Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hematopoiesis is a cellular process in which self-renewing stem progenitor cells differentiate into mature blood cells, which carry out specific biologic functions. These functions include oxygen delivery, clot formation, and immune responses, including defense of the host from infection and inflammation. Homeostasis of the whole hematopoietic system in vivo requires a tight control of systems and networks governing proliferation, cell fate, cell death, differentiation, cell–cell interaction, and migration. Imbalance in or dysregulation of these processes results in pathologic alterations. For example, uncontrolled cell proliferation is a signature of leukemias, and defective lymphocyte differentiation can lead to immunodeficiency, or tumor immune responses will determine cancer progression. A better understanding at the molecular level of these biologic events will help to identify new therapeutic targets for the design of better drugs to treat hematologic diseases.
Because of the diversity in cellular types and their respective, specific biologic functions, hematopoietic cells respond to a broad array of extrinsic and intrinsic signals transduced through signaling and metabolic pathways. It is therefore important to recognize that these pathways serve to ultimately define a specific functional response and activity in each cell type. These regulatory signals ( Table 6.1 ) can be general, such as growth factors (e.g., insulin growth factor [IGF], fibroblast growth factor [FGF]) or amino acids that control proliferation, or highly specific, such as the antigen signaling response in immune cells or 2,3-diphosphoglycerate in erythrocytes. Importantly, the action of these signals, as well as their integration inside the cell, is needed to accomplish a specific cellular task (either a physiologic or cellular fate decision). Moreover, as will be discussed later in this chapter, these signals also serve to tightly control metabolites in hematopoietic cells, defining a metabolomic profiles involved in processes such as anaerobic glycolysis for energy generation in red blood cells (RBCs) or immune and inflammatory responses in tumors.
| Types of Ligands | Examples |
|---|---|
| Peptide or Protein | |
| Soluble | Growth factors or cytokine |
| ECM | Fibronectin, collagen |
| Cell surface bound | ICAM, Kit ligand |
| Small organics | Thyroid hormone |
| Nucleotides | |
| Soluble | ADP |
| DNA | Double-strand breaks |
| Lipids | Eicosanoids, LPS |
| Gases | H 2 O 2 , nitric oxide a |
Extrinsic cellular signals, often polypeptides, are recognized by plasma membrane receptors that trigger a phosphorylation cascade (using tyrosine and/or serine/threonine residues) that propagates through the cytoplasm and cellular organelles, including the nucleus. Thus the sequential activation of this cascade occurs in a temporal and spatial manner to define the specific biologic response. In general, there are two types of signals ( Fig. 6.1 ): (1) signals that transduce immediate- or short-term biologic outputs without changes in gene expression, and (2) signals that transduce medium- and long-term biologic outputs with changes in gene expression. In the first case, for example, chemoattractants induce the phosphatidylinositol 3-kinase (PI3K) and Cdc42 pathways to rapidly establish neutrophil polarity. One example in the second case is the signaling transduced through Frizzled (Fz) receptors and the transcription factor T cell–specific transcription factor (TCF)-1 necessary for T-cell development. Another example is the programmed death-1 (PD-1) ligand in tumor cells that binds to the PD-1 receptor in T cells controlling the immune response. In these cases, the signals transduced are amplified through a series of physical interactions and chemical modifications on proteins, the most common being phosphorylation, but others such as ubiquitination, acetylation, and sumoylation also play important roles.
In this chapter, a general survey of the different key signaling and changes in energy metabolism that operate in hematopoietic cells will be reviewed. The goal is to provide the molecular basis by which signals are transduced and control fundamental cellular processes, including energy metabolism, in different lineages of the hematopoietic system.
Hematopoietic cells use general signaling transduction pathways that are common to most human cell types. The specificity in these signaling transduction pathways is often established at the beginning of the pathway’s activation (e.g., by specific antigen-binding or ligand–membrane receptor complexes) ( Table 6.2 ) and at downstream targets, including transcription of the specific genes that will serve to define a particular biologic process and response (see Fig. 6.1 ). Here we will review these general signaling transduction pathways, illustrating some of the specific components of hematopoietic cells.
| Types of Receptors | Examples | Types of Ligands |
|---|---|---|
| RTK | Insulin, Kit, Fms | Kit ligand, M-CSF |
| RSK | TGF-β receptors | Activin, BMPs, TGF-β |
| GPCR | Thrombin receptor, CXC, CC receptors | Thrombin chemokines |
| PTK-associated MIRR | Cytokine receptors BCR/TCR/FcR | Epo, interleukins, IFN peptide/MHC, Fc domains |
| TNF family | Fas, TNFR, CD40 | Fas, TNF, CD40L |
| Notch | Notch | Delta-serrate-LAG-2 |
| Frizzled family | Wnt receptors | Wnts |
| Toll receptors | TLR1-10 | Bacterial DNA, LPS |
| RPTP | CD45 | Unknown |
| Nuclear receptors | AR, RAR | Testosterone, retinoids |
| Adhesion receptors | Integrins | Fibronectin, collagen |
Receptor tyrosine kinases (RTKs) are enzyme-linked receptors localized at the plasma membrane containing an extracellular ligand-binding domain, a transmembrane domain, and an intracellular protein–tyrosine kinase domain. In general, the ligands for RTKs are proteins such as IGF, epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and FGF. Ephrins that bind to Eph receptors also form a large subset of RTK ligands. Colony-stimulating factor 1 (CSF-1), which is important for macrophage function, is another example of an RTK ligand. RTKs can function as monomers or multimeric subunits assembled at the plasma membrane that, upon ligand binding, cause oligomerization or conformational changes followed by tyrosine (trans)-phosphorylation in the kinase activation loop. Activation of RTKs results in phosphorylation of additional sites in the cytoplasmic part of the receptor, leading to docking of protein substrates, which initiates the intracellular signaling cascade. These substrates bind to RTK-phosphorylated tyrosines through Src homology-2 (SH2) domain or phosphotyrosine-binding (PTB) domains. Examples of these types of proteins are insulin receptor substrates or the p85 regulatory subunit of PI3K. RTKs recruit, assemble, and phosphorylate different proteins, including adaptors and enzymes.
There are mechanisms to terminate ligand-induced RTK activity through cellular processes including receptor-mediated endocytosis and/or through a family of regulated protein tyrosine phosphatases (PTPs), some of which are transmembrane and have extracellular domains, suggesting the possibility of ligand-mediated regulation. Interestingly, there is also intracellular regulation of PTPs through negative-feedback loops to attenuate the signal or direct control through reactive oxygen species (ROS) (see later discussion).
One of the key signaling components associated with RTKs is the PI3K signaling transduction pathway. This pathway is also activated by cytokine receptors and G protein–coupled receptors (GPCRs). Among the many functions of this pathway in hematopoietic cells, the interleukin-3 (IL-3)-dependent survival of these cells largely depends on activation of the PI3K pathway. PI3K is a heterodimeric complex formed of a regulatory and a catalytic subunit. The regulatory protein subunits are encoded by isoforms (which include p85α and p85β) that contain SH3-binding domains that mediate binding to activated RTKs. This binding allows additional recruitment and activation of the PI3K catalytic subunits (p110α, p110β, and p110*). At the plasma membrane, activated PI3K phosphorylates phosphoinosite-2 (PIP2) at position 3 of the inositol to produce PIP3. In addition, Ras, a small GTP-binding protein and potent oncogene, also activates PI3K. PTEN, an important lipid phosphatase and tumor suppressor, dephosphorylates PIP3, counteracting PI3K and decreasing the intensity of the pathway. Accumulation of PIP3 at the plasma membrane recruits several pleckstrin homology domain (PHD)-containing proteins, among them PDK and AKT serine/threonine kinases, which are key components in transducing PI3K signaling. Activated AKTs target different protein substrates for initiation of a biologic response. For example, the Bad protein, phospho-Bad, does not bind Bcl-2 and functions as an antiapoptotic mechanism, promoting cell survival. Other key targets of AKTs are the Forkhead transcription factors (FoxOs) ( Fig. 6.2 ). When phosphorylated by AKT, phospho-FoxOs are sequestered and inactive in the cytoplasm through direct binding to 14-3-3 proteins. In contrast, dephosphorylated FoxOs activate gene expression associated with stress resistance and cell growth arrest. Another major component downstream of Akt is mammalian target of rapamycin (mTOR, a kinase that belongs to the PI3K-related protein kinase family), which is involved in metabolism, growth, and proliferation. Akt phosphorylates TSC2, which forms a complex with TSC1, decreasing its GTPase-activating protein (GAP) activity for the small GTPase Rheb; as a consequence, the increases in GTP-Rheb activate mTORC1 (one of the mTOR complexes). Among the key downstream targets of mTOR are S6K and 4EBP1, which control protein translation. mTOR can also be activated independently of RTKs through nutrients including branched chain amino acids. mTORC1 forms an amino acid–sensing complex at the lysosomal membranes called the pentameric Ragulator complex that contains Rags, small GTPases that are controlled by the GATOR complex. Interestingly, mTORC1 inhibitors such as rapamycin are used as immunosuppressors in organ transplantation.
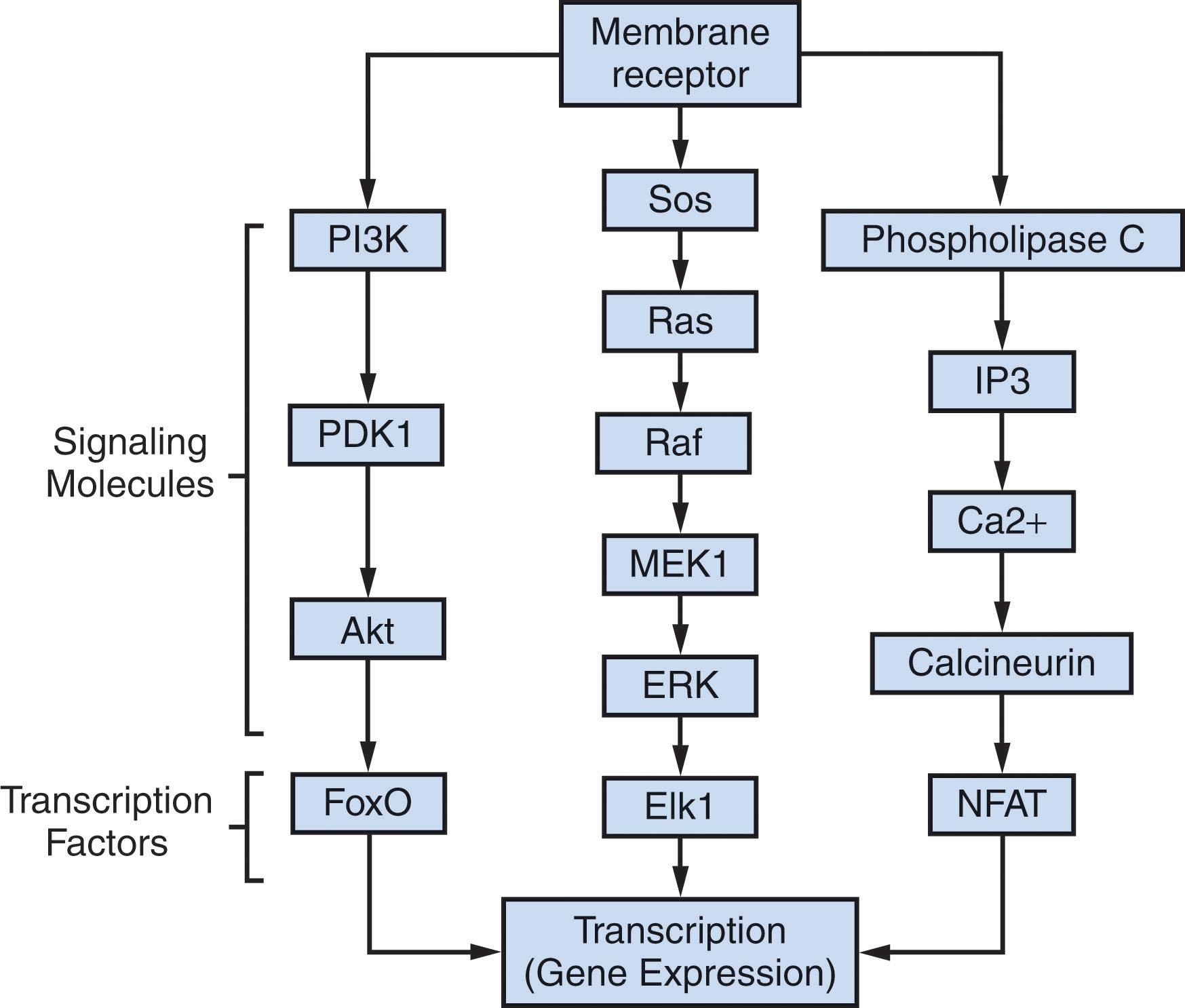
Activated RTKs recruit docking proteins, such as Grb2 and SOS, that allow binding of GTP to Ras to become active and trigger a kinase signaling cascade. Ras activates RAF kinase that, in turn, triggers a series of MEKs, which finally activate MAPK or EEK kinases. ERK phosphorylates many proteins involved in cell growth, including ribosomal S6K, which is involved in protein translation, and AP-1 and c-myc transcription factors, which increase many different cell cycle and antiapoptotic-related genes (see Fig. 6.2 ). Other MAPKs include the stress-activated kinases JNK and p38. Constitutive MAPK in hematopoietic stem cells is known to induce myeloproliferative disorders.
The transforming growth factor (TGF)-β family of cytokines contains two subfamilies: the TGF-β/Activin/Nodal and the bone morphogenetic protein (BMP)/growth and differentiation factor (GDF)/müllerian-inhibiting substance (MIS) subfamilies. At the plasma membrane, TGF-β ligands bind with high affinity to the ectodomain of type II receptors, which then recruit type I receptors. This forms a large ligand-receptor complex involving a ligand dimer and four receptor subunits. Upon ligand binding, the type II receptor phosphorylates multiple serine and threonine residues in the cytoplasmic GS-rich region of the type I receptor, leading to its activation. The phosphorylated TGF-β type I receptor binds to and phosphorylates Smad2 and Smad3 transcription factors, which are critical mediators of TGF-β signaling and function. Upon phosphorylation, Smad proteins translocate to the nucleus to activate gene expression through binding to specific DNA-binding sites. There are several mechanisms to terminate Smad activation, which include proteasomal degradation and dephosphorylation. TGF-β-1 has been shown to be associated with active centers of hematopoiesis and lymphopoiesis in the developing fetus.
Here, three different types of receptors and their signaling are included: (1) cytokine receptors, (2) multichain immune recognition receptors, and (3) integrin receptors.
The cytokine receptor superfamily mediates many of the central specific responses in hematopoietic cells. Ligands for these receptors include interleukins, thrombopoietin, erythropoietin, and so on. Cytokine receptors possess a conserved extracellular region (cytokine receptor homology domain [CDH]) and several structural modules, including extracellular immunoglobulin or fibronectin type III–like domains, transmembrane domains, and intracellular homology regions. Based on the divergence of the CHD, cytokine receptors are classified into two classes: class I and class II receptors. Class I receptors contain two pairs of cysteines linked through a disulfide bond and a C-terminal WSXWS motif within the CHD. This class is further subdivided into three families: IL-2R, IL-3R, and IL-6R. All three receptor families share similar receptor chains. The class I cytokine receptors are formed by one chain containing two motifs (Box 1 and Box 2), which transduce signaling through binding to Janus-activated kinase (JAK; see later discussion). Also included in this class are the homomeric receptors that form homodimers upon ligand binding. Examples of these receptors include the erythropoietin, thrombopoietin, prolactin, and growth hormone receptors. Class II receptors also have two pairs of cysteines but lack the WSXWS motif found in class I receptors. There are pools of 12 class II receptor chains that are capable of forming a total of 10 receptor complexes. This class is functionally divided into antiviral receptors (three receptor complexes that bind interferons) and non-antiviral receptors, which bind to several interleukins such as IL-10 and IL-20.
The oligomeric structures of cytokine receptors are complex and cannot be generalized. Cytokine binding often induces oligomerization, which activates protein tyrosine kinases in the JAK family that are constitutively associated with the Box 1 and 2 motifs of the cytokine receptor. Oligomerization brings JAKs in close enough proximity to trans phosphorylate on Tyr residues. This activates JAK, which results in the phosphorylation of other cytokine receptors as well as other substrate proteins. Among these substrates, the signal transducer and activator of transcription (STAT) family of transcription factors are pivotal to JAK-mediated cytokine signaling. STATs are phosphorylated on Tyr residues by JAKs upon cytokine binding to the receptor. Phospho-STATs homodimerize or heterodimerize and translocate to the nucleus to activate gene expression. STATs are also phosphorylated on a serine residue via MAPK, which serves to strengthen the intensity of the signal. As part of the cytokine signaling attenuation, STATs induce genes encoding for suppressors of cytokine signaling (SOCS) proteins, which bind to phosphotyrosine residues of the cytokine receptor and JAK through SH2-binding domains.
JAK inhibitors, based on their ability to block cytokine signaling, are used in allergic and rheumatoid arthritis disease therapy.
This family of receptors includes antigen receptors in B and T lymphocytes, activating receptors in natural killer (NK) cells, and immunoglobulin E (IgE) and Fc receptors. This class of receptors contains different integral membrane subunits that bind the ligand at the cell surface and transduce the signal. Ligand binding induces oligomerization of receptor subunits that contain immunoreceptor tyrosine-based activation motifs (ITAMs) within their cytoplasmic domains. These domains become phosphorylated on tyrosine residues upon receptor activation. These phosphotyrosines are involved in activation of a series of protein tyrosine kinases containing SH2 domains that include Src (Src family kinase [SFK]), Syk (Syk or ZAP-70), and Tec (Btk, Itk, Rlk), which mediate immune signaling through downstream pathways that include MAPK, calcium signaling, and nuclear factor (NF)-κB, among others. In Tec kinases, additional downstream targets include enzymes such as phospholipase C γ (PLCγ). The precise mechanism of this activation is not completely understood, and in some cases, such as T-cell receptors, a PTP (CD45, which counteracts the action of SFKs) is regulated upon ligand binding.
The activities of some of these receptors are the basis of immunotherapy in cancer. For example, PD-1 mediates tumor-induced immunosuppression. Cancer cells express the PD-1 ligand, which activates the PD-1 receptor present in tumor-infiltrated lymphocytes, suppressing the immune response. Signaling transduction through the PD-1 receptor involves tyrosine-depending binding to SHIP1/2 phosphatases. Blockade of PD-1 activation with monoclonal antibodies has been successful in treating several human tumors such as melanoma. Mechanistically, T cells are activated through the T-cell receptor upon binding of major histocompatibility complex (MHC) plus peptides on an antigen-presenting cell (APC; in this case in the tumor cell), and binding of APC CD80/86 to T cell CD28. Activation of the T-cell receptor increases expression of PD-1 to suppress the immune/inflammatory response. Cancer cells activate this pathway, upregulating the PD-1 ligand to promote survival and suppress the immune-mediated death of tumor cells.
Integrin receptors are involved in cell adhesion, migration, survival, and growth. This signaling is central in hematopoietic cell function (e.g., at places of inflammation or infection, where integrins trigger a cascade by which leukocytes exit the vasculature). Interestingly, these receptors signal bidirectionally through the plasma membrane in pathways referred to as inside-out and outside-in signaling . Integrins are a class of receptors that comprise heterodimeric type I transmembrane proteins consisting of α and β subunits. These subunits contain a large extracellular domain, a single transmembrane domain, and a short cytoplasmic tail. There are 18 α and 8 β subunits that are associated and form 24 different integrins with different affinities for ligands. Most of the ligands are extracellular matrix (ECM) proteins containing one of the two motifs: arginine-glycine-aspartate (RGD) or leucine-aspartate-valine (LDV). Examples of integrin ligands are ICAM-1, which is present at the plasma membrane of APCs and binds to the integrin receptor LFA-1 to promote cell-cell adhesion.
Ligand binding to the extracellular domain induces clustering of integrins, allowing separation of the different subunits cytoplasmic portions forming interactions with cytoskeleton proteins involved in actin polymerization (outside-in signaling). Signals arising from the cellular interior, including phosphorylation, can also separate these cytoplasmic domains and can affect ligand binding (inside-out). Ligand binding to integrin receptors also signals to protein tyrosine kinases such as the SFKs and focal adhesion kinase (Fak). This part of the signaling is not completely understood but appears to involve a domain in the β-integrin tail (NPXY motif) that binds talin, which in turn recruits paxillin that binds Fak, which, once activated, phosphorylates SFKs to mediate integrin response. Paxilin-independent integrin signaling that also mediates survival and migration have also been observed in intracellular endosomal compartments.
Tumor necrosis factor receptors (TNFRs) influence inflammation, innate immunity, lymphoid organization, and T-cell responses. There are approximately 19 different ligands for TNFRs that mediate cellular responses through 29 TNFRs. TNFRs are a family of single membrane–spanning proteins that contain an extracellular tumor necrosis factor (TNF)-binding region and a cytoplasmic tail. As in the case of other cytokine receptors, ligand binding causes oligomerization and the formation of a mature receptor complex that is required to transduce the signal. TNFRs fall into three classes: (1) death domain (DD)-containing receptors (fatty acid synthase [FAS], TNFR1, and DR3), which activate the caspase cascade via the DD-initiating extrinsic apoptotic pathway; (2) decoy receptors, which lack a cytoplasmic tail and therefore cannot transmit the signal, making these receptors ligand sequesters; and (3) TNFR-associated factor (TRAF) receptors such as TNFR2, which lack the DD-recruiting TRAF proteins. In general, TRAFs are associated with either proapoptotic or survival pathways through activation of the NF-κB family of transcription factors and MAPK signaling (ERK, JNK, and p38). TRAFs activate NF-κB through ubiquitin-mediated degradation of their inhibitor IκBα, which retains NF-κB inactive in the cytoplasm. This process is initiated by phosphorylation of IκBα by the IκBα kinase (IKK) complex, mainly by the IKK-β catalytic subunit, and requires a regulatory subunit (also known as NEMO). Upstream of IKKs are other kinases including NF-κB-inducing kinase (NIK), which binds to TRAFs. Nuclear-activated NF-κB modulates gene expression, which mediates TNF biologic responses.
Toll-like receptors (TLRs) play essential roles in the innate immune response. Ten TLRs have been identified and can be grouped into two classes based on their extracellular domain: (1) TLRs with leucine-rich repeats and (2) TLRs with immunoglobulin domains. The ligands for TLRs are diverse and include the different constituent components of the microorganism, such as lipopolysaccharides (LPSs) and heat shock proteins (which bind to TLR2 and TLR4). Host defense against microorganisms mainly relies on signals originating from the TIR (Toll/IL-1) intracellular domain (a domain present in TLRs and IL-1Rs). The TLR signaling pathway is similar to the one triggered by the IL-1R. Ligand binding induces TLR multimeric receptor complexes, recruiting adaptor proteins such as MyD88, which contains a TIR domain and a DD, that in turn binds to the IL-1R–associated kinase (IRAK). IRAK is activated by phosphorylation and then associates with TRAF6, leading to activation of mainly two different pathways, JNK and NF-κB to activate the innate immune response, including release of inflammatory cytokines.
Wnt proteins are lipid-modified, secreted proteins of approximately 400 amino acids that bind to Wnt cell surface transmembrane receptors, Fz, to initiate the canonical Wnt signaling transduction pathway. At the plasma membrane, binding of Wnt ligands to Fz receptors connect through direct binding to several intracellular proteins including Disheveled (Dsh), glycogen synthase kinase-3β (GSK3β), Axin, and adenomatous polyposis coli tumor suppressor (APC), inhibiting proteasome-mediated degradation of the transcriptional protein β-catenin. This degradation is regulated through GSK3β-mediated phosphorylation of β-catenin. As a consequence, β-catenin accumulates in the cytoplasm and translocates to the nucleus, where it interacts with transcription factors such as lymphoid enhancer-binding factor 1 (LEF)/TCF to modulate gene expression.
Notch ligands are plasma single-pass transmembrane proteins named Delta-like and Jagged. Thus cells expressing the ligands are adjacent to cells expressing the Notch receptors, which are also transmembrane proteins. The Notch receptor interacts with a Notch ligand on a contacting cell; this interaction produces Notch receptor cleavage, which releases the Notch intracellular domain (NICD). The NICD translocates to the nucleus, where it binds to several DNA-binding proteins including CBF1/Suppressor of Hairless/LAG-1 (CSL). As a result of this interaction between NICD and CSL, changes in Notch target genes occur. In contrast to the other signaling pathways discussed in this chapter that mainly function through phosphorylation, there is no amplification from the initial Notch ligand binding to the receptor. Moreover, this core pathway is modulated through auxiliary proteins that influence the response to the Notch ligand. Among these proteins are acute myeloid leukemia 1 (AML1), discoidin domain receptor family (DDR1), NECD, Notch extracellular domain, and CBF1-interacting protein.
Hedgehog (Hh) signaling is a ligand-dependent signaling pathway. There are three different protein ligands—Sonic, Desert, and Indian—that are secreted and produce an N-terminal active fragment. Indian appears to be highly expressed in hematopoietic tissue. These ligands bind to Patch transmembrane receptors and are internalized, and Smoothened (a GPCR member) translocates to the plasma membrane of the primary cilium and promotes activation of the Gli family of zinc finger transcription factors. Hh targets include genes involved in differentiation, apoptosis, and the cell cycle. Abnormal activation of Hh signaling occurs in hematologic malignancies and maintains stem cell expansion. Because these cells are resistant to conventional chemotherapy, Hh antagonism is considered a plausible target in these malignancies.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here