Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Adenocarcinoma: inactivation of multiple antioncogenes, as well as issues with deoxyribonucleic acid mismatch repair ( BRCA2 ). Smoking, diet high in meat and solvent exposure are risk factors.
Neuroendocrine tumors: multiple chromosomal losses. Associated with Von-Hippel Lindau syndrome and multiple endocrine neoplasia.
Pancreatic lymphoma: usually non-Hodgkin lymphoma.
Acinar cell carcinoma: mutations in adenomatous polyposis coli beta-catenin gene and loss of chromosome 11.
Detects and characterizes the lesion based on the enhancement pattern on dynamic imaging.
Pancreatic adenocarcinomas are hypodense on pancreatic phase whereas neuroendocrine tumors are hyperdense.
Venous phase imaging allows for evaluation of vascular invasion and metastases, including regional lymph nodes, hepatic and omental metastases.
Used as primary imaging tool for staging of pancreatic cancer at most institutions.
Problem solving tool.
Good for detection of small tumors and metastases.
Used as primary imaging tool for local staging at some institutions.
Magnetic resonance (MR) angiography can be used to assess vascular involvement.
MR cholangiopancreatography (MRCP) can be used to visualize the effect of the tumor on the biliary tree.
Secretin enhanced MRCP can improve assessment of ductal stenosis and help differentiate benign from malignant strictures.
Operator dependent with limitations based on bowel gas and patient body habitus.
Endoscopic ultrasound usually performed by gastroenterologists. Provides high resolution images of pancreas and allows biopsy (fine needle aspiration) of lesions.
Positron emission tomography (PET)/computed tomography (CT).
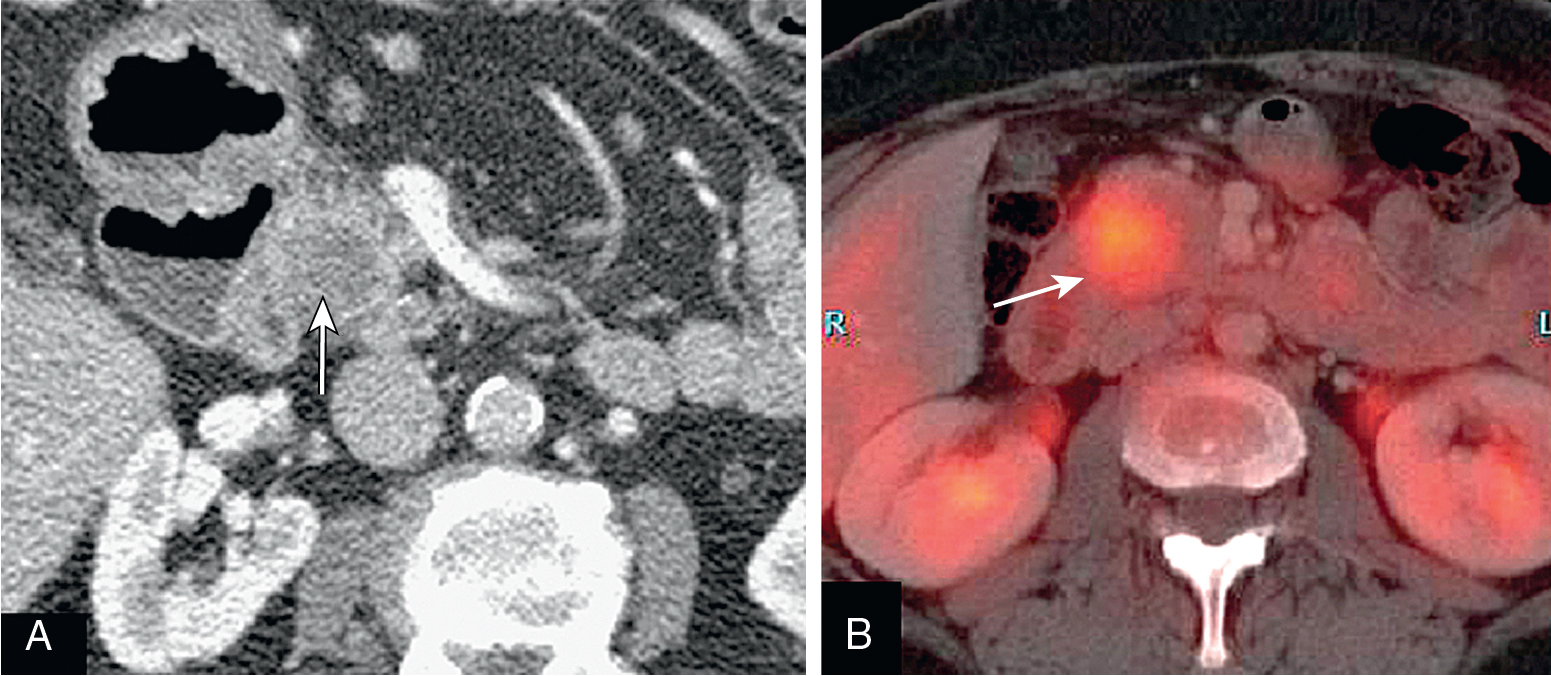
Normal pancreas should not have significant fluorodeoxyglucose (FDG) uptake.
Focal uptake is abnormal and could represent a primary malignancy.
90% of malignant pancreatic tumors.
Fifth leading cause of death in Western countries.
Five-year survival of 4%.
Males more than females between seventh and eighth decade.
Presentation: painless jaundice (75% of patients), new onset diabetes (10%), vague abdominal pain, weight loss.
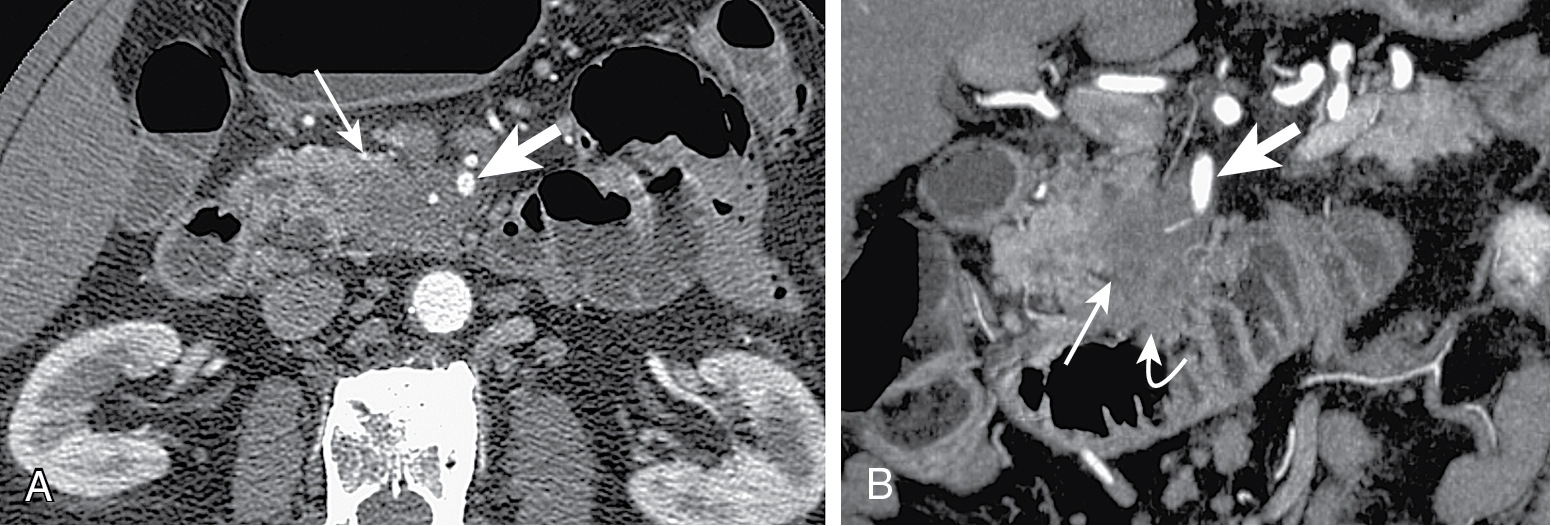
Tumors at head are more common (two-thirds) ( Figs. 13.1–13.3 ) and have better prognosis. Smaller at presentation with average size of 3 cm.
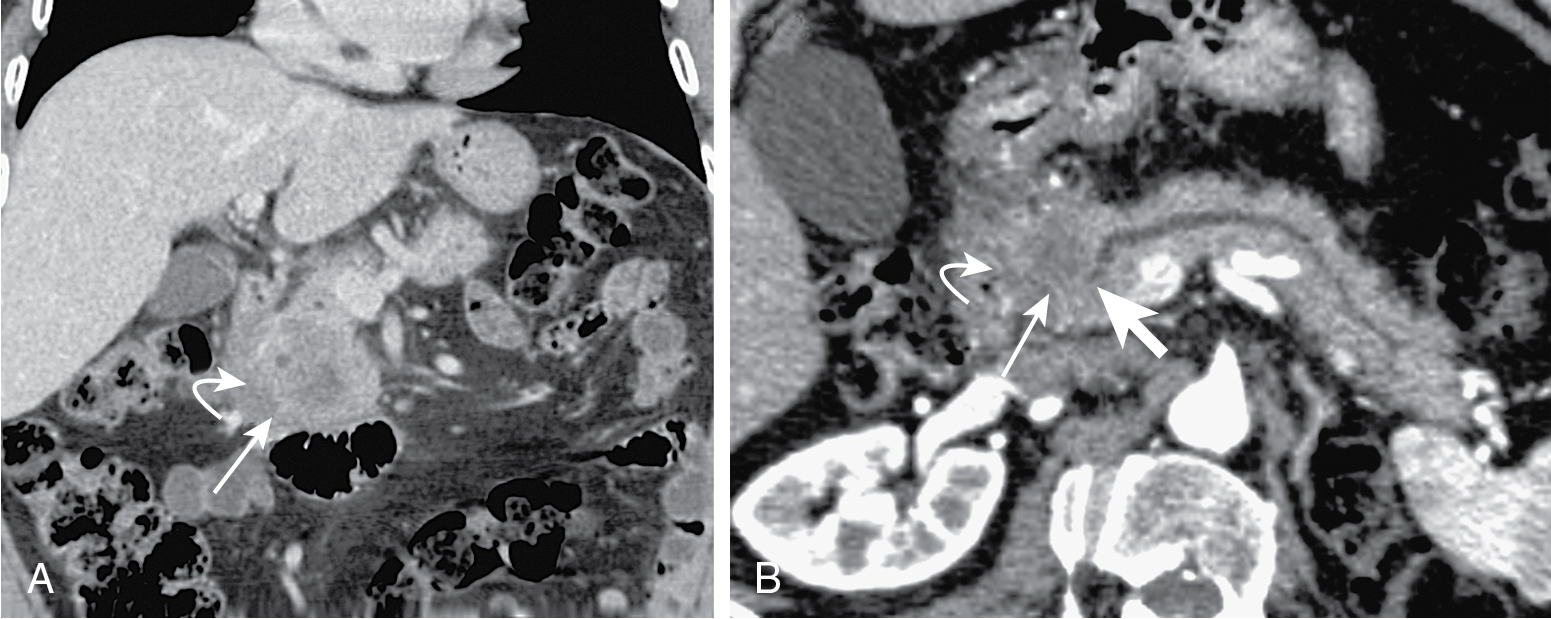
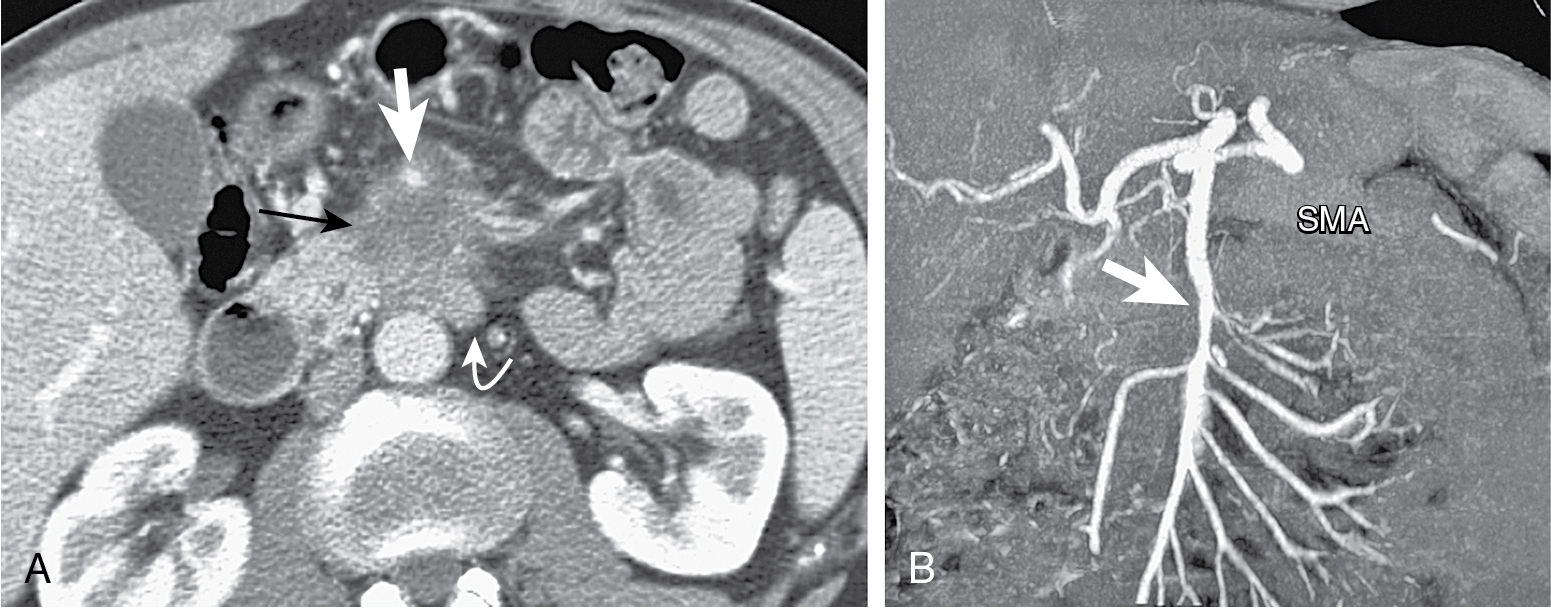
Tumors in body (5%–15%) or tail (10%–15%) may present with back pain. Worse prognosis. Larger at prognosis with average size of 5 cm ( Fig. 13.4 ).
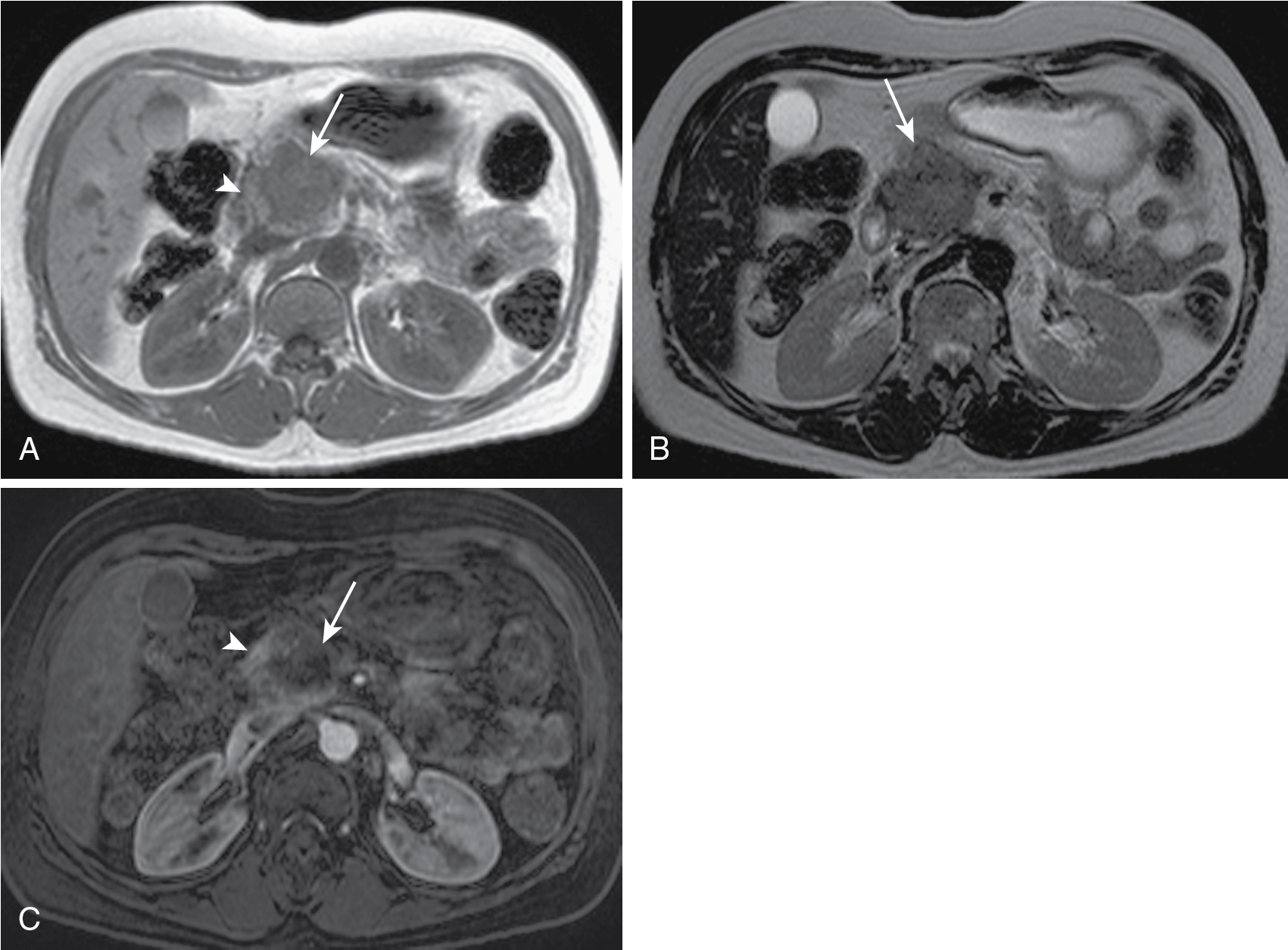
May be focal masses or may be infiltrative.
Cystic changes may happen because of necrosis or ductal obstruction.
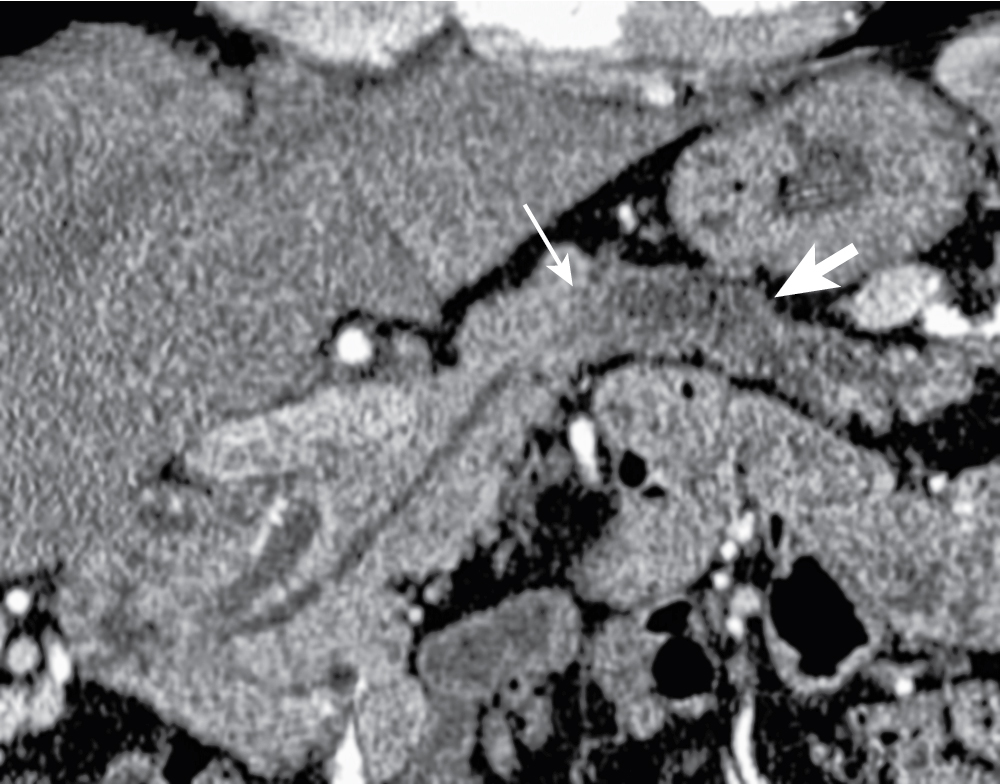
Incites extensive desmoplastic reaction leading to main pancreatic ductal (MPD) obstruction ( Fig. 13.5 ), pancreatitis, and/or parenchymal atrophy.
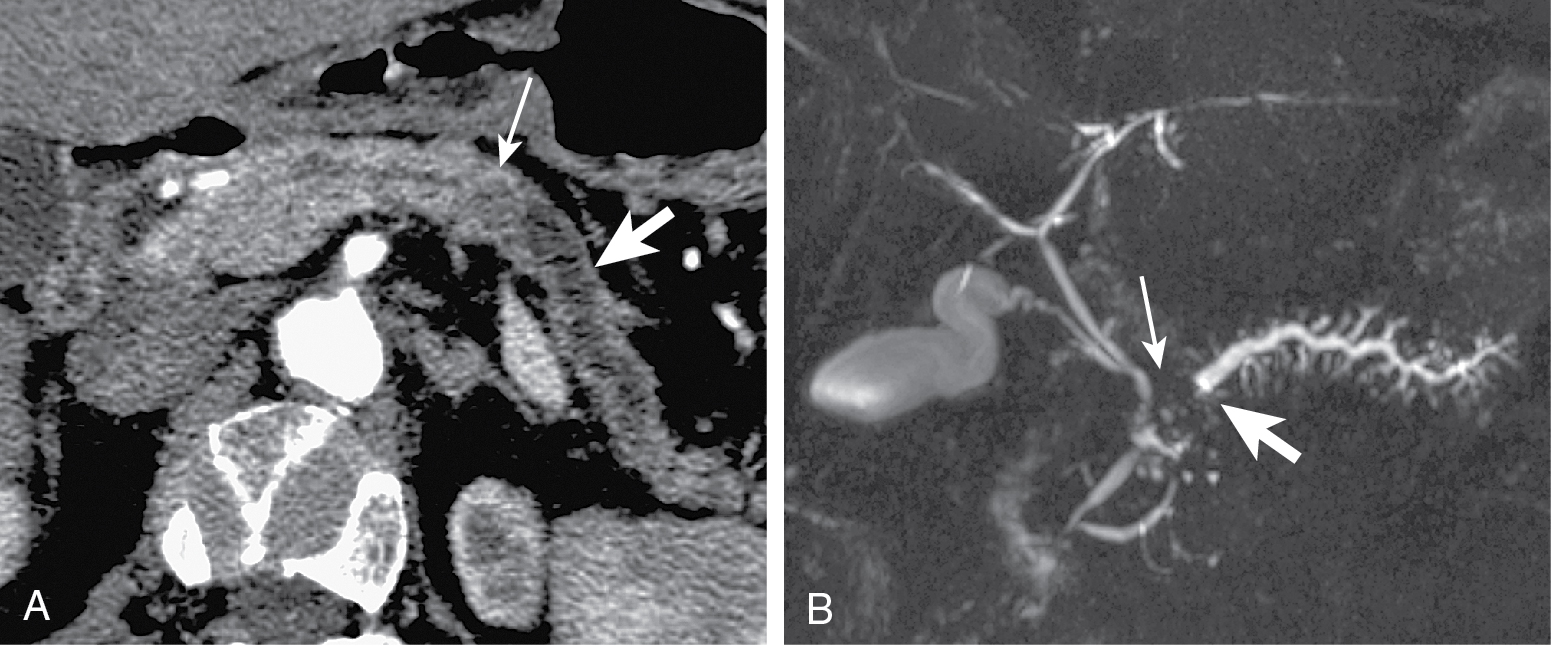
Mode of spread: local ( Fig. 13.6 ), retroperitoneum, peritoneal lymph nodes ( Fig. 13.7 ), and liver.
Mass conspicuity higher in pancreatic phase. Appears as low density mass compared with surrounding parenchyma but may be isoattenuating in 10% of cases.
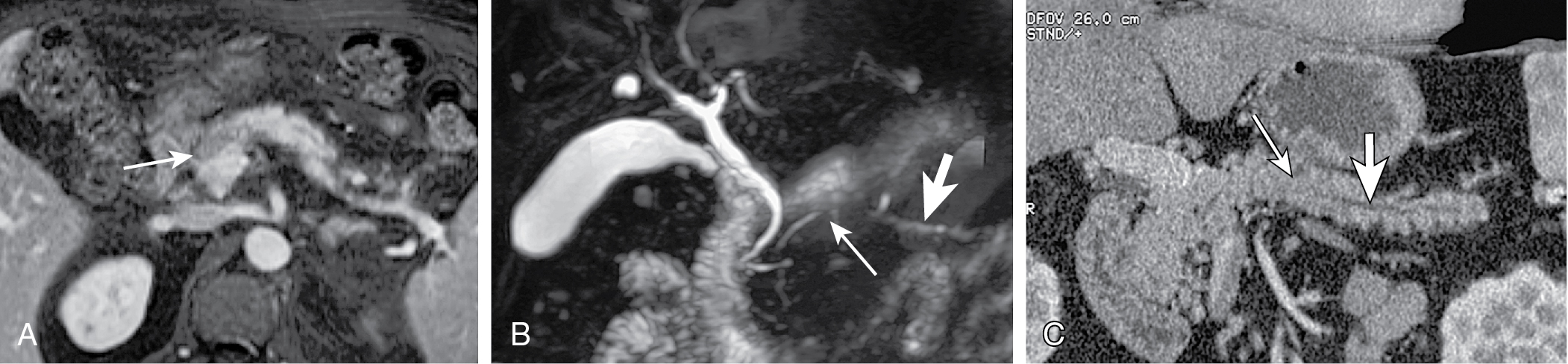
Indirect evidence: ductal dilation ( Fig. 13.8 ), parenchymal atrophy, double duct sign (common bile duct and MPD dilation) ( Fig. 13.9 ).
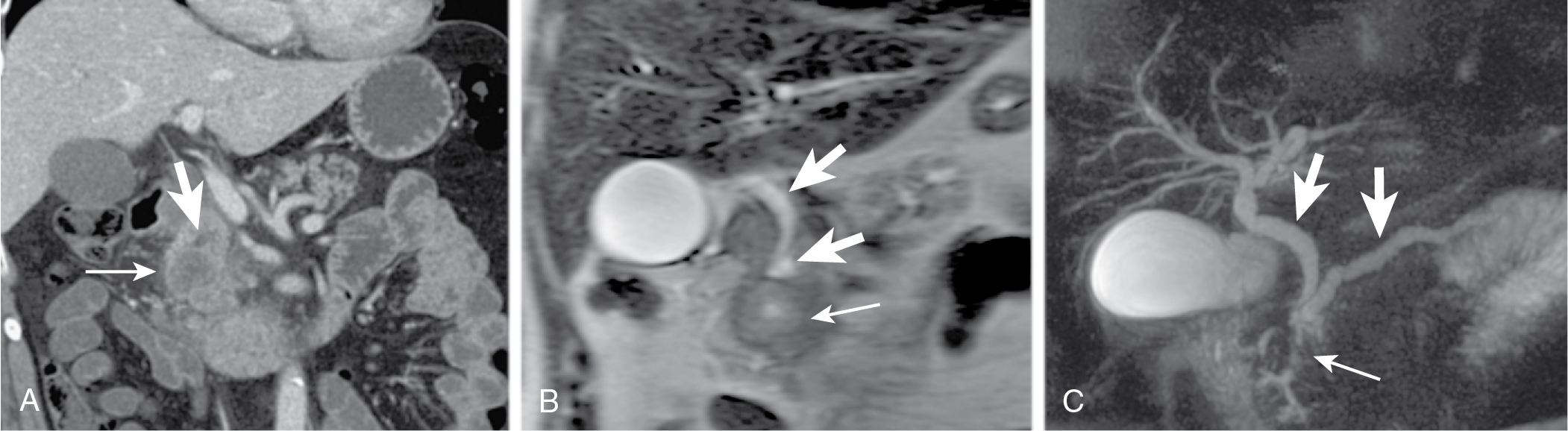
Portal venous phase important for evaluation of metastases, visualization of tumor in respect to vasculature.
Vessel encasement evaluation on CT is important prognostic factor in resection.
Less than 90 degrees, less than 3% infiltration; 90 to 180 degrees, 29% to 57% infiltration; more than 180 degrees, more than 80% infiltration.
T1 hypointense, variable T2 signal (usually hypointense), enhances less than parenchyma but demonstrates progressive enhancement.
MRCP can demonstrate ductal abnormalities, including stenosis, double duct sign, etc. Secretin MRCP better at characterization than MRCP.
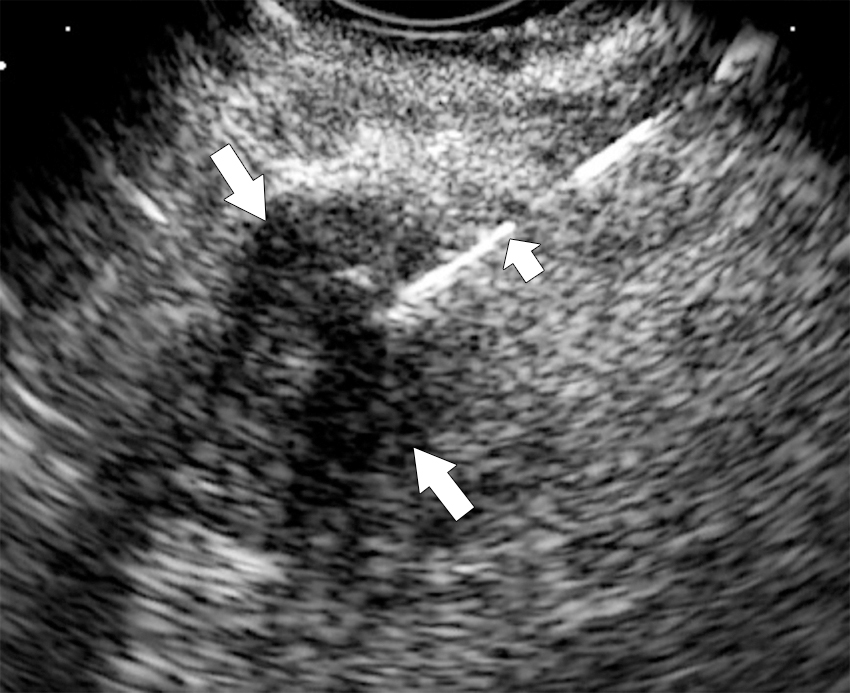
On ultrasound, pancreatic mass appears hypoechoic.
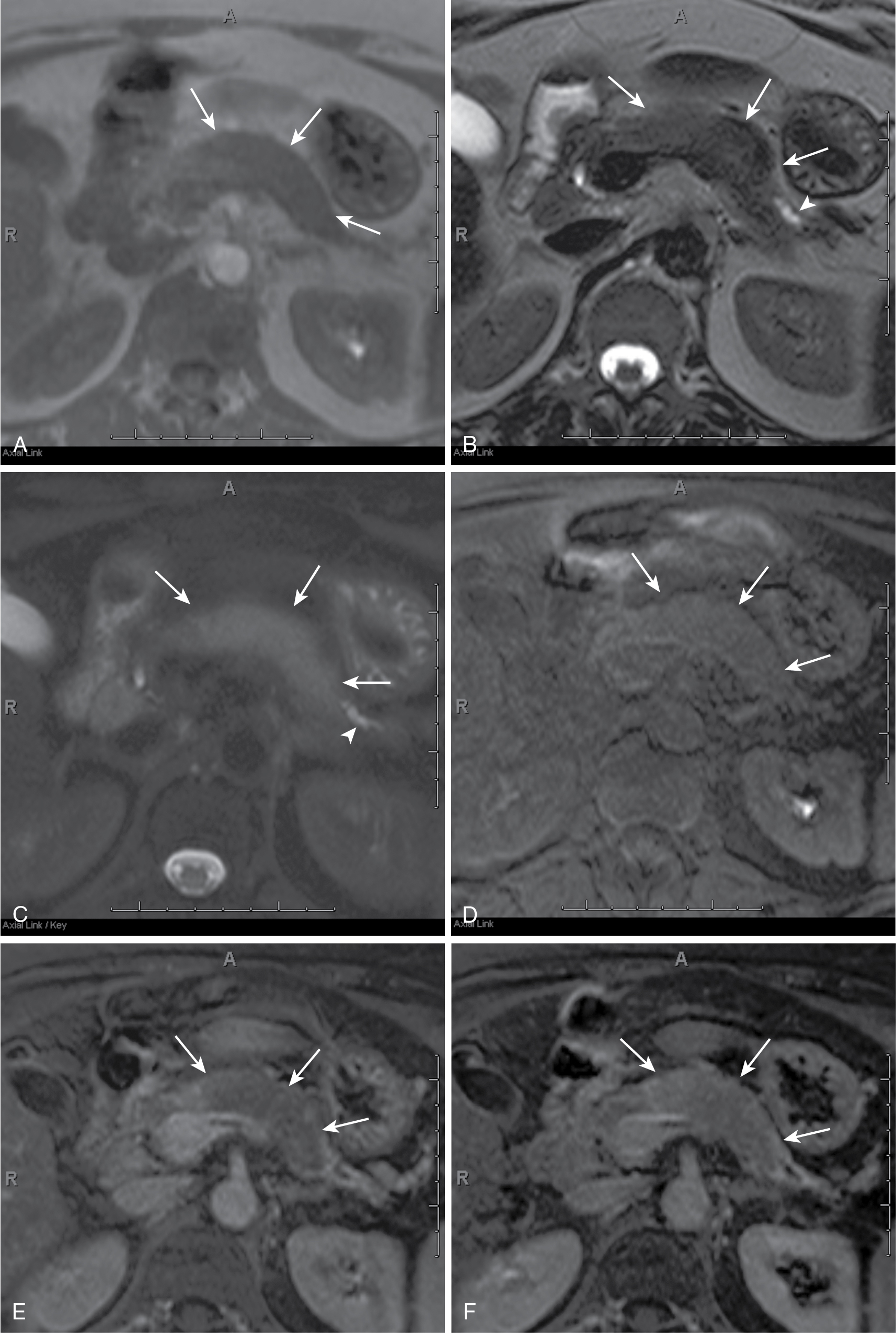
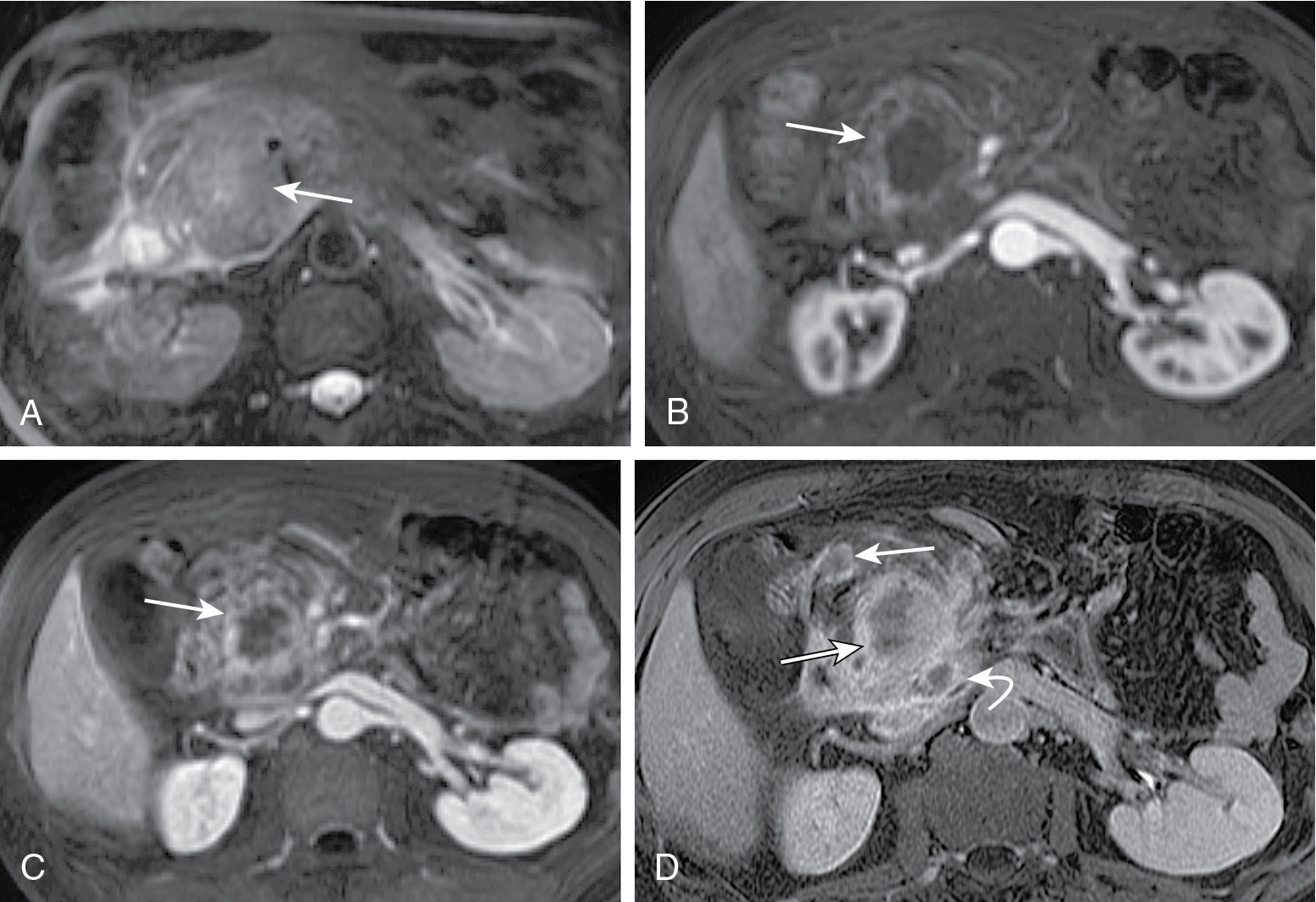
Endoscopic ultrasound is most accurate for detection of duodenal infiltration and lymph node staging ( Fig. 13.10 ).
Normal pancreas does not have significant activity. Increased FDG activity raises concern ( Fig. 13.11 ).
Imaging variants mimicking adenocarcinoma: collapsed duodenum, small bowel diverticula.
Alcohol mass forming pancreatitis (MFP): lesion blends with surrounding parenchyma. MPD obstruction is uncommon. Side-branches usually dilated. If MPD transverses the mass without stenosis, mass is highly likely to be MFP.
Autoimmune MFP: lesion is better defined. MPD obstruction uncommon. Side branches maybe dilated. If MPD transverses the mass without stenosis, mass is highly likely to be MFP ( Fig. 13.12 ).
Other malignancies: lymphoma, endocrine tumors, and so on.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here