Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The National Lung Screening Trial has definitively shown a reduction in lung cancer mortality in a research setting but effective implementation of a lung cancer screening program needs to be evaluated in different health-care communities.
Selection of high-risk participants for low-dose (radiation) computed tomography screening is improved by the use of multivariate risk prediction models.
A range of recruitment strategies will be required based on available health infrastructure and the distribution of the high-risk population.
Simplified management algorithms for pulmonary nodules , incorporating risk prediction models and image analysis techniques, will likely lead to reduction in downstream investigations and surgery for benign disease.
Overdiagnosis represents an important potential harm for participants in any lung cancer screening program. Rates of overdiagnosis vary with histologic subtype of the screen-detected cancer and with the phenotype of the screened population.
Smoking cessation is critical to the overall benefit and cost-effectiveness of a lung cancer screening program. The best strategy to optimize the intervention and integrate it into a program is not known.
The precision and cost-effectiveness of a lung cancer screening program is likely to be improved with probabilistic risk modeling and protocol-driven nodule management. The cost-effectiveness of a program will likely be a key determinant in federal or national decision making to adopt a lung cancer screening program.
Biomarkers have the potential to further refine a risk-based approach to screening through identification of high-risk phenotypes or following identification of an indeterminate nodule. At present, there are no molecular biomarkers approved for clinical practice in lung cancer screening.
Lung cancer is the most common cancer and the greatest cause of cancer death in our world. According to GLOBOCAN, there were an estimated 1.8 million cases of lung cancer diagnosed in 2012 and there were 1.59 million lung cancer–related deaths. The association between smoking and lung cancer was first described more than 50 years ago. Worldwide, smoking accounts for 80% of lung cancers in men and for 50% in women. About 30% of the world population reaching adulthood will start smoking, and the majority will continue to smoke throughout their lives. Although smoking rates are decreasing in most high-income countries, they are increasing or persistent in low- to middle-income countries. In some high-income countries with lower current smoking rates, a greater proportion of lung cancer is occurring in former smokers. The excess risk of lung cancer in former smokers is influenced by the age of smoking cessation, where 90% of lung cancer risk can be avoided if cessation occurs before 40 years of age.
The fatality rate (ratio of mortality to incidence) for lung cancer is high, estimated to be 0.87 in the GLOBOCAN report. The 5-year survival rate is generally low at less than 15% with a relative lack of variability amongst different world regions. Most patients have advanced and incurable disease at the time of diagnosis. In the United States (US), 56% of patients have distant metastasis and 22% have regional spread of disease; 15% of lung cancers are localized at the time of initial diagnosis. The reason for this low percentage of early-stage disease is that it is asymptomatic; most early-stage lung cancers are currently detected by chance imaging procedures performed for other reasons.
Until recently, there has been no role for lung cancer screening. Screening trials in which chest radiography and sputum cytology were evaluated did not demonstrate a decrease in lung cancer–related mortality. In the 1990s, single-arm screening trials with low-dose (radiation) computed tomography (LDCT) of the chest demonstrated an increase in sensitivity for detecting lung cancer compared with chest radiography. Authors of the initial trials reported that 60% to 80% of detected lung cancers were stage I disease. These studies led to a number of randomized screening trials to compare LDCT with either chest radiography or observation alone.
There are a number of European randomized control trials that include an LDCT screening arm and a control arm. However, these trials are likely underpowered to detect a clinically plausible benefit in terms of lung cancer–related mortality. Subsequently, two larger randomized studies have been undertaken, the National Lung Screening Trial (NLST) in the US and the Dutch-Belgian Randomised Lung Cancer Screening Trial (NELSON). The NLST study has been published and determined that LDCT screening was associated with a 20% reduction in lung cancer mortality compared with chest radiography. The NELSON study is nearing completion.
The NLST has definitively shown a reduction in all-cause and disease-specific mortality in a research setting but effective implementation of a lung cancer screening program needs to be clarified in different health-care communities. The International Association for the Study of Lung Cancer Strategic Screening Advisory Committee published position statements in 2012 and 2014 to inform the process of implementation of lung cancer screening. They have recommended incorporation of a multidisciplinary group of experts and identified a number of specific issues that need to be addressed for broader community implementation. These include identification of high-risk individuals, uniform radiology standards, standardized reporting and management of CT findings, and integration of smoking cessation programs.
The US Preventive Services Task Force (USPSTF) published recommendations on LDCT screening for lung cancer in the US in 2014. Following the final decision on February 5, 2015, by the Centers of Medicare and Medicaid Services to cover lung CT screening of Americans aged 55 years to 77 years who had smoked at least 30 pack-years, lung cancer screening is being implemented in the US health-care system. Many other countries are evaluating the translation of LDCT screening into clinical practice and implementation of coordinated programs in their respective health systems.
In this chapter, we summarize the current knowledge for lung cancer screening with LDCT and discuss the issues that require ongoing clarification.
Rather than a population-based strategy, as exists for breast and colorectal cancer, proposed lung cancer screening programs involve screening identified high-risk groups. A significant proportion of our community are current or former smokers who may be eligible for screening. There are an estimated 1.1 billion smokers worldwide. , To develop cost-effective lung cancer screening programs, an improved definition of a high-risk individual to target screening efforts is needed.
It is also important to consider comorbidities in participants who are potential candidates for screening. To achieve the benefits of screening, both at an individual and at a community level, participants must be able to undergo curative treatments and have a reasonable life expectancy. Smokers are at an increased risk of other comorbidities that may limit curative therapy or life span. USPSTF guidelines for screening in the US recommend discontinuing screening if a person develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery.
Selection criteria for LDCT screening used in research trials have largely been based on age and smoking history, but lung cancer screening is most effective when applied to people at highest risk. The identification of high-risk individuals using age and smoking criteria alone was used in the NLST and NELSON trials and is the basis of current eligibility criteria in the US (USPSTF criteria; Table 7.1 ).
| Age (y) | Smoking History | |
|---|---|---|
| NLST | 55–74 | ≥30 pack-years Quit <15 years |
| NELSON | 50–74 | ≥15 cigs/d for 25 years ≥10 cigs/d for ≥30 years Quit ≤10 years |
| USPSTF | 55–80 | ≥30 pack-years Quit <15 years |
Selection of individuals for lung cancer screening using risk prediction models is superior to selection using age and smoking criteria alone. Multiple risk prediction models exist but one of the best studied is the prostate, lung, colorectal, and ovarian (PLCO) model that is based on prospectively collected data from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. The use of this model in analysis of NLST data showed improved performance compared with NLST enrollment criteria. In 2014, Tammemagi and colleagues presented an evidence-based risk threshold based on the PLCO m2012 model and NLST mortality outcomes. At PLCO m2012 risk of 1.51% or more over 6 years there was consistently lower lung cancer mortality in those NLST participants screened with LDCT compared with chest x-ray. This risk threshold performed better than USPSTF criteria. More lung cancers were detected and fewer were missed using the risk model. The number needed to screen to prevent one lung cancer death was 255 compared with 963 in the lower-risk category. Importantly, the use of USPSTF criteria alone resulted in the screening of a substantial number of low-risk individuals and exclusion of some high-risk individuals.
External validation and comparison of four published risk prediction models were performed in 20,700 ever-smokers of the EPIC-Germany cohort (Bach model, Spitz model, Liverpool Lung Project, and PLCO m2012 ). The results revealed that the PLCO m2012 model showed the best performance. All of the models, except the Spitz model, showed better prediction and performance than screening trials eligibility criteria using age/smoking criteria alone.
The use of multivariate risk prediction models to select participants who will most benefit from screening is likely to be the most cost-effective strategy. The IASLC High Risk Working Group has recommended the use of these prediction models in a coordinated program.
The success of a screening program is dependent on uptake by the target population. Recruitment research from other screening programs such as breast and colorectal cancers has shown that there are many different barriers to uptake of screening, particularly in some minority groups and deprived populations, and there is no universal approach.
There is no disease-specific evidence available to advise on the best method of recruitment to a lung cancer screening program. Most published lung cancer screening trials used a combination of various media advertising, mailed invitations, and approach via general practitioners or primary practice databases to recruit participants. Analysis of eligible participants who declined LDCT screening in the trial setting revealed a variety of factors contributing to the decision. These included practical barriers (e.g., traveling, carer responsibilities, too difficult/too much effort), emotional barriers (e.g., fear of diagnosis, anxiety), fatalistic beliefs, avoidance, low perceived risk and/or benefits, knowledge barriers, and dislikes of health-care systems.
In the community, current smokers generally have lower socioeconomic status and lower education levels compared with former smokers or never-smokers. In the US they also were less likely to have a regular general practitioner and have reduced access to health care. Respondents to lung cancer screening trials are more likely to be younger, former smokers, better educated, more health conscious, and have better access to medical care.
Attitudes toward lung cancer screening vary in different cohorts. In the US, published surveys of different populations have highlighted some of these variations. In one cross-sectional telephone survey of 2000 individuals from the general population, less than 25% of current smokers and 7.7% of former smokers believed they were at increased risk of lung cancer. Current smokers were less willing to undergo surgery and had less perceived benefit of screening. Other cohorts have shown greater awareness of risk and willingness to undergo screening. In a separate cross-sectional written survey of war veterans in the US, 80% of current smokers and 16% of former smokers believed they were at an increased risk of lung cancer. The majority of participants were willing to take part in screening, have surgery, and had higher perceived benefits of screening. This cohort differed from the general population in that the majority had good access to health-care facilities. In an ethnically diverse cohort recruited from a US primary care center uptake of screening was affected by fatalistic beliefs, sense of self-efficacy, understanding of impact of lung cancer, concerns about radiation effects, and mistrust of health-care workers. Costs also play a role in screening uptake in health-care systems that are not fully publicly funded. It was noted in this cohort that a requirement to pay for a CT scan would be likely to reduce screening uptake.
A cross-sectional survey in the United Kingdom specifically addressed smokers in socioeconomically deprived communities and also revealed complex attitudes to screening. Participants were positive in principle about screening, had high levels of fear of lung cancer but had low perceived benefits of screening, felt stigmatized, and held avoidant and fatalistic beliefs, particularly amongst current smokers.
These findings suggest that implementation of a lung cancer screening program in a general population may have some difficulty recruiting current smokers, would need to educate current and former smokers about their perceived risks of lung cancer and benefits of screening, and would need to design targeted education and recruitment strategies to allay fears and anxiety in certain community groups. CT costs likely need to be fully covered, and programs would preferably have multiple access points in the community (i.e., noncentralized LDCT access).
A range of recruitment strategies will be required based on available health infrastructure and the distribution of the high-risk population in each country. National support and a centrally organized coordinated program are more likely to result in higher uptakes of screening. Approaches using invitations customized to the characteristics of the target group and involvement of the primary care team are also likely to have some benefit. Therefore to target different high-risk populations in the community, to maximize uptake in a screening program, and to ultimately reduce lung cancer mortality, we face a variety of challenges both within and between countries.
Published single-arm, observational screening trials since 1999 have established that lung cancer screening with LDCT is feasible. The majority of cancers detected by LDCT are at an early stage, making them amenable to curative techniques. The NLST and the NELSON were established to assess the mortality benefit of lung cancer screening but had notable differences in design ( Table 7.2 ). In addition, there are a number of smaller randomized studies in Europe including the Discontinuation of Antihypertensive Treatment in Elderly People (DANTE), Multicentric Italian Lung Detection (MILD), Danish Lung Cancer Screening Trial (DLCST), Italian Lung Cancer Screening Trial (ITALUNG), UK Lung Cancer Screening (UKLS), and German Lung Cancer Screening Intervention Trial (LUSI) studies that are at varying degrees of follow-up and maturity ( Table 7.2 ). One particularly notable difference is that the NELSON and other randomized European studies have predominantly male participants compared with the NLST study ( Table 7.2 ). This may influence the final mortality benefit, as subset analysis of the NLST data suggested greater mortality benefit for LDCT screening in women.
| Trial | NLST | NELSON | ITALUNG | MILD | LUSI | DANTE | DLCST | UKLS |
|---|---|---|---|---|---|---|---|---|
| Country | United States | The Netherlands, Belgium | Italy | Italy | Germany | Italy | Denmark | Great Britain |
| Enrollment | ||||||||
| Age (years) | 55–74 | 50–75 | 55–69 | 49–75 | 50–69 | 60–74 | 50–70 | 50–75 |
| Quit (years) | ≤15 | ≤10 | <10 | <10 | ≤10 | <10 | <10 | LLP risk ≥5% |
| Pack-years | ≥30 | >15 | ≥20 | ≥20 | ≥15 | ≥20 | ≥20 | |
| Screening | ||||||||
| Screen interval (y) | 1 | 1, 2, 2.5 | 1 | 1 or 2 | 1 | 1 | 1 | 0 |
| Rounds | 3 | 4 | 4 | 5 or 10 | 5 | 5 | 5 | 1 |
| Arms | LDCT vs. CXR | LDCT vs. usual care | LDCT vs. usual care | LDCT vs. usual care | LDCT vs. usual care | LDCT vs. usual care a | LDCT vs. usual care | LDCT vs. usual care |
| Participants | ||||||||
| Total number | 53,454 | 15,822 | 3206 | 4099 | 4052 | 2450 | 4104 | 4055 |
| LDCT arm | 26,309 | 7582 | 1406 | 2376 | 2028 | 1264 | 2502 | 1994 |
| Men (%) | 59 | 84 | 65 | 68 | 65 | 100 | 55 | 75 |
| Mean age (y) | 61 | 59 | 61 | 59 | NR | 65 | 57 | 67 |
| Mean pack-years | 56 | 38 | 42 | 39 | NR | 47 | NR | NR |
| Current smoker (%) | 48 | 56 | 65 | 69 | 62 | 57 | 76 | 39 |
| LDCT-Detected Lung Cancer | ||||||||
| Detection of lung cancer (%) | 2.6 | 2.6 | 2.7 | 2.1 | 2.9 | 5.2 | 2.7 | 2.1 |
| Stage I (%) | 59 | 71 | 66 | 65 | 72 | 64 | 68 | 67 |
| Surgery for benign disease (%) | 24 | 29 | 10 | 9 | NR | 19 | NR | 10 |
The NLST was powered to show a mortality benefit of at least 20% and enrolled 53,456 participants with three annual screening scans ( Fig. 7.1 ). The NELSON study was designed to show a mortality benefit of at least 25% but only enrolled about 15,822 participants with repeat screening scans at increasing intervals. The European CT screening trials collaborative group will pool the smaller randomized European studies with the NELSON study data for mortality evaluation ( Table 7.2 ).
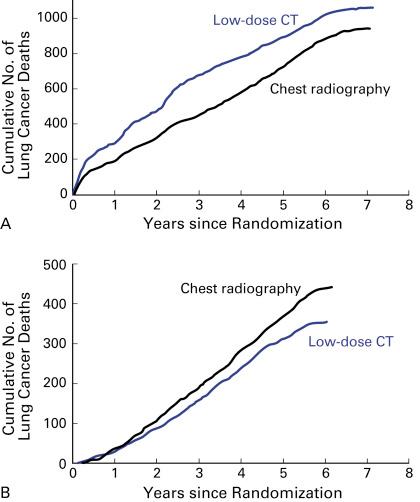
Because the random assignment of eligible participants in the NELSON study took place from 2004 to 2006, 10 years of follow-up will be reached for all participants in 2016. To perform the mortality analyses, data linkages with national cancer and death registries must be undertaken and complete data will become available in early 2019. The final mortality analyses of NELSON and pooling of data with other trials within the European consortium will then be performed.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here