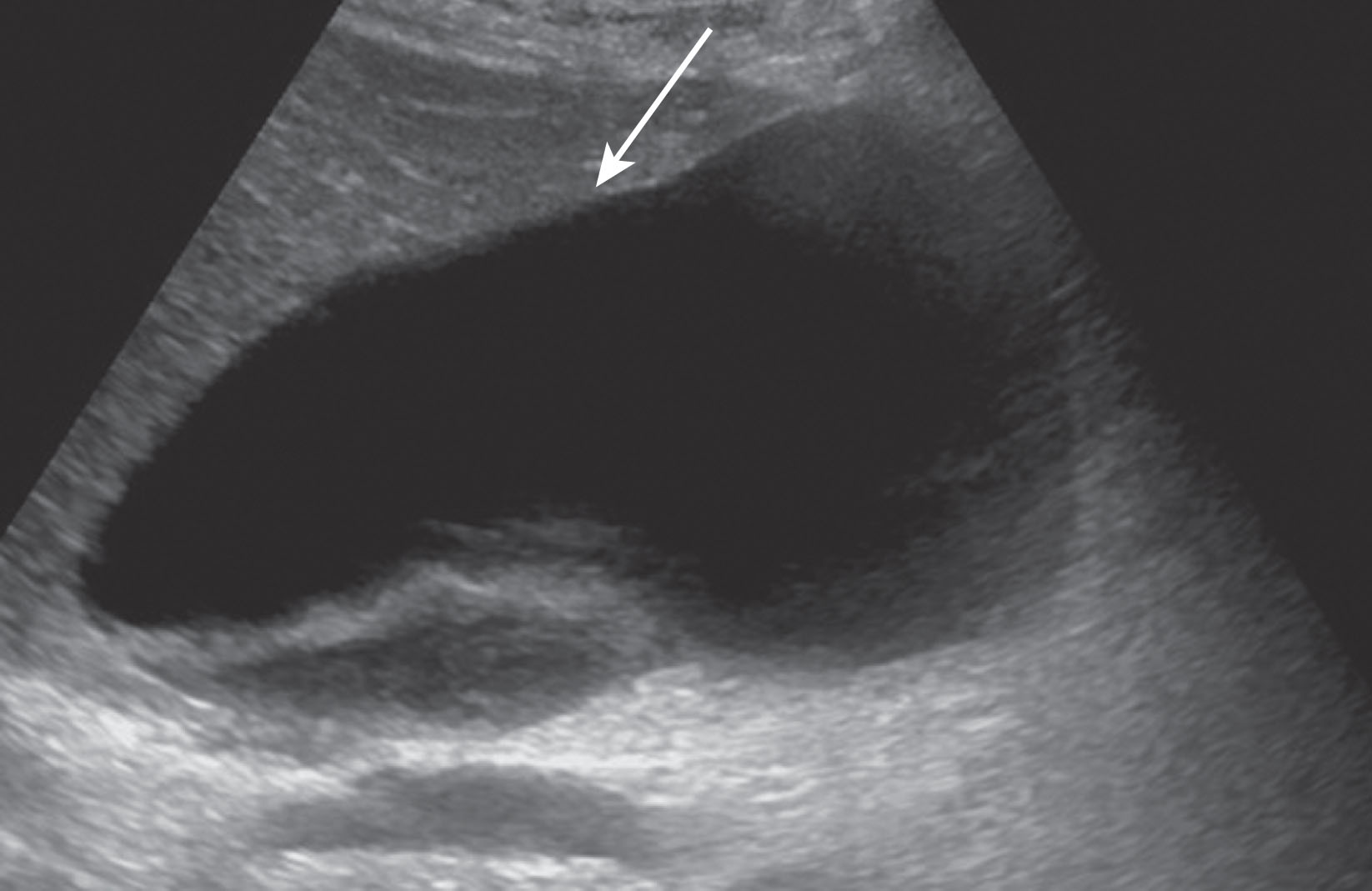
A surgical disruption of lymphatic channels causes lymph fluid leakage into the soft tissue space creating a lymphocele. These are typically found in the groin or retroperitoneal space. On ultrasound imaging, debris or septations within the fluid collection may be seen. Ultrasound is very sensitive to diagnose a fluid collection, although the contents are difficult to distinguish. CT or MRI is very helpful in differentiating among lymph, blood, bile, pus, or serosanguineous fluid. The most common complication of venovenous bypass during liver transplantation is a lymphocele, which can be seen between 10% and 30%. Spontaneous resolution is rare. Treatment includes percutaneous drain placement used in conjunction with sclerotherapy. Surgical repair is the optimal choice of treatment. Methylene blue dye injected through the lymphatic system makes it possible to identify the leak source during an open repair. This complication fortunately is now only rarely seen, as the venovenous bypass is no longer routinely used for liver transplantation surgery.
Bile leaks occur in about 5% of liver transplant patients. These typically present in the early postoperative period with more than 70% within the first month of transplantation. Leaks are most often located at the biliary tube site with rare occurrences at the anastomotic site. Bile seeps into the peritoneal cavity and may form a contained perihepatic collection, or biloma. Treatment includes stent placement over the biliary leak and possibly placement of a percutaneous drainage catheter. On ultrasound imaging, a biloma is a discrete, round, hypoechoic or anechoic fluid collection demonstrating no vascular flow. Ultrasound may have a difficult time differentiating biliary leaks from other fluid collections such as hematomas, abscesses, seromas, or ascites. Cholangiography and cholescintigraphy are sensitive and specific modalities that are helpful in biliary leakage evaluation ( Fig. 20.23 ).
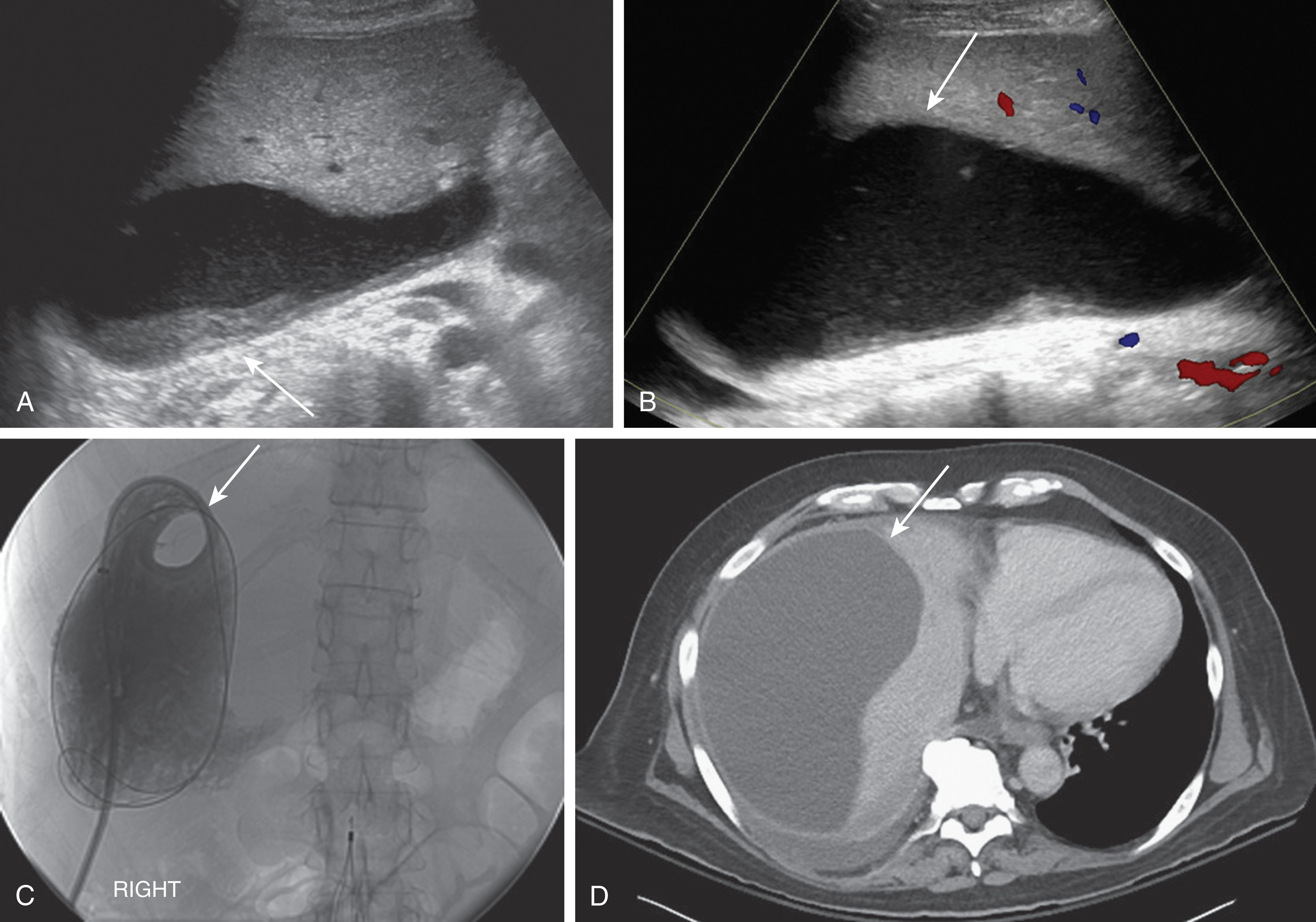
Ascites is usually present in small amounts in the early postoperative period. It may contain debris or blood products. The ascites commonly resolves in 7 to 10 days following transplantation. If the patient is having pain and remains uncomfortable with a large amount of free fluid, a therapeutic paracentesis can be performed ( Fig. 20.24 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here