Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
On completion of this chapter, you should be able to:
Identify the normal anatomy of the scrotum
Explain the vascular supply to the scrotal contents
Describe patient positioning, scanning protocol, and technical considerations for an ultrasound examination of the scrotum
Discuss the role of color and spectral Doppler in scrotal imaging
Describe the ultrasound characteristics of scrotal pathology
Ultrasound is the imaging modality of choice for evaluating the scrotum. High-frequency ultrasound imaging, combined with color and spectral Doppler, quickly and reliably provides valuable information in the assessment of scrotal pain or mass. In particular, color Doppler has a central role in the evaluation of suspected testicular torsion because it can demonstrate absence of flow in the affected testis. Color Doppler also plays a key role in the evaluation of testicular infection by demonstrating hyperemic flow on the affected side. Ultrasound imaging accurately differentiates intratesticular from extratesticular masses and cystic from solid masses. Advances in the development of ultrasound equipment have provided improved spatial and contrast resolution, reduced speckle artifact, and increased sensitivity to the display of scrotal perfusion. The steady progress in ultrasound image quality has enhanced our ability to clearly define the scrotal anatomy and to depict and differentiate abnormalities more accurately. This chapter covers the pertinent anatomy of the scrotum and its contents, including the vascular supply. The ultrasound scanning protocol is discussed, along with tips on scanning techniques and potential pitfalls. A review of the disease processes affecting the scrotum is provided, including a description of sonographic findings.
The testes are symmetric, oval-shaped glands residing in the scrotum . In adults, the testis measures approximately 3 to 5 cm in length, 2 to 4 cm in width, and approximately 3 cm in height. Each testis is divided into more than 250 to 400 conical lobules containing the seminiferous tubules. These tubules converge at the apex of each lobule and anastomose to form the rete testis in the mediastinum. The rete testis drains into the head of the epididymis through the efferent ductules ( Fig. 23.1 ). Sonographically, the testes appear as smooth, medium-gray structures with a fine echotexture.
The epididymis is a 6- to 7-cm tubular structure beginning superiorly and then coursing posterolateral to the testis. It is divided into head, body, and tail. The head is the largest part of the epididymis, measuring 6 to 15 mm in width. It is located superior to the upper pole of the testis ( Fig. 23.2 ). It contains 10 to 15 efferent ductules from the rete testis, which converge to form a single duct in the body and tail. This duct is known as the ductus epididymis. It becomes the vas deferens and continues in the spermatic cord. The body of the epididymis is much smaller than the head. It is difficult to see with ultrasound on normal individuals. It follows the posterolateral aspect of the testis from the upper to the lower pole. The tail of the epididymis is slightly larger and is positioned posterior to the lower pole of the testis. The appendix of the epididymis is a small protuberance from the head of the epididymis. Postmortem studies have shown the appendix epididymis in 34% of testes unilaterally and 12% of testes bilaterally. The normal epididymis usually appears as isoechoic or hypoechoic compared with the testis, although the echotexture is coarser.
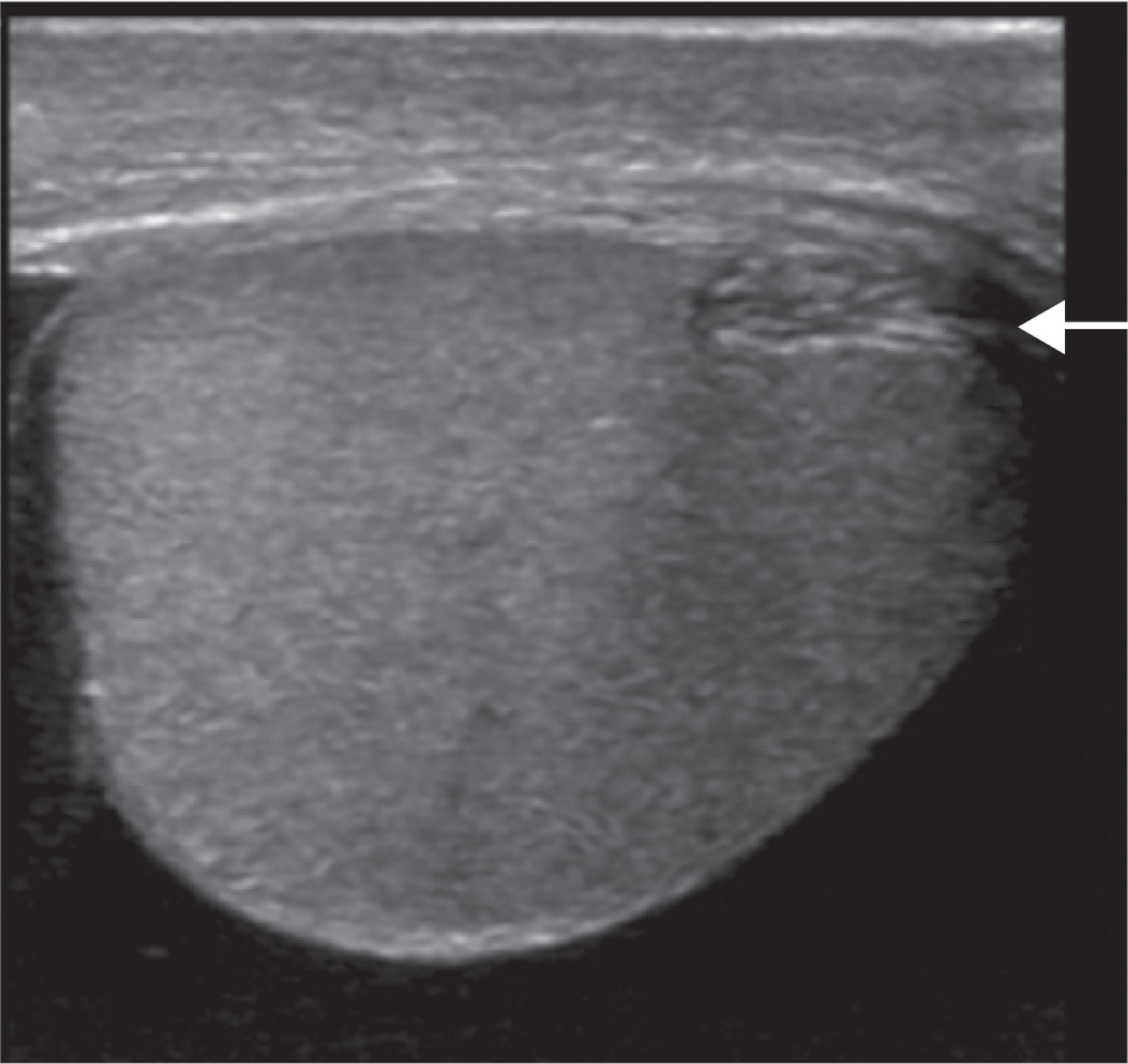
At the upper pole of the testis, the appendix testis is attached. It is located between the testis and the epididymis. Postmortem studies have shown the appendix testis to be present in 92% of testes unilaterally and 69% bilaterally ( Fig. 23.3 ).
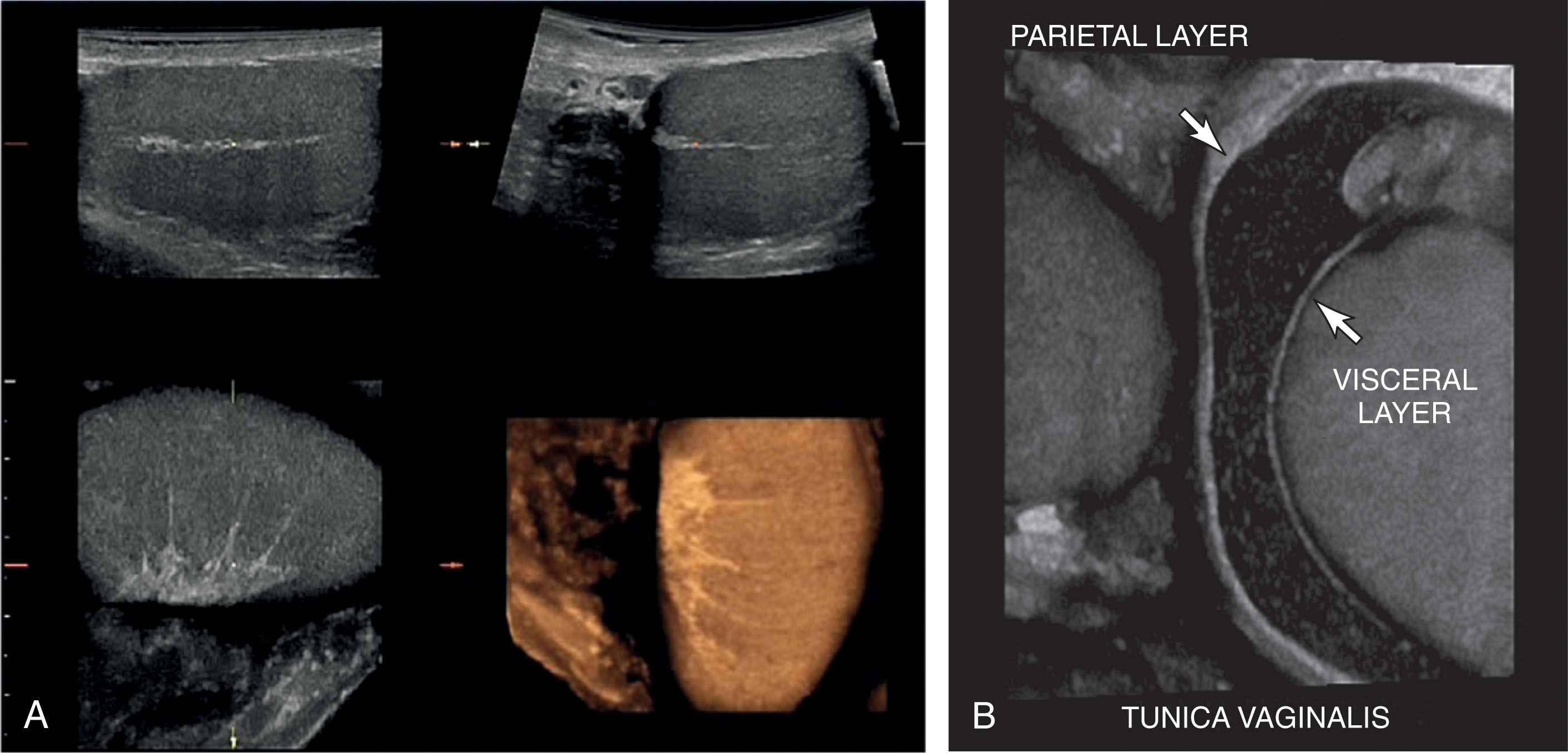
The testis is completely covered by a dense, fibrous tissue termed the tunica albuginea . The posterior aspect of the tunica albuginea reflects into the testis to form a vertical septum known as the mediastinum testis . Multiple septa ( septa testis ) are formed from the tunica albuginea at the mediastinum. They course through the testis and separate it into lobules. The mediastinum supports the vessels and ducts coursing within the testis. The mediastinum is often seen on ultrasound as a bright hyperechoic line coursing craniocaudal within the testis ( Fig. 23.4 ). The tunica vaginalis lines the inner walls of the scrotum, covering each testis and epididymis. It consists of two layers: parietal and visceral. The parietal layer is the inner lining of the scrotal wall. The visceral layer surrounds the testis and epididymis. A small bare area is posterior. At this site, the testicle is against the scrotal wall, preventing torsion. Blood vessels, lymphatics, nerves, and spermatic ducts travel through the area (see Fig. 23.1 ). The space between the layers of the tunica vaginalis is where hydroceles form. It is normal to see a small amount of fluid in this space.
The vas deferens is a continuation of the ductus epididymis. It is thicker and less convoluted. The vas deferens dilates at the terminal portion near the seminal vesicles . This portion is termed the ampulla of the deferens. The vas deferens joins the duct of the seminal vesicles to form the ejaculatory duct , which, in turn, empties into the urethra . The junction of the ejaculatory ducts with the urethra is termed the verumontanum . The urethra courses from the bladder to the end of the penis. In men, the urethra transports both urine and semen outside the body.
The vas deferens, testicular arteries, venous pampiniform plexus, lymphatics, autonomic nerves, and fiber of the cremaster form the spermatic cord . The cord extends from the scrotum through the inguinal canal and internal inguinal rings to the pelvis. The spermatic cord suspends the testis in the scrotum.
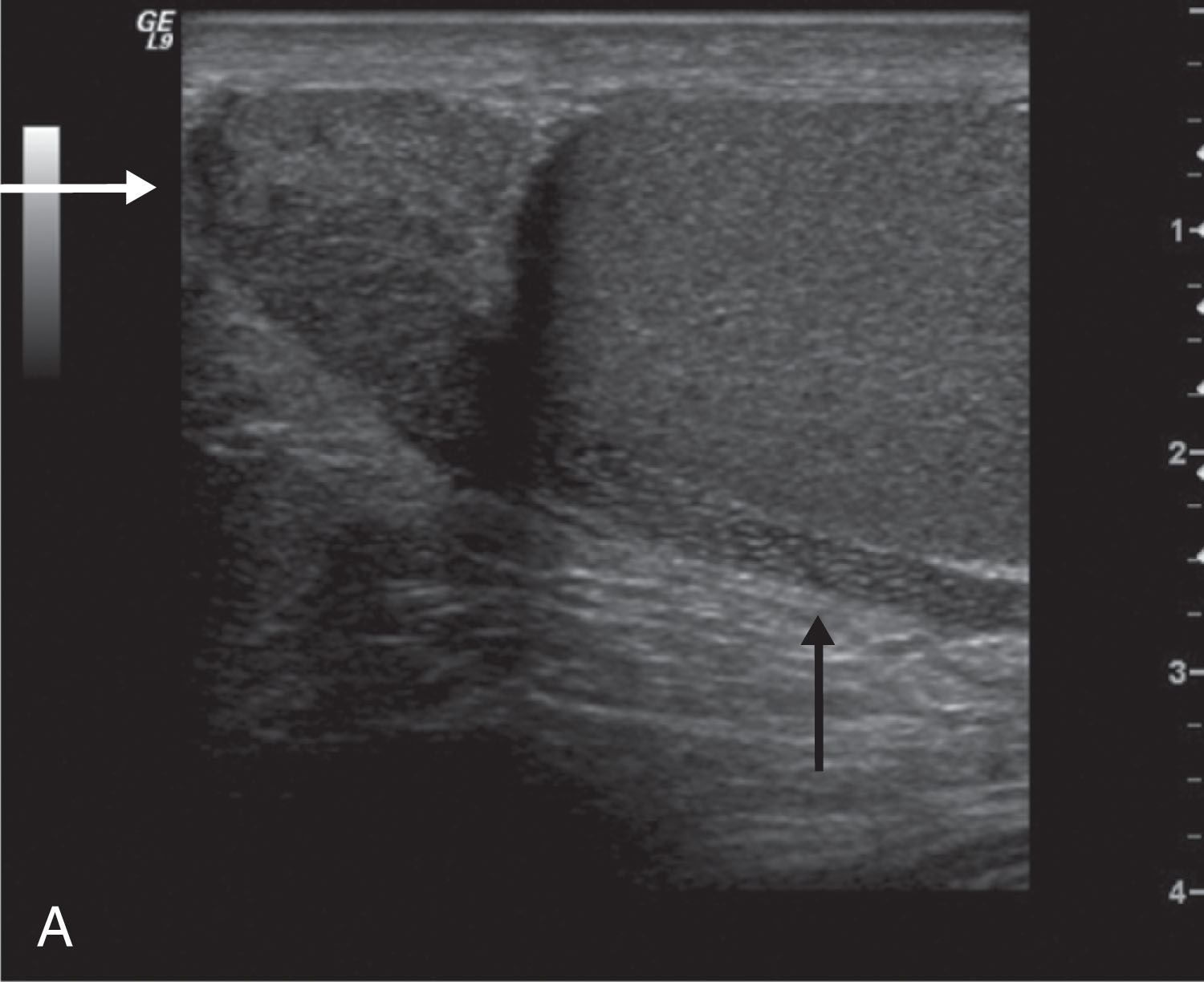
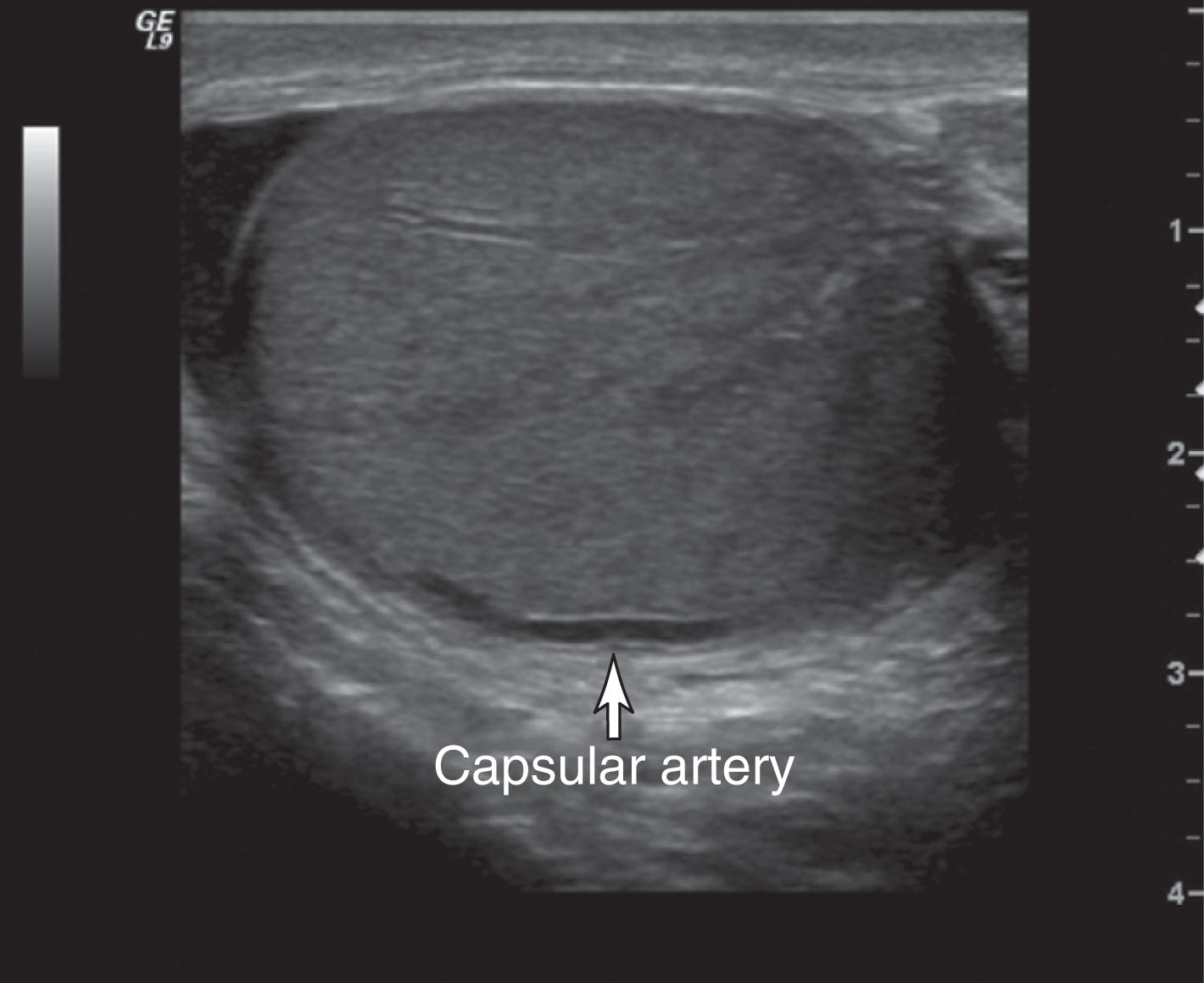
Right and left testicular arteries arise from the abdominal aorta just below the level of the renal arteries. They are the primary source of blood flow to the testis. The testicular arteries descend in the retroperitoneum and enter the spermatic cord in the deep inguinal ring. Then they course along the posterior surface of each testis and pierce the tunica albuginea, forming the capsular arteries, which branch over the surface of the testis. With high-frequency ultrasound imaging, the capsular artery is sometimes seen as a hypoechoic linear structure on the surface of the testis. Color Doppler can be used to confirm its identity ( Fig. 23.5 ). The capsular arteries give rise to centripetal arteries , which course from the testicular surface toward the mediastinum along the septa. Before reaching the mediastinum, they curve backward, forming the recurrent rami (centrifugal arteries) ( Fig. 23.6 ). These centrifugal arteries branch farther into arterioles and capillaries. With sensitive color Doppler settings, the recurrent rami may be seen giving a candy cane appearance ( Fig. 23.7 ).
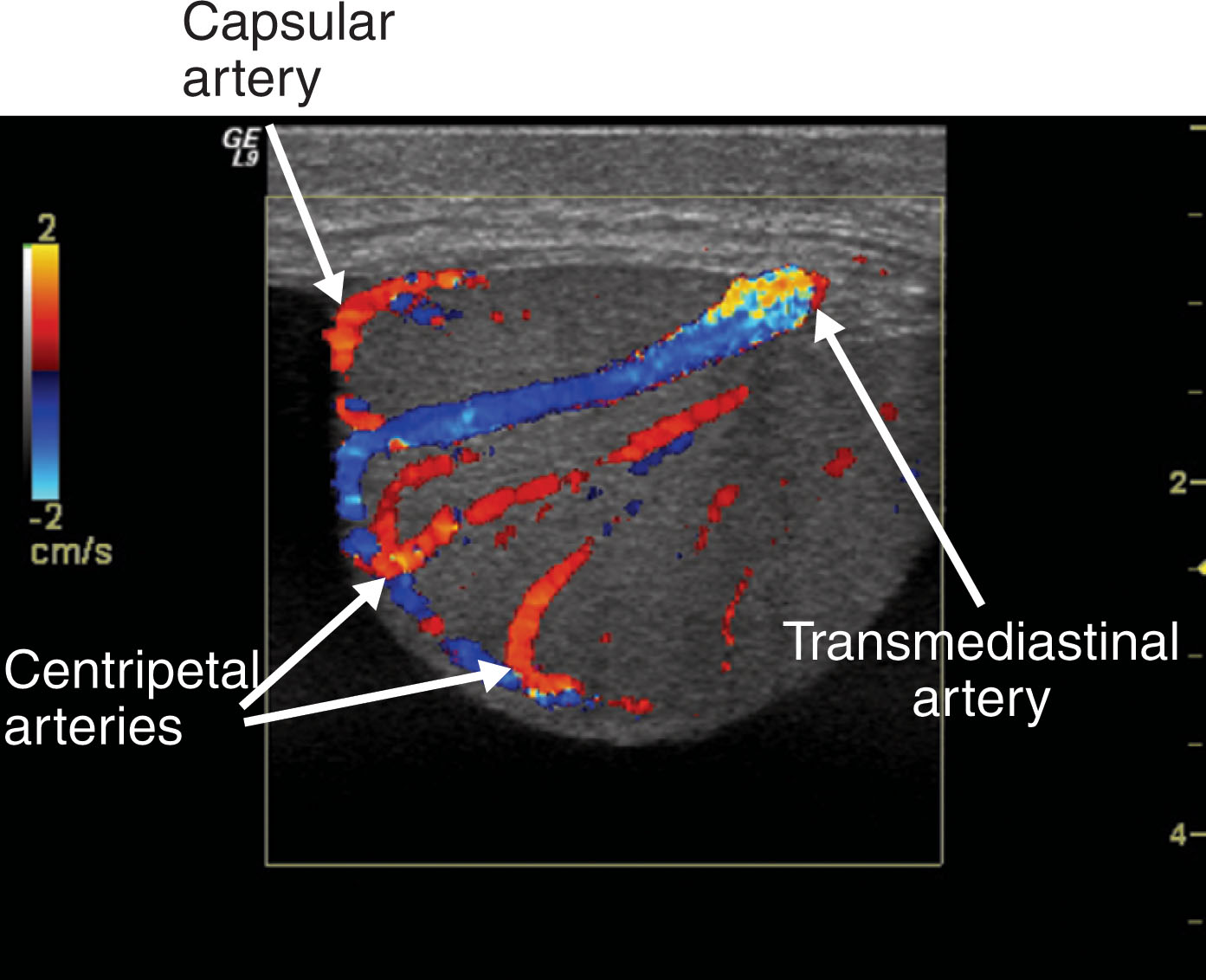
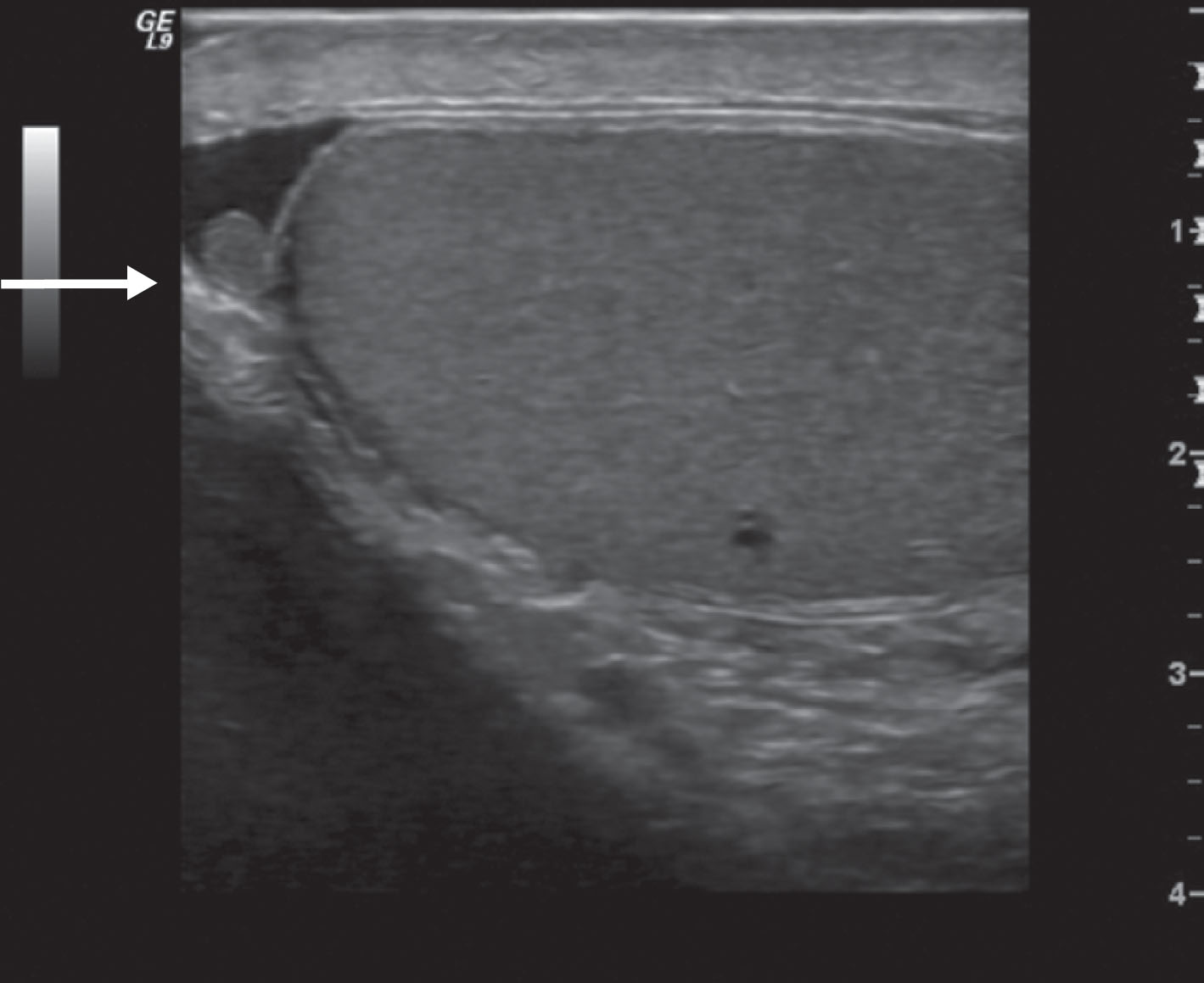
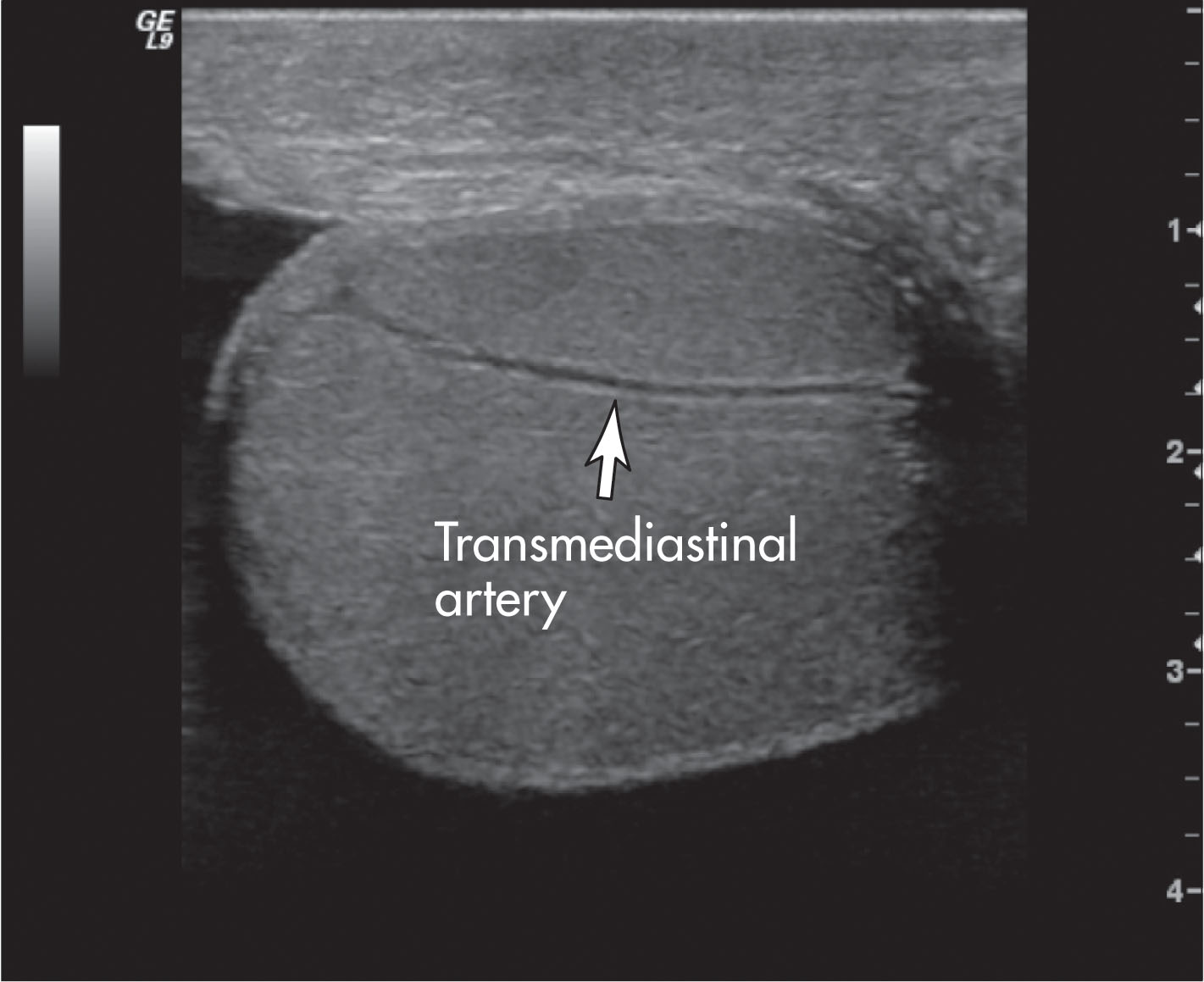
In approximately one-half of normal testes, a transmediastinal (or transtesticular) artery is visualized coursing through the mediastinum toward the testicular capsule. A large vein is often identified adjacent to the artery ( Fig. 23.8 ). On color Doppler, the transmediastinal artery will have a different color than the centripetal arteries because its flow is directed away from the mediastinum and toward the capsule. On reaching the testicular surface opposite the mediastinum, the transmediastinal artery courses along the capsule as capsular arteries. Spectral Doppler waveforms obtained from the capsular, centripetal, or transmediastinal arteries show a low-resistance waveform pattern in normal individuals ( Fig. 23.9 ). Box 23.1 diagrams arterial branching in the testicles.
Testicular artery
↓
Capsular artery
↓
Centripetal artery
↓
Recurrent rami
The cremasteric artery and deferential artery accompany the testicular artery within the spermatic cord to supply the extratesticular structures. They also have anastomoses with the testicular artery and may provide some flow to the testis. The cremasteric artery branches from the inferior epigastric artery (a branch of the external iliac artery). It provides flow to the cremasteric muscle and peritesticular tissue. The deferential artery arises from the vesicle artery (a branch of the internal iliac artery). It mainly supplies the epididymis and vas deferens. The scrotal wall is also supplied by branches of the pudendal artery .
Venous drainage of the scrotum occurs through the veins of the pampiniform plexus . The pampiniform plexus exits from the mediastinum testis and courses in the spermatic cord. It converges into three sets of anastomotic veins: testicular, deferential, and cremasteric. The right testicular vein drains into the inferior vena cava, and the left testicular vein joins the left renal vein. The deferential vein drains into the pelvic veins, and the cremasteric vein drains into tributaries of the epigastric and deep pudendal veins.
High-resolution ultrasound imaging is the primary screening modality for most testicular pathology. Applications include inflammatory processes of the testes and epididymis, tumors, trauma, torsion, hydrocele, varicocele, hernias, spermatoceles, and undescended testes.
Patient preparation: none.
Transducer selection: 8- to 12-MHz linear array.
Patient position: supine (Valsalva maneuver or upright position to check for varicocele).
Images and observations should include the following:
Gray scale.
Long testicle (include medial, mid, and lateral).
Long epididymis.
Anteroposterior (AP) and long measurements of above anatomy.
Transverse scan of each testis (upper, mid, lower).
Transverse scan of head of epididymis.
Include AP and transverse measurements of the middle pole of the testicles.
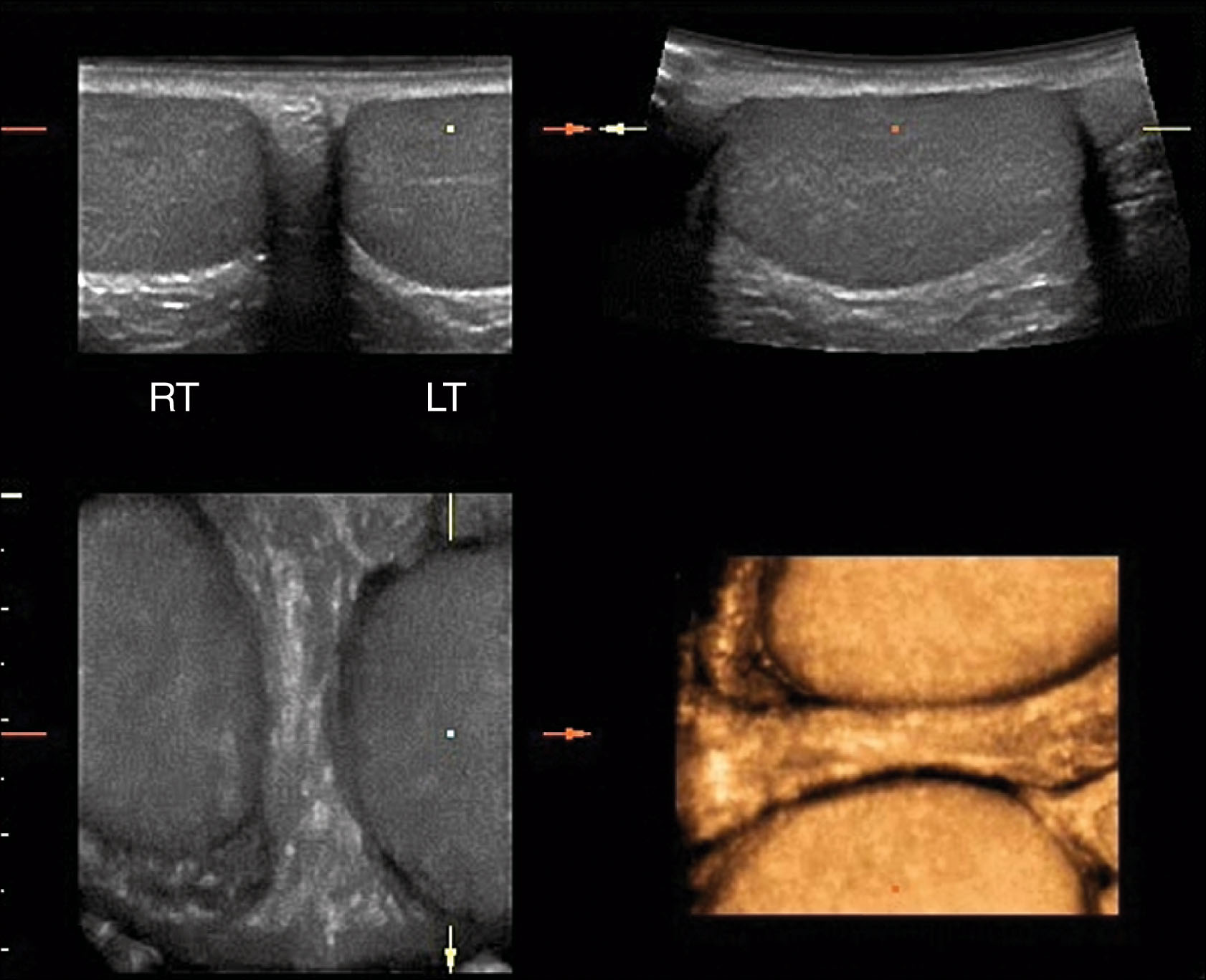
If possible, a split-screen image should be obtained to compare the echogenicity of each testis.
Images of the extratesticular area should be obtained to determine the presence of hydrocele, hernia, or other conditions.
Doppler flow analysis of the scrotal area:
When indicated, color and pulsed Doppler analysis of intratesticular flow with resistance measurements should be obtained.
The scanning instrument should be optimized for slow flow detection (e.g., decrease pulse repetition frequency/scale, lower filters, increase gain or power).
Ultrasound examination of the scrotum is performed with the patient in the supine position. The penis is positioned on the abdomen and covered with a towel. The patient is asked to place his legs close together to provide support for the scrotum. Alternatively, a rolled towel placed between the thighs can support the scrotum. It is often unnecessary to place a towel for support if the legs are positioned close together. This may be more comfortable for the patient in pain.
A generous amount of warmed gel is applied to the scrotum to ensure adequate probe contact and eliminate air between the probe and the skin surface. Rarely, a stand-off pad may be necessary to improve imaging of very superficial structures such as a tunica albuginea cyst. However, with the use of high-frequency probes (10 to 14 MHz), this is usually not necessary. Instead of a stand-off pad, an extra-thick mound of gel may be adequate to improve near-field imaging.
Before beginning the scrotal ultrasound, it is necessary to determine clinical findings. Was this patient referred because of a palpable mass, scrotal pain, swollen scrotum, or other reasons? It is important to ask the patient to describe his symptoms, including history, location, and duration of pain. Can he feel a mass? If so, ask the patient to find the lump. Then place the probe exactly over this location to examine the site. Did the patient experience trauma? When did the trauma occur? Ask him to describe what happened. Has he had a vasectomy? When? Not only is this information helpful in guiding the examination, but it is important to the interpreting physician and gives confidence to the patient regarding the quality of the ultrasound study. Box 23.2 lists important tips when performing an ultrasound examination of the scrotum.
Explain procedure and preparation to patient, and then allow the patient to get ready in private.
Be sure to take an image of right and left testicles together for comparison in both gray-scale and color Doppler.
Perform Valsalva maneuver when a varicocele is suspected.
Sensitize color Doppler for slow flow when evaluating torsion.
Torsion is a surgical emergency; perform the examination in a timely manner.
Scrotal ultrasound is always a bilateral examination, with the asymptomatic side used as a comparison for the symptomatic side. To begin, it is best to perform a brief survey scan to determine what abnormalities, if any, are present. Each testis is scanned from superior to inferior and is carefully examined to determine whether abnormal findings are present. The size, echogenicity, and structure of each testis are evaluated. The testicular parenchyma should be uniform with equal echogenicity between sides. Think of these questions as you scan: Is the parenchyma homogeneous or heterogeneous? Is there a mass? If so, is it cystic or solid? Is it intratesticular or extratesticular? Is one testis much larger than the other? Which side is swollen, or is one side shrunken? All testes should appear similar in size and shape. Is the epididymis normal? Is the skin thickened? Turn on color Doppler to assess the flow. Is there an absence of flow in the testis, or is it hyperemic? How does the color Doppler compare between sides? Testes should show about the same amount of flow when the same color Doppler setup is used. Check the flow in each epididymis. Again, compare between sides. They should be similar. After the survey scan, images are obtained that demonstrate the findings.
Representative images are obtained in at least two planes—transverse and sagittal—with additional imaging planes scanned as needed to demonstrate the findings. In transverse, images are taken that show the superior, mid, and inferior portions of each testis. The width of the testis is measured in the midtransverse view. A transverse view of the head of the epididymis is included. Superior to the epididymal head, an image is obtained to demonstrate the area of the spermatic cord. In the sagittal plane, images are taken to show the medial, mid, and lateral portions. A long axis measurement of testicular length is obtained in the midsagittal image. Again, additional images may be taken to demonstrate abnormal areas. An image is obtained of the epididymal head superior to the testicle . The body and tail of the epididymis can be demonstrated coursing posteriorly on each side. Scrotal skin thickness is evaluated and compared from side to side. At least one image is taken to show both testes at the same time, so the interpreting physician can compare size and echogenicity ( Fig. 23.10 ). Additional views may be taken in patients with suspected varicocele. These include upright positioning and the Valsalva maneuver. Color and spectral Doppler are used in all examinations, with representative images taken to demonstrate both arterial and venous flow in each testis. Table 23.1 lists the scanning protocols for scrotal ultrasound.
| Transverse Image | Sagittal Image |
|---|---|
| Spermatic cord area | Spermatic cord area |
| Epididymal head | Epididymal head with superior testis |
| Superior testis | Long axis mid-testis with measurement |
| Mid testis with measurement | Medial long axis |
| Inferior testis | Lateral long axis |
| Transverse view showing both testes | Color Doppler of epididymal head |
| Color Doppler of mid-testis | |
| Spectral Doppler of artery | |
| Spectral Doppler of vein |
High-frequency linear-array transducers are preferred for scrotal imaging because they provide the best spatial resolution. However, the field of view is limited with linear arrays. Occasionally, a larger field of view is required to measure anatomy or display anatomic relationships. Ultrasound systems provide numerous methods to meet this need, including virtual convex imaging, panoramic imaging, stitching images together, and using a curved-array transducer.
Real-time imaging of the scrotum is performed with a high-frequency linear-array probe of at least 7.5 MHz. Because high-frequency transducers have better spatial and contrast resolution compared with lower-frequency transducers, they are preferred for scrotal imaging. Probes with frequencies of 10 to 15 MHz are usually best. Because there is a tradeoff between frequency and penetration, the highest frequency providing adequate penetration should be used. In patients with considerable wall edema and thickening, frequencies as low as 5 to 7.5 MHz may be necessary to adequately penetrate the testis.
Many ultrasound systems have a trapezoid or virtual convex feature that can be selected with the linear-array probe. This is very helpful for measuring the long axis of the testis or when an abnormal area cannot be entirely imaged with the standard linear format ( Fig. 23.11A ). It is best to use this feature selectively instead of routinely because steering the beam to create the wider format has a negative impact on image quality; steering widens the distance between scan lines and degrades lateral resolution.
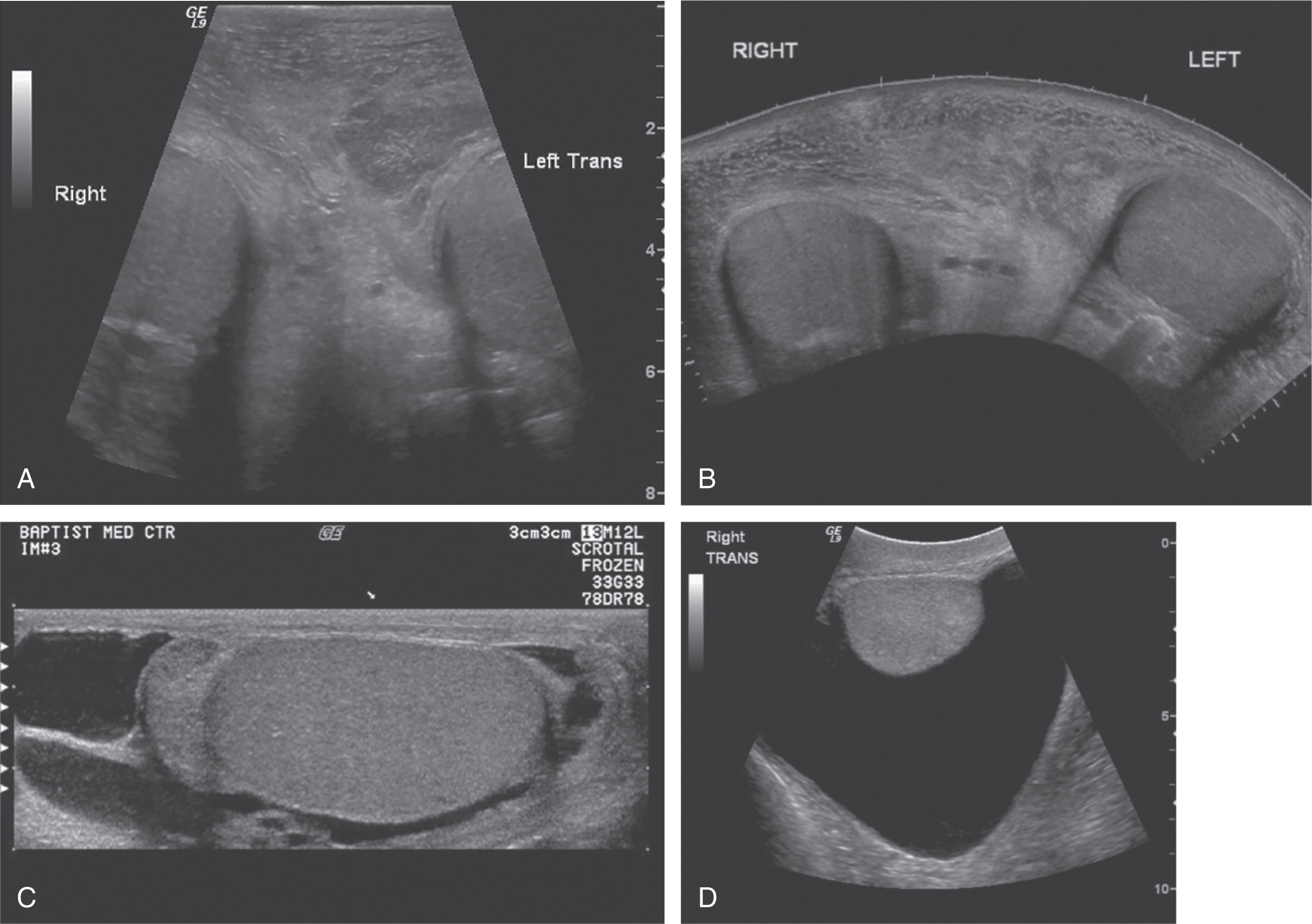
In cases of large hydroceles, hematomas, or swelling, an even larger field of view may be required. In these cases, a panoramic tool may be useful. This tool allows the image to build as the probe is moved over the skin surface. A very long image can be obtained that shows anatomic relationships (see Fig. 23.11B ). Images may also be stitched together in a combined mode. The first image is obtained in one window; then the probe is moved, and another image is obtained by attempting to match the boundaries of the first image (see Fig. 23.11C ). Another way to obtain a larger field of view is to use a 5- to 7.5-MHz curved-array transducer for a portion of the examination to demonstrate the entire scrotal contents. Again, this should be done selectively to obtain the necessary images and should be followed by a return to the high-frequency linear-array probe for further evaluation of each testis (see Fig. 23.11D ).
Most modern ultrasound scanners offer additional features that enhance the quality of the ultrasound image. These features include, but are not limited to, compound imaging, harmonics, extended field-of-view imaging, virtual convex, speckle reduction algorithms, and use of multiple focal zones ( Table 23.2 ). All these controls may be adjusted to improve image quality.
| Feature | What Is It? | Advantage | Disadvantage |
|---|---|---|---|
| Harmonics | Selective reception of penetration (uses frequencies generated within tissue) |
|
Less harmonic penetration (uses higher frequency) |
| Compound imaging | Uses multiple-angled firings to create one image |
|
|
| Speckle reduction algorithms | Sophisticated algorithms applied to the image to reduce speckle (salt and pepper appearance of ultrasound image) |
|
None |
| Extended field of view imaging | Image builds up as probe is moved across anatomy | Improved ability to show anatomic relationships of structures too large to fit in linear-array format | May be difficult to perform on uncooperative patient or over sharply curving interface |
| Trapezoid or virtual convex imaging | Steering of linear-array probe to create sector format | Larger field of view with linear-array probes | Reduced lateral resolution |
| Multizone focus | Use of multiple focal zones to create an extended area of focus on one image | Improved lateral resolution | Slowed frame rate |
Color and spectral Doppler play an important role in scrotal ultrasound. The typical color/spectral Doppler frequencies used for scrotal ultrasound are between 4 and 8 MHz. The upper-frequency range is used to improve sensitivity to slow flow. This is important in evaluation of testicular torsion or tumor vascularity. Penetration is decreased with higher frequencies, so it is important to make sure that the color penetrates to the depth of interest. Color and spectral Doppler findings on the symptomatic side are always compared with the asymptomatic side.
Power Doppler is often used to quickly get to a sensitive setting that will demonstrate slow flow. Power Doppler shows the amplitude or power of the moving signal, whereas color Doppler shows the frequency shift. Power Doppler does not demonstrate flow direction or aliasing and, to some, offers a more straightforward display of blood flow. Presets for power Doppler are often set at a lower pulse repetition frequency (PRF) than color Doppler because aliasing is not an issue, so pushing the power Doppler button may show more flow with fewer adjustments to the controls. This often provides a quick way to get to a more sensitive flow setting. Persistence is usually much greater with power Doppler, requiring a steady hand and slower movement of the probe. To further enhance power Doppler, the same parameters are adjusted as for color Doppler.
Familiarity with color Doppler controls is very important when performing scrotal ultrasounds. The sonographer may need to adjust some of the following color Doppler parameters throughout the study to enhance the visibility of scrotal perfusion ( Table 23.3 ):
Gain —The color gain control is used to amplify the reflected color Doppler signal. Whenever the expected amount of color is not visible in the image, the color gain should be increased until noise is present. Once color noise is visible, the gain can be decreased until it just disappears. At this point, the color gain setting is optimized.
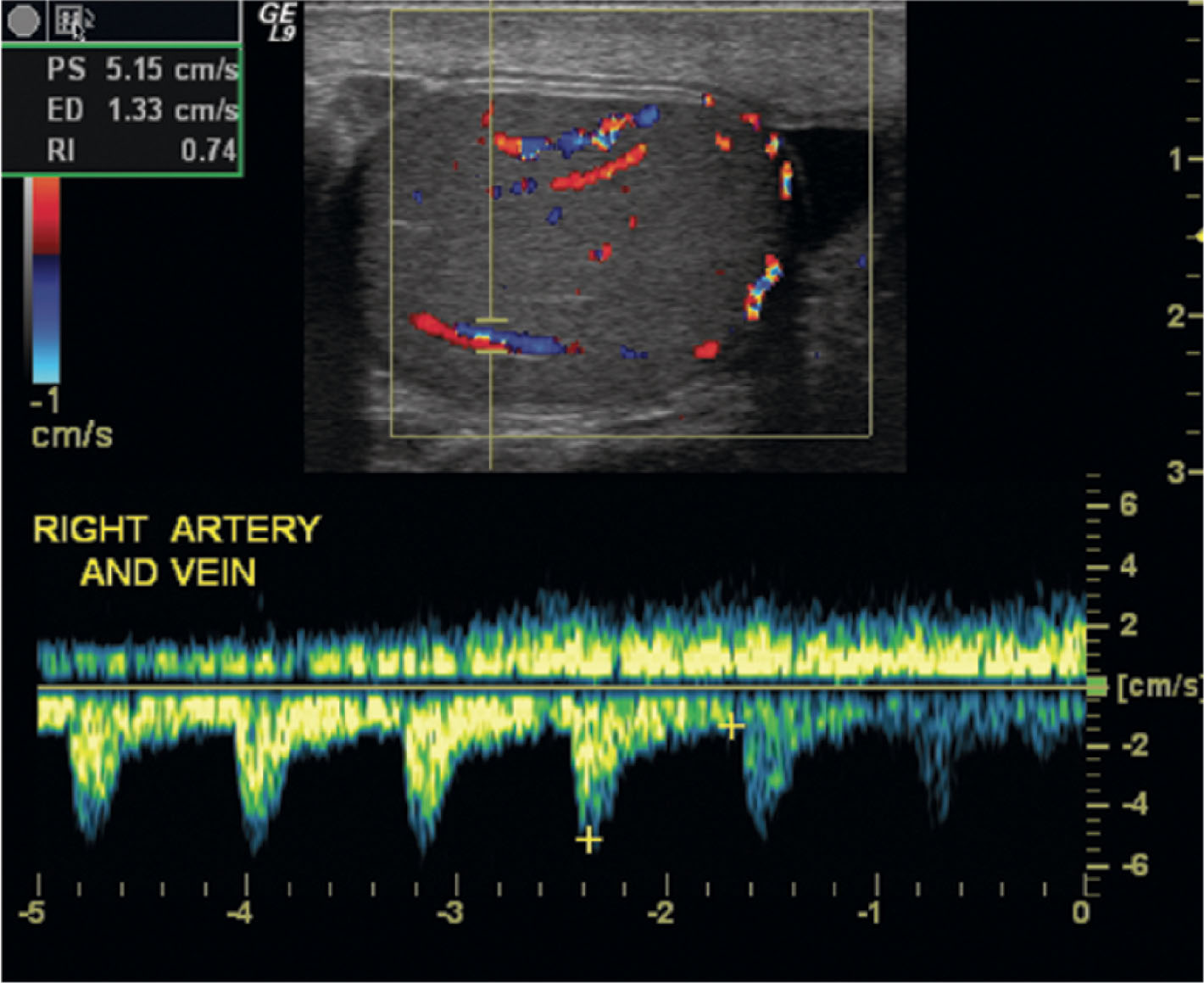
Scale/PRF —The PRF is the number of pulses transmitted in 1 second. This important color parameter affects the sensitivity of the system in displaying slow flow. It also sets the point at which color aliasing occurs (Nyquist limit). The control has different names depending on the ultrasound equipment being used. It is variably named scale, PRF, or flow rate. The PRF is reduced to improve sensitivity to slow flow. This is critical when ruling out testicular torsion. If the PRF is set too high, slow flow may not be visible. When the PRF is set too low, excessive color aliasing occurs, which makes it impossible to determine flow direction or to assess flow quality. Neither of these factors is significant in scrotal ultrasound, so it is common to use low PRF settings. However, flash artifact from patient motion is more apparent with very low PRFs and may make scanning difficult. It is recommended to adjust the PRF so that the asymptomatic testicular flow is well demonstrated without excessive flash artifact. Then compare the same settings on the contralateral side ( Fig. 23.12 ).
Wall filter —The wall filter acts as an electronic eraser. Color echoes that fall below the filter cutoff do not appear on the image display. The wall filter is adjusted downward to enhance flow sensitivity. It is turned up to reduce flash artifact. On most ultrasound systems, the wall filter is automatically adjusted with the PRF. But in some instances, it may be beneficial to make further adjustments.
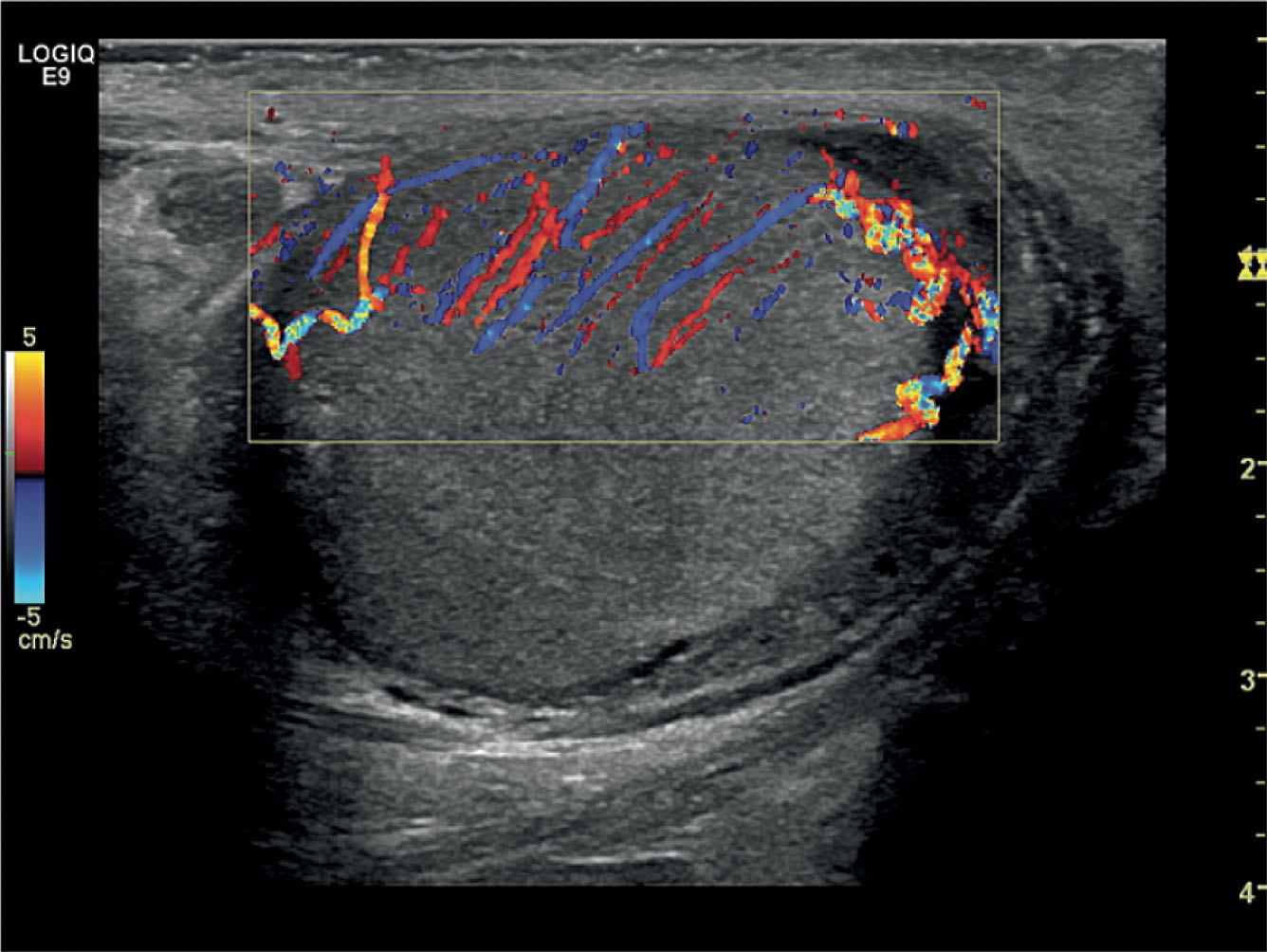
Line density —The line density is the number or density of scan lines contained within the color box. It affects the lateral resolution of the color display. As line density is increased, lateral resolution is improved. The size of the intratesticular arteries is displayed more accurately when line density is high. Frame rate becomes slower as line density is increased because more transmitted pulses are required to create each image frame. If the frame rate becomes too slow, the line density can be decreased. The user must choose the tradeoff between resolution and frame rate ( Fig. 23.13 ).
Threshold or color/tissue priority —The B/color threshold is used to determine whether a gray scale or a color pixel is displayed in any given location on the image. Color and power Doppler images are color overlays on top of an existing gray-scale image. A problem arises when gray-scale and color information are received for the same pixel location. The threshold control allows the user to prioritize either gray scale or color. For ultrasound of most small parts, including scrotal imaging, it is best to set the threshold so that color is prioritized. Based on the setting, when color and gray-scale information is received for an identical pixel location, the one displayed is determined by the brightness (amplitude) of the gray-scale dot and the frequency shift and/or power level of the color signal. This feature is not as important when looking at large vessels, such as the common carotid artery, because the vessel lumen typically does not contain gray-scale information, and color can be freely displayed in those pixels. However, in small parts imaging, most vessels are so small that the lumen is not visible or may contain gray-scale echoes caused by volume averaging. In these instances, color will not be displayed unless the threshold control is set to a level that prioritizes color.
Packet size —The packet size is the number of sound pulses transmitted on each scan line within the color box. The packet size is usually set between 8 and 20 pulses for each scan line. The packet size affects the signal-to-noise ratio, improving color sensitivity when more pulses are used. The frame rate gets slower as the number of packets (pulses) is increased on each scan line. The packet size can be reduced to raise the frame rate when necessary or increased to improve color sensitivity. The key factor affecting color sensitivity, however, is the PRF.
Color box/region of interest —The color box or region of interest is the area within the gray-scale image where flow is color encoded. The width of the color box affects the frame rate. If the color box is very wide, more scan lines are required to complete each image frame. This means that a greater number of pulses must be transmitted. This takes more time, so the frame rate is reduced. Color box depth also affects frame rate. When the color box is placed deep in the image, the round-trip time for the sound is increased. This slows down the frame rate.
| Parameter | What Is It? | How to Adjust |
|---|---|---|
| Gain | Amplification of selected frequency shift signal | Turn up until noise is present and then decrease until noise goes away |
| Pulse repetition frequency (PRF) | PRF is the number of pulses transmitted per second; sets the Nyquist limit; main control affecting sensitivity to flow | Adjust on the asymptomatic side so that flow is visible without too much flash or motion artifact; decrease to improve sensitivity to slow flow; increase to reduce aliasing |
| Wall filter | Color signals received below the wall filter setting do not appear on the image | Decrease to improve sensitivity and to reduce flash/motion artifact |
| Line density | Density of scan lines contained within the color box | Turn up to improve lateral resolution of vessels; turn down to increase frame rate |
| Threshold | Level of gray-scale brightness that is allowable to be overwritten by color when both gray-scale and color information are obtained for the same pixel location within the image | Turn up so that color information is prioritized compared with gray-scale information; if the threshold (also known as color/write priority) is set too low, small intratesticular vessels will not be filled with color |
| Packet size | Number of pulses on each color scan line sensitivity | Turn up to improve signal-to-noise ratio and turn down to improve frame rate |
| Color box size | Region of interest that is color encoded within the image | Set just over the area of interest; increasing color box size or depth will slow frame rate |
An understanding of the factors affecting color frame rate, sensitivity, and resolution allows the sonographer to optimize the color parameters for each clinical situation. Most systems have specific presets for each ultrasound application. Selection of the scrotal preset will set the color parameters near an optimal setting for a typical normal examination. However, the user must further adjust the controls to enhance the visibility of scrotal perfusion in abnormal states.
Table 23.4 lists the pathology and sonographic appearance associated with scrotal trauma, infection, and fluid collection. Although the sonographer is not responsible for the interpretation of ultrasound images, an understanding of the various differential considerations is useful to fully evaluate the lesion. Table 23.5 lists many of the common and uncommon masses or fluid collections found within or surrounding the testes.
| Pathology | Sonographic Appearance |
|---|---|
| Infection | |
| Epididymitis | Enlarged epididymis |
| Heterogeneous texture | |
| Hypoechoic, may contain hyperechoic areas | |
| Blood flow in the epididymis | |
| Focal orchitis | Hypoechoic area within testis |
| Blood flow in the testis | |
| Diffuse orchitis | Enlarged, hypoechoic testis |
| Echogenicity of the whole testis | |
| Trauma | |
| Rupture | Irregular contour |
| Focal alteration in echogenicity | |
| Hematoma | Heterogeneous area |
| Becomes hyperechoic as the blood clot ages | |
| Avascular | |
| Torsion | Gray-scale image of testis normal when duration <4 h |
| Testis enlarged and hypoechoic 4–12 h | |
| Testis heterogeneous after 24 h | |
| Absence of testicular flow | |
| Fluid Collections | |
| Hydrocele | May be anechoic, but often contains low-level echoes |
| Surrounds anterolateral aspect of testis | |
| Spermatocele | Located in head of epididymis |
| May contain internal echoes and/or septations | |
| Smooth walls | |
| Posterior acoustic enhancement | |
| Epididymal cyst | May be located anywhere in epididymis |
| Usually small, anechoic | |
| Ultrasound cannot differentiate between spermatocele and epididymal cyst | |
| Posterior acoustic enhancement | |
| Varicocele | Tortuous, dilated veins |
| Increased size with Valsalva maneuver or patient standing | |
| Dilated veins fill with color on Valsalva maneuver | |
| Spectral Doppler confirms venous flow | |
| Hematocele | Contains low-level echoes |
| May contain septations and loculations | |
| Scrotal Masses | |
| Common | Hydrocele |
| Varicocele | |
| Ascites | |
| Hematocele | |
| Spermatocele | |
| Epididymitis | |
| Uncommon | Cysts |
| Pyoceles | |
| Herniated bowel | |
| Metastasis | |
| Polyorchidism | |
| Extratesticular | |
| Seminoma | |
| Extratesticular Cystic Mass | |
| Common | Hematocele |
| Spermatocele | |
| Uncommon | Pyocele |
| Epididymal cyst | |
| Herniated bowel | |
| Hypoechoic Lesion | |
| Common | Seminoma |
| Embryonal cell carcinoma | |
| Choriocarcinoma | |
| Mixed cell tumor | |
| Lymphoma | |
| Leukemia | |
| Uncommon | Teratoma |
| Torsion | |
| Metastasis | |
| Epididymal tumor | |
| Abscess | |
| Enlarged Testicle | |
| Common | Tumor |
| Edematous testis caused by trauma | |
| Torsion | |
| Uncommon | Myeloma of testicle |
| Idiopathic macro-orchidism | |
| Enlarged Epididymis | |
| Common | Epididymitis |
| Sperm granuloma | |
| Uncommon | Polyorchidism |
| Lipoma | |
| Hypoechoic Band in Testis | |
| Common | Normal mediastinum testis |
| Normal vessels | |
Scrotal trauma presents a challenge to the sonographer because the scrotum is often painful and swollen. Trauma may be the result of motor vehicle accident, athletic injury, direct blow to the scrotum, or straddle injury. The most important goal of the ultrasound examination in testicular trauma is to determine whether a rupture has occurred. Rupture of the testis is a surgical emergency that requires prompt diagnosis. If surgery is performed within 72 hours following injury, up to 90% of testes can be saved, but only 45% can be saved after 72 hours. Hydrocele and hematocele are both complications of trauma. However, neither is specific to trauma. Hematoceles contain blood and are also found in advanced cases of epididymitis or orchitis.
The sonographic findings associated with scrotal rupture include focal alteration of the testicular parenchymal pattern, interruption of the tunica albuginea, irregular testicular contour, scrotal wall thickening, and hematocele . These findings may also be associated with abscess, tumor, or other clinical conditions. When combined with a history of trauma, they suggest rupture.
The sonographic appearance of hematoceles varies with age. An acute hematocele is echogenic with numerous, highly visible echoes that can be seen to float or move in real-time. Over time, hematoceles show low-level echoes and develop fluid-filled levels or septations. The presence of a hematocele does not confirm rupture. Hematoceles result from bleeding of the pampiniform plexus or other extratesticular structures.
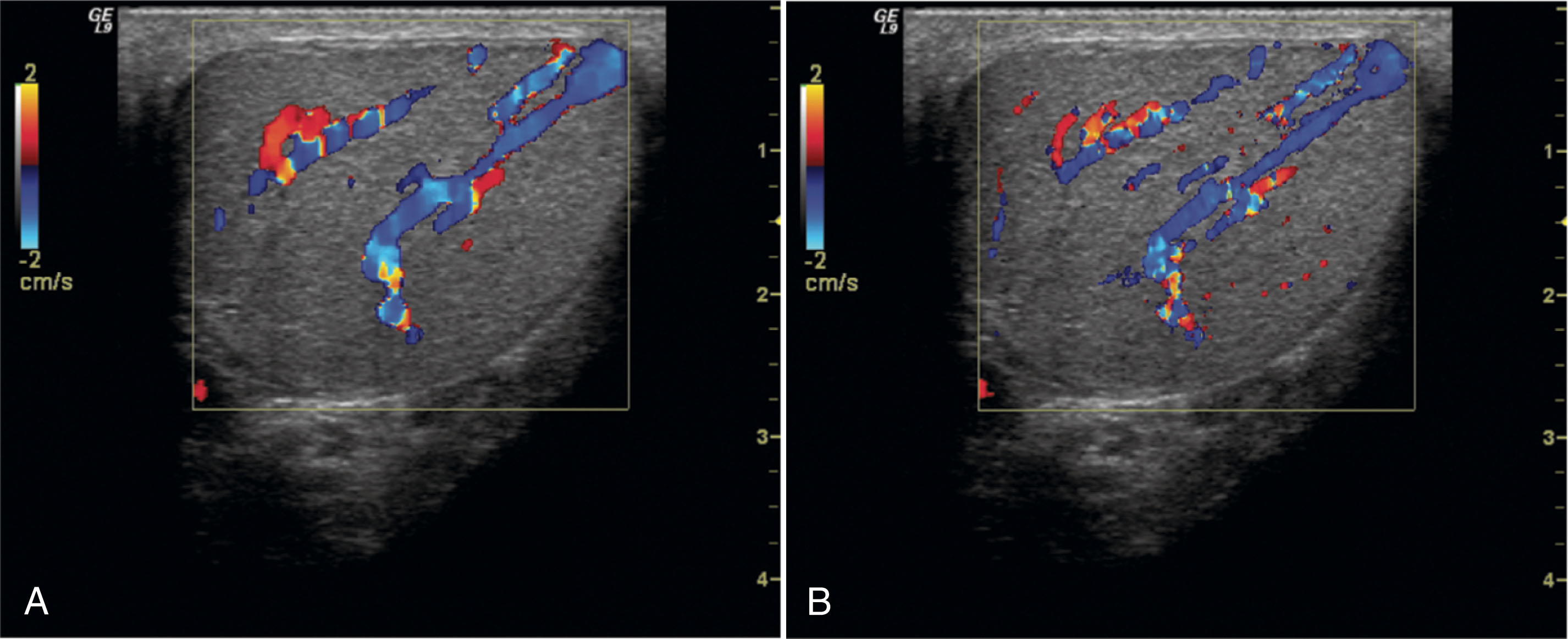
Hematomas associated with trauma may be large and may cause displacement of the associated testis. Hematomas appear as heterogeneous areas within the scrotum. They tend to become more complex over time, developing cystic components. Hematomas may involve the testis or epididymis, or they can be contained within the scrotal wall. Because hematomas are avascular, color Doppler is helpful in identifying them as areas with no flow ( Fig. 23.14 ).
Other uses of color Doppler in testicular trauma include identification of blood flow disruption across the surface of the testis. This is an indication of rupture. Color Doppler can aid in separating a normally vascularized testis from one that is disrupted by hematoma. Epididymitis may result from trauma, and color Doppler imaging can be used to identify the associated increased vascularity in the epididymis . Torsion may also be associated with trauma. Color Doppler is used to confirm the absence of flow in the testis with torsion.
Epididymo-orchitis is infection of the epididymis and testis. It most commonly results from the spread of a lower urinary tract infection via the spermatic cord. Less common causes include mumps, syphilis, tuberculosis, viruses, trauma, and chemical causes. Epididymo-orchitis represents the most common cause of acute scrotal pain in adults. The epididymis is the organ primarily involved with infection, which spreads to the testis in about 20% to 40% of cases. Orchitis almost always occurs secondary to epididymitis. Patients typically have increasing scrotal pain over 1 or 2 days. The pain may be mild or severe. Symptoms may also include fever and urethral discharge.
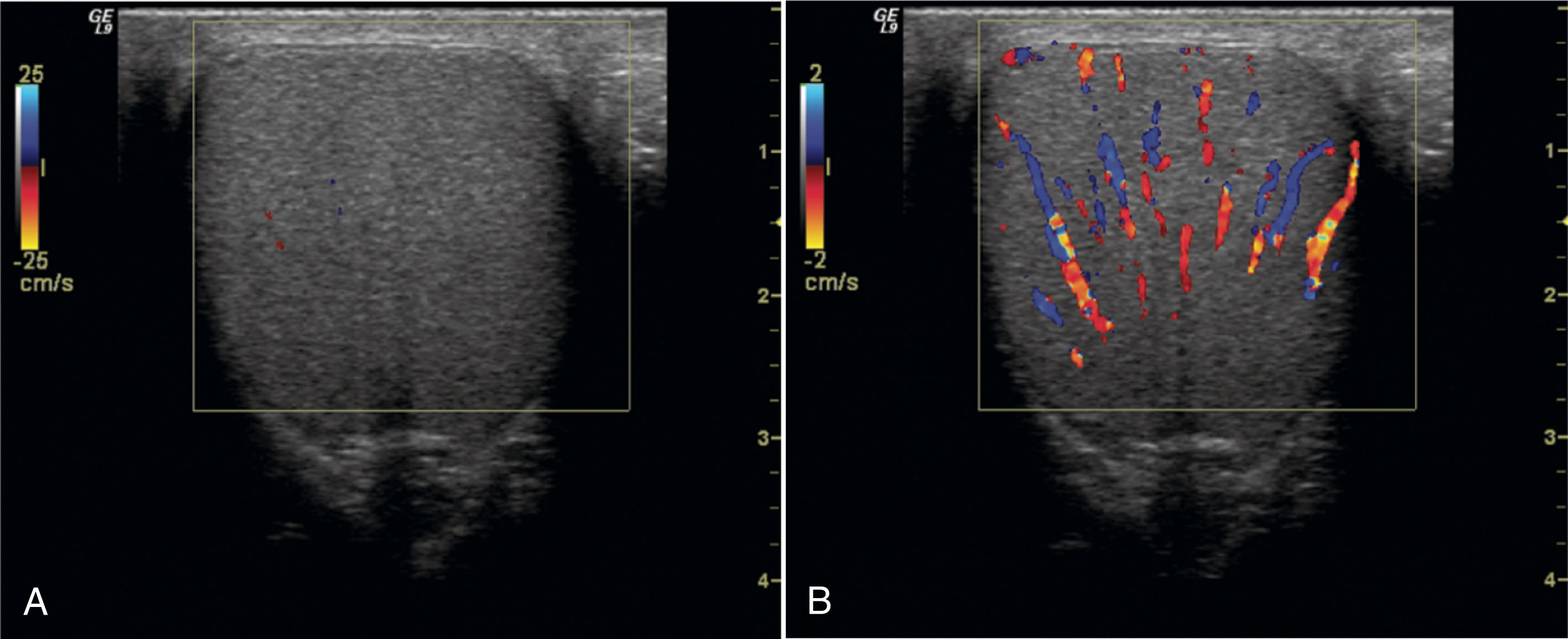
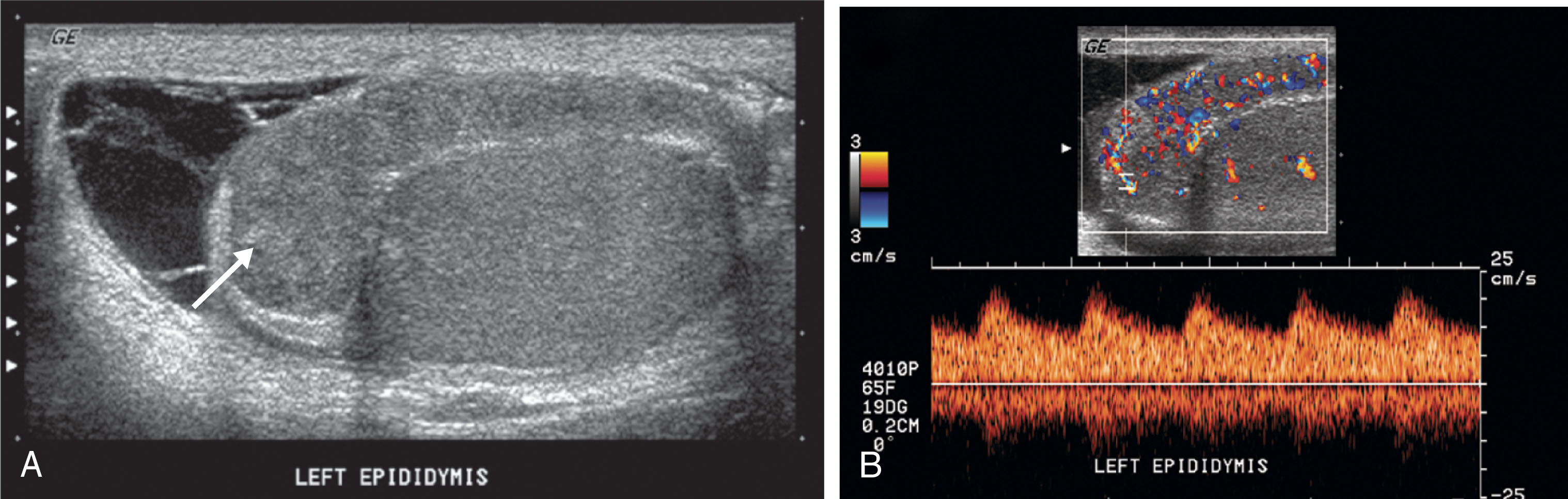
Epididymitis appears as an enlarged, hypoechoic gland. If secondary hemorrhage has occurred, the epididymis may contain focal hyperechoic areas. Hyperemic flow is confirmed with color Doppler ( Fig. 23.15 ). The normal epididymis shows little flow with color Doppler. The amount of color flow signal should be compared between sides. The affected side shows significantly more flow than the asymptomatic epididymis. It is important to use the same color Doppler settings when comparing the amount of flow between sides.
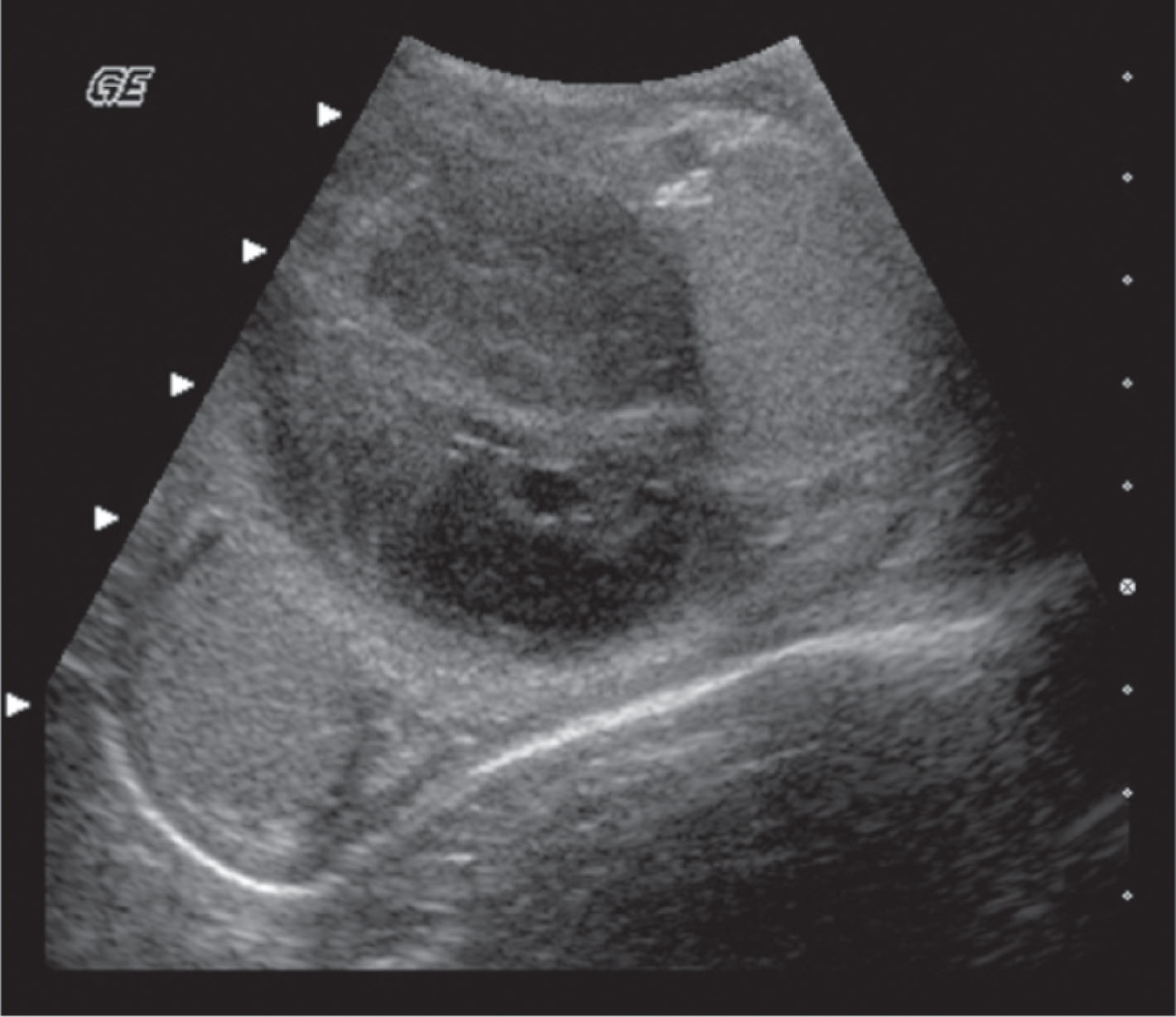
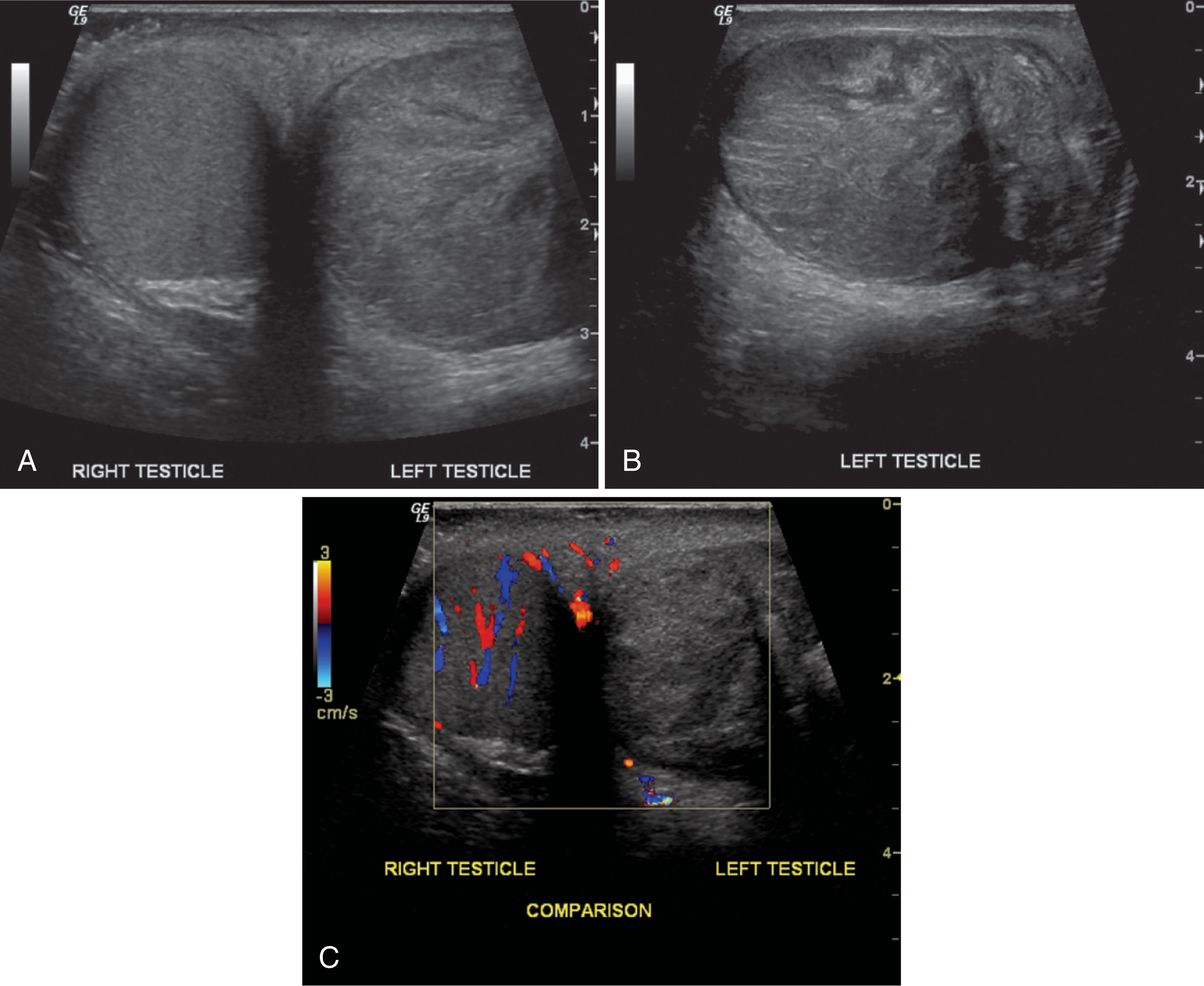
With epididymitis, Doppler waveforms demonstrate increased velocities in both systole and diastole. A low-resistance waveform pattern is present (see Fig. 23.15 ). If the infection is isolated to the epididymis, the testis will appear normal. When orchitis has developed, ultrasound imaging will show an enlarged testis. The infection may be focal or diffuse, and affected areas may appear hypoechoic compared with the surrounding tissue. Focal areas of infection within the testis will result in a heterogeneous appearance on ultrasound. A diffusely infected testis will appear enlarged and homogeneous with a hypoechoic echogenicity ( Fig. 23.16 ). Up to 20% of cases will have a normal-appearing epididymis and testis on ultrasound. Ultrasound gray-scale findings associated with epididymo-orchitis are not specific and may also be seen with torsion or tumor. Color and spectral Doppler are key tools in differentiating between epididymo-orchitis and torsion in the patient with acute scrotal pain.
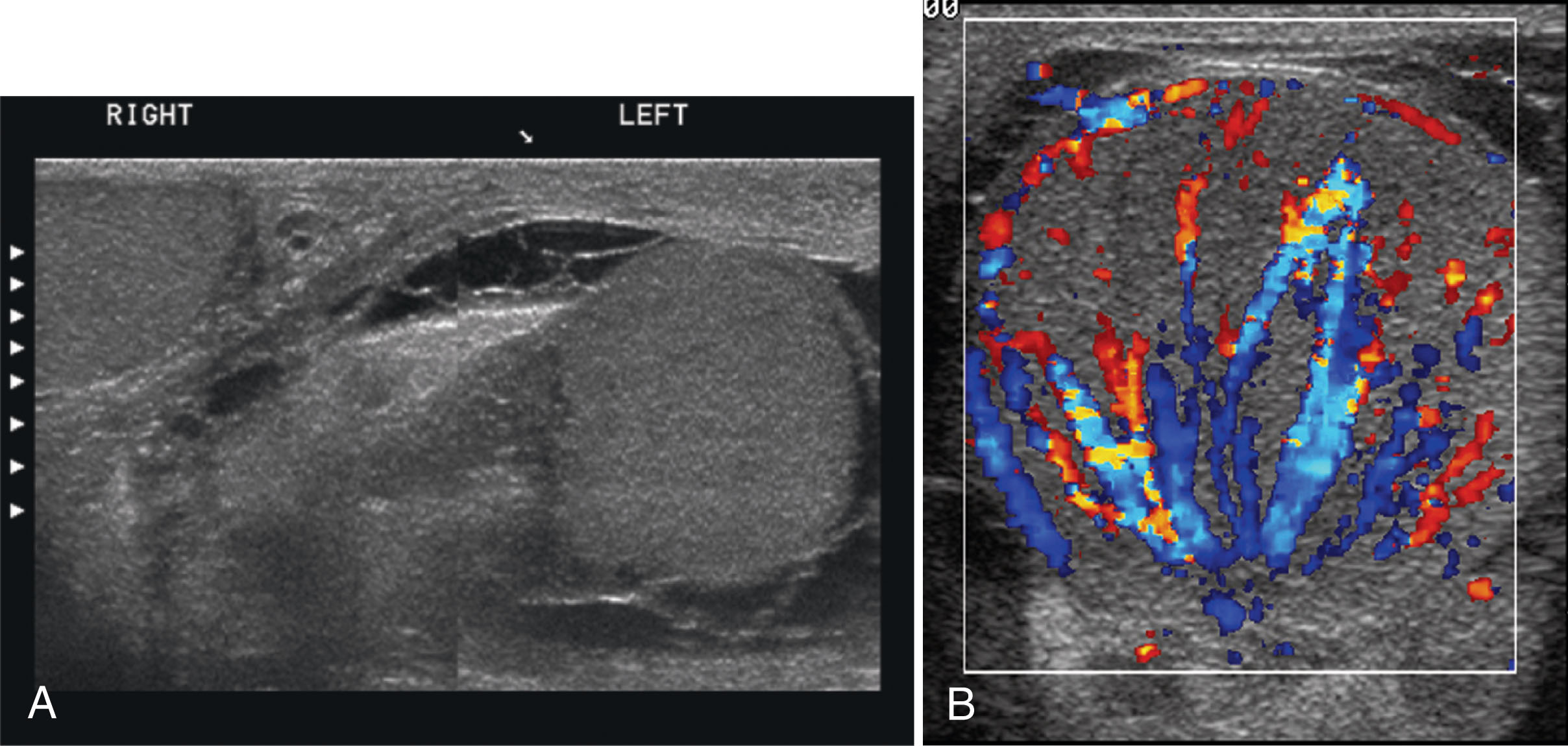
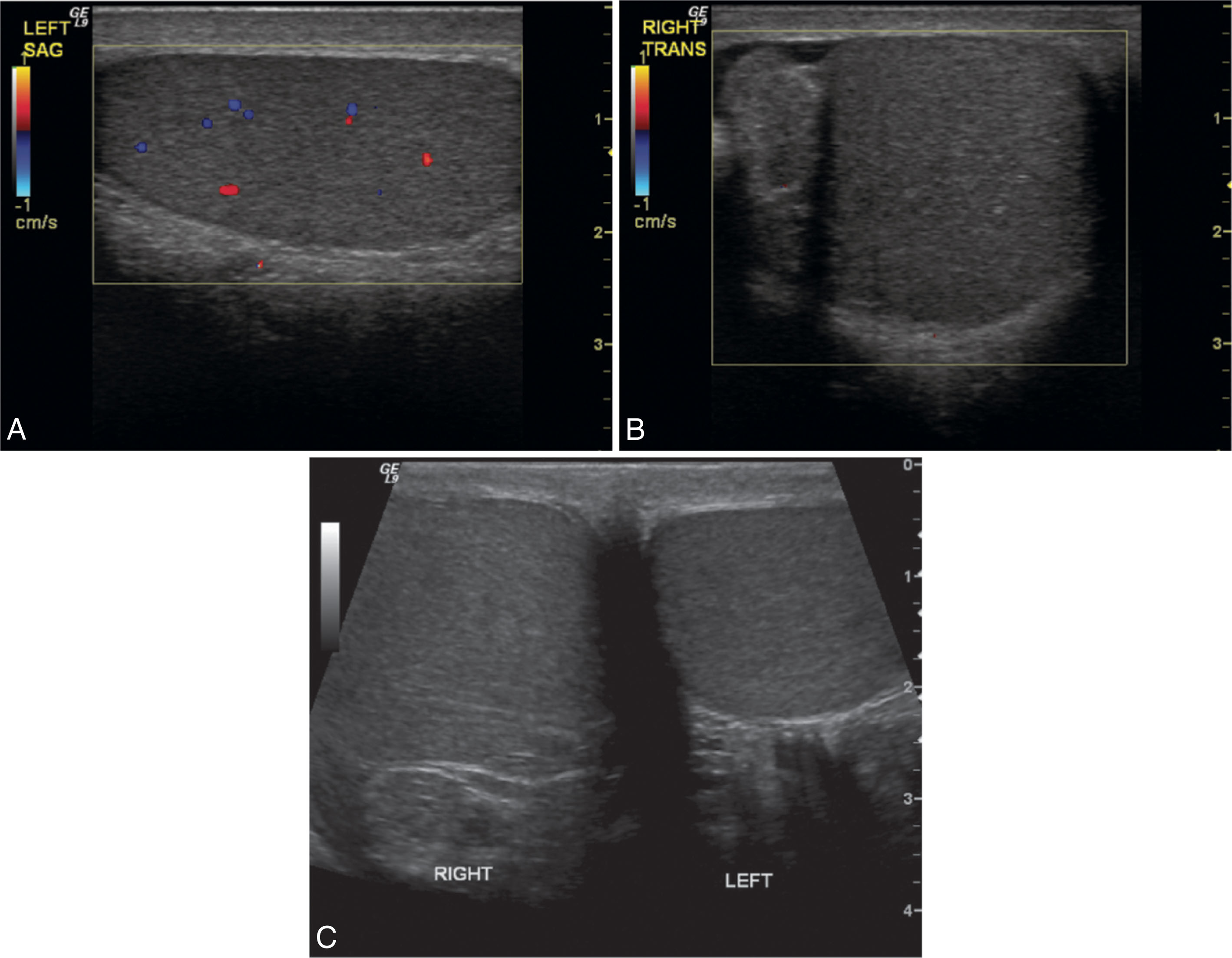
Epididymo-orchitis causes hyperemic flow with a significantly greater number of visible vessels on color Doppler compared with the asymptomatic side. Hyperemic flow is seen in the epididymis and testis when both are involved but is isolated to the epididymis when the testis is normal. Documentation of findings on ultrasound must include an image showing both testes, so the size and echogenicity can be compared. It is also recommended to obtain an image with the color box opened wide enough to show portions of both testes so that the amount of flow between sides can be easily compared.
Other findings associated with epididymitis and epididymo-orchitis include scrotal wall thickening and hydrocele . Hydroceles are found around the anterolateral aspect of the testis. They may appear anechoic or may contain low-level echoes. Complex hydroceles may be associated with severe epididymitis and orchitis. These have thick septations and contain low-level echoes. In severe cases, a pyocele may be present. A pyocele occurs when pus fills the space between the layers of the tunica vaginalis. It usually contains internal septations, loculations, and debris. This same appearance may be noted following trauma or surgery.
In severe cases of orchitis, testicular infarction may occur. The swollen testis is confined within a rigid tunica albuginea. Excessive swelling can cause obstruction to the testicular blood supply. Color Doppler will show decreased or absent flow compared with the contralateral testis. With decreased flow, spectral Doppler waveforms will have high resistance with little or no diastolic flow. A Doppler waveform demonstrating reversed diastolic flow is a serious finding, indicating threatened testicular infarction ( Fig. 23.17 ). Infarction can affect the entire testis or may be confined to a focal area. With focal infarction, the color will show perfusion only in portions of the testis that have an absence of color signals in the affected areas. Gray-scale imaging will depict a heterogeneous pattern. Areas of infarction tend to appear hypoechoic compared with the surrounding testicular parenchyma. If the entire testis becomes infarcted, findings cannot be differentiated from testicular torsion.
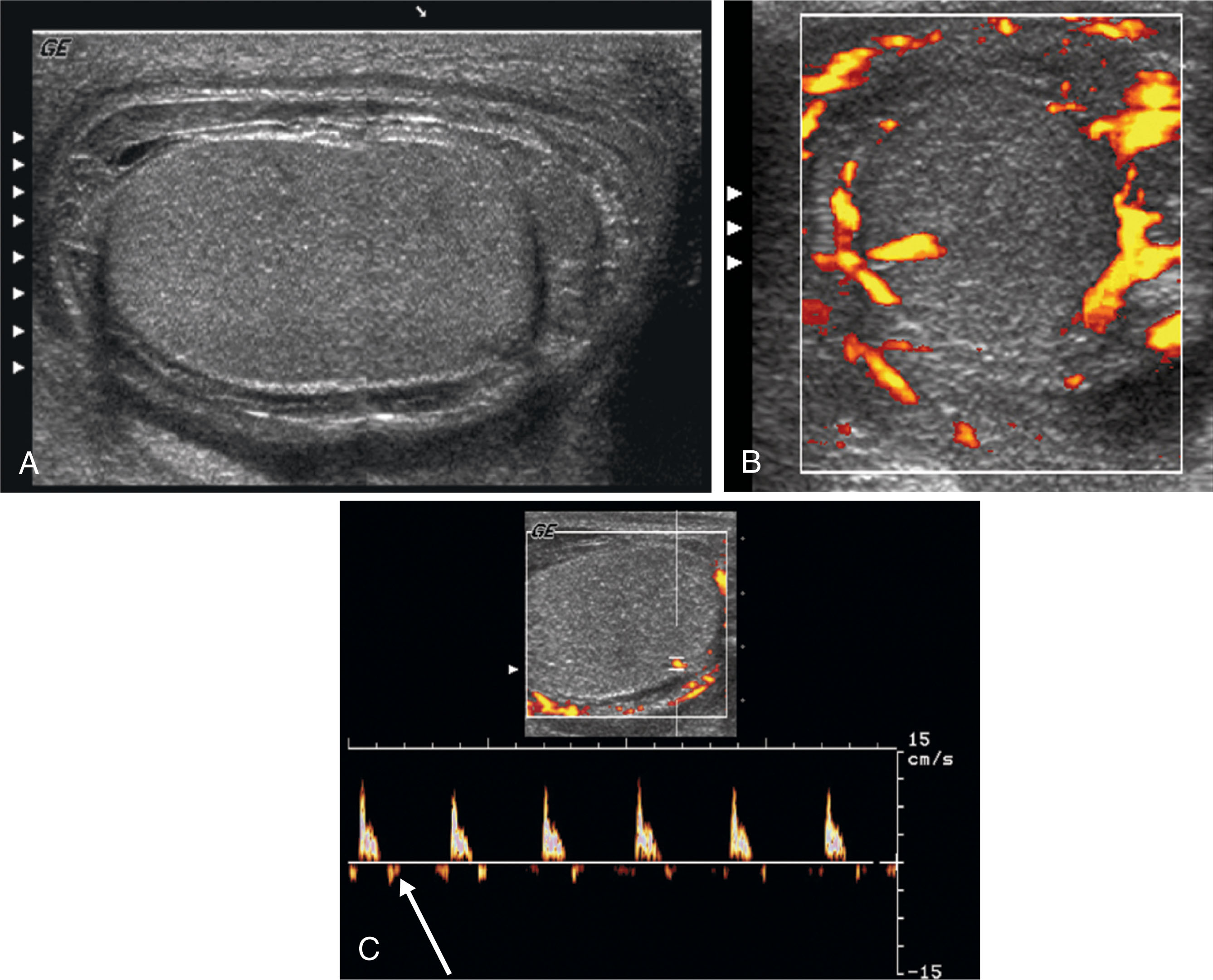
Torsion of the spermatic cord occurs as a result of abnormal mobility of the testis within the scrotum. An anomaly termed the bell clapper deformity is the most common cause of this condition. Normally, the testis and epididymis are surrounded by the tunica vaginalis, except at the bare area where they are attached to the posterior scrotal wall. The bell clapper anomaly occurs when the tunica vaginalis completely surrounds the testis, epididymis, and distal spermatic cord, allowing them to move and rotate freely within the scrotum. This movement is similar to that of a clapper inside a bell, hence the name. Torsion results when the testis and epididymis twist within the scrotum, cutting off the vascular supply within the spermatic cord. Up to 60% of patients with torsion will have an anatomic anomaly on both sides. Undescended testes are 10 times more likely to be affected by torsion than normal testes. Torsion compromises blood flow to the testis, the epididymis, and the intrascrotal portion of the spermatic cord. Venous flow is affected first, with occluded veins causing swelling of the scrotal structures on the affected side. If torsion continues, the arterial flow is obstructed, and testicular ischemia follows.
Torsion of the spermatic cord is a surgical emergency. It is important to obtain diagnostic images as quickly as possible because the salvage rate of the testis depends on the elapsed time since torsion. If surgery is performed within 5 to 6 hours of the onset of pain, 80% to 100% of testes can be salvaged. Between 6 and 12 hours, the salvage rate is 70%, but after 12 hours, only 20% will be saved. The degree of torsion (or the number of twists) also affects testicular salvage.
Torsion is the most common cause of acute scrotal pain in adolescents. Although it is more common in young adults and adolescents, torsion can occur at any age, with peak incidence at age 14. Patients with torsion most often present with sudden onset of scrotal pain accompanied by swelling on the affected side. The severe pain causes nausea and vomiting in many patients. Patients with torsion frequently report previous episodes of scrotal pain. The clinical differentiation between torsion and epididymo-orchitis is difficult in that patients have similar symptoms. Ultrasound plays a key role in helping to differentiate these entities.
Gray-scale findings on ultrasound depend on how much time has passed since the torsion occurred. In the early stages, scrotal contents may have a normal sonographic appearance. After 4 to 6 hours, the testis becomes swollen and hypoechoic ( Fig. 23.18 ). The lobes within the testis are usually well identified during this time as a result of interstitial and septal edema. After 24 hours, the testis becomes heterogeneous as a result of hemorrhage, infarction, necrosis, and vascular congestion ( Fig. 23.19 ).
The epididymal head appears enlarged and may have decreased echogenicity or may become heterogeneous. In some cases, the twisted spermatic cord knot may be seen as a round or oval extratesticular mass that can be traced back to normal spermatic cord. Other findings may include scrotal skin thickening and reactive hydrocele.
Because ultrasound gray-scale findings are similar to those noted with epididymo-orchitis, Doppler evaluation in testicular torsion is very important. Color Doppler imaging is used to make diagnostic images of torsion. The absence of perfusion in the symptomatic testis with normal perfusion on the asymptomatic side is diagnostic of torsion. Color or power Doppler parameters must be adjusted for optimal detection of slow flow. The PRF and wall filter should be set at a low level. Flow around the ischemic testis will appear normal or decreased.
Spontaneous detorsion can produce a very confusing picture both clinically and by ultrasound. Depending on how long the testis was torsed and how long it has been since relief was attained, the intratesticular flow may be minimal or hyperemic. Extratesticular flow is usually increased. This is very difficult to differentiate from epididymo-orchitis.
Torsion of the appendix epididymis and the appendix testis also occurs and further complicates the clinical picture. The clinical presentation is similar to that of testicular torsion and epididymo-orchitis. Ultrasound may show a small, hypoechoic mass located between the head of the epididymis and the superior testis. Color Doppler shows increased flow around the mass. Hemorrhage may cause the mass to appear hyperechoic.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here