Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Snoring affects at least 40% of men and 20% of women and often accompanies sleep-disordered breathing. However, only 6% of women and 13% of men older than 50 years of age have symptomatic obstructive sleep apnea (OSA).
OSA is defined by five or more respiratory events per hour of sleep—apneas, hypopneas, or respiratory effort–related arousals—in association with excessive daytime somnolence; waking with gasping, choking, or breath holding; or witnessed reports of apneas, loud snoring, or both.
Negative health effects have been attributed to untreated OSA; these include increased mortality, an increase in cardiovascular disease, and neurocognitive difficulties. In addition, untreated OSA has been demonstrated to be an independent risk factor for insulin resistance, gastroesophageal reflux disease, motor vehicle accidents, and decreased attention, working memory, and executive function.
The most common symptoms of OSA include loud snoring, restless sleep, and daytime hypersomnolence. However, polysomnography is required and is considered the gold standard for diagnosis of OSA.
Fiberoptic laryngoscopy is an important tool to identify whether the level of obstruction is nasal, retropalatal, or retrolingual. Most patients exhibit multilevel obstruction.
Uvulopalatopharyngoplasty is the most commonly performed surgical procedure for OSA and is often misused as the first-line surgical therapy, regardless of coexistent patient factors such as obesity, retrognathia, and the existence of other sites of obstruction. As a result, uvulopalatopharyngoplasty is often unsuccessful in treating OSA in unselected patients.
Partial midline glossectomy, lingualplasty, and radiofrequency tongue base ablation are procedures that have been developed in an attempt to address the retrolingual collapse or narrowing that occurs in OSA.
Upper airway stimulation offers a new form of therapy with promising long-term results for patients who are CPAP intolerant and meet appropriate criteria.
Surgical treatment of the hypopharyngeal area comprises procedures designed to prevent tongue collapse into the airway during sleep. These include genioglossal advancement and hyoid myotomy and suspension, which are both used to create an enlarged retrolingual airway.
Of patients whose OSA was diagnosed in a sleep disorder center, 31% were found to have coexistent sleep disorders, with the most common being inadequate sleep hygiene (15%) and periodic limb movement disorder (8%).
Insomnia is defined as difficulty with sleep initiation, maintenance, consolidation, or quality that causes daytime dysfunction; it is recurrent despite adequate occasion and opportunity for sleep.
Circadian rhythm sleep disorders occur when personal sleep-wake patterns are misaligned with the societal clock on a persistent or recurrent basis, leading to excessive daytime sleepiness or insomnia and resulting in impaired function.
Parasomnias are undesirable movements or subjective phenomena that occur during sleep and while falling asleep or waking up.
Over the past decade, interest in sleep and sleep disorders has increased substantially. Most of this renewed interest within the otolaryngology community has been focused on obstructive sleep apnea (OSA), a sleep-related breathing disorder. As the waistline of the average American has grown, so too has the incidence of OSA. Population-based estimates published in 1997 suggested that 2% of women and 4% of men over age 50 have symptomatic OSA, but more recent estimates have demonstrated an increasing prevalence with estimates of moderate-to-severe OSA in 13% to 50% of middle-aged men and 6% to 23% of middle-aged women. Advances have also been made in the understanding of the pathophysiology of OSA, diagnostic methods, and medical and surgical treatments. The recognition that sleep disorders require a multidisciplinary approach has led to the development of a multidisciplinary subspecialty of sleep medicine with teams made up of internists, pulmonologists, otolaryngologists, neurologists, pediatricians, psychiatrists, oral/maxillofacial surgeons, dentists, behavioral psychologists, and nutritionists who work together to take care of patients with sleep disorders. Considerable advances have been made in the diagnosis and management of sleep disorders, and otolaryngologists are at the forefront of this new field, providing a host of both traditional and novel operative techniques to facilitate treatment.
The idea of obesity being intimately associated with daytime somnolence seems to have been first written about by Charles Dickens in The Posthumous Papers of the Pickwick Club , originally published in 1837. Dickens gives a vibrantly descriptive picture of Joe, a boy so obese that he has difficulty breathing, sounds as if he is snoring even when awake, and frequently falls asleep while standing. In addition, there is evidence to suggest that some of our most noted leaders and dictators suffered from this problem. The 20th President of the United States, William Howard Taft, had a body mass index (BMI) of 42 kg/m 2 while in office and was reported to snore, to fall asleep during the day frequently, and to have hypertension. Chouard and colleagues describe several reasons to suspect Napoleon I (1769–1821) had OSA in the last decade of his life: he was obese and retrognathic, his neck was short and thick, he had nasal obstruction, he frequently slept during the day, he complained of declining energy and intellect, and he was quite tired looking and somewhat disheveled. In 1956, Bickelmann and colleagues drew on Dickens's description in their case report, titled “Extreme Obesity Associated with Alveolar Hypoventilation: A Pickwickian Syndrome.” This frequently cited paper served as the first description of the pickwickian syndrome in an academic journal. Sleep apnea was first described as a clinical entity in 1965, but it was not until the 1970s that Elio Lugaresi's group provided a complete description of the OSA syndrome with its potential adverse cardiovascular effects.
In the infancy of this new diagnosis, treatment options were limited to tracheostomy or weight loss. This changed in the early 1980s with the introduction of the uvulopalatopharyngoplasty (UPPP), as described by Fujita and colleagues and Simmons and colleagues. Not long after the introduction of UPPP, it was recognized that although this new surgical intervention was helpful in many cases, it left half of the patients with persistent sleep apnea and could be a painful procedure. It was during this same time that Colin Sullivan, a young Australian medical researcher, developed nasal continuous positive airway pressure (CPAP), which today remains the first-line treatment for adult OSA.
As treatment for OSA has evolved so too has therapy aimed at treating snoring. The development of office-based surgical treatments, over-the-counter devices, and pharmacologic interventions has increased considerably in recent years.
Sleep-related breathing disorders range from partial airway collapse and increased upper airway resistance to episodes of hypopnea or complete airway collapse with sleep apnea ( Table 15.1 ). In addition, a number of indices are used to describe sleep-disordered breathing ( Table 15.2 ).
| Respiratory Event | Definition |
|---|---|
| Apnea | A cessation of airflow for at least 10 s |
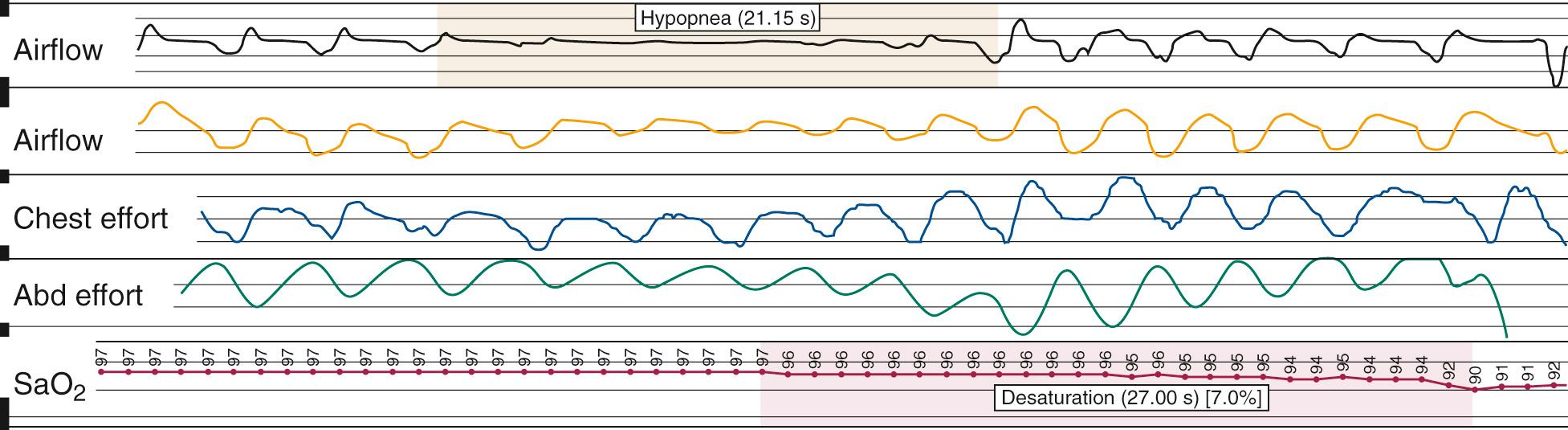
| Hypopnea | A reduction in airflow (≥30%) at least 10 s with ≥4% oxyhemoglobin desaturation |
| OR a reduction in airflow (≥50%) at least 10 s with ≥3% oxyhemoglobin desaturation or an electroencephalogram (EEG) arousal | |
| Respiratory effort–related arousal (RERA) | Sequence of breaths for at least 10 s with increasing respiratory effort or flattening of the nasal pressure waveform, leading to an arousal from sleep when the sequence of breaths does not meet the criteria for an apnea or a hypopnea |
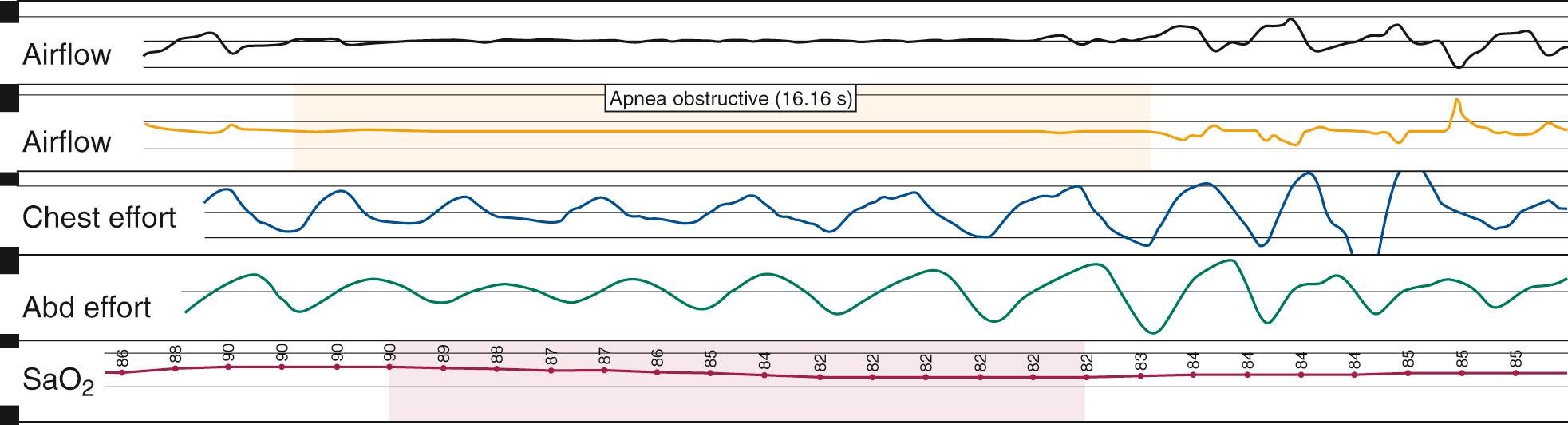
| Obstructive | Continued thoracoabdominal effort in the setting of partial or complete airflow cessation |
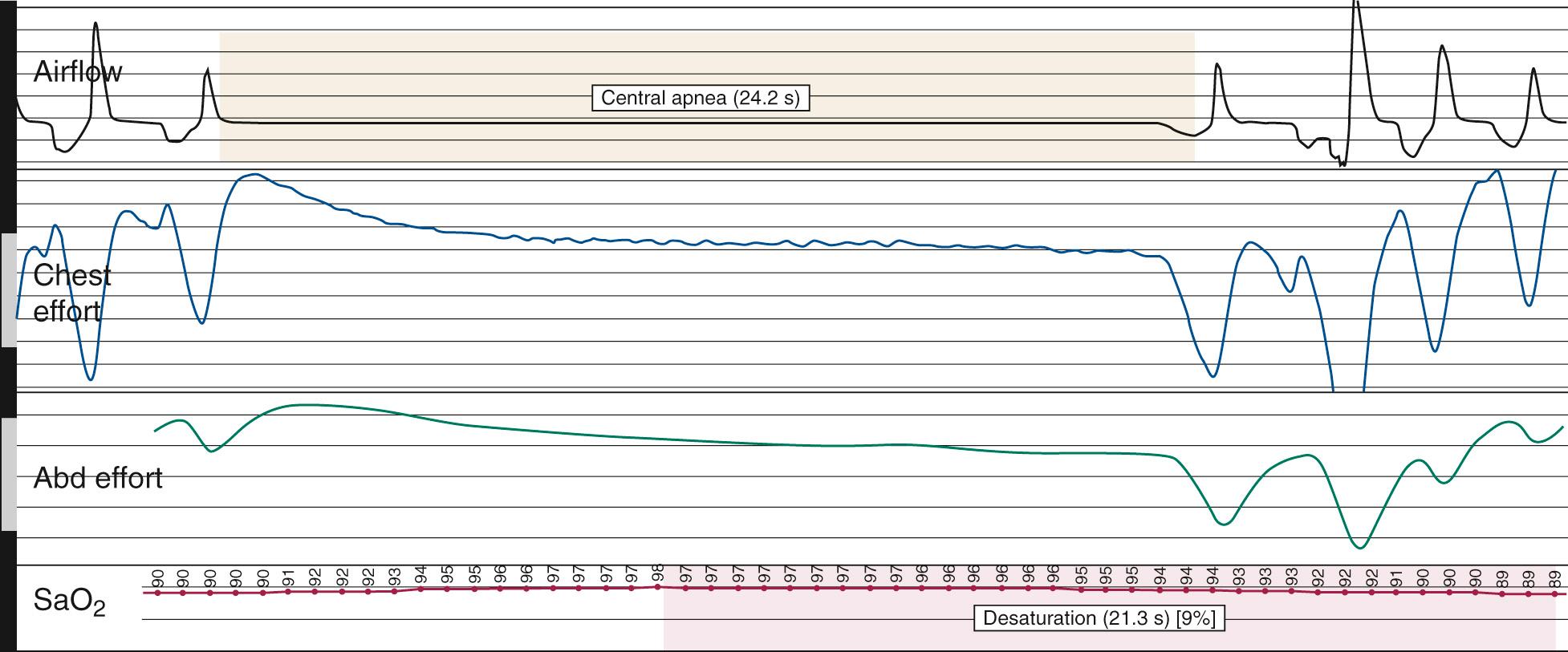
| Central | The lack of thoracoabdominal effort in the setting of partial or complete airflow cessation |
| Mixed | A respiratory event with both obstructive and central features, with mixed events generally beginning as central events and ending with thoracoabdominal effort without airflow |
| Index | Definition |
|---|---|
| Apnea index | Number of apneas per hour of total sleep time |
| Hypopnea index | Number of hypopneas per hour of total sleep time |
| Apnea-hypopnea index | Number of apneas and hypopneas per hour of total sleep time |
| Respiratory effort–related arousal (RERA) index | Number of RERAs per hour of total sleep time |
| Respiratory disturbance index | Number of apneas, hypopneas, and RERAs per hour of total sleep time |
| Central apnea index | Number of central apneas per hour of total sleep time |
| Mixed apnea index | Number of mixed apneas per hour of total sleep time |
Snoring is sound generated by the vibration of the pharyngeal soft tissues. It is often louder during inspiration than expiration. It affects at least 40% of men and 20% of women and often accompanies sleep-disordered breathing. However, snoring can occur in isolation and by definition is not associated with symptoms of excessive daytime sleepiness (EDS) or insomnia. In the absence of OSA, snoring is documented when habitual audible snoring occurs with an apnea hypopnea index (AHI) of less than five events per hour without daytime symptoms. Polysomnography (PSG) is not required for diagnosis, but when performed it reveals an audible microphone signal not associated with arousals, desaturations, airflow limitation, or arrhythmias.
The term upper airway resistance syndrome (UARS) was first used to describe patients who do not meet the criteria for OSA syndrome but who experience excessive daytime somnolence and other debilitating somatic complaints. UARS is characterized by respiratory effort–related arousals (RERAs), defined as a sequence of breaths over at least 10 seconds with increasing respiratory effort that terminates with an arousal. A RERA is detected using esophageal pressure manometry, which reveals a pattern of progressively increasing negative esophageal pressure followed by an arousal. PSG reveals frequent arousals associated with snoring, abnormally negative intrathoracic pressure, or increased diaphragmatic electromyogram activity. The current American Academy of Sleep Medicine (AASM) International Classification of Sleep Disorders 3 (ICSD-3) does not consider UARS to be a separate sleep disorder, but recommends that it be included under the definition of OSA, because the pathophysiology is similar.
Diagnostic criteria for OSA in adults requires a polysomnogram or home sleep apnea test (HSAT) that demonstrates either (1) 5 or more predominately obstructive respiratory events (obstructive and/or mixed apneas, hypopneas, or RERAs) per hour of sleep, in association with symptoms or comorbidities, or (2) 15 or more predominately respiratory events per hour of sleep regardless of symptoms or comorbidities. Symptoms related to OSA include excessive daytime somnolence, waking with gasping, choking, or breath holding; or witnessed reports of apneas, loud snoring, or both. Comorbidities include hypertension, a mood disorder, cognitive dysfunction, coronary artery disease, stroke, congestive heart failure, atrial fibrillation, or type 2 diabetes mellitus. Diagnosis may be made on in-lab PSG or by HSAT. Each episode of apnea or hypopnea must last a minimum of 10 seconds, is commonly accompanied by reductions in blood oxygen saturation of at least 3% to 4%, and is usually terminated by brief, unconscious arousals from sleep. Thus OSA causes fragmentation and disruption of the normal sleep cycle, resulting in symptoms of EDS and difficulty with concentration and alertness. Snoring between apneas is a frequent complaint of bed partners and is often the symptom that prompts these patients to seek medical attention, although excessive daytime somnolence is a common initial complaint. Many OSA sufferers complain of awakening from sleep with morning headache, sore throat, and fatigue or a feeling of being unrefreshed regardless of the duration of sleep ( Box 15.1 ). OSA is exacerbated by ingestion of alcohol, sedative use, and weight gain. The AASM classifies mild OSA as 5 to 15 events per hour, moderate as 15 to 30 events, and severe as 30 events or more.
Restless sleep
Loud snoring
Observed apnea, choking, or gasping episodes
Excessive daytime sleepiness
Morning fatigue or irritability
Memory loss
Decreased cognitive function
Depression
Personality or mood changes
Decreased libido and impotence
Morning and nocturnal headaches
Nocturnal sweating
Nocturnal enuresis
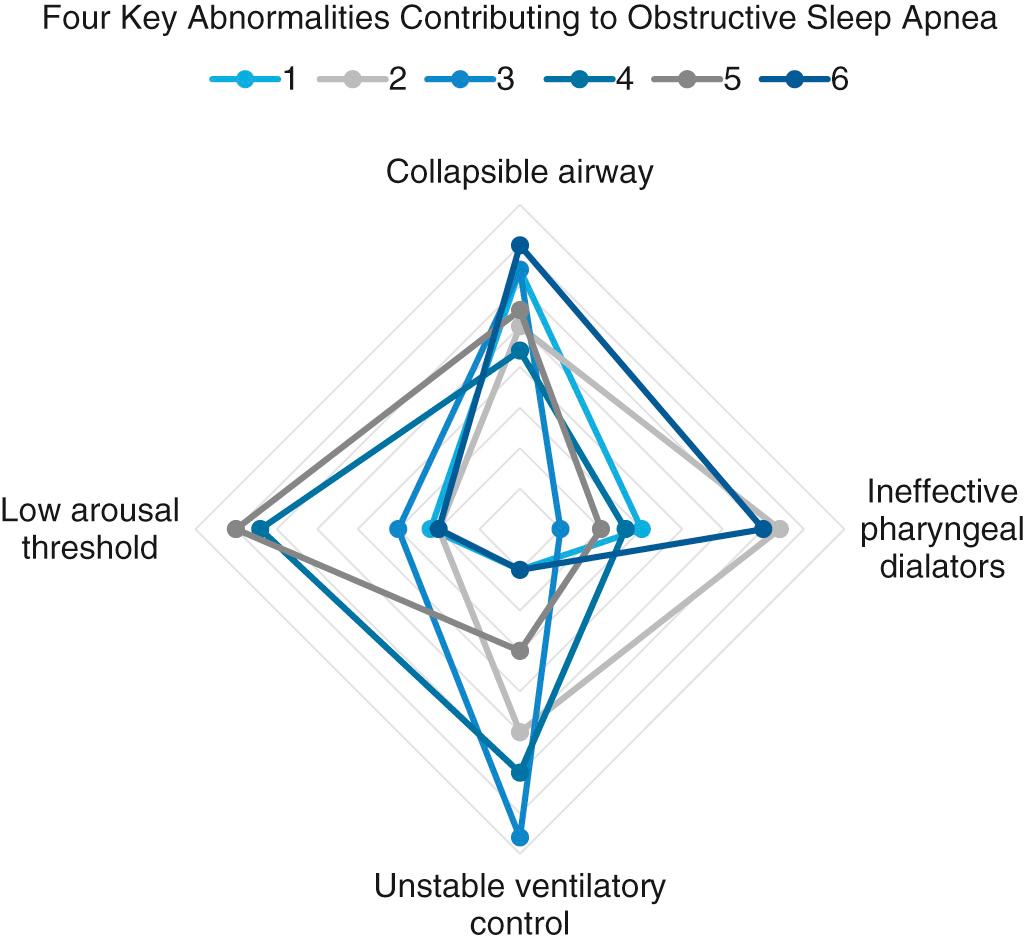
The obstruction that occurs in OSA results from collapse of the pharyngeal airway during sleep. In the last decade, there have been significant advances in the understanding of the pathophysiology of OSA. There are four key traits or phenotypes that contribute to OSA: (1) impaired upper airway anatomy that is narrow or collapsible, (2) low respiratory arousal threshold, (3) inadequate responsiveness of upper airway dilator muscles during sleep, and (4) unstable or overly sensitive respiratory control, a concept referred to as high loop gain ( Fig. 15.1 ). Recognition of these different traits and the specific contribution of each in an individual patient offers the potential for tailored treatment according to a precision medicine approach. Obesity, soft tissue hypertrophy, and craniofacial characteristics such as retrognathia contribute to the impaired upper airway anatomy by increasing the extraluminal tissue pressures surrounding the upper airway. However, structural compromise of the airway alone is not always sufficient to produce OSA. Notably, patients without anatomic abnormalities may also have OSA. This may occur because the complex reflex pathways from the central nervous system to the pharynx, which control the action of pharyngeal dilator muscles, may fail to maintain pharyngeal patency. The three major areas of obstruction are the nose, palate, and hypopharynx, although OSA associated with laryngeal obstruction from bilateral laryngeal paralysis, laryngomalacia, and obstructing laryngeal lesions has also been reported.
Fujita classified the patterns of obstruction by anatomic location: type I is collapse in the retropalatal region only; type II is collapse in both retropalatal and retrolingual regions; and type III is collapse in the retrolingual region only. Subsequent studies have evaluated the prevalence of retropalatal versus retrolingual obstruction in patients with OSA and snoring and found that both retrolingual (77% vs. 40%) and retropalatal (100% vs. 70%) obstruction are increased in OSA patients, confirmed by PSG, compared with snorers.
Nasal obstruction contributes to increased airway resistance and may worsen OSA, but it is rarely the sole cause. Nasal obstruction may contribute to open-mouth breathing during sleep, which increases upper airway collapsibility and may decrease the efficacy of dilator muscles. Snoring is often a presenting symptom of OSA and can be caused by nasal obstruction. Surgical procedures aimed at improving nasal breathing have demonstrated subjective improvement in snoring following correction of nasal obstruction. Even though isolated treatment of the nasal airway rarely leads to cure of OSA, it may allow for decreased CPAP levels to be used for subsequent treatment. Nasal obstruction can be caused by bony and cartilaginous deformation or from soft tissue changes. Because many causes of nasal obstruction are possible, they all warrant investigation when evaluating a patient with OSA.
Obesity is a major risk factor for OSA. The increased fat deposition around the neck and parapharyngeal spaces is postulated to narrow and compress the upper airway and may offset the effects of dilator muscles that maintain airway patency. Obesity is also thought to contribute to OSA through its deleterious effects on metabolism, ventilation, and lung volume, resulting in a mismatch between alveolar ventilation and pulmonary perfusion. Obesity can significantly reduce lung volume, which results in a reduction of functional residual capacity. It has been observed that changes in lung volume significantly reduce pharyngeal upper airway size through the mechanical effect of tracheal and thoracic traction, referred to as tracheal tug , increasing the risk for airway collapse.
Adenotonsillar hypertrophy is the major cause of OSA in children. Tonsillar hypertrophy has also been observed to be a significant contributor to OSA in some adults as well, though multiple structural characteristics are associated with OSA. Craniofacial variations that have been associated with OSA include increased distance of the hyoid bone from the mandibular plane, decreased mandibular and maxillary projection, a downward and posterior rotation of mandibular and maxillary growth, increased vertical facial length, increased vertical length of the posterior airway, and increased cervical angulation.
These anatomic features predispose to OSA, but they do not by themselves reflect the dynamic collapse that occurs during sleep. To quantify the functional collapsibility of the upper airway, the passive critical closing pressure (P crit ) is measured using a mask attached to a device that can deliver both positive and negative airway pressure. The P crit is the luminal pressure at which the upper airway collapses after a prolonged period of therapeutic positive pressure, such that there is minimal recruitment of the pharyngeal dilator muscles when the airway pressures are suddenly reduced. Individuals with OSA have been shown to have a P crit above atmospheric pressure, while those without OSA had a P crit below −5 cm H 2 O.
Neuromuscular tone contributes to the patency of the upper airway. During sleep this tone decreases, and the airway tends toward collapse. The genioglossus muscle is considered to be the most important muscle in maintaining airway patency in OSA. Elevated genioglossal and tensor palatini muscle activity has been observed in awake OSA patients compared with normal awake subjects who have lower levels of activity. This suggests that even while the patient is awake, airway dilator muscle activity compensates for a more anatomically compromised upper airway in the OSA patient. Conversely, an inability to increase the airway dilator muscle activity in response to negative collapsing pressure contributes to OSA. One study noted that more than one-third of OSA patients generated less than 0.1% increase in their maximal genioglossus activity in response to a 1 cm H 2 O decrease in negative pharyngeal pressure. The concept of loop gain, the sensitivity of the respiratory control system to perturbations in CO 2 level, also plays an important role in OSA pathogenesis. A high loop gain indicates an unstable respiratory control system that is prone to overcompensation, resulting in excessive changes in ventilation in response to small changes in CO 2 . Conversely, a low loop gain indicates a more stable respiratory control system where responses to perturbations are less prone to overcompensation, resulting in a more rapid return to homeostasis. High loop gain can contribute to OSA by causing rapid increases in respiratory drive in response to small increases in CO 2 , resulting in large negative luminal negative pressures and increasing the likelihood of airway collapse. Individuals with a low arousal threshold are particularly sensitive to even small changes in intrathoracic pressure, waking too easily in response to airway narrowing. This prevents progression to deeper stages of sleep associated with higher genioglossus muscle activity and more stable pharyngeal dilator response.
A number of negative health effects have been attributed to untreated OSA, including increased mortality, an increase in cardiovascular disease, and neurocognitive difficulties. In a retrospective study, He and colleagues found that untreated OSA patients with an apnea index (AI) greater than 20 had a statistically significant increase in mortality compared with patients with an AI less than 20, and they also found that untreated patients with an AI greater than 20 had a 63% probability of surviving 8 years, compared with 96% in those with an AI less than 20. In addition, untreated OSA is reported to increase the risk of fatal and nonfatal motor vehicle accidents by 2.5-fold. A significant proportion of the mortality and morbidity related to OSA occurs through its effects on the cardiovascular system, which can result in hypertension, coronary heart disease, congestive heart failure, arrhythmias, pulmonary hypertension, stroke, and sudden death. Untreated moderate and severe OSA has been reported to result in a threefold increase in fatal and nonfatal cardiovascular events, compared with both healthy men without OSA and men with CPAP-treated OSA. Treatment of OSA with CPAP has also been reported to lower blood pressure by 10 mm Hg.
Untreated OSA has been demonstrated to be an independent risk factor for insulin resistance. Recently it has been suggested that OSA may contribute to the development of diabetes and metabolic syndrome , the term used to describe the commonly occurring conditions of obesity, insulin resistance, hypertension, and dyslipidemia. However, further research is needed to determine whether there is an independent link between OSA and metabolic abnormalities.
The prevalence of gastroesophageal reflux disease in OSA patients is significantly higher than in the general population. Even though these disorders commonly occur together, no temporal or causal relationship has ever been demonstrated between the two. This may reflect the fact that they share similar risk factors. Treatment of OSA with CPAP has been demonstrated to decrease the occurrence of gastroesophageal reflux disease.
Aside from the obvious physical effects of OSA, such as EDS and impaired mood, neurocognitive deficits have also been associated with OSA. Untreated OSA has been documented to cause problems with attention, working memory, and executive function, all of which are improved with CPAP treatment. Bed partner dissatisfaction is also a common complaint among OSA patients, and treatment has been shown to improve the quality of life in both the treated individuals and their bed partners. Thus the benefits of treating OSA are substantial and well documented.
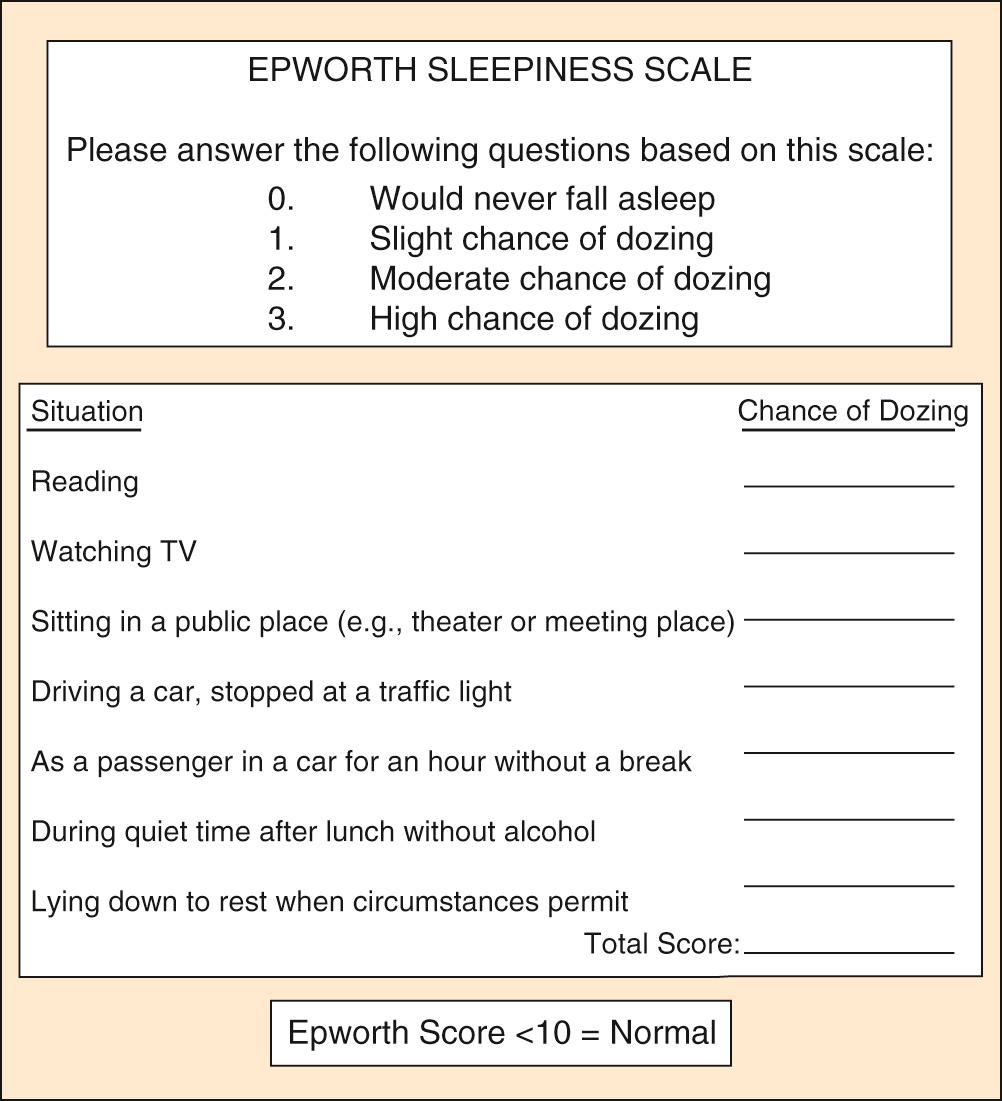
The most common symptoms of OSA include loud snoring, restless sleep, and daytime hypersomnolence. However, a broad range of signs and symptoms have been reported to occur in OSA (see Box 15.1 ). Obesity is a common finding in patients with OSA, and 70% of adult patients with OSA are reportedly obese. Screening that includes a detailed sleep history and physical examination is recommended for all obese patients. The Epworth Sleepiness Scale (ESS) is a widely used tool that assesses daytime sleepiness ( Fig. 15.2 ). OSA may be suspected in patients with an ESS score greater than 10. Another commonly used questionnaire for OSA screening is the STOP-BANG questionnaire, consisting of eight yes/no questions where three or more positive responses is considered high risk for OSA ( Box 15.2 ). Though considered too inaccurate to substitute for objective testing in establishing a diagnosis of OSA, this questionnaire has demonstrated high sensitivity but low specificity for the detection of OSA. Sleepiness or fatigue may also be precipitated by a number of other medical conditions that should be considered when evaluating patients for possible OSA ( Box 15.3 ). In addition, the presence of additional sleep disorders should be considered.
S: Do you snore loudly?
T: Do you often feel tired, fatigued, or sleepy during the day?
O: Has anyone observed you stop breathing during your sleep?
P: Do you have or are you being treated for high blood pressure?
B: Is your body mass index >35 kg/m 2 ?
A: Are you at least 50 years old?
N: Is your neck circumference >40 cm (female) >43 cm (male)?
G: Are you male?
Eight simples questions with yes or no answers. A score of 5–8 indicates a high probability of moderate to severe obstructive sleep apnea.
Severe anemia
Endocrine dysfunction, including hypothyroidism and Addison disease
Chronic fatigue syndrome
Pulmonary disease, including asthma, emphysema, and pickwickian syndrome
Cardiovascular disease, including congestive and left heart failure
Neoplasms, including disseminated and central nervous system lesions
Anticancer chemotherapy
Collagen vascular diseases
Chronic infections, including mononucleosis, hepatitis, and influenza
Depression and other psychiatric disorders
Malnutrition
Neurologic disorders, including Parkinson disease and multiple sclerosis
Medication side effects
Because of the increased prevalence of OSA in patients with hypertension, coronary artery disease, congestive heart failure, cerebrovascular accident, and diabetes mellitus, these populations must be carefully screened for the signs and symptoms of OSA. It has also recently been recognized that OSA may be underdiagnosed in women because of a lower index of suspicion among physicians or underreporting of the classic symptoms by female patients. Women with OSA are more likely to report symptoms of insomnia, heart palpitations, and ankle edema.
Although the presence of daytime somnolence and loud snoring are frequently the signs that prompt OSA sufferers to seek medical attention, the physical examination findings strengthen the likelihood of the diagnosis. The BMI calculation, blood pressure measurement, and neck circumference are important general assessment parameters. In addition, body habitus, mandible and maxilla position and size, and facial characteristics should be noted. Assessment of the nose should include evaluation of any external deformity, adequacy of the nasal valve, septal position, turbinate size, nasal mucosa swelling, and the presence or absence of polyps, purulence, and rhinorrhea. In the oral cavity, appraisals of the tongue size and position, elongation of the palate and uvula, tonsil size, modified Mallampati score, dentition, and crowding of oropharynx should follow. In the neck, its size, hyoid position, and jaw position, including retrognathia, should be evaluated ( Box 15.4 ).
Septal deviation
Turbinate hypertrophy
Nasal valve collapse
Adenoid hypertrophy
Nasal tumors or polyps
Large soft palate
Palatine tonsillar hypertrophy
Posterior pharyngeal wall banding
Macroglossia
Large mandibular tori
Narrow skeletal arch
Lateral pharyngeal wall collapse
Omega-shaped epiglottis
Hypopharyngeal tumor
Lingual tonsillar hypertrophy
Retrognathia and micrognathia
True vocal cord paralysis
Laryngeal tumor
Increased neck circumference
Redundant cervical adipose tissue
Obesity
Achondroplasia
Chest wall deformity
Marfan syndrome
Arterial hypertension, especially morning hypertension
Peripheral edema
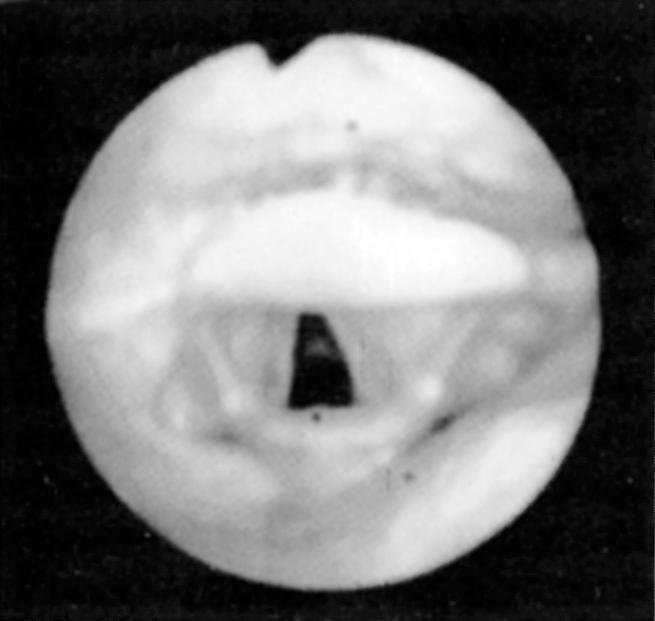
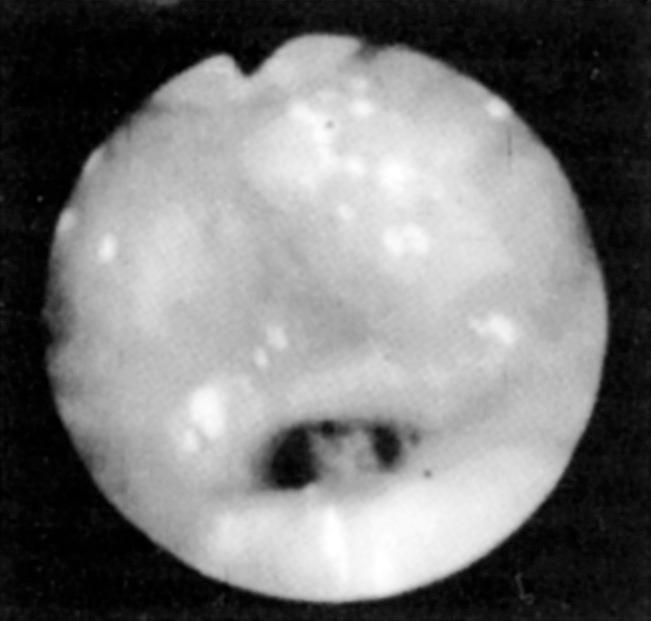
Fiberoptic nasopharyngoscopy and laryngoscopy are important for evaluation of the airway. This examination can be performed in multiple positions—nasal, retropalatal, or retrolingual—in awake and asleep patients and is an important tool to identify level of obstruction ( Figs. 15.3 and 15.4 ). Numerous studies have described the benefits and limitations of the addition of the Müller maneuver to this examination for preoperative prediction of the effectiveness of surgical interventions. The Müller maneuver is performed in an awake patient, who generates negative pressure by inhaling against a closed glottis with the nose and mouth closed to trigger airway collapse ( Figs. 15.5 and 15.6 ). Sher and associates performed both seated and supine examinations using the Müller maneuver in OSA patients, selected those patients who experienced palatal collapse, and found that 73% had at least a 50% reduction in the respiratory disturbance index (RDI) after UPPP. Aboussouan found that using the Müller maneuver to guide the decision on UPPP resulted in an AHI reduction of 50% in 78% of patients who had velopalatal collapse, compared with 36% for multilevel obstruction. Thus even though the Müller maneuver can help guide surgical decision making when the area of collapse is purely retropalatal, it appears to be less useful in patients who have multilevel obstruction, which is the majority of patients.
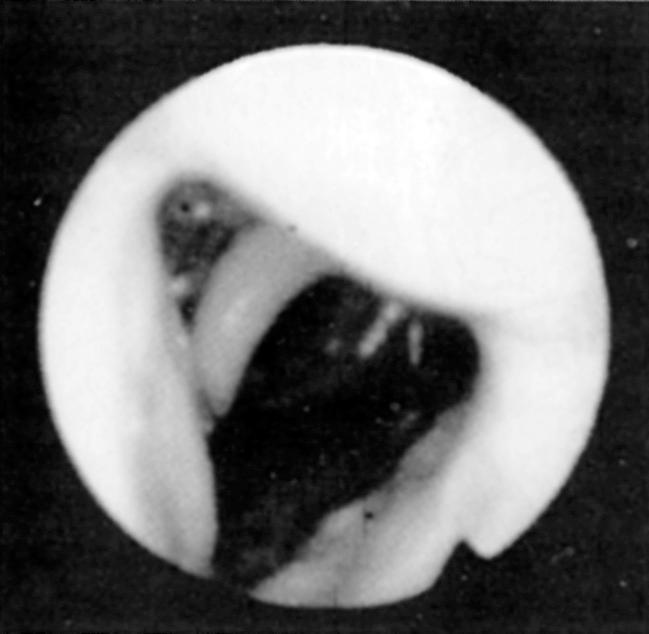
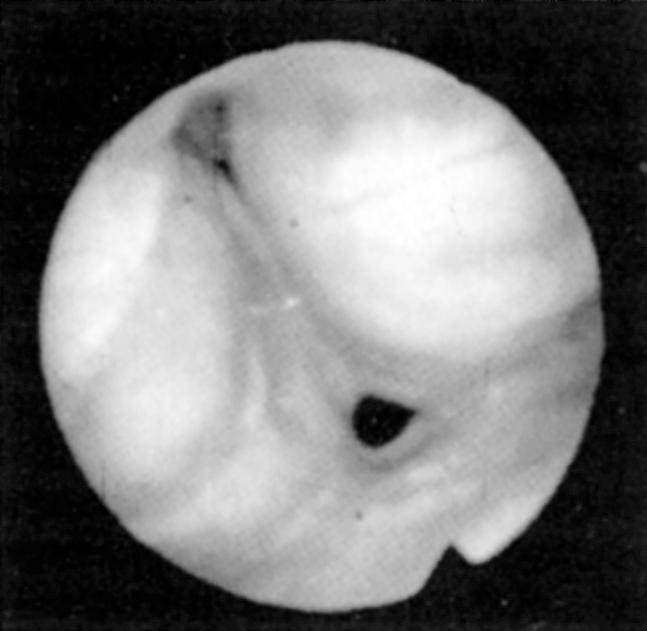
To better identify the site or sites of obstruction in patients with OSA, drug-induced sleep endoscopy (DISE) has been used to guide more effective surgical intervention. DISE involves the use of fiberoptic nasopharyngoscopy to evaluate the site of airway collapse during pharmacologically induced sleep. It is thought that the dynamic obstruction observed during sedated endoscopy may more accurately reflect airway obstruction during natural sleep than during an awake examination. Several studies have noted poor correlation between the findings during DISE and awake endoscopic examination, and one recent meta-analysis found that a DISE assessment resulted in a change in the surgical plan in more than half of cases, primarily with respect to management of hypopharyngeal and laryngeal structures. Although this evaluation is most commonly performed using some combination of midazolam and propofol for sedation, some authors who perform DISE primarily in children have suggested using dexmedetomidine as the primary agent for sedation. This is because it causes less respiratory depression and cardiovascular instability and thus better mimics natural sleep. It has been observed that sedation with propofol may result in greater obstruction in general, which may facilitate preoperative planning, though it remains unclear to what extent sedated endoscopic findings can be generalized to natural sleep. Nevertheless, this technique has been demonstrated to be a useful tool for assessing the location, severity, and pattern of airway obstruction during sleep. A proposed method of standardizing DISE findings called the velum , oropharynx , tongue base , epiglottis (VOTE) classification has demonstrated good intrarater and interrater reliability. Recent studies have used this system to prognosticate outcomes of surgical intervention. While the VOTE classification remains the most widely used scoring method, a variety of other scoring schemes have been proposed to standardize the reporting of DISE findings in both adults and in children. Most investigators have found that multilevel collapse and complete collapse at the velum or tongue base predispose to worse outcomes of surgical intervention. Others have attempted to use image processing software to objectively measure the percent change in airway cross-sectional area during waking and sleep.
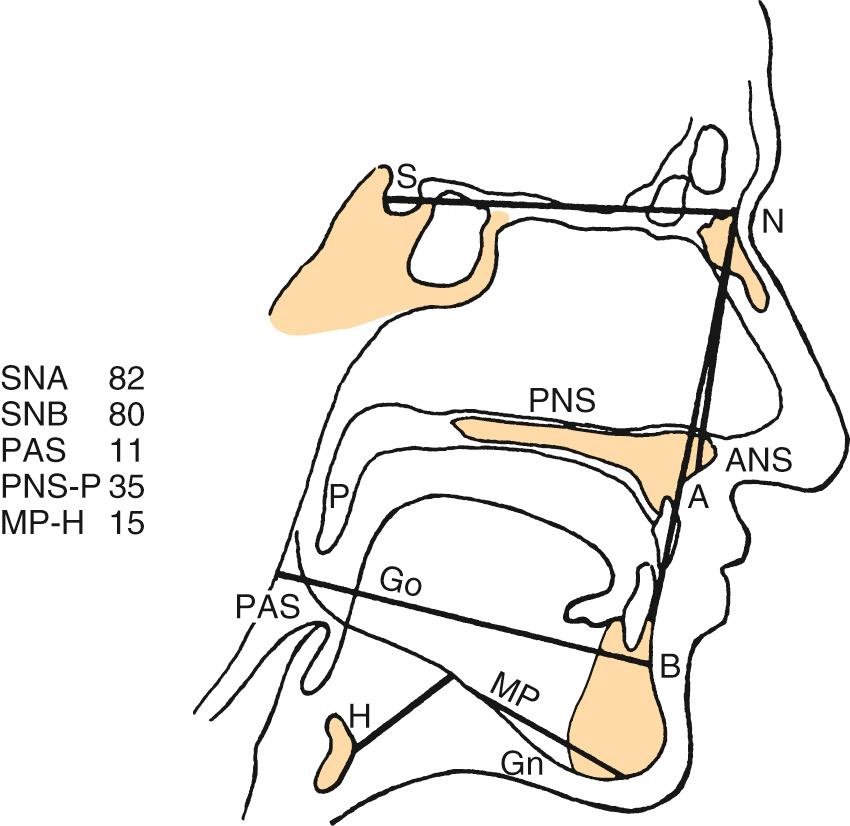
A number of radiologic techniques have been used to aid in the identification of the site and severity of upper airway obstruction or collapse in OSA. Historically, most of the radiologic techniques for evaluation of OSA were performed in awake patients. Thus limited information could be gained about obstruction during sleep. One such modality is the cephalometric radiograph. The cephalogram is a two-dimensional representation of the airway, a standardized evaluation system with broad availability and relatively low cost ( Fig. 15.7 ). These films provide information on both the bony skeleton and the overlying soft tissues. Multiple studies using cephalometry have confirmed that OSA patients have inferior displacement of the hyoid, a smaller posterior airway space, and longer soft palates than do non-OSA patients ( Fig. 15.8 ). Even so, the differences between OSA and non-OSA patients noted on cephalometry have not been significant enough to allow for the use of lateral cephalograms as a sole diagnostic tool.
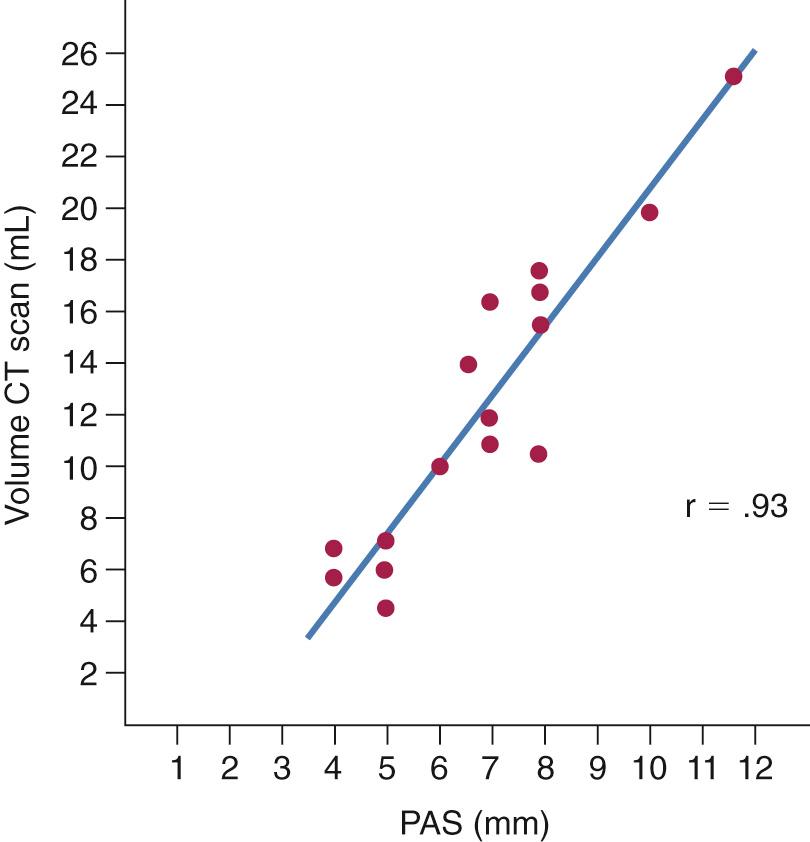
Awake computed tomography (CT) provides good anatomic detail of the bone and soft tissue. In general, like the cephalogram, awake CT has poor sensitivity for diagnosing OSA; however, multiple studies have demonstrated that it is a better modality to illustrate postoperative anatomic changes that correlate with improved PSG parameters. Recent advances in CT technology have allowed detection of dynamic upper airway changes during sedated sleep, which has been used to help guide multilevel sleep surgery. The primary advantage of this diagnostic modality over other methods is high anatomic resolution of dynamic airway movement during sleep without the presence of an endoscope that might potentially alter airflow. A similar technique has been used in children with Down syndrome to generate dynamic three-dimensional CT images of the upper airway during sedated sleep, with radiation doses comparable to standard facial CT scans.
Magnetic resonance imaging (MRI) provides excellent soft tissue differentiation and does not require radiation exposure. Similar to dynamic CT imaging, cineMRI was developed as a method to rapidly capture images at short time intervals to allow assessment of dynamic movement of soft tissues. This has been applied to the assessment of the upper airway in sedated patients with OSA, in both adults and children.
Lastly, fluoroscopy has been used as a dynamic airway examination to directly evaluate the sites of obstruction. Somnofluoroscopy, a study done while the patient is asleep, has been shown to improve UPPP success rates, when the site of initial obstruction is identified (67% vs. 42%); however, these studies are time intensive and expose patients to increased radiation, which limits their utility.
Nocturnal PSG is the gold standard for diagnosis of OSA. A full-night diagnostic study is considered to be the most accurate instrument for measuring the presence and severity of OSA. The parameters monitored during PSG include electroencephalogram, electrooculogram, submental electromyogram, electrocardiogram, airflow, thoracoabdominal effort, and oximetry ( Box 15.5 ). The information obtained from the study is analyzed by a trained PSG technologist and interpreted by a sleep physician. Classification of disordered breathing events recorded during PSG have been standardized to guide practice ( Figs. 15.9 through 15.11 ). Recent guidelines have recommended that HSAT can be used instead of in-lab PSG in uncomplicated adults with signs and symptoms indicating an increased risk of moderate to severe OSA. However, because HSAT tends to be less sensitive than PSG with a high false negative rate, if a HSAT is negative or inconclusive, then in-lab PSG should be performed. Sleep studies are traditionally categorized into Types I-IV according to the number and type of sensors utilized ( Table 15.3 ). Type II studies use the same sensors as a full PSG but are unattended and therefore portable. An important distinction among the portable studies is that because type III and IV studies do not include EEG recording, wakefulness and sleep stage cannot be ascertained. Therefore, AHI cannot be accurately determined, but a minimum estimate can be made based on the available data.
Electroencephalogram (C3 or C4, O1 or O2, F1)
Electrooculogram
Nasal and oral airflow monitors: thermistors, nasal pressure transducers, or inductance plethysmography
Submental electromyogram
Anterior tibial electromyogram
Body-position monitors
Chest respiratory effort monitor
Abdominal respiratory effort monitor
Electrocardiogram
Pulse oximetry
Tracheal microphone
Optional:
End-tidal carbon dioxide monitor
Esophageal pressure monitor
Nasal continuous positive airway pressure and bilevel positive airway pressure
| Type | Portability | Channels (n) | Signals | Airflow/Effort Channels | Differentiates Wake/Sleep | Measures AHI |
|---|---|---|---|---|---|---|
| I | In-lab | 14–16 | EEG, EOG, EMG/ ECG/HR, SaO 2 | Airflow and effort | Yes | Yes |
| II | Portable | 7 | EEG, EOG, EMG, ECG/HR, SaO 2 | Airflow and effort | Yes | Yes |
| III | Portable | 4–6 | ECG/HR, SaO 2 | Airflow and/or effort | No | No |
| IV | Portable | 1–3 | Usually only HR and SaO 2 | none | No | No |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here