Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The skeletal system is formed from highly specialised types of supporting/connective tissue. The tissues are made up of collagen and acellular matrix, as well as the cells which synthesise them. Bone provides a rigid protective and supporting framework, the rigidity resulting from the deposition of calcium salts within the collagen and matrix. Cartilage occurs in different forms and provides a smooth articular surface at bone ends, as well as structural support in special areas (e.g. trachea, pinna). It is also important in one form of new bone formation.
Joints are composite structures which join the bones of the skeleton and, depending on the function and structure of individual joints, permit varying degrees of movement.
Ligaments are robust but flexible bands of collagenous tissue which contribute to the stability of joints. Tendons provide strong, pliable connections between muscles and their points of insertion into bones.
The functional differences between the various tissues of the skeletal system relate principally to the different nature and proportion of the ground substance and fibrous elements of the extracellular matrix. The cells of all the skeletal tissues, like the cells of the less specialised supporting/connective tissues, have close structural and functional relationships and a common origin from primitive mesenchymal cells (see Ch. 4 ).
CARTILAGEThe semi-rigid nature of cartilage stems from the predominance of proteoglycan ground substance in the extracellular matrix.
Proteoglycans (see Ch. 4 ), disposed in proteoglycan aggregates of 100 or more molecules, make up the ground substance and account for the solid, yet flexible, consistency of cartilage. Sulphated glycosaminoglycans (GAGs, chondroitin sulphate and keratan sulphate) predominate in the proteoglycan aggregates, with molecules of the non-sulphated GAG hyaluronic acid forming the central back-bone of the complex. The different types of cartilage vary in the amount and nature of fibres in the ground substance: hyaline cartilage contains few fibres, fibrocartilage contains abundant collagen fibres and elastic cartilage contains elastin fibres.
Cartilage formation commences with the differentiation of stellate shaped primitive mesenchymal cells (see Fig. 4.2 ) to form rounded cartilage precursor cells called chondroblasts . Subsequent mitotic divisions give rise to aggregations of closely packed chondroblasts which grow and begin synthesis of ground substance and fibrous extracellular material. Secretion of extracellular material traps each chondroblast within the cartilaginous matrix, thereby separating the chondroblasts from one another. Each chondroblast then undergoes one or two further mitotic divisions to form a small cluster of mature cells separated by a small amount of extracellular material.
Mature cartilage cells, known as chondrocytes , maintain the integrity of the cartilage matrix. Most mature cartilage masses acquire a surrounding layer called the perichondrium , composed of collagen fibres and spindle-shaped cells which resemble fibroblasts. These have the capacity to transform into chondroblasts and form new cartilage by appositional growth . There is also very limited capacity in mature cartilage masses for interstitial growth . This occurs by further division of chondrocytes trapped within the previously formed matrix and subsequent deposition of more matrix material. The hyaline cartilage of the articular surfaces of joints does not have perichondrium on the surface and has no capacity to regenerate new cartilage after damage. In general, mature cartilage has a very limited capacity to repair and regenerate, partly because of its poor blood supply.
Most cartilage is devoid of blood vessels and, consequently, the exchange of metabolites between chondrocytes and surrounding tissues depends on diffusion through the water of the ground substance. This limits the thickness to which cartilage may develop while maintaining viability of the innermost cells. In sites where cartilage is particularly thick (e.g. costal cartilage), cartilage canals convey small vessels into the centre of the cartilage mass.
The role of cartilage in bone formation is discussed in Figs 10.18 to 10.21 .
The various different forms of cartilage are specialised to perform distinct functions. The nature of the extracellular matrix differs in these subtypes.
Hyaline cartilage is found on the articular surfaces of many joints, lying over the surface of the bone ends and forming a smooth, firm, but slightly flexible surface. Hyaline cartilage has very abundant gel-like ground substance.
Fibrocartilage is found in the intervertebral discs as well as in sites such as the pubic symphysis. Fibrocartilage resembles dense connective tissue and has very abundant collagen fibres but with intervening bands of extracellular matrix.
Elastic cartilage is important in the structure of the external ear and the larynx, being firm but very flexible due to the presence of many elastin fibres within an extracellular matrix which is similar to that of hyaline cartilage.
Cb chondroblast Cc chondrocyte L lipid droplet M cartilage matrix N nucleus P perichondrium
Bone is composed of cells and a predominantly collagenous extracellular matrix (type I collagen) called osteoid which becomes mineralised by the deposition of calcium hydroxyapatite , thus giving the bone considerable rigidity and strength. The cells of bone are:
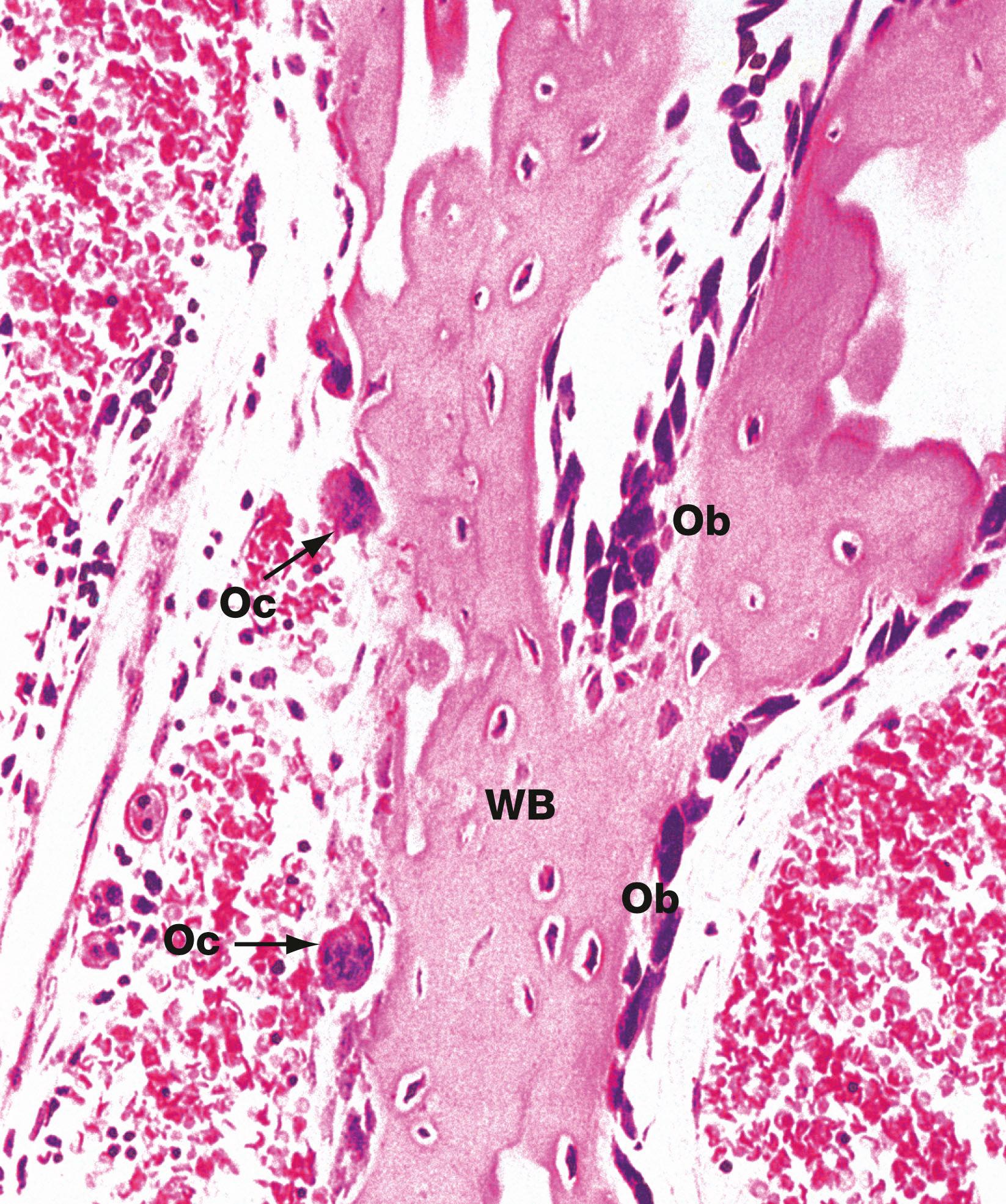
Osteoblasts which synthesise osteoid and mediate its mineralisation. These are found lined up along bone surfaces.
Osteocytes which represent largely inactive osteoblasts trapped within formed bone. These may assist in the nutrition of bone.
Osteoclasts are phagocytic cells which are capable of eroding bone. These are important, along with osteoblasts, in the constant turnover and refashioning of bone.
Osteoblasts and osteocytes are derived from a primitive mesenchymal (stem) cell called the osteoprogenitor cell .
Osteoclasts are multinucleate phagocytic cells derived from the macrophage–monocyte cell line.
Bone forms the strong and rigid endoskeleton to which skeletal muscles are attached to permit movement. It also acts as a calcium reservoir and is important in calcium homeostasis. Bone is heavy and its architecture is optimally arranged to provide maximum strength for the least weight. Most bones have a dense, rigid outer shell of compact bone, the cortex , and a central medullary or cancellous zone of thin, interconnecting narrow bone trabeculae. The number, thickness and orientation of these bone trabeculae is dependent upon the stresses to which the particular bone is exposed. For example, there are many thick intersecting trabeculae in the constantly weight-bearing vertebrae, but very few in the centre of the ribs, which are not subjected to constant stress. The space in the medullary bone between trabeculae is occupied by haematopoietic bone marrow (see Fig. 3.3 ).
C chondrocyte G Golgi apparatus H Howship lacuna M matrix material MM extracellular matrix material O osteoclast Ob osteoblast Oc osteocyte Os osteoid P perichondrium rER rough endoplasmic reticulum
The normal maintenance and refashioning of bone is the result of coordinated activity of osteoblasts depositing new bone and osteoclasts eroding redundant bone. Some diseases are the result of excessive unbalanced activity of one or other of the cell types, commonly osteoclasts.
In hyperparathyroidism, excessive uncontrolled secretion of parathyroid hormone by the parathyroid gland (see Fig. 17.11 ) stimulates an increase in numbers and erosive activity of osteoclasts. This leads to diffuse destruction of bone, producing radiological areas of lucency (‘brown tumours’) and predisposition to fracture. A serious side effect of excessive bone erosion is the release of large amounts of ionic calcium into the bloodstream, producing severe symptoms of hypercalcaemia .
Paget disease is a disease of unknown cause in which there is random and haphazard excessive osteoclastic erosion of bone occurring in waves, followed by increased osteoblastic activity attempting to replace eroded bone ( Fig. 10.6 ).
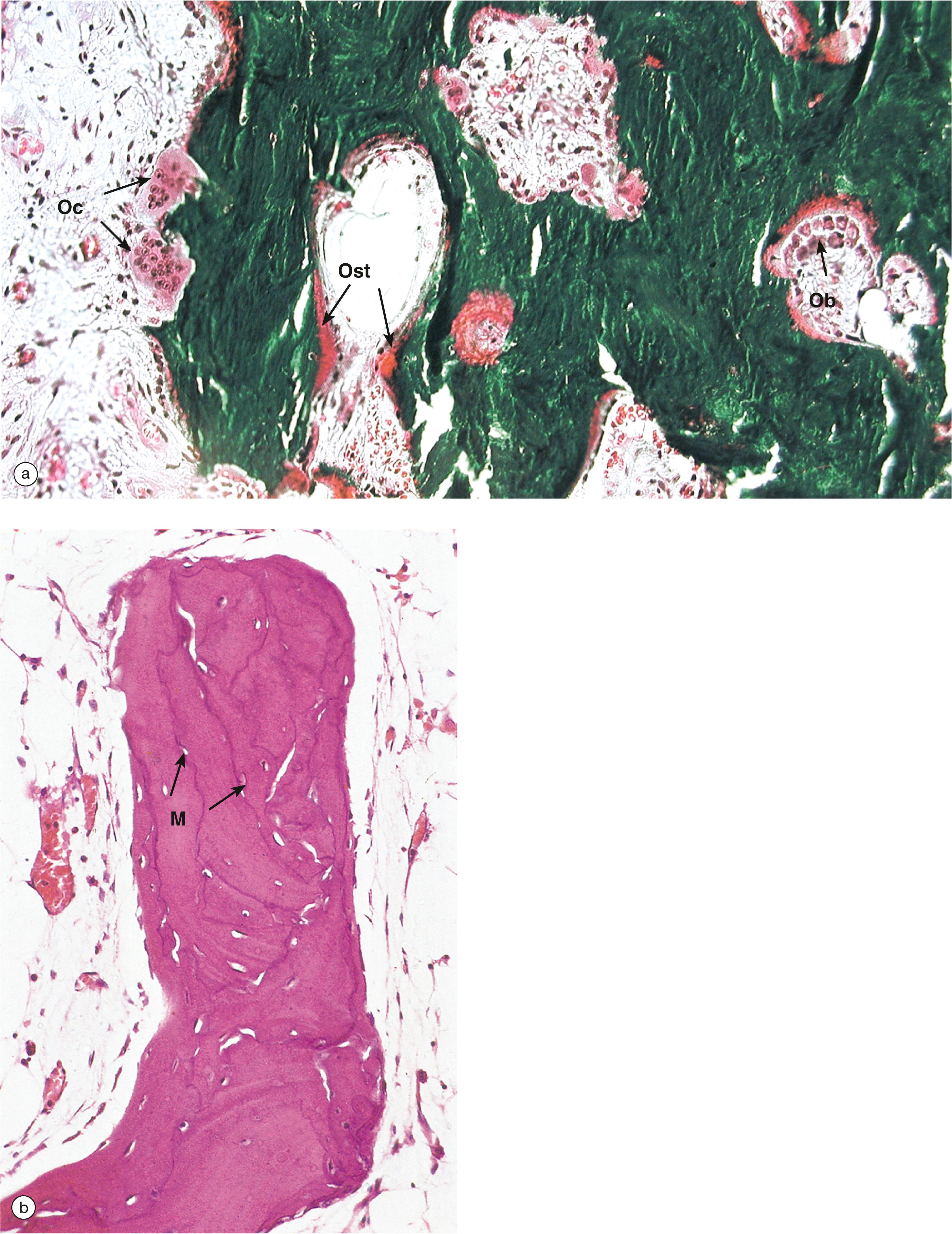
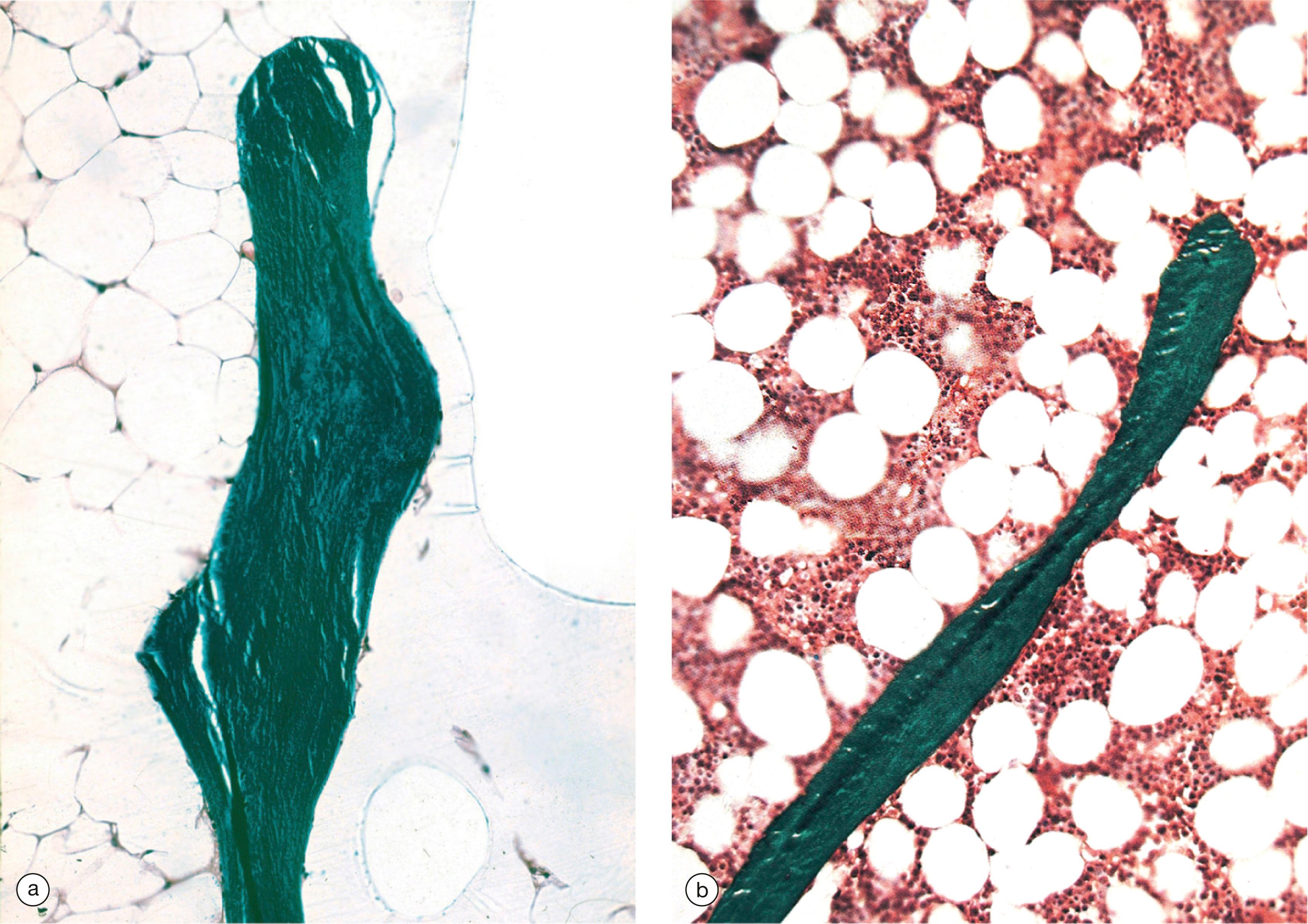
However, the new osteoid and bone formation does not always occur where bone has previously been eroded, so the architecture of the bone is grossly distorted (usually with woven bone , Fig. 10.7 ) and the bone is structurally weak.
Bone fractures (broken bones) are common and usually occur due to trauma. Sometimes fractures occur in abnormal or weakened bone, termed pathological fractures , and may be due to a wide range of underlying disorders, including osteoporosis , Paget disease and metastatic cancer.
Fractures can be described as closed or simple (with no damage to the overlying skin) versus open or compound (where there is an overlying skin wound exposing the broken bone). In comminuted fractures, the bone is broken into a number of pieces.
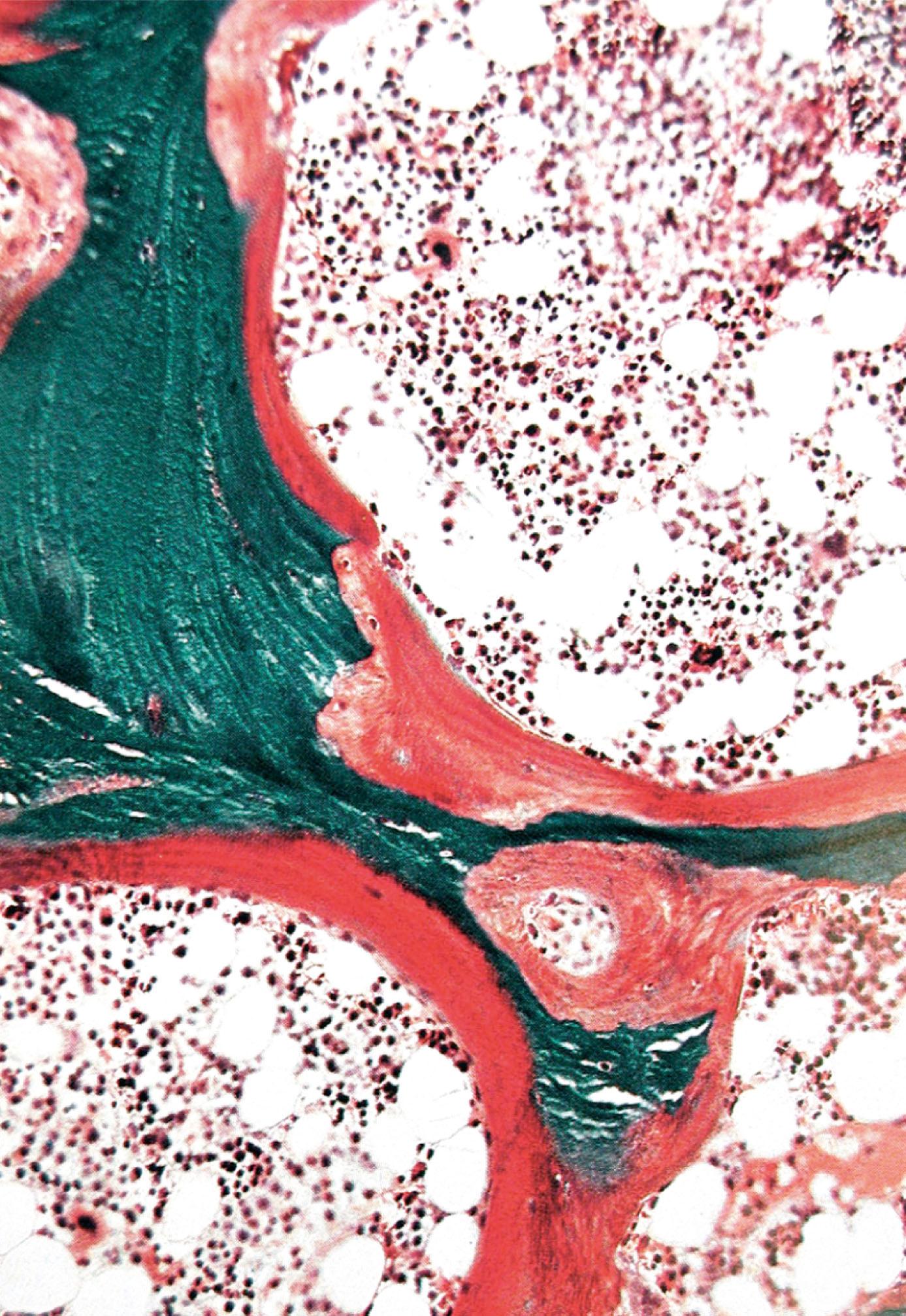
Healing and repair of fractures is a specialised process which involves the formation of callus between the damaged bone ends ( Figs 10.9 and 10.23 ). Bleeding occurs immediately at the fracture site to form a haematoma .
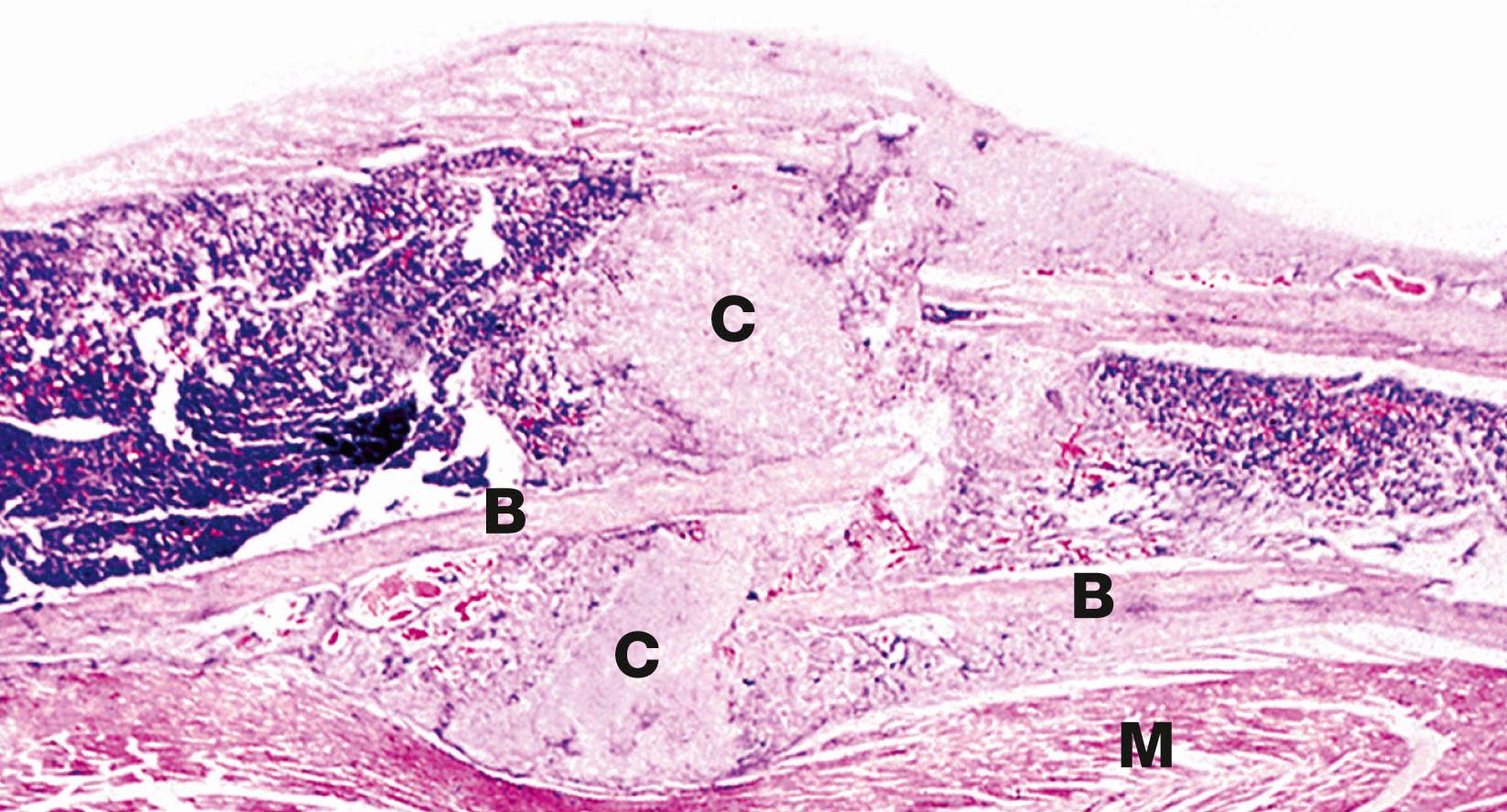
Fibroblasts and blood vessels then grow into this haematoma to form granulation tissue . The fibroblasts and other cells then develop into osteoblasts and chondroblasts, which produce woven bone and hyaline cartilage. This callus acts to temporarily unite the bone ends, and over time there is remodelling into mature lamellar bone.
B bone C callus L lamellar bone M striated muscle W woven bone
AC articular cartilage C canaliculus CB cortical bone CL cement line FM fatty marrow H Haversian system HC Haversian canal HM haematopoietic marrow I interstitial system L lacuna Ob osteoblast Oc osteocyte Op osteoprogenitor cell OCL outer circumferential lamellae P periosteum T trabecular bone V Volkman canal
Mature compact bone is made up of about 70% inorganic salts and 30% organic matrix by weight. Collagen makes up over 90% of the organic component, the remainder being ground substance proteoglycans and non-collagen molecules which appear to regulate bone mineralisation. The collagen of bone is almost exclusively in the form of type I fibres. Spaces within this three-dimensional structure, called hole zones , are the initial site of mineralisation.
Ground substance proteoglycans contribute a much smaller proportion of the matrix than in cartilage and mainly consist of chondroitin sulphate and hyaluronic acid in the form of proteoglycan aggregates. As well as controlling the water content of bones, ground substance is probably involved in regulating formation of collagen fibres. The remaining non-collagen organic material includes osteocalcin ( Gla protein ), involved in binding calcium during the mineralisation process, osteonectin , which may serve some bridging function between collagen and the mineral component, and sialoproteins .
The mineral component of bone mainly consists of calcium and phosphate in the form of hydroxyapatite crystals. These are conjugated to a small proportion of magnesium carbonate, sodium and potassium ions but also have affinity for heavy metals and radioactive pollutants.
Collagen and the other organic matrix constituents are synthesised by the rough endoplasmic reticulum of osteoblasts, packaged by the Golgi apparatus and then secreted from the cell surface, resulting in the production of osteoid . After a maturation phase lasting several days, amorphous (non-crystalline) calcium phosphate salts begin to precipitate in the hole zones of the collagen. These mineralisation foci expand and coalesce into hydroxyapatite crystals by further remodelling. Around 20% of the mineral component remains in the amorphous form, as a readily available buffer in calcium homeostasis. The concentration of calcium and phosphate ions in bony extracellular fluid is greater than that required for spontaneous deposition of calcium salts and a variety of inhibitors, including pyrophosphate , play a crucial role in controlling bone mineralisation. Deposition of calcium appears to be associated with membrane-bound vesicles derived from osteoblast plasma membrane called matrix vesicles . These contain the enzyme alkaline phosphatase and other phosphatases which may neutralise the inhibitory effect of pyrophosphate.
Osteomalacia is a disease which results from failure of normal mineralisation of newly formed osteoid . Successful mineralisation requires adequate concentrations of calcium and phosphate ions. If there is deficiency (e.g. due to poor diet, malabsorption, etc.), mineralisation of osteoid cannot take place and osteomalacia develops. Severe osteomalacia produces bone trabeculae which are only mineralised at their centre, the bulk being soft non-rigid osteoid. The bone is soft and prone to fracture.
The fetal development of bone occurs in two ways, both of which involve replacement of primitive collagenous supporting tissue by bone. The resulting woven bone is then extensively remodelled by resorption and appositional growth to form the mature adult skeleton, which is made up of lamellar bone . Thereafter, resorption and deposition of bone occur at a much reduced rate to accommodate changing functional stresses and to effect calcium homeostasis.
The long bones, vertebrae, pelvis and bones of the base of the skull are preceded by the formation of a continuously growing cartilage model which is progressively replaced by bone. This process is called endochondral ossification and the bones so formed are called cartilage bones .
In contrast, the bones of the vault of the skull, the maxilla and most of the mandible are formed by the deposition of bone within primitive mesenchymal tissue. This process of direct replacement of mesenchyme by bone is known as intramembranous ossification and the bones so formed are called membrane bones . Bone development is controlled by growth hormone, thyroid hormone and the sex hormones.
Achondroplasia is a common cause of dwarfism. Patients with achondroplasia have short stature, mainly due to a marked reduction in the lengths of the long bones of the limbs. The trunk and skull bones tend to be of more normal size. This reflects differences in bone development in these different parts of the skeleton.
The primary defect in achondroplasia affects the process of endochondral ossification, the type of bone development necessary for normal long bone growth. The processes of intramembranous and periosteal ossification are normal in achondroplastic individuals. As a result, those parts of the skeleton such as the skull which develop by intramembranous ossification are of normal size. Similarly, whilst limb bones tend to be short they are also broad, since growth in the diameter of long bones usually occurs via the process of subperiosteal new bone formation.
B woven bone FM fatty marrow HM haematopoietic marrow M primitive mesenchyme Ob osteoblast T bone trabecula V sinusoidal blood vessels
B newly forming bone C cortical bone CD zone of cartilage degeneration D diaphysis E epiphysis GP growth plate H zone of hypertrophy and calcification M zone of maturation O osteogenic zone P zone of proliferation R zone of reserve cartilage SC secondary ossification centre V blood vessels
Joints may be classified into two main functional groups, synovial and non-synovial , both of which may show wide morphological variations.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here