Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
To effectively care for an affected newborn and provide information for anxious parents, an organized diagnostic approach is essential. The physical exam requires special attention to ectodermal involvement by assessing the hair, teeth, nails, palms and soles of the feet. Moving beyond the skin, a child should be examined for dysmorphic features, associated major or minor congenital anomalies and accompanying illness. Results of a newborn hearing screen and pediatric ophthalmology exam are often valuable. Finally, a detailed family history including ethnic background and miscarriage history is beneficial. After gathering clinical information, superb resources are available online to consider diagnostic possibilities. One example is Online Mendelian Inheritance in Man (OMIM; http://www.ncbi.nlm.nih.gov/omim ).
OMIM is maintained by the National Center for Biotechnology Information (NCBI) and can be searched using a list of the clinical features in an affected patient. Each disorder is assigned a number based on broad categories such as inheritance pattern. ‘GeneTests’ ( http://www.ncbi.nlm.nih.gov/sites/GeneTests ) and a separate database, ‘GeneReviews’ offers a frequently updated list of internationally available DNA-based genetic tests and concise, yet comprehensive overviews of selected disorders. For effective coordination of testing and advising the patient about the complexities of genetic testing and reproductive options, the GeneTests site provides a database of genetics services that is searchable by country and province or state. Increasingly, individual mutations that cause familial genetic disorders are recognized to affect important regulatory pathways. This chapter highlights pathways that are particularly important to growth and development of the skin.
Several genetic skin disorders are caused by mutations in genes in the RAS-MAPK signaling pathway ( Fig. 29.1 ) and have been coined the ‘RASopathies’. The RASopathies include neurofibromatosis-1 (NF-1), NF-like syndrome (Legius syndrome), Noonan syndrome, Noonan with multiple lentigines (formerly LEOPARD syndrome), cardiofaciocutaneous syndrome, Costello syndrome, capillary malformation–arteriovenous malformation (see Chapter 22 ), and hereditary gingival fibromatosis ( Table 29.1 ). Neurofibromatosis and Legius syndrome are also discussed in Chapter 24 .
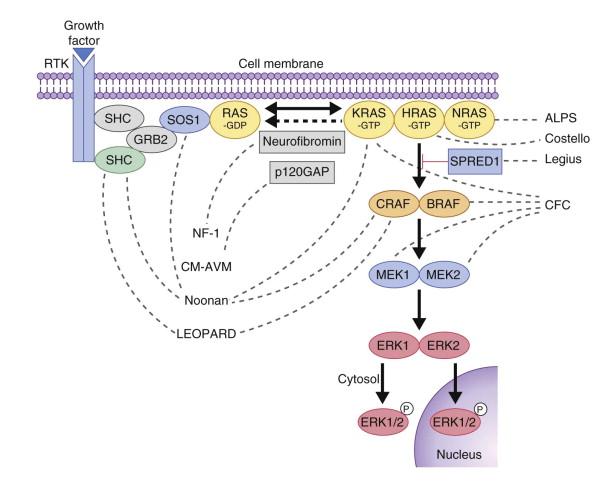
| NF-1 | Cardiofacio-cutaneous syndrome | Costello syndrome | Noonan syndrome | Multiple lentigines syndrome | Capillary malformation- arteriovenous malformation | |
|---|---|---|---|---|---|---|
| Gene | NF1 | BRAF, MEK1, MEK2, KRAS | HRAS | PTPN11, SOS1, KRAS, NRAS, RAF1, SHOC2, CBL and BRAF | PTPN11, RAF, BRAF | RASA1 |
| Dermatologic features | Café-au-lait macules Intertriginous freckling Neurofibromas Plexiform neurofibroma |
Ulerythema ophryogenes Keratosis pilaris Melanocytic nevi Infantile hemangiomas |
Papillomata of the nose and perianal region Palmoplantar hyperkeratosis Redundant skin on the hands and feet Acanthosis nigricans |
Congenital lymphedema Café-au-lait macules Keratosis pilaris (most common with SOS1 mutations) |
Lentigines Café noir patches |
Capillary malformations Arteriovenous malformations of the brain, spine, skin, muscle and bone |
| Cardiac features | Uncommon | Pulmonic stenosis Hypertrophic cardiomyopathy |
Pulmonic stenosis Hypertrophic cardiomyopathy |
Pulmonic stenosis Hypertrophic cardiomyopathy |
EKG abnormalities Pulmonic stenosis Hypertrophic cardiomyopathy |
Rare |
| Tumor predisposition | Yes: Optic glioma Hematologic malignancies Meningioma and other brain cancers Malignant peripheral nerve sheath tumor Pheochromocytoma |
Rare: Possible risk for ALL and lymphoma |
Yes: Malignant solid tumors (rhabdomyosarcoma, neuroblastoma, transitional cell carcinoma of the bladder) |
Rare: Hematologic malignancies |
Rare: Hematologic malignancies |
Rare: Possible increased risk for neural tumors |
| Other | Lisch nodules Learning disability Pseudoarthrosis Macrocephaly Sphenoid wing dysplasia Renal artery stenosis Hypertension |
Failure to thrive (severe) Developmental delay |
Failure to thrive (mild) Developmental delay Sociable, outgoing personality |
Low posterior hairline Webbed neck Pectus excavatum Bleeding tendency Short stature |
Ocular hypertelorism Sensorineural hearing loss Short stature |
Risk for intracranial and spinal arteriovenous malformation Parkes–Weber syndrome |
Neurofibromatosis 1 (NF-1, MIM #162200) is a multisystem disorder characterized by age-related abnormalities of tissue proliferation. NF-1 is one of the most common autosomal dominant genetic conditions with an incidence of approximately 1 in 3000 individuals. Consensus criteria for the diagnosis of NF-1 were established at the 1988 NIH conference to be used as a guideline for clinical diagnosis ( Box 29.1 ). In 95% of affected individuals, a diagnosis can be made by age 11 through the use of clinical evaluation alone. However, NF-1 can be a difficult condition to diagnose in some infants due to the high incidence of sporadic mutations, variability of clinical expression, and age-related penetrance of individual clinical manifestations. Hence, anticipatory guidance counseling should be provided in both established and suspected cases of NF-1.
≥6 café-au-lait macules >5 mm in greatest diameter in prepubertal individuals or >15 mm in greatest diameter after puberty
≥2 neurofibromas of any type, or ≥1 plexiform neurofibromas
Axillary/inguinal freckling (Crowe's sign)
Tumor of the optic nerve pathway (optic glioma)
≥2 Lisch nodules (iris hamartomas)
Distinctive osseous changes (e.g., sphenoid wing dysplasia or pseudoarthrosis)
First-degree relative with NF-1
Café-au-lait macules (CALM) are light to dark brown sharply defined oval macules and patches which can occur on almost any skin surface ( Fig. 29.2A ). Infants with ≥6 café-au-lait macules >5 mm in diameter that are not confined to a single segmental region, should be evaluated and managed as though they have NF-1, even without other signs of NF-1, because of the high likelihood that they will develop other diagnostic signs with time. At puberty, if other features are lacking, the approach and diagnosis can be reconsidered.
Intertriginous freckling of the axillary and inguinal regions may occasionally be present in infancy, but most often present between 2–10 years of age ( Fig. 29.2B ). Although freckling on the neck and trunk is common in NF-1, it is not accepted as a diagnostic criterion.
Peripheral neurofibromas are infrequent in early childhood NF-1, occurring in only 14% of children less than 10 years of age. Often the first sign of dermal neurofibromas are 3–6 mm, light blue, minimally raised papules which are most easily detected with side lighting. It has been suggested that a subset of children with large deletions of the NF-1 gene typically present with multiple neurofibromas in early childhood.
Plexiform neurofibromas may be apparent at birth or soon thereafter; however because they are often internal, may be difficult to detect. They occur in at least 25% of affected individuals ( Fig. 29.2C ). Most have overlying hyperpigmentation; some – but not all – have increased vascularity resembling a vascular anomaly or hypertrichosis resembling a congenital melanocytic nevus. Plexiform neurofibromas may grow rapidly and interdigitate with and surround normal structures. Radiologic imaging and neurosurgical consultation should be considered if lesions are extensive or close to major nerve bundles.
Optic gliomas are astrocytomas arising anywhere along the optic pathway. These lesions tend to arise in infancy or early childhood. Half of the tumors are symptomatic, causing loss of visual acuity, decreased field of vision, proptosis, or interference with the hypothalamopituitary axis. Symptomatic optic gliomas are often diagnosed by 6 years of age.
Lisch nodules are pigmented iris hamartomas that rarely present in infants, and only 20% of individuals under 5 years of age with NF-1 have Lisch nodules. They are best seen on slit-lamp examination and do not result in functional disability. Congenital glaucoma occurs in less than 0.05% of individuals with NF-1 and may present with an ipsilateral neurofibroma of the eyelid.
Relatively short stature and large head size are frequent findings and more common than the more diagnostic skeletal changes, pseudoarthrosis and sphenoid wing dysplasia. Pseudoarthrosis represents the failure of union after fracture. It is always unilateral and most commonly presents in the tibia as anterolateral bowing. Ultimately, pseudoarthrosis can progress to severe deformity. Sphenoid wing dysplasia is a unilateral defect of the orbit present in approximately 5% of individuals with NF-1 and results in a change in orbit structure. Approximately half of those cases with sphenoid wing dysplasia develop an ipsilateral temporal–orbital plexiform neurofibroma, and half of individuals presenting with a temporal–orbital tumor also have an underlying plexiform neurofibroma. Learning disabilities occur in approximately 50% of children with NF-1. The main learning difficulties include reading, deficits in perceptual skills (visuospatial) and executive functioning. Attention issues are also common.
NF-1 is due to an autosomal dominant mutation localized to chromosome 17 that results in defects in neurofibromin, a tumor suppressor protein that stimulates hydrolysis of guanosine triphosphate (GTP) bound to ras .
Café-au-lait macules may be found in many other conditions, including segmental pigmentary disorder and McCune–Albright syndrome (see Chapter 24 ). Additional differential diagnoses for multiple café-au-lait macules are shown in Box 29.2 . Genetic testing for NF-1 is available from several commercial and research laboratories (see: www.genetests.org , for specific information). Decisions regarding whether laboratory-based NF testing is appropriate are best made in conjunction with a geneticist and genetic counselor.
Legius syndrome
Neurofibromatosis type 2
Schwannomatosis
Noonan syndrome
Noonan syndrome with multiple lentigines (LEOPARD syndrome)
Multiple endocrine neoplasia 2B
Bannayan–Riley–Ruvalcaba syndrome
Piebald trait
Patients with neurofibromatosis require age-related anticipatory guidance counseling and regular follow-up with a pediatrician, ophthalmologist and geneticist. Additional specialists can be included, based on symptoms or complications and may include orthopedics, neurology, dermatology, neuropsychology, and neurosurgery ( Box 29.3 ). Ophthalmologists and neurologists should evaluate for optic nerve pathway tumors and glaucoma. Anterolateral tibial bowing when present require prompt orthopedic evaluation, because the critical time for fracture and poor healing is infancy to early childhood. Periodic evaluation for scoliosis is also necessary. Regular physical examinations should include careful measurement of blood pressure because of a higher incidence of hypertension secondary to renovascular disease, vasoactive secreting tumors, and coarctation of the aorta. Head circumference should be monitored because of the risk of hydrocephalus and the occurrence of macrocephaly without hydrocephalus. Careful developmental assessment is a key part of management, as the risk of neurologic abnormalities and learning disabilities is increased.
Routine history and physical examination by a pediatrician
Yearly blood pressure monitoring
Baseline and annual ophthalmologic examination
Routine neurologic and developmental evaluation
Regular head circumference monitoring
Genetic counseling with discussion of genetic testing
Imaging based on neurologic signs and symptoms
Dermal neurofibromas are benign and should only be excised if they are symptomatic or disfiguring. Plexiform neurofibromas require close evaluation. Skin should be palpated carefully as plexiform neurofibromas may remain below the surface. If there are symptoms or neurologic abnormalities on clinical exam, MRI scans should be obtained to determine the extent of plexiform neurofibromas. Serial imaging or volumetric MRI may be necessary to assess potential growth. Pain and/or growth may herald malignant transformation, but this is exceedingly rare in infancy. Neurologic compromise may result from perineural extension, however, and neurology and/or neurosurgery may need to be consulted for plexiform lesions near neurovascular structures (such as the neck, axilla and spinal area). Orbitotemporal neurofibromas may be better managed by numerous surgical procedures over time; plastic surgery should be involved early for management of these tumors.
Legius syndrome (MIM #611431) was first recognized in 2007 as an NF-1-like syndrome in families with multiple café-au-lait macules, but negative NF-1 gene testing. The phenotype is milder than NF-1 and seems to lack the propensity for tumor development. The clinical features include a mild NF-like phenotype and 50% of these patients meet diagnostic criteria for NF-1. Of the diagnostic criteria for NF-1 ( Box 29.1 ), the features that have been reported in Legius include >6 CALM >5 mm, intertriginous freckling, positive family history, macrocephaly, short stature and learning disabilities. Neurofibromas, plexiform neurofibromas, bone dysplasia and scoliosis have not been associated with Legius syndrome. Legius syndrome is caused by loss of function (LOF) mutations in the SPRED1 gene. Like neurofibromin, SPRED1 is a negative regulator of the RAS-MAPK pathway ( Fig. 29.1 ).
Noonan syndrome (MIM #163950) is an autosomal dominant multisystem disorder characterized by congenital lymphedema, broad or webbed neck, low posterior hairline, short stature, and cardiac malformations ( Box 29.4 ).
Webbed neck
Cutis vertices gyrata
Ulerythema ophryogenes
Koilonychia
Thick, curly and wooly hair
Prominent fetal finger pads
Short stature
Craniofacial:
Ptosis
Downslanting palpebral fissures
High palate
Cardiac pulmonic stenosis
Cryptorchidism
The neonate with Noonan syndrome is unlikely to have skin manifestations other than nuchal webbing and peripheral lymphedema that suggest the diagnosis. Keratosis pilaris atrophicans faciei (ulerythema ophryogenes), characterized by horny, whitish, hemispherical, or acuminate papules at the opening of pilosebaceous follicles, is generally noted in older children, but may manifest in the external third of the eyebrows by a few months after birth. Some children with Noonan syndrome have multiple CALM and/or lentigines.
Neonates have a characteristic facial appearance consisting of a tall forehead, low posterior hairline, hypertelorism, downslanting palpebral fissures, epicanthal folds, short and broad, depressed nasal root, deeply grooved philtrum and micrognathia. The chest shape is unique with superior pectus carinatum and inferior pectus excavatum. Feeding difficulties, gastroesophageal reflux and failure to thrive are frequent problems in infancy. Unilateral or bilateral cryptorchidism are common in boys. Infants are at risk for transient monocytosis, thrombocytopenia and myeloproliferative disorder. Coagulation defects occur in approximately one-third of patients with Noonan syndrome and may present with easy bruising or prolonged bleeding times. There is a slightly increased risk of hematologic malignancy (most commonly juvenile myelomonocytic leukemia, JMML) compared with the general population. The classic congenital heart defect in Noonan syndrome is pulmonic stenosis.
The incidence of Noonan syndrome is approximately 1 in 1000–2500 live births. Noonan syndrome may be caused by a mutation in one of several genes in the RAS-MAPK signaling pathway, including PTPN11 , SOS1 , KRAS , NRAS , RAF1 , SHOC2 , CBL, and BRAF . PTPN11 and CBL mutations have a higher rate of bleeding diathesis and JMML. Patients with SOS1 mutations have a lower rate of intellectual disability. SHOC2 mutations have been associated with a Noonan phenotype with loose anagen hair. PTPN11 mutations are also the genetic basis of Noonan with multiple lentigines (formerly known as LEOPARD syndrome; see Chapter 24 ).
In the neonatal period, the RASopathies can be very difficult to distinguish as they share the features of congenital heart defects, severe feeding difficulties and developmental delay. Later in infancy, facial and ectodermal features become more distinct for each of the RASopathies. Cardiofaciocutaneous syndrome and Costello syndrome are described in more detail below.
Patients with Noonan syndrome should be monitored for growth deficiency and developmental delay. Electrocardiogram and echocardiography should be performed at the time of diagnosis and repeated as indicated based on findings. A renal ultrasound is recommended at diagnosis. Coagulopathy work-up should be performed if the patient has a history of either easy bruising or prolonged bleeding.
Cardiofaciocutaneous (CFC) syndrome (MIM #115150) is characterized by short stature, congenital heart defects, intellectual disability, ectodermal abnormalities and a characteristic coarse facial appearance ( Table 29.2 ). Numerous cutaneous findings have been reported in CFC. In 2010, Siegel and colleagues evaluated the cutaneous manifestations in 61 mutation-positive individuals with CFC syndrome. All had dermatologic findings. One of the striking features identified in this study was a high number of melanocytic nevi. In the study, 23% of participants had over 50 nevi and 36% of those patients reported over 100 nevi. The amount of nevi increased with age and were not a prominent feature in infancy. Keratosis pilaris and ulerythema ophryogenes were very common and affected the majority of individuals in childhood and adolescence ( Fig. 29.3 ). Infantile hemangiomas occurred at a greater frequency when compared with the general population. Additional features in CFC include macrocephaly, characteristic facial appearance, growth retardation, cardiac defects, neurologic impairment, gastrointestinal dysfunction, and ocular abnormalities.
| Percentage of cases | ||
|---|---|---|
| Cardiofaciocutaneous | Costello | |
| Curly hair | 93.4% | 95.7% |
| Eyebrow density | 90.0% sparse | 47.8% thick |
| Keratosis pilaris | 80.3% | 32.6% |
| Infantile hemangioma | 26.2% | 10.9% |
| Papillomas | 4.9% | 71.7% |
| More than 50 nevi | 23.0% | 4.0% |
| Palmoplantar keratoderma | 36.1% | 76.1% |
CFC syndrome is caused by mutations in BRAF , MEK1 or MEK2 . These mutations were discovered in part because of the similarity of the phenotypic features of Noonan and Costello syndromes, both of which were known to have mutations involving the RAS-MAPK pathway. Given insights into genetics and pathogenesis, it is not surprising that the main differential diagnoses for CFC syndrome include Noonan and Costello syndromes.
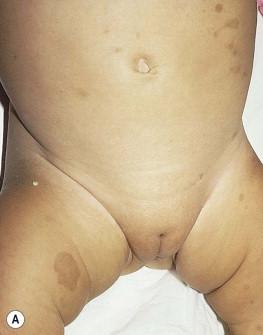
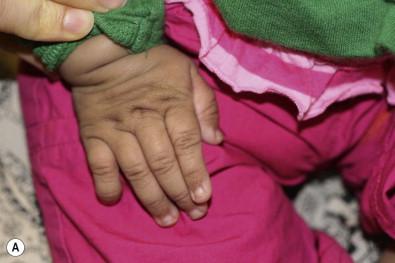
Costello syndrome (CS) (MIM #218040) is a rare, autosomal dominant, multiple congenital anomaly syndrome associated with failure to thrive, developmental delay, and an increased risk of malignancy. The features of Costello syndrome in the neonatal and infantile period include macrosomia, severe feeding problems, developmental delay, coarse facial features, gingival hyperplasia, osteopenia, hypertrophic cardiomyopathy and atrial arrhythmias. The most common malignancies include rhabdomyosarcoma, neuroblastoma and transitional cell cancer of the bladder. CS is caused by mutations in the HRAS gene, at 11p15.5, leading to constitutive activation of the RAS-MAPK pathway. The cutaneous findings include curly hair, papillomas on the perinasal, perioral and perianal skin, palmoplantar keratoderma, unusual body odor and heat intolerance. The skin on hands is loose and redundant ( Fig. 29.4A ). Acanthosis nigricans has been reported in 37% of the cases ( Fig. 29.4B ). In the neonatal period, it can be difficult to distinguish Costello, CFC and Noonan syndromes, as all three conditions can manifest with neonatal macrosomia, coarse facial features, failure to thrive and developmental delay.
CM-AVM is an autosomal dominant disorder characterized by multiple, small, oval capillary malformations on the face, body and limbs, which are associated with arteriovenous malformations in about 30% of cases. The AVMs can occur in the brain, spine, muscle or skin. CM-AVM is caused by mutations in the RASA1 gene. See Chapter 22 for a more in-depth discussion about this condition.
Neurofibromatosis type 2 (NF-2) (MIM #101000) is an autosomal dominant condition characterized by a high burden of schwannoma and meningioma tumor development.
Presentation in infancy is rare, although cutaneous schwannomas have been reported. Cutaneous features which may be present in infancy or early childhood include CALM and cutaneous schwannomas ( Fig. 29.5 ). The age range at the onset of NF-2-related symptoms is 5–55 years. The initial symptoms that lead to the diagnosis include hearing loss, visual disturbance, and enlarging subcutaneous mass. The incidence of NF-2 is difficult to predict due to a high rate of mosaicism, but is estimated at about 1 : 25 000. NF-2 is caused by mutations in the merlin (also called schwannomin) tumor suppressor gene on chromosome 22q12. The differential diagnosis for NF-2 includes NF-1 and schwannomatosis.
Overgrowth syndromes and human cancer share disruption of similar critical regulatory pathways ( Table 29.3 ). Intensive cancer research, therefore, has improved our understanding of these rare but important disorders. The phosphatidylinositol 3-kinase (PI3K)-AKT pathway, in particular, critically guides cell growth and metabolism ( Fig. 29.6 ). The PI3K enzymes are a family of highly conserved enzymes that regulate cell growth, migration and survival and disruption in the embryonic period typically impacts vascular, limb or brain development. Mutations cause recognizable patterns of increased cell number, hypertrophy, increased interstitium, or a combination of these. Typical neonatal clues of an overgrowth syndrome are abnormally increased body length, macrosomia, and macrocephaly, dysregulated growth of a body part or asymmetry ( Fig. 29.7 ). Almost all overgrowth syndromes are associated with neoplasms, especially solid tumors.
| Syndrome | Causative mutation | Cutaneous features | Extracutaneous features | Associated malignancy |
|---|---|---|---|---|
| Tuberous sclerosis | TSC1, TSC2 | Hypomelanotic macules, angiofibromas, forehead plaques, shagreen patches, periungual fibromas | Seizures, infantile spasms, intellectual disability. Renal cysts and angiomyolipomas, cardiac rhabdomyomas |
Malignant angiomyolipoma, renal cell cancer; sub-ependymal giant cell astrocytoma (SEGA) |
| Proteus | Mosaic AKT1 | Cerebriform connective tissue nevi (CCTN), epidermal nevi, vascular malformations, soft subcutaneous masses, patchy dermal hypoplasia, macrodactyly and lipomas | Disproportionate, relentless segmental overgrowth of body parts; skeletal asymmetry, lung cysts, thromboembolism, eye problems, ovarian cysts, epididymal cysts. Overgrowth generally after neonatal period |
Mostly benign tumors |
| Hemihyperplasia-multiple lipomatosis (HH-ML) | Unknown | Superficial capillary vascular malformation, lipomas. Lacks deep vascular malformation and cerebriform connective tissue nevi of Proteus. | Non-distorting overgrowth present at birth | Risk for embryonal malignancies is unknown. Screening for Wilms tumor, adrenal cell carcinoma, hepatoblastoma is recommended |
| Megalencephaly-capillary malformation (MCAP) | Mosaic PIK3CA | Patchy capillary malformations; stretchy skin and joints | Large brain, growth dysregulation, asymmetry, syndactyly, polydactyly, developmental delay, hypotonia, frontal bossing | Mildly increased risk of cancer ; Wilms tumor, leukemia; meningioma reported |
| Mosaic overgrowth with fibroadipose hyperplasia (MOFH) | Activating PIK3CA | Lacks cutaneous features of Proteus | Distorting or non-distorting, segmental overgrowth of muscles, skeleton and fibroadipose tissue that is present at birth | Unknown |
| SOLAMEN | PTEN | Signs of Cowden: trichilemmoma, acral keratoses, oral papillomas. Lacks cerebriform connective tissue nevi of Proteus | Features of Cowden: macrocephaly, breast and thyroid hamartoma | Breast, thyroid, endometrial |
| CLOVES | Activating PIK3CA | Vascular anomalies, truncal lipomas, epidermal nevi. May be wrinkled skin on the palms and soles, but not CCTN of Proteus | Non-progressive overgrowth at birth. Overgrown feet, hands; ‘sandal gap’ toes, severe scoliosis; high vascular-flow masses; phlebectasia; thromboembolism |
Unknown |
| Encephalocraniocutaneous lipomatosis (ECCL) | Unknown, sporadic | Nevus psiloliparus overlying lipomatous overgrowth; angiofibromas, connective tissue nevi cutis aplasia, nodular skin tags | Proportionate, non-distorting overgrowth at birth. Ocular abnormalities, CNS lipomas, heart defects, lytic bone lesions, hypospadias, cryptorchidism, seizures, jaw osteomas | Mostly benign tumors. Low-grade glioma reported |
| Beckwith–Wiedemann syndrome (BWS) | Imprinting error affecting chromosomal region 11p15.5 | Nevus flammeus, distinctive ear creases, posterior helical pits | Abdominal wall defects (omphalocele), placental overgrowth, macrosomia, macroglossia, abnormal kidney, cardiomegaly, hypoglycemia | Tumor risk increased, especially embryonal tumors; requires monitoring |
| Simpson–Golabi–Behmel syndrome type I | Glypican-3 (GPC3) | Supernumerary nipples, characteristic index finger and nail hypoplasia | Macrosomia, macrocephaly, macroglossia, coarse square-shaped face, abdominal hernias, broad hands, normal intelligence | Risk of embryonal tumors increased; requires monitoring |
| Sotos syndrome | Nuclear receptor set domain containing protein 1 gene (NSD1) | Frontotemporal sparse hairs, malar flushing | Learning disabilities, distinct facies, macrocephaly, tall stature | Overall cancer risk slightly increased. Age appropriate cancer screening recommended |
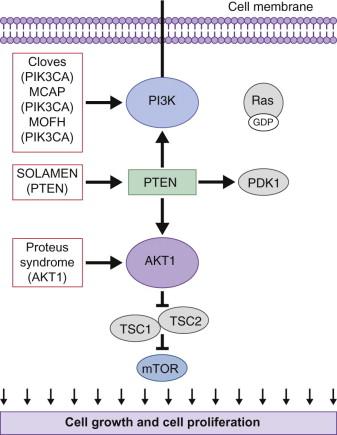
PI3K-AKT activity is considered so essential that disruptive germline mutations are often presumed fatal. For example, an activating PI3K pathway mutation appears only in the mosaic form. In addition to impacting embryonic development, the PI3K-AKT pathway participates in normal cellular function by regulating glucose metabolism and apoptosis. Therefore, mutations affecting this pathway may cause congenital anomalies as well as ongoing complications. Improved understanding of the PI3K pathway, especially by revealing therapeutic targets for inhibitory drugs like rapamycin, gives hope for future trials and treatment.
Tuberous sclerosis (TSC, MIM #191100) is a multisystem disorder characterized by tumors and hamartomas affecting the skin, brain, heart, kidneys and lungs, often in association with seizures and developmental delay ( Box 29.5 ). TSC is discussed in further detail in Chapter 23 .
Facial angiofibromas or forehead plaque
Nontraumatic ungual fibroma
≥3 hypomelanotic macules
Shagreen patch
Multiple retinal nodular hamartomas
Cortical tuber a
a Cerebral cortical dysplasia and cerebral white matter migration tracts count as one feature rather than two when they occur together.
Subependymal nodule
Subependymal giant cell astrocytoma
Cardiac rhabdomyoma
Renal angiomyolipoma or pulmonary lymphangiomyomatosis b
b Other features of TSC must be present for a definite diagnosis when lymphangiomyomatosis and renal angiomyolipomas are both present.
Multiple randomly distributed pits in dental enamel
Hamartomatous rectal polyps
Bone cysts
Cerebral white matter radial migration lines a
Gingival fibromas
Nonrenal hamartoma
Retinal achromic patch
‘Confetti’ skin lesions
Multiple renal cysts
Definite diagnosis: two major features or one major and two minor features
Probable diagnosis: one major and one minor feature
Possible diagnosis: one major or two minor features
There is a very high prevalence of cutaneous findings in TSC. Hypomelanotic macules are usually present at birth or appear within the first few months of life and ultimately are present in approximately 90% of TSC patients. Classically, they present as an ‘ash leaf macule,’ an oval area with reduction in pigment ( Fig. 29.8A ). These areas can also be irregular in outline and shape, or very small and guttate (confetti-like) ( Fig. 29.8B ). In fair-skinned infants, a Wood's lamp examination may be necessary for detection. They can also occur on the scalp, with lightening of the hair within the patch. A single, hypopigmented macule in an infant, without other features of TSC, should not cause concern, but multiple lesions (≥3) should lead to further investigation.

Classic facial angiofibromas typically appear after 4 years of age, however fibrous plaques resembling coalescence of angiofibromas may be present over the scalp, face, or neck at birth or appear shortly thereafter, as firm slightly raised patches or plaques that are commonly erythematous ( Fig. 29.8C ). Shagreen patches – firm, palpable thickened dermal plaques – can occur in the lumbar region as a roughened area of erythematous skin with a rubbery consistency or develop in other areas of the torso ( Fig. 29.8D ). They range in size from a few millimeters to 15 cm in diameter, and generally appear by adolescence but are infrequent in young infants. Periungual fibromas are similarly uncommon in the first decade. Gingival fibromas are a less common feature, but occasionally develop in young children ( Fig. 29.8E ).
Seizures occur in more than 60–80% of patients with TSC. Conversely 4–50% of infants with infantile spasms have TSC. TSC patients with early onset of seizures (<2 years of age) or infantile spasms have an elevated risk for intellectual disability. The most common lesions in the brain include tubers, subependymal nodules and subependymal giant-call astrocytomas (SEGA). Renal cysts, angiomyolipomas, and cardiac rhabdomyomas are findings in newborns and infants that suggest TSC. Cardiac rhabdomyomas are often discovered on routine antenatal ultrasound; 30–50% of infants with TSC have cardiac rhabdomyomas, and 80–90% of infants with these lesions have TSC. They are rarely symptomatic and typically regress spontaneously. An expert panel at the Tuberous Sclerosis Consensus Conference recommended a baseline electrocardiogram both at the time of diagnosis and prior to surgery, as cardiac rhabdomyomas can be associated with pre-excitation and arrhythmias on the electrocardiogram. Angiomyolipomas are the most common renal manifestation of TSC.
TSC may be caused by an autosomal dominant mutation in the TSC1 gene (encoding hamartin) or the TSC2 gene (encoding tuberin). The two proteins form a complex that is involved with the phosphoinositide-3-kinase (PI3K) signaling pathway, which regulates cell growth and proliferation ( Fig. 29.6 ).
The differential diagnosis for the hypopigmented macules seen in the neonatal period includes vitiligo, nevus depigmentosus, nevus anemicus, and piebaldism. A single lesion, or several lesions occurring along the lines of Blaschko suggests the diagnosis of nevoid hypopigmentation rather than TSC (see Chapter 23 ). Connective tissue nevi may be sporadic and have also been associated with Buschke–Ollendorff or Proteus syndrome. Angiofibromas have been described in MEN1 and Birt–Hogg–Dubé syndrome, and also as an isolated autosomal dominant disorder.
Consensus recommendations for screening and evaluation of TSC were proposed in 1998 and updated in 2000 ( Box 29.6 ). The Scottish Clinical Genetics Service and the UK Tuberous Sclerosis Association have also created clinical guidelines. Neurology and/or neurosurgery should be consulted for management of seizures, brain tumors, and shunting of obstructive hydrocephalus. Ophthalmology should also examine patients to assist in confirming the diagnosis. Renal ultrasound or renal MRI are important to screen angiomyolipomas. This may also help to identify patients with coexisting polycystic kidney disease due to a contiguous gene deletion syndrome involving the TSC2 and PKD1 genes. Echocardiography and electrocardiogram are recommended at diagnosis for confirmation (to detect cardiac rhabdomyomas and arrhythmias) and to screen for aortic aneurysms.
Age-appropriate neurologic and developmental assessment
Dermatologic examination
Ophthalmic examination
Neurologic consultation
Cardiac evaluation (ECG and echocardiogram)
Renal MRI or renal ultrasound
Head magnetic resonance imaging
Additional information for recommended screening, and surveillance and management can be found at: http://www.tsalliance.org/pages.aspx?content=731 (Accessed February 2014)
Pulsed dye laser has been recommended for flat erythematous angiofibromas, and both Potassium titanyl phosphate (KTP) laser and carbon dioxide laser are used to treat more elevated lesions, but are rarely indicated in infants. Fibrous forehead plaques are generally left untreated, but may also be treated with lasers or surgery. Several case reports have recently described the utility of topical rapamycin for the treatment of angiofibromas. This approach holds promise, but further study is needed to establish the safety, efficacy and dosing guidelines.
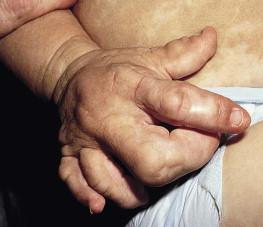
Proteus syndrome (MIM #176920) is a very rare condition characterized by dramatic segmental or mosaic overgrowth. Common complications include skeletal asymmetry, characteristic overgrowth of the palms or soles referred to as ‘cerebriform connective tissue nevi’ (CCTN), linear epidermal nevi, deep or superficial vascular malformations, dysregulated adipose tissue (formerly referred to as lipomas) and tumor predisposition (see also Chapter 22 ).
Almost all individuals with Proteus syndrome have a dermatologic manifestation. Over 40% of affected neonates will demonstrate at least some evidence of the disease at birth, such as epidermal nevi or vascular malformations. The three main cutaneous findings are epidermal nevi, vascular malformations, and soft subcutaneous masses. Epidermal nevi are usually linear and verrucous, but may be macular and hyper- or hypopigmented. Malformations may be venous, capillary, and/or lymphatic. The cerebriform connective tissue nevus, when on the sole of the foot, is caused by hyperplasia of cutaneous and subcutaneous tissues and considered virtually pathognomonic. The tissue is very firm; similar lesions may also occur on the hands, perinasal area, or near the canthus. Prominent cutaneous venous structures may occur as a result of patchy dermal hypoplasia. Macrodactyly and adipose overgrowth may also be observed.
Overgrowth in Proteus syndrome is disproportionate, asymmetric, progressive, distorting, and persistent (see Fig. 29.7 ). Overgrowth usually presents between 6 and 18 months of age and can occur in areas that were completely normal at birth. Overgrowth affects most tissues including bones, cartilage, muscle, and connective tissues. At least some overgrowth and asymmetry is present at birth in 17.5% of cases. Orifices may be affected, causing respiratory obstruction, conductive hearing loss, or gastric outlet obstruction. Cystic degeneration of the lungs may lead to pneumonia. Affected individuals are predisposed to deadly deep venous thrombosis and pulmonary embolism. The central nervous system is commonly affected by hemimegalencephaly (unilateral enlargement of the brain), but most patients are asymptomatic. Eye complications are common and range from strabismus to epibulbar hamartomas. Ovarian cystadenomas and cystic lesions of the epididymis are also common.
The cause of Proteus syndrome is a de novo post-zygotic activating mutation in the AKT1 oncogene. Proteus features are an example of somatic mosaicism that is lethal in the non-mosaic state. Because Proteus is a mosaic disorder, biopsy of affected tissue is required to make a genetic diagnosis.
Proteus syndrome has been overdiagnosed, prompting the creation of diagnostic criteria ( Box 29.7 ) to separate Proteus syndrome from other overgrowth syndromes, many of which share asymmetric hypertrophy as a feature but almost always to a less severe degree. Proteus stands apart by having fairly rapid postnatal progression, relentless deforming overgrowth and a poor prognosis.
General criteria: Mosaic, and progressive, and sporadic
Category A: Cerebriform connective tissue nevus
Category B:
Epidermal nevus
Disproportionate overgrowth in one: limbs, skull, external auditory canal, vertebrae, or viscera
Bilateral ovarian cystadenomas or monomorphic adenomas of the parotid gland in children
Category C:
Dysregulated adipose tissue (lipoatrophy or lipomas)
Vascular malformations (capillary, venous, or lymphatic)
Facial phenotype, all: long face, dolichocephaly, downslanting palpebral fissures, low nasal bridge, wide or anteverted nares, open mouth at rest
All three general criteria plus either one from A, two from B, or three from C are required to make a diagnosis of Proteus syndrome.
This is most commonly misdiagnosed as Proteus syndrome. HH-ML includes superficial capillary vascular malformation (similar to port-wine stain), but lacks progressive, distorting overgrowth, deep vascular malformations and cerebriform connective tissue nevi on the palms or soles. In non-distorting overgrowth that characterizes HH-ML, a bone is normally shaped and larger than expected, but without growths or odd edges. In HH-ML, non-distorting overgrowth is present at birth, whereas, the distinctive distorting overgrowth that characterizes Proteus may not be apparent until age 2 or 3. Lipomas may recur after surgical removal. Therefore, removal of symptomatic lipomas only is recommended.
Sometimes also referred to as M-CM, it has previously been termed macrocephaly-capillary malformation and macrocephaly-cutis marmorata telangiectatica congenital. MCAP sometimes includes markedly large brain size with variable cortical malformation, growth dysregulation with variable asymmetry, patchy capillary malformations frequently on the philtrum, upper lip and nose as well as the limbs and trunk, and distal limb abnormalities such as syndactyly and polydactyly, and mild connective tissue dysplasia (hyperextensible joints and skin). Congenital Chiari I malformation had been associated with MCAP, however, the term ‘cerebellar tonsil herniation’ (CTH) is now preferred to describe acquired herniation caused by cerebellar overgrowth that occurs in up to 70% of MCAP cases. With capillary vascular malformation and asymmetric overgrowth, children often meet the criteria for Klippel–Trenaunay syndrome. Affected children typically have developmental delay (85%) and neonatal hypotonia (68%). Frontal bossing and prominent nevus simplex are additional facial features. There is also a mildly increased risk of cancer (about 3%). MCAP has been associated with postzygotic mutations in the PIK3CA gene.
This is an example of an overgrowth syndrome defined by its newly identified causative mutation, an activating mutation in the PIK3CA gene. Affected children have segmental overgrowth that affects the muscles, skeleton, and fibroadipose tissue without cutaneous features of Proteus syndrome. Both distorting and non-distorting overgrowth are reported. The features of MOFH often begin at birth, in contrast to Proteus syndrome which generally appears later.
Segmental overgrowth, lipomatosis, arteriovenous malformation and epidermal nevus (SOLAMEN) is a ‘Proteus-like’ syndrome, now known to be a mosaic form of Cowden disease. Affected individuals carry a germline PTEN mutation and a mosaic second hit to the PTEN gene on the opposite allele in affected tissues. Absence of the cerebriform connective tissue nevi on the palms and soles distinguishes SOLAMEN syndrome. In addition, characteristic features of Cowden disease such as macrocephaly, breast and thyroid hamartomas and skin changes are likely to be present.
Congenital lipomatosis, overgrowth, vascular malformations, epidermal nevi and skeletal anomalies (CLOVES) describes another subset of patients, most of whom were initially misdiagnosed with Proteus syndrome. In CLOVES syndrome, overgrowth is present at birth with truncal vascular anomalies, truncal lipomatous masses and overgrown feet with acral or musculoskeletal anomalies. In contrast to Proteus, the overgrowth is not progressive. Patients may have wrinkled skin on the palms and soles, but lack the firm rubbery CCTN of Proteus syndrome. Since CLOVES syndrome was suspected to be a mosaic disorder at the outset, massive DNA sequencing of affected tissues revealed causative activating mutations in PIK3CA . Severe scoliosis, large truncal masses, high flow vascular lesions, phlebectasia with thromboembolism and orthopedic problems of the hands and feed may all require early, aggressive intervention in affected individuals. Prognosis seems better compared to Proteus syndrome but this condition can still be very severe, even life-threatening.
The so-called ‘nevus psiloliparus’ is the cutaneous hallmark, with a localized non-scarring patch of alopecia on the scalp which may or may not overlie a fatty tissue mass. ECCL, while once thought to be a localized form of Proteus syndrome, does not meet the diagnostic criteria of Proteus, lacking disproportionate growth as a salient feature (see also Chapter 31 ).
Maffucci syndrome consists of multiple enchondromatosis (which may be confused with hyperostosis) and vascular malformations. Klippel–Trenaunay (MIM #149000) syndrome consists of vascular malformations with overgrowth, typically in the same segment. Axillary freckling and neurofibromas help distinguish neurofibromatosis.
Management of these syndromes depends on the specific disease; if overgrowth is severe – as in Proteus syndrome – management is often very difficult. A multidisciplinary approach best addresses all aspects of the disease adequately. Ongoing ophthalmology, neurology, and developmental assessment exams are recommended. Caregivers should be informed of the risk of pulmonary embolism and stroke so that healthcare providers consider it if an emergency arises. Orthopedic surgery should be consulted before functional deficits appear and before the patient becomes too debilitated to undergo surgery. Cancer surveillance is an important part of management as dictated by clinical symptoms; routine imaging is not recommended.
Isolated hemihyperplasia places individuals at increased risk (5.9%) for embryonal malignancies such as Wilms tumor, adrenal cell carcinoma and hepatoblastoma. A study of 260 children with hemihyperplasia reported that the risk in truly isolated idiopathic hemihyperplasia is lower (1.2%) and in syndrome-related hemihyperplasia higher (10%) than previously reported. The risk of malignancy in specific syndromes with hemihyperplasia, including HH-ML and excluding Klippel–Trenaunay, is not clear. Until further information becomes available, screening with abdominal ultrasound every 3–6 months until age 8, checking of blood alpha fetoprotein level every 3 months until age 4 and referral to a geneticist for identifying associated syndromes is recommended. Individuals with SOLAMEN syndrome have a risk of malignancies associated with Cowden (PTEN hamartoma) syndrome.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here