Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
These tumors (SCSTs), which account for ~5% of all primary ovarian tumors, are classified primarily on the basis of the constituent recognizable cell types ( Table 16.1 ): granulosa and theca cells, Sertoli cells, Leydig cells, and fibroblasts.
| Granulosa−stromal tumors |
|
| Sertoli−stromal cell tumors |
| Sex cord tumor with annular tubules |
| Mixed sex cord–stromal tumors |
| Steroid cell tumors |
|
a Retiform and heterologous elements or both may be seen in intermediate and poorly differentiated tumors.
Certain distinctive features (sclerosis, microcystic change, signet-ring cells, annular tubules) are also incorporated within the designations of three stromal neoplasms and one sex cord tumor.
These account for most clinically malignant SCSTs and are divided into adult (AGCTs) and juvenile (JGCTs) categories.
The adult and juvenile designations are terms of convenience to connote a spectrum of appearances typically seen in adults or juveniles, although AGCTs occasionally occur in young individuals and JGCTs occasionally (but less often) occur in older patients.
Tumors of each type are usually pure but occasional tumors have significant components of each type.
AGCTs, which account for ~2% of malignant ovarian tumors and 95% of all GCTs, peak between age 50 and 55 years but occur at all ages; they are rare in the first decade.
The usual presentation is related to an adnexal mass, endocrine manifestations (see below), or both. Acute abdominal symptoms from tumor rupture and hemoperitoneum occur in 10% of cases.
AGCT is the most common ovarian tumor with estrogenic manifestations, which include isosexual pseudoprecocity, menometrorrhagia, postmenopausal bleeding, amenorrhea, endometrial hyperplasia, and, in <5% of patients (who are usually postmenopausal), endometrial adenocarcinoma (usually low-grade endometrioid carcinoma).
Progestagenic or androgenic manifestations occur rarely; a disproportionate number of androgenic GCTs have been cystic. Progestagenic tumors tend to be luteinized.
95% of the tumors are stage I. The rest are mostly stage II, only rarely are they stage III.
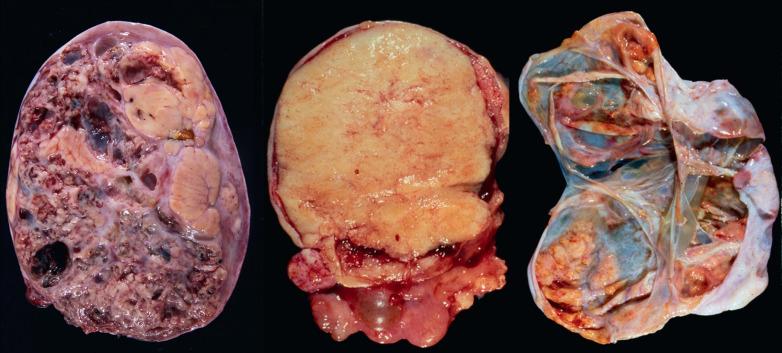
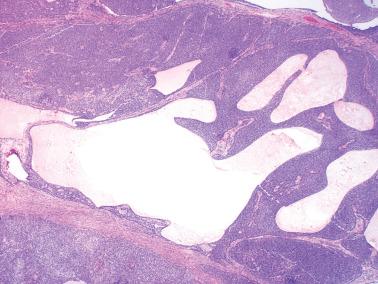
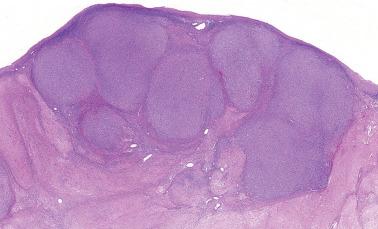
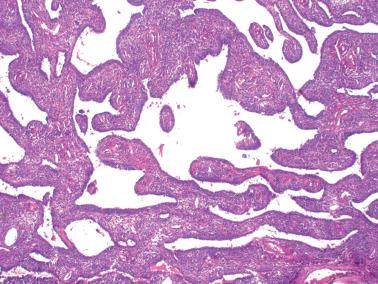
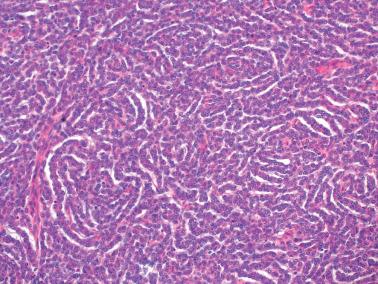
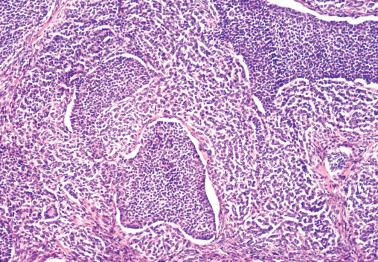
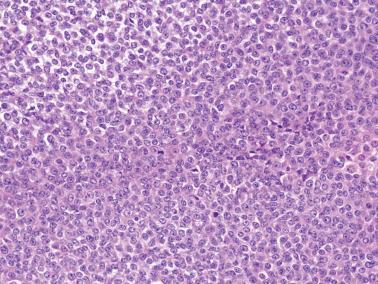
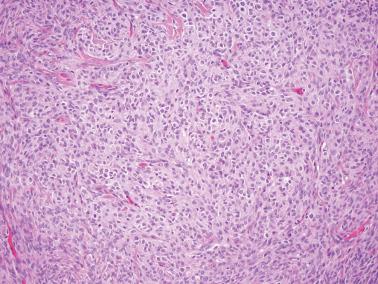
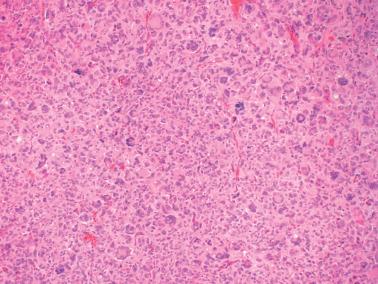
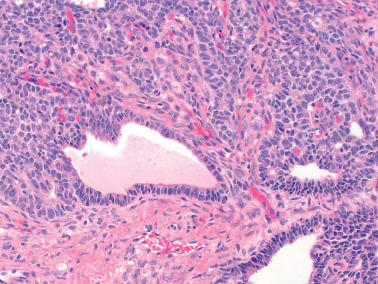
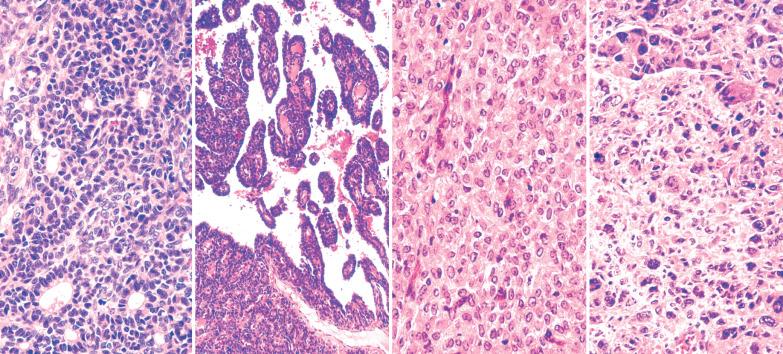
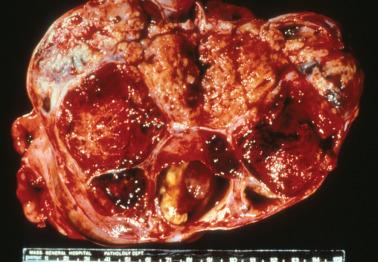
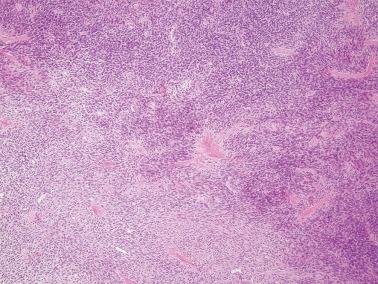
AGCTs have a mean diameter of 12 cm and >95% are unilateral. The cut surfaces are typically solid and cystic with fluid or blood-filled cysts separated by solid, yellow to white, soft to firm tissue.
Less common appearances are entirely solid with a white to yellow cut surface; multilocular or unilocular with fluid-filled, thin-walled cysts; cystic with thick walls sometimes having a ‘beefy’ appearance; diffusely hemorrhagic; and partially cystic with cysts lined by shaggy neoplastic tissue sometimes mimicking a surface epithelial carcinoma.
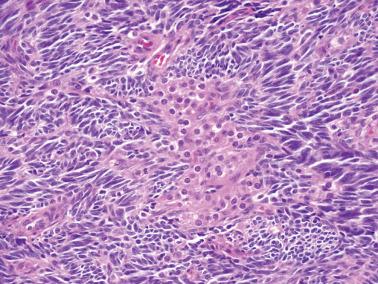
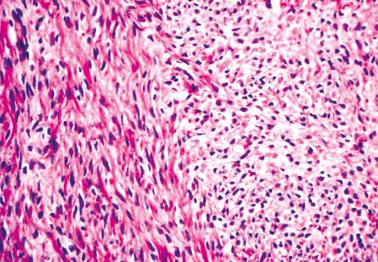
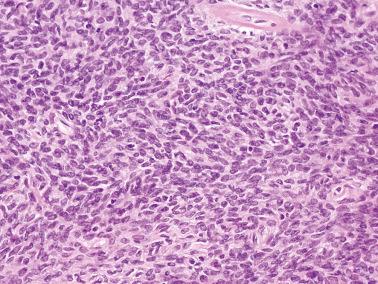
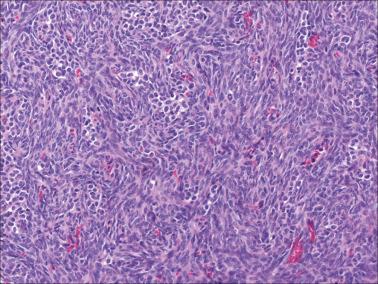
Granulosa cells are arranged in a wide variety of patterns, usually admixed, including diffuse, nodular, trabecular and corded, insular, follicular, watered silk, gyriform, and sarcomatoid. Follicular patterns, although emphasized in the literature, are much less common than the others in aggregate. The various patterns are considered here in approximate order of frequency. The overall appearance is also often contributed to by a variably prominent stromal component, considered after the epithelial patterns.
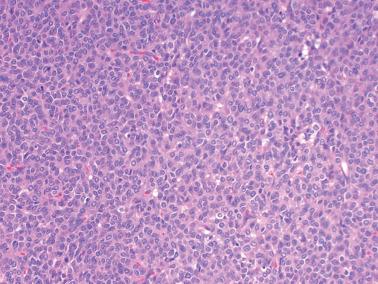
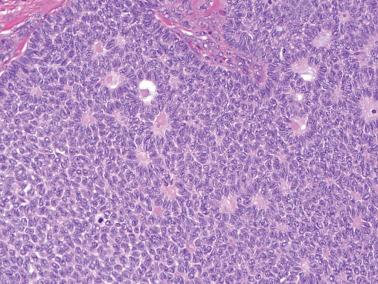
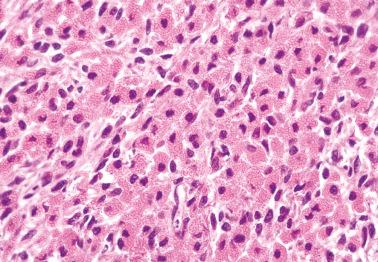
Diffuse: densely cellular sheets of cells with scanty cytoplasm imparting a ‘small blue cell tumor’ appearance. Careful scrutiny usually shows subtle foci of epithelial-type patterns, sometimes most evident at the periphery.
Nodular: a striking nodular pattern with a diffuse of arrangement of cells within the nodules.
Insular: discrete nests usually surrounded by a conspicuous stroma.
Trabecular and corded: thick columns or thin cords with a regular to irregular arrangement.
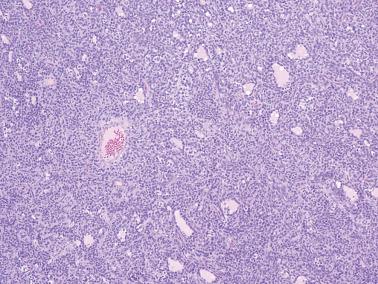
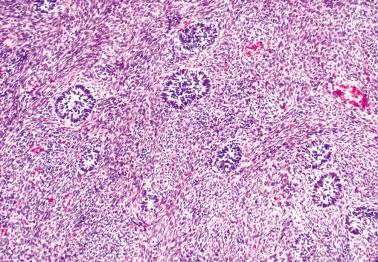
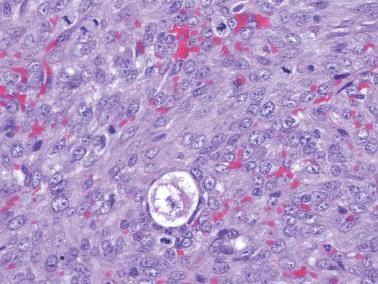
Microfollicular: small cavities (Call–Exner bodies) that may contain eosinophilic fluid, degenerating nuclei, hyalinized basement membrane material, or rarely, basophilic fluid. These are rarer than often suggested in the literature and only occasionally dominate.
Watered-silk (moiré-silk) and gyriform: undulating parallel rows and zigzag cords of granulosa cells, respectively.
Sarcomatoid: fusiform cells that may suggest cellular fibroma.
Granulosa cells usually have scanty cytoplasm (exceptions are noted below) and pale, uniform, angular to oval, often grooved nuclei that are typically arranged haphazardly in relation to one another. The conspicuousness of nuclear grooves is highly variable. Nucleoli may be conspicuous, particularly in luteinized tumors (see below).
The mitotic rate is variable, but is <3 mf/10 hpf in 75% of cases. A diagnosis of AGCT should be made with caution in the presence of numerous and/or abnormal mitoses, but such findings occasionally occur.
The stromal component varies from scanty in tumors with a diffuse pattern to accounting for most of the neoplastic tissue in others. In most tumors it is seen in some degree and often prominent but is generally less abundant than the sex cord component.
The stroma may be richly vascular and ranges from fibrous to more cellular. It commonly contains theca externa-like cells, or occasionally, luteinized theca interna-like cells with moderate to abundant eosinophilic cytoplasm or more often pale lipid-rich cytoplasm. The cells with eosinophilic cytoplasm can rarely be identifiable as Leydig cells if Reinke crystals are present.
Reticulin in granulosa elements is seen predominantly around cellular aggregates and vessels, whereas reticulin invests theca cells individually or in small groups.
Nonspecific stromal fibrosis, chronic inflammation, old or recent hemorrhage, hemosiderin, and nonspecific cysts are often present and may complicate the appearance.
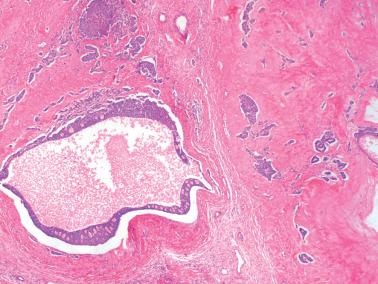
Macrofollicular pattern. Large rounded spaces are lined by granulosa cells, and occasionally, an outer layer of theca cells.
Tubular pattern. Hollow or solid tubules are lined by cells that usually retain typical granulosa cell features. Rarely the tubules may have a gland-like appearance.
Pseudopapillary pattern. This is more common in JGCTs, and is considered under that heading.
Pregnancy-related findings. These may occur in the last trimester of pregnancy and include prominent edema and/or extensive luteinization that may obscure the usual features of the tumor.
Cells with bizarre, enlarged hyperchromatic nuclei, including multinucleated forms, in 2% of cases. Such cells are typically focal but may be numerous and divert attention from diagnostic areas.
Cells with appreciable pale cytoplasm and tinctorial properties reminiscent of the cells seen in thecomas. These cells often grow in large nodules and when they dominate, minor foci of typical AGCT, often seen at the periphery, can be of crucial diagnostic aid. Reticulin stains can further aid ( ).
Luteinized granulosa cells with moderate to abundant eosinophilic cytoplasm and rounded nongrooved nuclei with a prominent nucleolus. These cells predominate in 2% of tumors, potentially mimicking a steroid cell tumor. One luteinized AGCT was associated with sclerosing peritonitis.
Myxoid stroma. This is rare in AGCTs being most common in luteinized variants.
Exceptionally rare findings include hepatic-cell differentiation (distinguish from luteinized/Leydig cells by more granular cytoplasm that stains with hepatocytic markers but not inhibin) and sarcomatous transformation.
A GCT in a patient with the Li–Fraumeni syndrome and an unusual germline p53 mutation exhibited multifocal intrafollicular (‘in situ’) growth in which the tumor cells had bizarre p53+ nuclei, numerous mitoses, and hyaline globules.
One AGCT was associated with mucinous heterologous elements.
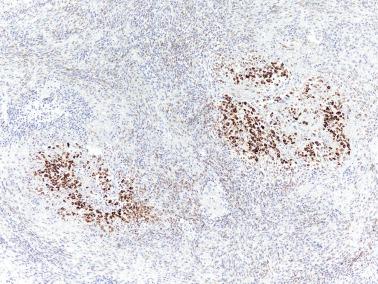
AGCTs are usually reactive for markers of sex cord differentiation. The best markers are inhibin, calretinin, CD99, steroidogenic factor [SF-1], and WT1. Occasional AGCTs are inhibin−. Immunoreactivity for FOXL2 (see below) and for 14-3-3 sigma has been found in 97% and 100% of AGCTs respectively.
Other usually positive markers include CD56, vimentin, CK (punctate pattern), S100 protein, SMA, and CD10 (the last usually showing weak staining). The stromal component may be desmin+.
In contrast to JGCTs, AGCTs are typically EMA−.
found that although 74% of AGCTs were immunoreactive for EGFR, no mutations were found in the EGFR gene in hot-spots of therapeutic significance.
identified a TERT C228T promoter mutation in a subset of AGCTs and suggested its possible role in recurrence.
found a recurrent somatic missense mutation (402C→G) in FOXL2 , a gene encoding a transcription factor required for granulosa-cell development, in 97% of AGCTs (86 of 89 tumors).
found the same mutation in 50% of granulosa–theca cell tumors that they defined as tumors with 10–50% granulosa cell component within a prominent fibrothecomatous background.
FOXL2 (C402G) mutation is absent or rare in other SCSTs (even in those immunoreactive for FOXL2) as well as other ovarian tumors.
The varied patterns of AGCTs can cause many issues in diagnosis ( 8 , 9 , 10 , 11 , 12 , 13 ).
Other SCSTs, mixed germ cell–SCSTs, and non-neoplastic lesions.
Thecoma and cellular fibroma. Thecomas have cells with more abundant cytoplasm than AGCTs with occasional exceptions as noted above; the cells in cellular fibromas resemble fibroblasts. Both tumors lack the epithelial patterns of AGCTs, and unlike the latter, typically have pericellular reticulin.
Stromal tumors with minor sex cord elements. These tumors are otherwise typical fibromas or thecomas with minimal sex cord differentiation confined to a few microscopic foci.
Steroid cell tumor vs luteinized AGCT. The latter has focal areas with the architectural and cytologic features of typical (nonluteinized) AGCT.
Gonadoblastomas and sex cord tumors with annular tubules (SCTATs).
Hyaline deposits in these tumors are typically larger than Call–Exner bodies, are often calcified, and often merge with thickened basement membranes. Also, gonadoblastomas contain germ cells and usually arise in the background of intersex.
SCTATs typically exhibit prominent ring-shaped simple and complex tubules, and if bilateral and/or microscopic in size, are usually associated with the Peutz–Jeghers syndrome.
Germ cell−SCST tumor, unclassified ( Chapter 15 ). The sex cord component of these rare tumors may be granulosa-like but definitionally also contain germ cells.
Follicle cysts, including the large solitary luteinized follicle cyst of pregnancy and the puerperium. The follicle cysts are almost invariably lined by cells with more abundant eosinophilic cytoplasm than the cells of cystic AGCTs. The large pregnancy-associated follicle cysts typically contain cells with large bizarre nuclei. The latter may be seen in the AGCT but almost never in cells lining cysts.
Carcinomas.
Endometrioid carcinomas with sex cord-like patterns ( Chapter 14 ).
Undifferentiated carcinoma ( Chapter 14 ).
Small cell carcinoma of hypercalcemic type. Features favoring or diagnostic of this tumor include hypercalcemia, no estrogenic manifestations, hyperchromatic nongrooved nuclei, a high mitotic rate, no thecomatous elements, and a p53+/inhibin−/calretinin− immunoprofile.
Other primary tumors.
Endometrioid stromal sarcoma ( Chapter 14 ).
Transitional cell carcinomas vs AGCTs with a pseudopapillary pattern (see JGCT).
Carcinoid tumors. Features favoring or diagnostic of carcinoid tumor include cells with abundant cytoplasm surrounding lumens containing densely eosinophilic, sometimes calcified secretion, nongrooved nuclei with coarse chromatin, and chromogranin and/or synaptophysin positivity.
Metastatic tumors.
Malignant melanoma. Features favoring or establishing this diagnosis include the history of an extraovarian melanoma, bilateral ovarian involvement, malignant nuclear features, melanin pigment, and positivity for melanoma markers.
Metastatic breast carcinomas (especially lobular type). This tumor (which rarely presents as an ovarian metastasis) is distinguished from AGCT by the absence of the typical cytologic features of granulosa cells, the presence in some tumors of mucin-containing intracytoplasmic vacuoles, and positivity for one or more of EMA, GCDFP, and GATA3.
Metastatic endometrial stromal sarcoma. The history, frequent bilaterality, and distinctive vascularity point to this diagnosis as does like tongue-like growth often seen in foci of extraovarian disease. Immunohistochemical differences will also be helpful.
AGCTs are tumors of low malignant potential typically manifested by late abdominopelvic recurrence.
The main prognostic factor is stage: stage I tumors have an 86–96% 10-year survival vs 26–49% for higher-stage tumors; IC lowers the 25-year survival from 86% to 60% ( ).
, using the National Cancer Database, found that each 1 cm increase in tumor size in stage I tumors was associated with a 4% increased risk of death. They also found that the hazard ratio for death associated with incomplete staging was 1.77 (both findings, p = <0.001).
Histologic patterns, grade, bizarre nuclei, mitotic activity, proliferation indices, and ploidy have not been consistently shown to be prognostic in stage I tumors.
Other prognostic markers:
D'Angelo et al. found that AGCTs with FOXL2 mutation and high mRNA expression (>72RU) were associated with a worse prognosis. However, no correlation was seen regarding level of immunoreactivity and survival.
found that decreased β-catenin expression in primary AGCTs may correlate with increased risk of recurrence and earlier recurrence.
found that low immunohistochemical expression of ERβ in the primary tumor was associated with a higher risk of recurrence.
found that in stage I AGCTs, high expression of SMAD3 (a transcription factor in the TGF-β signaling pathway) and lack of chemotherapy were independent predictors of recurrence. They also found that high expression of CD56 predicted a greater risk of recurrence.
As previously mentioned, suggest a possible role of TERT C228T promoter mutation in recurrence of AGCTs.
These tumors account for only 5% of GCTs; >90% occur in the first three decades. Isosexual pseudoprecocity occurs in 80% of prepubertal patients. The presentation in postpubertal cases includes abdominal pain or swelling, menstrual irregularities, amenorrhea, or combinations thereof.
Uncommon presentations include an acute abdomen (due to tumor rupture and hemoperitoneum), androgenic manifestations, and an occasional association with Ollier's disease (enchondromatosis) and Maffucci's syndrome (enchondromatosis and hemangiomatosis). One JGCT was associated with tuberous sclerosis.
Other uncommon intraoperative findings include ascites and extraovarian spread, usually confined to the pelvis.
The gross features are similar to those of AGCTs, with tumor dimensions ranging from 3 to 32 (mean, 12.5) cm; 2% of tumors are bilateral.
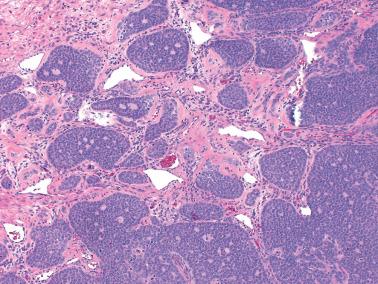
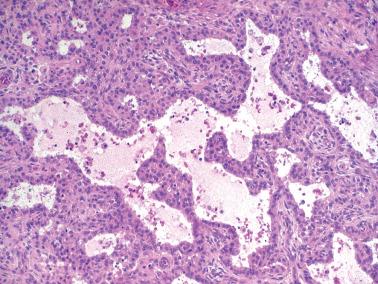
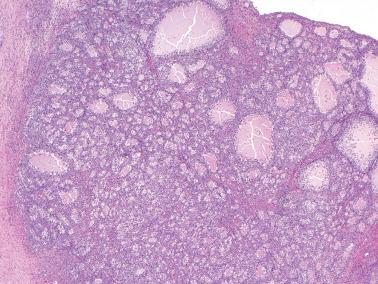
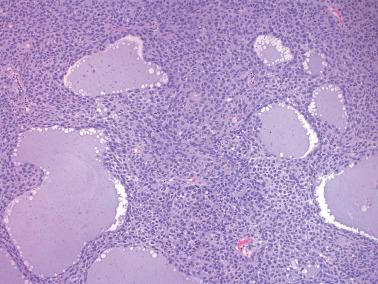
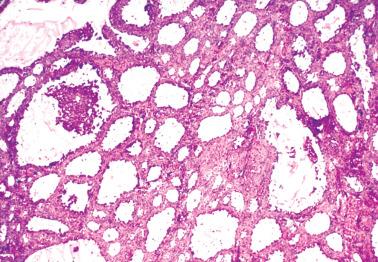
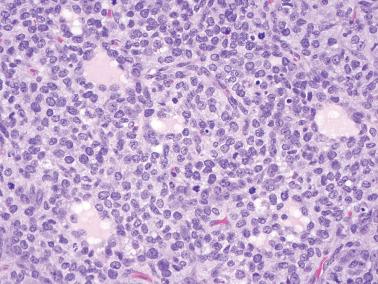
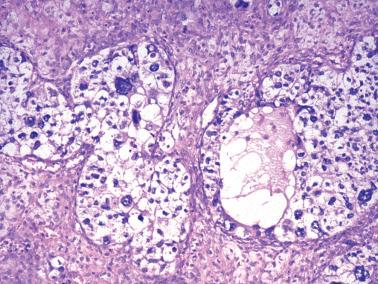
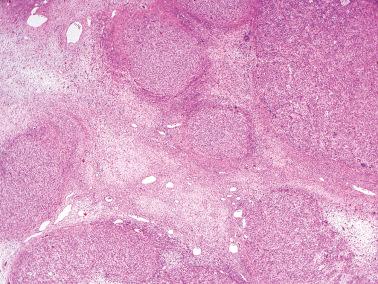
The most common pattern is that of sheets of cells interrupted by follicles that range from few to numerous. They are often of variable size and shape with luminal eosinophilic to basophilic fluid that may be mucicarminophilic. A uniformly solid (‘afollicular’) pattern, that often is nodular, may occur. Call–Exner bodies are seen only rarely.
The follicles are lined by granulosa cells, sometimes with an outer mantle of theca cells, but more commonly, the lining granulosa cells blend into the intervening solid areas. Granulosa cells typically predominate, but there may be an admixture of granulosa and theca cells or a predominance of theca cells.
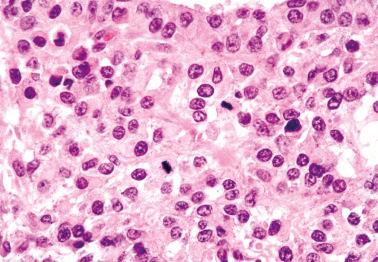
The granulosa cells typically have abundant eosinophilic or vacuolated cytoplasm and generally round, nongrooved, euchromatic or hyperchromatic nuclei with minimal to severe nuclear atypicality, the latter being present in 15% of cases. Occasionally, hobnail-type cells line follicles. The mitotic rate is often brisk.
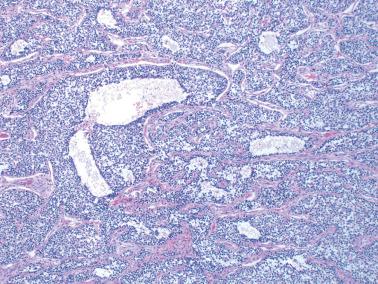
An uncommon finding is the presence of pseudopapillae, which can also occur in AGCTs but are more common in JGCTs.
The tumors are usually grossly cystic with multiple papillary structures projecting from the cyst lining.
The pseudopapillae represent projections of neoplastic cells with surrounding necrotic debris and/or undulating folds of neoplastic cells without appreciable necrosis.
The correct diagnosis rests on the presence of the typical architectural features of JGCT (or AGCT) elsewhere in the tumor and typical cytologic features of granulosa cells throughout the tumor.
Other uncommon findings include small foci of AGCT, a prominent fibrothecomatous stromal component, areas of sclerosis, and sheet-like aggregates of anaplastic cells exhibit striking nuclear atypia. The correct diagnosis in tumors with these unusual features may require thorough sampling to reveal typical foci of JGCT with follicles.
The neoplastic cells are typically positive for inhibin, calretinin, FOXL2, 14-3-3 sigma, CD56, and WT1. McCluggage has found focal EMA positivity in 50% of JGCTs, a finding that could suggest carcinoma, including the hypercalcemic small cell carcinoma (see below).
found consistent immunoreactivity for Fli-1 and CD99, findings similar to those in Ewing's sarcoma, but FISH found no chromosomal rearrangements of EWSR1.
AGCT. Features favoring JGCT include follicles that are irregular in size and shape, numerous luteinized cells, round nongrooved hyperchromatic nuclei, brisk mitotic rate, and no Call–Exner bodies
Yolk sac tumor (YST) (including polyvesicular vitelline variant) and embryonal carcinoma. Features favoring or diagnostic of these tumors include an association with other germ cell elements (e.g., dermoid cyst), a typical reticular pattern (YST), primitive embryonal carcinoma-type glands and nuclei, Schiller–Duval bodies (YST), syncytiotrophoblastic elements, positivity for AFP, glycipan 3, or hCG, and negativity for inhibin and calretinin.
Hypercalcemic small cell carcinoma (especially its large cell variant; Chapter 17 ). Features favoring or diagnostic of this tumor include hypercalcemia, no estrogenic manifestations, more disorderly architecture, at least some cells with scanty cytoplasm, no theca cells, and a p53+/inhibin−/calretinin− immunoprofile. EMA and WT1 staining may be seen in both tumors.
Thecoma (vs JGCTs with absent or rare follicles). Sample well to demonstrate follicles or cellular clusters of granulosa cells. Although thecoma-like areas may be seen rarely in the JGCT, expansive areas with the typical features of thecoma are absent. An age <30 years and more than slight mitotic activity favor JGCT.
Clear cell, undifferentiated and serous carcinomas with prominent transitional cell carcinoma-like patterns (the last vs JGCTs with pseudopapillae). These carcinomas are rare in the young, and while they may focally exhibit some overlapping features, they lack true follicles, have their own distinctive features when well sampled, and lack positivity for inhibin and calretinin.
Steroid cell tumor and pregnancy luteoma. These lesions, unlike JGCTs, tend to have more homogenous patterns and cytologic features. The steroid cell tumor lacks follicles with rare exceptions. The pregnancy luteoma may have follicle-like spaces but is frequently multiple, bilateral, or both.
Metastatic malignant melanoma. Difficulty may arise because some melanomas have oxyphilic cells and follicle-like spaces. In addition to those features helpful in the differential of melanoma with AGCT (see above), the age of the patient and immunohistochemical differences will aid.
The survival in stage I tumors is 97%. Higher-stage tumors are often fatal; recurrences in these cases almost always occur within the first 3 postoperative years.
The presence or absence of rupture, the mitotic rate, the degree of nuclear atypicality, DNA ploidy, and S-phase fraction have not been shown to be prognostic in stage I tumors. However, when cells with nuclear atypia are widespread and the normal follicular architecture is lost, we suspect that these findings may be prognostically adverse.
As in AGCTs, found that higher FOXL2 mRNA expression (>2RU) had a worse overall survival than those with lower FOXL2 mRNA expression. Of note, the mRNA expression levels seen in JGCTs were much lower than that in AGCTs. Additionally, while 14 of 19 JGCTs showed expression of FOXL2 by immunohistochemistry, none of the JGCTs harbored a FOXL2 mutation. Only rare reports of JGCT with a mutation exist in the literature.
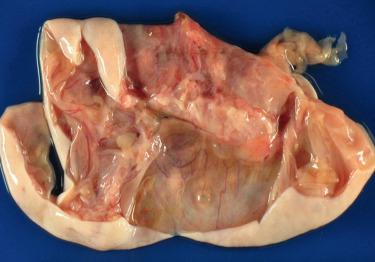
Fibromas, which account for 4% of all ovarian tumors, occur at all ages, but are most common during middle age (mean, 48 years); <10% occur in those <30 years of age, including occasional examples in children.
Typical fibromas are nonfunctioning, but occasional fibromas with luteinized cells or minor thecomatous foci may be associated with endocrine manifestations.
Meigs’ syndrome occurs in up to 1% of tumors and is defined as ascites and pleural effusion accompanying a fibrous ovarian tumor, usually a fibroma. The effusions resolve after removal of the tumor. Ascites alone occurs in ~10% of ovarian fibromas >10 cm in diameter.
Fibromas associated with the nevoid basal cell carcinoma syndrome (NBCCS) (Gorlin's syndrome) frequently differ from typical fibromas (see below).
Fibromas have a mean maximal diameter of 6 cm; 8% are bilateral, mostly in patients with the NBCCS in whom there may be multiple tumors in each ovary.
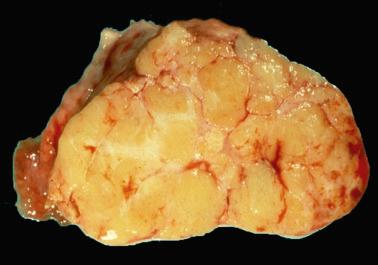
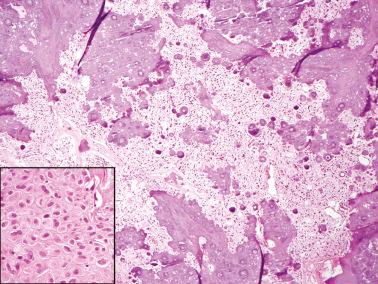
Gross examination reveals typically a hard, white to tan to pale yellow cut surface; some tumors are soft due to edema or high cellularity. Focal edema (sometimes with cystification) is common, and some tumors are predominantly cystic. Hemorrhage, and less often necrosis, may be present, especially in cellular neoplasms.
Focal or diffuse calcification occurs in <10% of the cases but is almost always present in NBCCS-related fibromas.
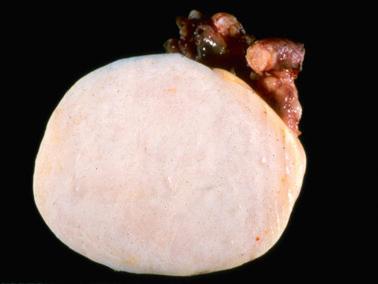
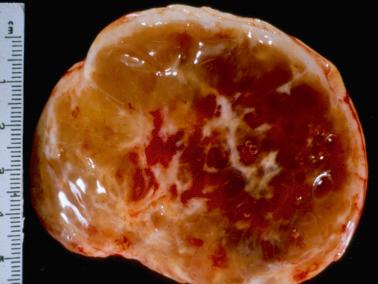
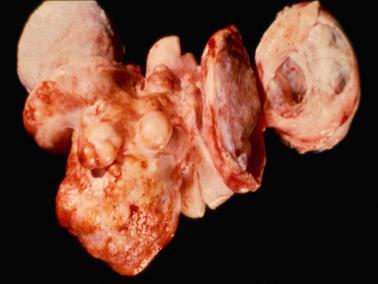
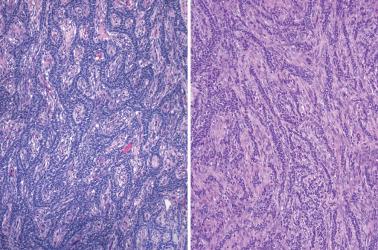
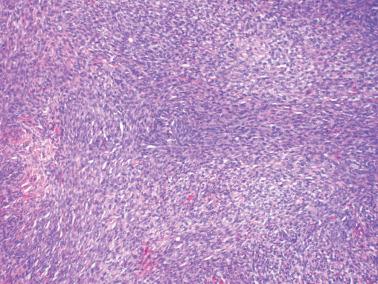
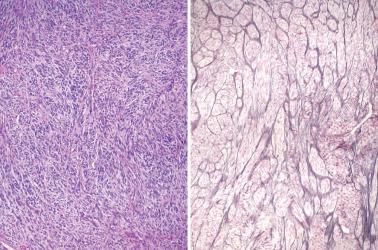
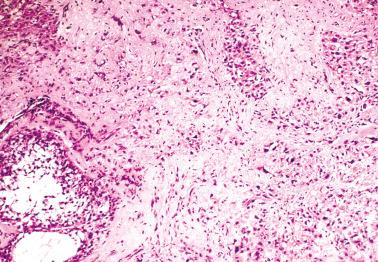
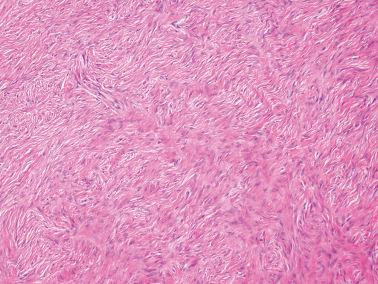
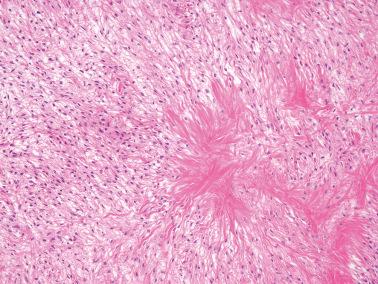
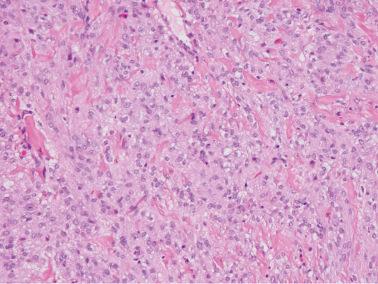
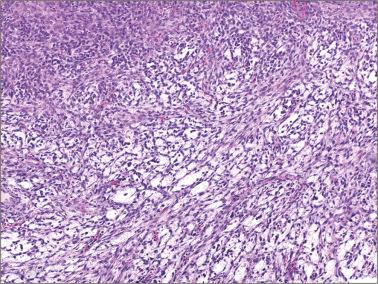
Sparsely to intensely cellular proliferations of spindle cells with scanty cytoplasm are arranged in intersecting fascicles or occasionally a storiform pattern. Cross-sectioning of the fascicles of cellular tumors may suggest the presence of rounded cells growing in nests and an erroneous diagnosis of AGCT.
The cells may contain small quantities of lipid and usually have uniform nuclei and only rare mitotic figures. Eosinophilic cytoplasmic or extracellular PAS+ hyaline globules are an occasional finding, and in some cases may be numerous. Some cells may have a signet-ring-like appearance.
The spindle cells in most tumors are separated by variable amounts of collagen that is frequently focally hyalinized. Focal to extensive intercellular edema is common, and may erroneously suggest the presence of thecomatous cells with pale cytoplasm.
About 10% of fibromas are densely cellular (cellular fibromas) but exhibit no more than mild atypia except for occasional foci of moderate atypia around foci of infarction (see below). Cellular fibromas have mitotic activity that is usually ≤3 mf/10 hpf but occasionally much higher (‘mitotically active cellular fibroma’).
Luteinized cells, minor foci (<10%) of sex cord elements (granulosa cells, indifferent sex cord-type cells, sertoliform tubules), and foci resembling thecoma may be seen.
The pseudocysts that occur in tumors with cystic degeneration may be striking and impart a confusing appearance. Hemorrhage and infarction are particularly common in cellular fibromas; the infarct-type necrosis should not be misconstrued as tumor cell necrosis.
Occasional tumors are somewhat vascular and if the background neoplasm has sharply contrasting cellular and paucicellular areas, a misdiagnosis of sclerosing stromal tumor may result.
Immunostains are rarely required for diagnosis, with typical staining for WT1, CD56, SF-1, and FOXL2 (but without FOXL2 mutations), and much less commonly, inhibin and calretinin. Other markers that are often positive include SMA, ER, and PR.
One cellular fibroma in a patient with Ollier's disease harbored an IDH1 mutation.
Thecoma (see corresponding heading).
Sclerosing stromal tumor (SST). Fibromas lack the jumbled admixture of fibroblasts and weakly luteinized cells seen in the cellular pseudolobules of SST.
Massive edema, fibromatosis, and stromal hyperplasia. Features favoring one of these lesions over fibroma are bilaterality, entrapment of follicles and their derivatives (massive edema and fibromatosis), and aggregates of closely packed, small stromal cells with minimal collagen formation (stromal hyperplasia).
Primary endometrioid or metastatic endometrial stromal sarcoma ( Chapter 14 ).
Fibrosarcoma (see next heading).
Krukenberg tumor ( Chapter 18 ).
Cystadenofibromas. These tumors, in contrast to cystic fibromas, contain glands and cysts with an epithelial lining.
Wolffian tumors ( Chapter 17 ). These rare neoplasms may have a dominant component of fusiform spindle cells imparting a cellular fibroma-like appearance but thorough sampling will show distinctive features of a Wolffian tumor.
A wide variety of tumors ( Appendix 13 ) may have foci that when viewed in isolation may suggest fibroma or thecoma. Thorough sampling is obviously crucial in their evaluation.
Cortical fibromatosis. It may be difficult to distinguish a localized focus of cortical fibromatosis from a fibroma but the former is usually less discrete.
Corpus albicans. Rarely a hyalinized old corpus albicans may suggest a fibroma but there is usually some evidence of a cerebriform contour in the former.
Up to 12% of cellular fibromas and mitotically active cellular fibromas are associated with implants at the time of oophorectomy, although that figure is almost certainly inflated from inclusion of consultation cases. Pedunculated tumors with focal necrosis are more commonly associated with extraovarian involvement, likely due to implantation of detached fragments.
Rare cellular fibromas recur locally, sometimes years postoperatively. Tumor rupture and/or adherence are risk factors for recurrence; tumor-related deaths are very rare.
reported a ‘cellular thecoma’ (1–2 mf/10hpf, minimal atypia) with peritoneal spread, although their description and illustrations in our opinion suggest a cellular fibroma.
These findings indicate that cellular fibromas should be regarded as tumors of low malignant potential when they are large, ruptured, or associated with adhesions.
These are the most common ovarian sarcoma, and are usually found in older women. They are rarely associated with Maffucci's syndrome and the NBCCS.
Gross examination typically reveals a unilateral large tumor with a solid cut surface, often with focal hemorrhage and necrosis.
Densely cellular proliferations of spindle cells usually have a less orderly fascicular pattern than seen in cellular fibromas; tumor cell necrosis may be seen.
The tumors usually exhibit moderate to less frequently severe nuclear atypia and an average mitotic count of ≥4 mf/10 hpf; abnormal mitoses are common.
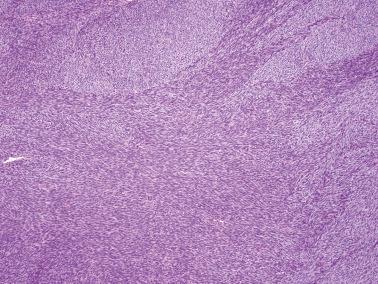
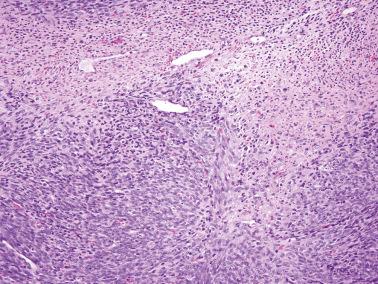
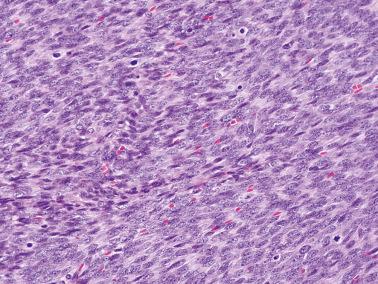
Cellular fibroma. In contrast to fibrosarcomas, cellular fibromas definitionally exhibit no more than mild atypia (other than moderate atypia around foci of necrosis) and usually exhibit <4 mf/10 hpf. However, occasional tumors exhibit a brisker mitotic rate and in the absence of other features of concern are usually benign (mitotically active cellular fibroma). The cellular areas of fibromas are often punctuated by less cellular, often collagenous, regions contrasting with the more diffuse hypercellularity of most fibrosarcomas.
Primary endometrioid stromal sarcoma or metastatic endometrial stromal sarcomas (ESSs). ESSs have smaller, less atypical cells with round nuclei and scanty cytoplasm, and a characteristic network of arterioles. Extraovarian metastatic tumor often present in these cases usually shows the typical tongue-like growth of ESS.
Other sarcomas ( Chapter 17 ). These are diagnosed using soft-tissue criteria.
Poorly differentiated Sertoli–Leydig cell tumor (see corresponding heading).
Thecomas, which are a third as common as GCTs, typically occur in peri- and postmenopausal women (mean age, ~60 years); 10% occur in those <30 years of age but prepubertal thecomas are very rare.
Thecomas are often associated with estrogenic changes; uterine bleeding occurs in 60% of postmenopausal women with a thecoma. Occasional, mostly postmenopausal, women have an associated endometrial (usually endometrioid) adenocarcinoma, or rarely an MMMT or ESS.
Most thecomas are small (mean 5 cm); only 3% are bilateral. The cut surfaces are typically solid and yellow, or occasionally mostly white and focally yellow. Cysts, hemorrhage, necrosis, and focal calcification may be seen; rare extensively calcified tumors tend to occur in young patients.
Typical microscopic features:
Sheets or nodules are composed of oval to rounded cells with ill-defined membranes (creating a syncytial appearance) and moderate to abundant pale gray cytoplasm. In about a third of tumors, some of the cells have a lipid-rich appearance (that may include lipid vacuoles) with more voluminous cytoplasm than the more typical cell type.
Lutein (steroid-type) cells with clear to eosinophilic cytoplasm may be present, so-called ‘luteinized thecoma’, a term no longer recommended except for a distinctive lesion considered below. The presence of lutein cells, however, may merit a comment, particularly if prominent, because of potential androgenic manifestations, rarely if ever seen in nonluteinized thecomas.
Rarely the lutein cells contain Reinke crystals, so-called ‘stromal Leydig cell tumors’, a term no longer recommended (Sternberg and Roth).
The nuclei are round to fusiform with little or no atypia, and rare to absent mitoses. Nuclear grooves are seen in occasional tumors but are rarely conspicuous.
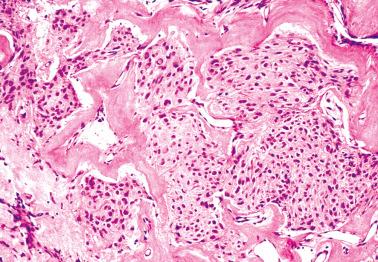
A fibromatous component is often present and may separate sheets and nests of theca cells. Conspicuous hyaline plaques or foci of calcification (see above) may be seen. Individual tumor cells (or occasionally small groups of cells) are usually surrounded by reticulin fibrils.
Unusual to rare findings include:
Striking zones of confluent hyalinization, marked calcification, myxoid change, and occasional adipocytes that may focally or extensively efface the typical morphology.
Cells with large bizarre nuclei with a degenerative appearance and absent to rare mitotic figures.
Minor sex cord elements, including granulosa cells, indifferent sex cord-type cells, or sertoliform tubules.
Marked cellularity, atypia, and increased mitotic activity (≥4 mf/10hpf) that, especially in combination, raise concern for malignancy.
Most so-called ‘malignant thecomas’ have been inadequately documented or better interpreted as fibrosarcoma, endometrioid stromal sarcoma, or granulosa cell tumor.
Rare bona fide clinically malignant thecomas ( , ) usually exhibit moderate to severe atypia and a high mitotic rate.
In the absence of firmly established criteria for malignant thecoma, and tentatively using criteria similar to those for cellular fibromatous tumors, we regard thecomas with ≥4 mf/10hp but no other worrisome features as probably benign, and a tumor with ≥4 mf/10hpf and ≥1 other worrisome features (atypia, necrosis, rupture, adhesions) as having a malignant potential.
Staining for WT1, CD56, and SF-1 is present in almost all cases; for inhibin, calretinin, ER, and PR in most cases; and for FOXL2 in 60% of cases (although the presence of a FOXL2 mutation is uncommon).
Fibromas.
As the distinction between thecomas and fibromas is imprecise and arbitrary, the designation ‘fibrothecoma’ has been used. We avoid this term and diagnose fibroma in the absence of appreciable numbers of typical thecoma cells and allow minor fibromatous zones in thecomas.
As noted above, edema in fibromas can sometimes be misinterpreted as tumor cells with clear or lipid-rich cytoplasm, resulting in a misdiagnosis of thecoma.
Granulosa cell tumor and sclerosing stromal tumor (see corresponding headings).
Steroid cell tumor. A diagnosis of steroid cell tumor is appropriate when an associated fibromatous or thecomatous component accounts for <10% of the tumor. The cells of a typical steroid cell tumor and typical thecoma are distinctly different.
Pregnancy luteoma. Aside from an association with pregnancy, these lesions, unlike thecomas, are multiple in ~50% of cases, lack a background of fibroma or typical thecoma, and may have follicle-like spaces. The luteomas typically have more abundant brightly eosinophilic cytoplasm than do thecomas, even those that are focally luteinized.
This rare ovarian lesion has been enigmatically associated in almost all cases with sclerosing peritonitis ( Chapter 20 ).
The ovarian lesions have also been referred to as ovarian edema, stromal hyperthecosis, fibromatosis, or sarcoma-like nodules, and the peritoneal lesions as fibroma-like proliferations, fibromatosis, or retractile mesenteritis.
Parenthetically, one case of luteinized AGCT was associated with sclerosing peritonitis.
The patients have ranged from 10 months to 85 years (median, 27 years) of age, usually presenting with abdominal swelling (due to ascites and adnexal masses) or occasionally bowel obstruction.
The ovarian lesions are bilateral in most cases, and range from large masses to normal-sized or slightly enlarged ovaries with a cerebriform appearance. The cut surface is red–brown and often edematous with occasional cysts. The process tends to involve the cortex, often sparing the medulla.
A dense proliferation of spindle cells is typically admixed with foci of small rounded luteinized cells with pale cytoplasm. The spindle cells may exhibit striking mitotic activity. Edema with microcyst formation is common. The stromal proliferation typically entraps normal ovarian structures, including follicles.
The luteinized cells are usually diffusely positive for inhibin, calretinin, CD56, and SF-1. The spindle cells usually express muscle markers (SMA, desmin), PR, SF1, and occasionally AE1/3, calretinin, CD56, and ER.
The sclerosing peritonitis may cause intermittent small bowel obstruction that has been rarely fatal. Treatment with one or more of antiestrogens, GnRH agonists, tamoxifen, and corticosteroids has ameliorated the peritonitis in some cases. The ovarian lesions have also responded to GnRH agonists in a few cases. None of the ovarian lesions have recurred or metastasized.
The differential diagnosis of luteinized thecoma includes the following although none of them are associated with sclerosing peritonitis.
Typical thecoma with luteinized cells. This tumor is almost always unilateral, often estrogenic, and has more defined nests of larger luteinized cells; microcystic change is rare.
Massive edema and fibromatosis. These are diffusely hypocellular lesions with either marked edema or collagen deposition.
Edematous cellular fibromas and sclerosing stromal tumors (SSTs). These tumors are typically unilateral, are generally mitotically inactive, and in the case of SSTs, have other distinctive features (see next heading). They both lack the distinctive features of the peritonitis-associated lesions.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here