Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Flap survival depends on adequate perfusion to meet the metabolic demands of the mobilized tissue.
The primary insult that affects flap survival is impaired vascular supply and the resultant ischemia.
Flap survival is the rule in the majority of flaps that are carefully designed and are accomplished in healthy patients.
Careful clinical evaluation and monitoring methods must be used in at-risk cases to identify failing flaps as early as possible, so flap salvage methods can be used.
Microvascular free flaps are becoming more commonly used for head and neck reconstruction; their survival can be optimized by coordinated care and monitoring.
Wound healing is a dynamic process that requires a series of tightly coordinated events for proper wound healing to occur.
Acute wounds can fail to heal for many reasons. An acute wound becomes a chronic wound when healing is delayed by more than 3 weeks or when a wound fails to return to functional and anatomic integrity in a timely fashion.
Advances in cellular and molecular biology have increased our understanding of wound physiology and have allowed for manipulation of host factors to aid in the healing process.
The most important aspect of planning and execution of a flap is ensuring vascular supply. All flaps, including pedicled flaps and free tissue transfer, depend on the maintenance of adequate perfusion to meet the metabolic demands of mobilized tissue for a period of time. An understanding of flap physiology and vascular anatomy allows the surgeon to achieve an optimal outcome. Often the distal flap is the most important region of the flap in providing coverage for a defect, but the distal flap is also most likely to fail. In free tissue transfers, flap survival is often an all-or-none proposition; although free flaps provide reconstructive options that were not possible with pedicled flaps, free flap failure can be disastrous. When flaps fail, the resultant defect is typically worse than if there was no attempt at all. Certain risk factors such as diabetes or irradiated tissue increase the chance of failure. Some methods to augment flap survival can be used to maximize the chance of a favorable outcome. Thus, a surgeon must know and employ every advantage possible in using a flap to close a defect; in some cases, a minimalist approach is the best option. This section will review the physics of flow and flap physiology and anatomy, which will help achieve an optimal clinical outcome.
In a homogeneous fluid that exhibits equal shear stress at different rates of shear, flow (Q) in tubing can be approximated by the Hagen-Poiseuille equation:
where r equals the fourth power of the vessel radius; Δ P is the difference between the proximal pressure and end pressure; η equals the dynamic viscosity; and l equals the tube length. For consideration of tissue pressure, Δ P can be further defined as:
where the difference in pressure that determines perfusion to the tissues is the difference between P DP , diastolic blood pressure, and P TP , tissue pressure. Tissue pressure approximates end intravascular pressure in most cases, but more closely describes the clinical value of perfusion pressure, also defined as P DP − P TP . Perfusion pressure (PP) is used extensively for management of cerebral edema and compartment syndrome.
Based on Ohm's law ( Q = Δ P /Resistance), resistance to flow in vessels can be calculated and simplified by removing the constants:
Basically, all clinical efforts should be focused on maintaining normal PP and a low resistance to flow. Thus, systemic blood pressure must be maintained (avoid hypotension and vasoconstriction, and assess vascular disease or injury), tissue pressure must be kept low by avoiding excess tension on the closure, excess tissue edema due to periods of ischemia, inflammation, or fluid overload. Resistance to flow is most affected by vessel radius. Large vessels and low resistance are ensured by designing the flap based on large blood vessels, when present, developing collateral flow when time allows by using flap delay, or by bringing large vessels into the field by free tissue transfer. Blood viscosity can be kept low by hydration and a low to normal hematocrit. Resistance to flow increases with the length of a tube—the length of a flap is well recognized to affect survival.
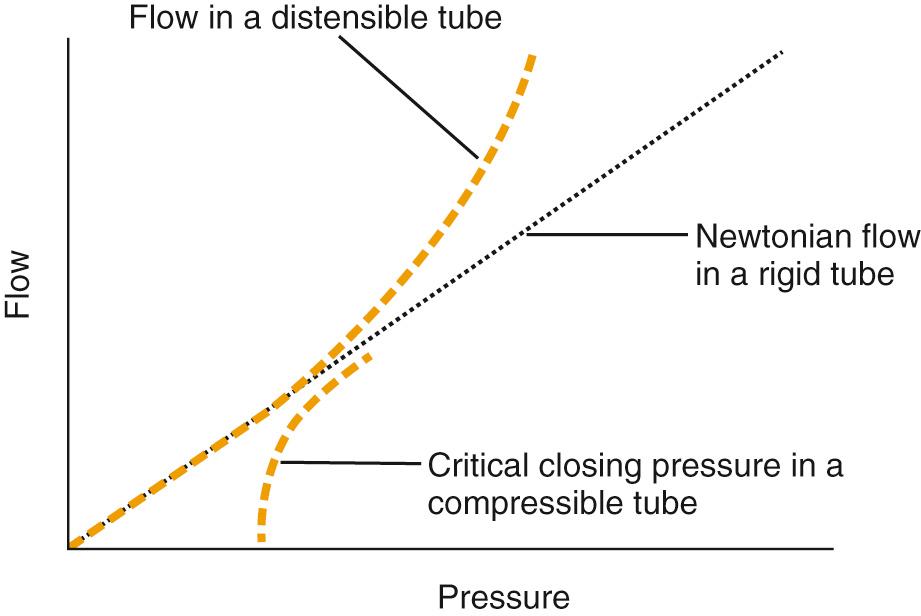
Critical closing pressure is a time-honored concept based on the idea that there is a point at which capillary blood flow will cease; this occurs when tissue pressure exceeds intracapillary pressure. It has been used in cerebral studies. LaPlace's law, which states that surface tension on the walls of a vessel increases with vessel radius, has been used to explain critical closing pressure, but LaPlace's law better explains dilation of blood vessels, and over time, production of collateral blood flow (see Fig. 77.1 ). According to Hagen-Poiseuille, flow in a rigid tube increases linearly as the intralumenal pressure difference between the original and end pressure increases. This occurs because blood vessels are distensible, causing an exponential rise in flow as described by LaPlace. When tissue pressure is high, capillary blood vessels collapse, causing blood flow to stop as described by the critical closing pressure. Relaxation of vessel walls occurs over time, and collapse of vessels can occur immediately.
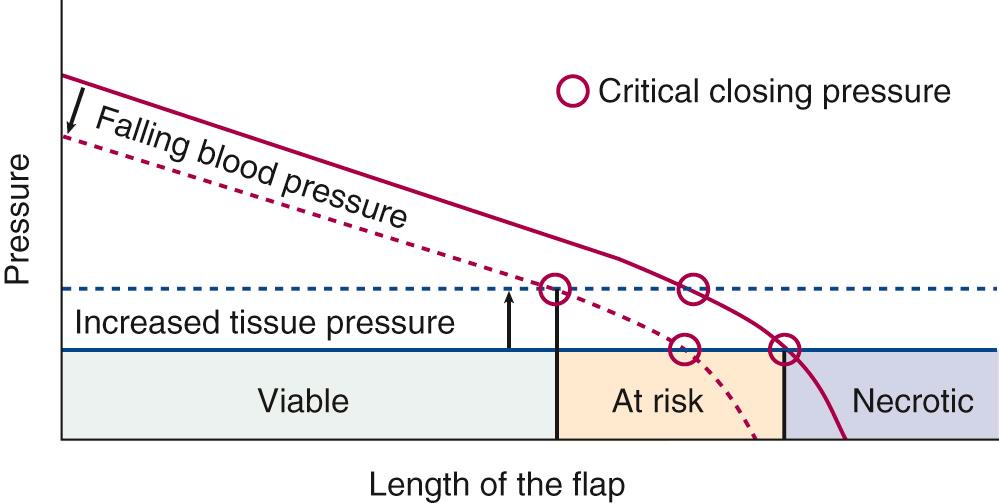
In the past, random cutaneous flaps were often designed according to a desired length-to-width ratio, a wider base being needed to successfully transfer a longer flap. The wider random flap includes only additional vessels with the same perfusion pressure. The relationship between perfusion pressure and critical closing pressure is not altered, and there is no change in survival length.
Clinically, these relationships are all intertwined. For example, raising a short flap is good, but putting too much tension on it can result in flap loss. Maintaining low viscosity may also result in fluid overload or reduced oxygen-carrying capacity of the blood. The fall in intravascular pressure along the flap length is the greatest in small vessels, and in high flow states, which are possible if arteriovenous shunting is present. Elevated tissue pressure will cause perfusion pressure to fall below the threshold for viability, and necrosis at the tip of the flap (see Fig. 77.2 ). All these factors and their interactions must be considered to optimize flap survival.
Skin flap biomechanics are governed principally by collagen and elastin. The interplay of these components has a direct influence on the results of flap wound closure. Collagens I and II constitute the principal structural framework of the extracellular matrix (ECM). Meanwhile, elastic fibers are primarily responsible for the elasticity and compliance of the skin. When skin is stretched, the fine elastin network initially allows easy deformation. This is demonstrated on the stress-strain curve. As patients age, there is a progressive decrease in elastic fibers. Thus, less force is necessary to produce lengthening. Older patients can therefore be expected to have less wound tension. As noted earlier, excessive tension in skin flaps is associated with greater flap necrosis. This is probably secondary to a drop in blood flow with increasing wound tension. Wound tension is an important factor in flap survival and scar formation.
Creep refers to the increase in strain (change in length relative to original length) seen when a constant stress (force) is applied to the skin. Stress relaxation , on the other hand, refers to the decrease in stress that occurs when skin is held under tension at a constant strain. Thus, the physiology of skin is altered over time by the application of constant stress. These properties are responsible for the effects seen with tissue expansion and serial excisions of lesions. Flap undermining is routinely performed during flap elevation and wound closure. It is the release of the attachments of the skin to the underlying tissue that results in a decreased wound tension when undermining is performed.
Maintenance of cell function and viability is the objective of the circulatory system at macrocirculatory, capillary, interstitial, and cellular levels. The importance of tissue vascularity is well recognized, but the complexity and diversity of tissue perfusion mechanisms require that the process be divided into components. Each level of perfusion has unique physiologic characteristics, and optimal management is possible only if these differences are recognized. Johnson and Barker described two levels of risk zones for flap failure, making a distinction between thrombosis at the arterial and capillary levels. By extension of this concept, four zones of the circulatory system can be considered for all hazards ( Fig. 77.3 ). Proper function of each zone is crucial to tissue viability.
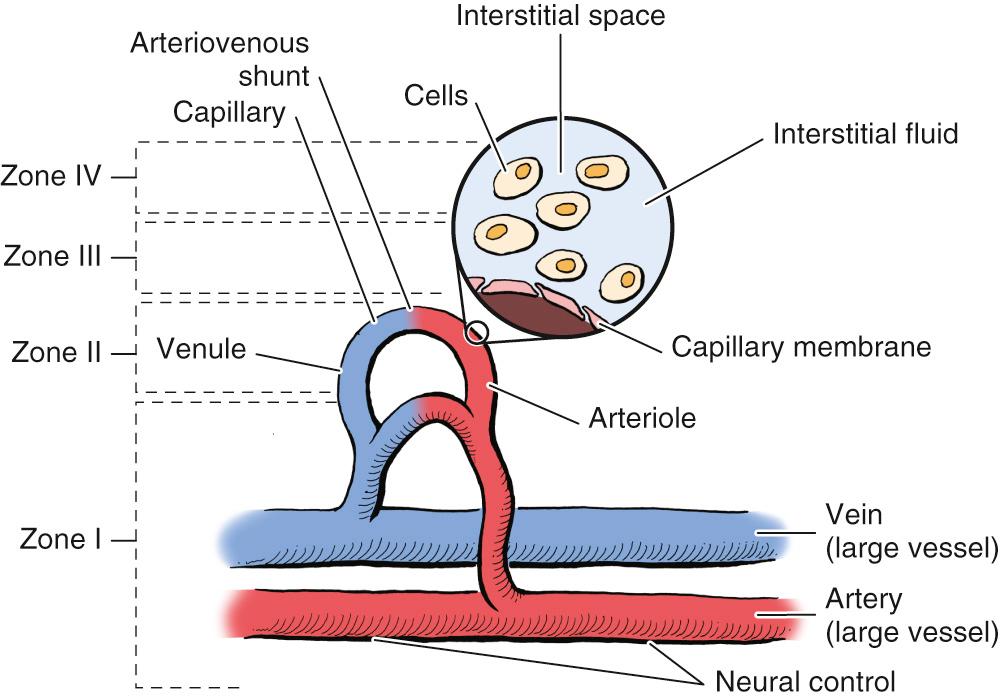
The macrovascular system consists of the cardiopulmonary system, the conduits for blood flow (arteries and veins), arteriovenous shunts, neural control of those conduits, and lymphatic vessels. The flow in Zone I is nonnutritive in that there are no mechanisms for exchange between the tissue and blood. Zone I has historically been recognized as essential for flap survival. The delay phenomenon is primarily a Zone I effect. The development of the pedicled flap underscores the importance of Zone I in extending flap survival time. Free microvascular tissue transfer is also a Zone I manipulation.
The capillary system comprises the microcirculation, which includes the arterioles, venules, capillaries, and lymphatic buds. This is the site of nutrient exchange between the blood and interstitial space. The importance of the capillary circulation, Zone II, is shown by the “no-reflow phenomenon,” wherein a loss of nutritive blood flow occurs in the presence of an adequate vascular supply. Critical closing pressure is also a Zone II phenomenon.
Zone III includes the interstitial space and its mechanisms of nutrient delivery. Nutrients and waste removal occur by diffusion and convection. Failure of metabolites to enter and traverse the interstitial space can result in loss of cell viability, and these processes are affected by raising a flap.
The cell and its membranes are the final step in maintaining cell viability. Ischemia changes cell membrane function. Drug treatment to optimize flap viability may require getting the drug into the cell, so failure of therapeutics, especially large agents or biologics, could be due to failure of this final step.
This zone classification framework will be referenced throughout the chapter. Survival of the flap is survival of the cells, so Zones I to IV must all be considered to optimize clinical outcomes.
The skin serves as a sensory organ and a protective organ. The epidermal layers are derived from ectoderm and are largely impermeable to gases and most liquids. The epidermis is a metabolically active, but avascular, stratified squamous epithelium. Melanocytes derived from neural crest cells are also found in the epithelium of skin and constitute a second cell type.
The neural supply to the skin originates from sensory nerves and sympathetic nerves. The sensory nerves are distributed in segmental fashion, forming dermatomes, and participate in the skin's protective function.
Blood vessels travel by one of two main routes to terminate in the cutaneous circulation. Musculocutaneous arteries pass through the overlying muscle to which they provide nutrition; septocutaneous arteries (also referred to as direct cutaneous arteries) travel through fascial septa that divide the muscular segments ( Fig. 77.4 ).
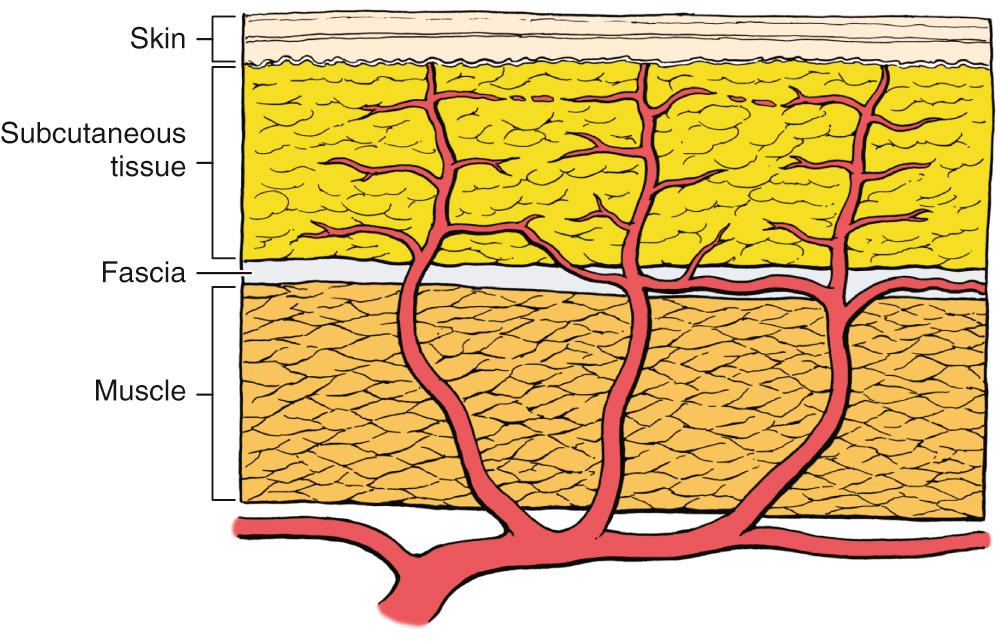
The cutaneous portion of a septocutaneous artery typically runs parallel to the skin surface, providing nutrition to a large area of skin. Septocutaneous arteries are usually accompanied by pairs of veins and run above the superficial muscular fascia. The more common musculocutaneous arteries leave the muscle and enter the subcutaneous tissue to supply a smaller region of skin. Septocutaneous and musculocutaneous arteries empty into a diffuse interconnecting vascular network of dermal and subdermal plexuses that provide a redundancy in the vascular supply to the skin. A collateral blood supply supports the vascular territory of each artery. Vascular tone is controlled by the autonomic neural system.
At the cutaneous levels, the dermis contains nerves, blood vessels, lymph vessels, and the base of the epidermal glandular appendages. The outer papillary layer of the dermis is characterized by a highly developed small vessel network. The reticular dermis and subcutaneous layer are less vascular. There is generally a subdermal vascular plexus.
The cutaneous capillary system and the arteriovenous shunts serve the two important functions of nutritional support and thermoregulation. Primarily because of its thermoregulatory function, the rate of blood flow through the skin is one of the most variable in the body. The postganglionic terminals of cutaneous sympathetic nerves contain the neurotransmitter norepinephrine and are found in the area of cutaneous arterioles.
Before entering the capillary bed, the arterioles branch into small vessels (e.g., terminal arterioles or metarterioles) that are surrounded by a discontinuous layer of smooth muscle. A simple ring of smooth muscle forms a sphincter at the point where the capillaries originate from the metarteriole. This sphincter can completely stop blood flow within the capillary. The capillary bed can be bypassed by arteriovenous shunts that allow the arterioles to empty directly into venules. During conditions of adequate systemic vascular pressure, preshunt and precapillary sphincters regulate the distribution of cutaneous blood flow. The preshunt sphincters are involved in regulating the changes in blood flow that affect thermoregulation and systemic blood pressure by vasoactive substances.
The precapillary sphincter controls the amount of nutritive blood flow to the skin, mainly as a thermoregulatory function. Capillary blood flow is also affected by elevated interstitial pressure, which can compress the capillary and decrease transcapillary flow. Conversely, as pressure decreases in the interstitium, the capillary expands and flow increases. The normalization of capillary blood flow is called autoregulation . Lymphatic vessels form a plexus running parallel and deep to the network of blood capillaries. The lymphatic capillaries, which end in blind sacs, conduct extracellular fluid back into the blood stream. Lymphatic function is affected by inflammation and loss of blood vessel pulsations. Blood viscosity will affect capillary flow and is influenced by the hematocrit, serum proteins, temperature, erythrocyte deformability, aggregation, and other factors.
The interstitial space is filled with proteoglycans and collagen. In many tissues, hyaluronic acid filaments make up the interstitial ground substance. These filaments are normally woven through the interstitium, producing a medium that exhibits high resistance to fluid movement unless the tissue is well hydrated. In tissues with excess edema, there may be planes of free fluid within the interstitium, which manifests itself clinically in pitting edema.
The makeup of interstitial fluid is determined by capillary permeability. Two processes by which substances can move across the interstitial space are diffusion and convection. Diffusion is probably the most important mechanism of mass transfer in the interstitial space, especially for small molecules during conditions of normal function. Large molecules diffuse more slowly than small ones. As edema occurs, diffusion distances increase, and interstitial concentrations can decrease significantly.
Convective flow, or bulk flow, is another way for molecules to move across the interstitium. Rather than diffusing to a lower concentration, an agent is swept along with the current of fluid that flows across the interstitial space in microchannels. The agent then diffuses the short distance from the microchannels to the cells.
Convective flow of nutrient-laden plasma out of the capillaries and across the interstitial space is produced by a combination of hydrostatic and osmotic pressures as described by Starling in 1896. The equation is as follows:
In descriptive terms, flow of plasma out of the capillary per unit area ( Jv / A ) is related to the product of the water permeability of the membrane ( Lp ) multiplied by the difference between hydrostatic pressure in the capillary ( P c ) and interstitial space ( P i ), minus the difference between osmotic pressure in the capillary (π c ) and the osmotic pressure in the interstitial space (π i ). The osmotic pressures are adjusted by the osmotic coefficient, (σ), a measure of the “leakiness” of a semipermeable membrane.
In functional terms, over the length of the capillary, the balance of hydrostatic and osmotic pressure favors outflow of fluid on the arteriolar end and inflow of fluid on the venular end of the capillary system. Pressure gradients are also responsible for bulk flow of fluid across the interstitial space in fluid microchannels. Elevated hydrostatic tissue pressure can prevent any nutritive outflow while flow through the capillary is maintained. Progressive increases in hydrostatic interstitial pressure can compress the venules and lymphatics, and eventually the capillaries and arterioles. A net increase of 2 mOsm in the interstitial space as a result of hyperosmosis of injury is sufficient to increase interstitial pressure beyond transmural capillary pressure, thereby compressing the capillary. Increased venous pressure limits interstitial resorption of fluid and contributes to formation of an interstitial transudate. Inflammation in a flap is probably a result of nociceptor pathway stimulation by handling of the flap. Ischemia also produces inflammation. Capillary hyperpermeability has been found throughout ischemic flaps. The lymphatic system has two functions that affect Zone III: to remove excess fluid and to remove interstitial protein. If the lymphatic system is malfunctioning, interstitial fluid and interstitial protein accumulate, which increases interstitial osmotic pressure.
The intracellular space is the endpoint for nutrient transport and the origin of metabolic waste. The cell wall is a lipid bilayer and the major barrier to movement of solutes across the membrane. Specific membrane proteins maintain cell homeostasis. Osmotic pressure in the cell is maintained to preserve normal cell volume by the sodium-potassium pump. A loss of energy substrate (oxygen and consequently adenosine triphosphate, or ATP ) produces an intracellular movement of sodium and an increase in intracellular osmotic pressure. These changes occur quickly, within 10 minutes after arterial occlusion. Within seconds of hypoxia, levels of ATP begin to decrease, and cells begin to swell. Relatively brief periods of ischemia result in a reversible swelling of the cell. If the ischemic insult is severe and prolonged, cell lysis and flap necrosis occur.
A number of changes detrimental to skin survival occur when a cutaneous flap is created. The primary insult affecting flap survival is impaired vascular supply and the resultant ischemia.
Partial interruption of the vascular supply (Zone I) to the skin is the most obvious and critical change that occurs with elevation of a cutaneous flap. Flap survival depends on the severity of ischemia and the amount of time before recovery of nutrient blood flow. Generally, tissues can tolerate 6 hours of complete ischemia. The ischemia time during the transfer of free flaps (called primary ischemia) appears to hinder the flap's ability to tolerate a second ischemic insult. The longer the period of perfusion after the microvascular anastomosis (called the reperfusion period), the better a flap will tolerate secondary ischemia.
Limitation of the vascular supply results in a local decrease in perfusion pressure to the skin. In arterial or myocutaneous flaps, the blood supply to the skin overlying the vascular pedicle is usually adequate. In random flaps or random extensions of flaps, the decrease in perfusion pressure becomes more pronounced with increasing distance from the base of the flap. When perfusion is reduced in one area of a random flap, the adjacent vascular territories supplied by a separate perforating vessel can provide a low-pressure blood supply through the subdermal plexus. A number of vascular territories can be compromised before necrosis results.
In free flaps, a secondary ischemia can occur when thrombus forms at the anastomosis. Porcine ventral arterial island flaps initially exposed to 2 hours of ischemia followed by 12 hours of blood flow had a 50% survival rate after a subsequent 7.2 hours of “secondary ischemia.” The same researchers found that 13 hours was required for the initial ischemic event to produce a 50% survival rate. Anastomosis failure is related to exposure of the vascular intima to platelets. Platelet activation is caused by superficially exposed subendothelium or, with deeper vessel injury, exposure to collagen types I and III. The geometry of the vascular arrangement is often a critical factor when experienced surgeons lose a free flap. The venous outflow from the skin is also impaired with flap elevation. Venous flow can occur through the subdermal plexus or via venous channels that accompany the feeding artery in the pedicle. Complete venous occlusion in the early postelevation period may be more damaging to flap survival than inadequate arterial supply. Fortunately, the subdermal plexus alone is often able to provide adequate venous outflow.
Sensory and sympathetic nerves are often severed in the process of flap elevation. Although loss of sensation may limit the usefulness of the flap after transfer, adrenergic denervation has implications for flap survival, producing vasocontriction. The stored transmitter is depleted within 24 to 48 hours, and blood flow increases as the concentration of norepinephrine declines. In critical areas of the flap, the time to recovery of nutrient blood flow may be delayed sufficiently to produce additional necrosis.
Necrosis in flaps is caused by prolonged loss of nutritional blood flow. Erythrocyte sludging has frequently been noted in capillaries undergoing ischemia, often without thrombus formation. The reason for the erythrocyte sludging is multifactorial. Erythrocytes become turgid and lose their flaccid, biconcave disk shape in the acidic environment of ischemia. Narrowing of the capillaries, presumably a result of external compression, also contributes to stacking of erythrocytes within the lumen of ischemic tissue capillaries. Sludging is seen less often if the hematocrit value is kept below 30%. Therefore, sludging is not necessarily caused by platelet activation unless a microvascular anastomosis or other vessel injury is involved, such as tearing, stretching, laceration, or crushing. Capillary blood flow is further hindered by leukocyte adherence to the capillary wall. Ischemic cells of all tissues swell because of the loss of energy substrate needed to continue maintaining the osmotic gradient. Swelling of extravascular cells causes compression of the vascular lumen, but endothelial cell swelling has a greater effect on capillary blood flow.
Impairment of lymphatic drainage with flap elevation also occurs, which leads to increased interstitial pressure, which decreases capillary perfusion. Alterations in the Starling forces result in further ischemic swelling of cells and the interstitial space, setting a positive feedback circle in motion.
The surgical trauma and ischemia associated with an acutely raised flap result in an inflammatory response. Histamine, serotonin, and kinins are released into the extracellular compartment after flap elevation, increasing the permeability of the microcirculation. The result is a rise in the concentration of proteins and cells within the extracellular space. The presence of nonbacterial inflammation beginning a few days before flap elevation has been shown to improve flap survival, presumably as a result of an increase in local blood flow. The inflammation created during flap elevation may have deleterious effects because of the resultant edema formation. Prostaglandins, thromboxane synthetase, and glucocorticoids all play a role in the inflammatory response after flap surgery.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here