Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cancer incidence is on the rise in the United States and worldwide. Beyond therapeutics, substantial reduction in cancer mortality necessitates improved understanding of cancer risk, implementation of effective preventive intervention strategies, and early detection of cancers that are likely to progress.
Genomic- and proteomic-based approaches have the potential to refine risk assessment and stratification, allowing for more tailored screening and early detection strategies. However, the use of such approaches is still in its early stages.
In the United States, population-based screening tests are currently recommended for breast, cervical, colon, and lung cancers. Individualized decision making after a discussion with a clinician is recommended for men in the case of prostate cancer screening.
Discordant professional society recommendations for screening have accentuated the need to implement risk-stratified screening for common cancers to improve the effectiveness of screening.
Overdiagnosis is finding cancers that would never become clinically relevant in a person's lifetime. This has been an issue mainly in breast and prostate cancer screening.
Systematic reviews of randomized controlled trials (RCTs) of screening mammography have demonstrated an approximate 20% mortality reduction in women ages 40 to 74 years.
Guaiac-based fecal occult blood tests (gFOBTs) have the strongest direct evidence supporting their use as a colorectal cancer screening modality, with a 32% reduction in colorectal cancer mortality with annual use.
Lung cancer screening with low-dose computed tomography resulted in an approximate 20% reduction in lung cancer mortality in the National Lung Screening Trial.
Human papillomavirus (HPV) vaccination does not remove the need for routine cervical cancer screening according to age and clinical history.
Because most risk factors for liver cancer in the United States and other developed countries are associated with cirrhosis, patients with cirrhosis are the target of cancer prevention and surveillance efforts for hepatocellular carcinoma.
An effective screening test for ovarian cancer has not been identified. The two tests commonly investigated for ovarian cancer screening are CA125 and transvaginal ultrasound.
Randomized clinical trials for skin cancer screening are unlikely to be practical. The US Preventive Services Task Force (USPSTF) statement on skin cancer is forward-looking because it opens the door to case-control designs that are more practical, while acknowledging that strong evidence of benefits and harms is lacking, including for nonmelanoma skin cancer.
Although substantial progress is currently being made in the development of novel and more effective therapeutics, cancer remains a largely unsolved clinical problem with high mortality. Cancer incidence is on the rise in the United States and worldwide. Between 2005 and 2015, the number of cancer cases increased by 33%, owing in part to population aging, which contributed 16%, and population growth and changes in age-specific rates, which contributed the remainder. Cancer incidence and the societal cancer burden are expected to increase further. Beyond therapeutics, substantial reduction in cancer mortality necessitates improved understanding of cancer risk, implementation of effective preventive intervention strategies, and early detection of cancers that are likely to progress to avoid the problem of overdiagnosis, all of which represent substantial challenges that can be overcome. Identifying individuals at increased risk of developing a particular cancer type would allow for a range of preventive interventions to be implemented, from altered lifestyle and health behaviors to the use of vaccines, and early detection of particular cancer(s) for which these persons are at risk. A case in point is obesity, which is a risk factor for at least 13 different cancers. Elucidation of metabolic, immune, and other molecular profiles that contribute to the increased risk would allow for more focused screening strategies for persons with these risk profiles and would allow implementation of targeted prevention strategies aimed at the cancer(s) for which they are at risk.
Guidelines are currently available for risk assessment in the clinical setting through several organizations. Guidelines allow a determination of a subject's risk for the common cancers and the associated options for screening. Organizations creating guidelines include the National Comprehensive Cancer Network (NCCN; https://www.nccn.org/ ), the American Cancer Society (ACS; https://www.acs.org/ ), and other advocacy and professional societies. Family history and other patient characteristics are often important determinants of risk. In general, individuals are first defined as being at either average risk or increased risk for a particular cancer. For example, the NCCN Version 2.2016 colorectal cancer (CRC) screening guidelines identify a person as being at “average risk” based on age, negative family history, and no prior personal history of inflammatory bowel disease, adenoma, or sessile serrated polyp or CRC. Risk is increased with a positive family or personal history of cancer or precancer. High-risk syndromes include Lynch syndrome and a number of other syndromes. Screening guidelines for CRC and the modalities used for screening vary based on the risk profile. Other common cancers for which risk assessment and screening guidelines are available include breast, cervical, lung, and prostate. Given the widespread use of the prostate-specific antigen (PSA) test for prostate cancer screening, the NCCN guidelines for early detection of prostate cancer stratify men based on their age and PSA levels.
There is currently substantial interest in bolstering traditional guidelines by adding genomic and other “omic” profiles to other personal characteristics and family history to determine the need for and frequency of screening. The field is still in its early stages. In the case of breast cancer, susceptibility genes include highly penetrant mutations in the BRCA1 and BRCA2 genes, the testing for which is indicated by a positive family history of breast cancer. Several other moderately penetrant mutations in other genes and more common genomic variants with a modest increase in risk have been identified. These may very well influence risk assessments and guide screening in the near future.
Although advances in screening and early detection are essential for progress in both the prevention and treatment of cancer, the incorporation of such advances into the clinic is challenging and demands a careful weighing of all potential risks and benefits. At least three criteria must be fulfilled for a cancer screening test to be useful :
The test must detect the disease earlier than routine methods.
Earlier treatment must lead to improved outcomes.
The benefits of screening must be greater than the risks of any subsequent diagnostic and therapeutic treatments.
A screening test is assessed by its performance characteristics, which include its sensitivity and specificity, its accuracy, and its positive predictive value (PPV) and negative predictive value (NPV) ( Table 23.1 ). Sensitivity can be considered the “true-positive” rate, and specificity can be considered the “true-negative” rate. If a screening test were 100% sensitive, every individual with the disease in question within the population would be identified as such; and if it were 100% specific, it would identify every healthy individual as not having the disease. In reality, however, there is no screening test that achieves this ideal. Sensitivity and specificity are inversely related, such that increasing the sensitivity of a test will result in more false positives, whereas increasing the specificity will result in more false negatives. Striking an acceptable balance between these two performance characteristics is challenging and depends on the disease in question. In the case of many types of cancers that are curable only in the earlier stages of the disease, it may be desirable to minimize false negatives, because these could result in a patient's delayed diagnosis and cancer-specific death, and accept more false positives (i.e., higher sensitivity than specificity). However, where the balance is struck ultimately depends on the nature and severity of the specific disease being screened for and the effectiveness of downstream interventions and treatments for that disease.
| Performance Characteristic | Definition | Formula | Practical Application |
|---|---|---|---|
| Accuracy | Proportion of correct test results | TPs + TNs/TPs + TNs + FPs + FNs | Have disease and have positive test result + do not have disease and have negative test result/all test results |
| Sensitivity | Ability of the test to correctly identify someone with the disease as positive | TPs/TPs + FNs | Have disease and have positive test results/true disease |
| Specificity | Ability of the test to correctly identify someone without the disease as negative | TNs/TNs + FPs | Do not have disease and have negative test results/true no disease |
| Positive predictive value (PPV) | Probability that someone with a positive test result actually has the disease | TPs/TPs + FPs | Have disease and have positive test results/all positive test results |
| Negative predictive value (NPV) | Probability that someone with a negative test result does not have the disease | TNs/TNs + FNs | Do not have disease and have negative test results/all negative test results |
The sensitivity and specificity of a screening test are critical to consider in the design of any population-based screening program. However, at least at the clinical level, it is also useful to appreciate the PPV and NPV of the screening test. The PPV is the proportion of patients who test positive who actually have the disease, and the NPV is the proportion of those who test negative who truly do not have the disease. Thus these values provide a clinician with an estimate of the probability that his or her patient does or does not have the disease, given the test result. The PPV is influenced by the prevalence of the disease in the population screened, such that the PPV will be higher at higher disease prevalence. In addition, when the disease in question is relatively rare within a population, as is the case with most cancers, the specificity of the test being used also matters greatly because most people who will be screened will not have the disease. In these patients, increasing the specificity of a screening test will improve its PPV.
Observational data supporting the use of screening tests do not rule out the potential for these tests to mislead as a result of at least two important potential biases: lead-time and length biases ( Fig. 23.1 ). To minimize the influence of these biases, the development of effective screening tests should ideally culminate in well-conceived and well-conducted randomized controlled trials (RCTs) that assess a cancer-related, if not overall, mortality end point.
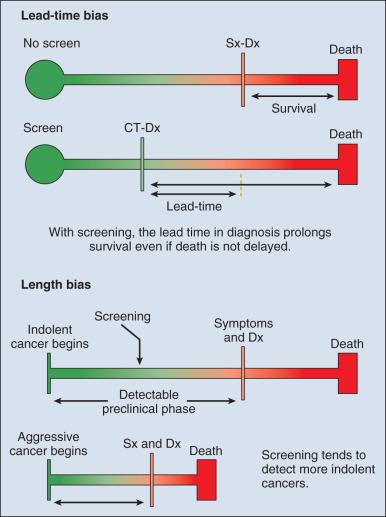
Implementation of screening programs for common cancers, notably cervical, breast, lung, and colon cancers, has been shown to reduce the mortality associated with these cancers. However, whenever there is a screenable disease that is prevalent but potentially indolent, there is the potential for overdiagnosis and overtreatment, resulting in a range of personal and social costs that may ultimately outweigh the intended benefits. Overdiagnosis is the identification by screening of a cancer that would never have caused symptoms or adversely influenced the health of an individual during his or her lifetime; and overtreatment is the treatment of such an identified cancer. Breast and prostate cancer screening are two examples in which these issues have challenged screening efforts. The use of the PSA test for prostate cancer screening has been particularly controversial. The American Urological Association recommended shared decision making for men ages 55 to 69 years considering PSA-based screening, a target age group for whom benefits may outweigh harms. On the other hand, the US Preventive Services Task Force (USPSTF) has discouraged (i.e., assigned a D recommendation to) the use of the PSA for prostate cancer screening, although the organization is now in the process of updating this recommendation. Nevertheless, it is viewed that the USPSTF decision affected the number and distribution of prostate cancer diagnoses in the United States. Mammography screening for breast cancer has been widely adopted in many Western countries. However, there has been growing debate and concern about the benefits and harms of screening with mammography. Guidelines and recommendations for screening mammography have varied among countries. A working group assembled by the International Agency for Research on Cancer (IARC) assessed the cancer-preventive and adverse effects of different methods of screening for breast cancer. The group recognized that the relevance of RCTs conducted more than 20 years ago should be questioned. After a careful evaluation of the balance between the benefits and adverse effects of mammographic screening, the group concluded that there is a net benefit from screening women ages 50 to 69 years of age. However, the merits of mammographic screening have been questioned by others. The ACS updated its 2003 breast cancer screening guidelines for women at average risk of breast cancer and issued qualified recommendations. The guidelines of 2015 state that women ages 45 to 54 years should be screened annually, although women ages 40 to 44 should have the opportunity to begin screening if they so choose, and women age 55 years and older should transition to biennial screening or have the opportunity to continue screening annually. Screening should continue as long as a woman's overall health is good and she has a life expectancy of 10 years or longer.
Clearly, guidelines for cancer screening are continually evolving. Discordant professional society recommendations for screening have accentuated the need to implement risk-stratified screening for common cancers to improve the effectiveness of screening. Risk stratification may be based on an individual's genotype or other types of molecular markers in addition to subject characteristics. For example, a novel approach to risk-based breast cancer screening has been proposed that integrates clinical risk factors, breast density, a polygenic risk score representing the cumulative effects of genetic variants, and sequencing for moderate- and high-penetrance germline mutations. The added costs versus benefits of molecular testing to guide screening for common cancers requires further evaluation.
Table 23.2 presents a summary of the USPSTF classification of the evidence for various cancer screening tests in average-risk individuals.
| Grade | Definition | Suggestions for Practice | Current Screening Test Recommendations |
|---|---|---|---|
| A | The USPSTF recommends the service. There is high certainty that the net benefit is substantial. | Offer or provide this service. | Cervical cancer screening for women ages 21–65 with pap smear or pap smear with HPV-testing in women ages 30–65 Colorectal cancer screening in adults ages 50–75 |
| B | The USPSTF recommends the service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. | Offer or provide this service. | Breast cancer screening (biennial) for women ages 50–74 Lung cancer screening in adults ages 55–80 with a history of smoking |
| C | The USPSTF recommends selectively offering or providing this service to individual patients based on professional judgment and patient preferences. There is at least moderate certainty that the net benefit is small. | Offer or provide this service for selected patients depending on individual circumstances. | Breast cancer screening for women ages 40–49 Colorectal cancer screening in adults ages 76–85 Prostate cancer screening in men ages 55–69 c |
| D | The USPSTF recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. | Discourage the use of this service. | Cervical cancer screening in women younger than age 30 with HPV testing alone or in combination with cytologic assessment Cervical cancer screening in women younger than age 21 Cervical cancer screening in women age 65+ who have had adequate prior screening Ovarian cancer screening Pancreatic cancer screening Prostate cancer screening in men age 70+ c |
| I | The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. | Read the clinical considerations section of USPSTF Recommendation Statement. If the service is offered, patients should understand the uncertainty about the balance of benefits and harms. | Breast cancer screening in women age 75+ Oral cancer screening Skin cancer screening |
a Based on grade definitions after July 2012.
b The USPSTF defines certainty as “likelihood that the USPSTF assessment of the net benefit of a preventive service is correct.” The net benefit is defined as benefit minus harm of the preventive service as implemented in a general, primary care population. The USPSTF assigns a certainty level based on the nature of the overall evidence available to assess the net benefit of a preventive service.
Breast cancer is the most commonly occurring cancer in women and the second leading cause of cancer death. Since 1989, the number of deaths from breast cancers has decreased owing to improvements in treatment as well as screening. Screening provides an opportunity for the early detection of breast cancer, which has been shown to reduce not only breast cancer mortality but also treatment morbidity.
Nonmodifiable risk factors include older age, a personal or family history of breast or ovarian cancer including women with a genetic mutation for breast cancer, a history of premalignant breast lesions such as atypical hyperplasia (AH) or lobular carcinoma in situ (LCIS), or a history of radiation exposure between the ages of 10 and 30 years.
Modifiable or potentially modifiable risk factors for breast cancer include increased breast density, moderate to heavy alcohol use, weight gain after the age of 18, being overweight or obese (for postmenopausal breast cancer), physical inactivity, and use of exogenous estrogen plus progesterone (e.g., in postmenopausal hormone therapy).
Reproductive factors that increase breast cancer risk include a long menstrual history (early age of menarche or late age of menopause) and nulliparity or late age of first pregnancy. These endocrine risk factors demonstrate the responsiveness of breast cancer to antiestrogen drugs and have led to the use of antiestrogen strategies for the prevention of breast cancer.
Breast cancer risk assessment is relatively sophisticated compared with other cancers. The Gail model, a multivariable model used to assess risk of breast cancer based on age, family history of breast cancer in first-degree relatives (FDRs), breast biopsy history, and the presence of AH and various other endocrine features, is often used to determine candidates for preventive therapy with antiestrogen drugs such as tamoxifen or raloxifene. Although this model is generally accurate for populations, its ability to predict if an individual woman will get breast cancer is limited. Newer risk assessment models such as the Breast Cancer Surveillance Consortium model also consider mammographic density in determining the risk of development of breast cancer.
For women with a strong family history of breast cancer (with or without a family history of ovarian cancer), other models may be more accurate. The Tyrer-Cuzick model estimates a woman's risk of developing breast cancer through use of many of the variables used in the Gail model but includes a more expansive family history. It also estimates her risk of a BRCA mutation and may be used to determine if genetic testing is recommended. Other models developed for the assessment of a genetic mutation in a specific woman include the following: Berry-Parmigiani-Aguilar (BRCA-Pro), Claus, Couch, and the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA). Geneticists and physicians specializing in cancer risk assessment commonly use these models to guide genetic testing decisions as well as recommendations for breast screening.
Breast self-examination (BSE) was a mainstay for decades in breast cancer screening recommendations, but this recommendation changed for most organizations in the early 2000s when findings from a large RCT of BSE in women in Shanghai, China noted no difference in breast cancer mortality between women performing BSE versus controls. This trial randomized 266,064 Chinese women to receive instruction on BSE or a topic unrelated to breast cancer. During the time this study was conducted, women in China had access to mammography only for diagnostic evaluation of a clinical finding. Women in the BSE arm received intensive instruction in BSE technique. Compliance was encouraged through feedback and reinforcement sessions as well as monthly reminders. After 10 to 11 years of follow-up, with 135 breast cancer deaths in the instruction group and 131 in the control group, there was no statistically significant difference between the two arms in breast cancer mortality (RR, 1.04; 95% confidence interval [CI], 0.82–1.33; P = .72). In addition, no statistically significant difference in breast cancer incidence or stage was seen. However, the BSE group had a higher rate of false positives.
Nonetheless, it is recognized that women are the most likely person to find a palpable breast cancer, with most being found during normal activities of daily living (e.g., showering, dressing). For this reason, BSE has been replaced with the concept of breast awareness, which recommends that women be familiar with their breasts and promptly report any change. Differing from BSE, breast awareness does not involve formalized instruction in the clinical setting or reminders (such as shower cards).
There are limited data on the effectiveness of clinical breast examination (CBE). Randomized trials comparing CBE versus no screening have not been performed. A review of controlled trials and case-control studies that included CBE as part of the screening modality found sensitivity of CBE to be 54% and specificity, 94%.
Whereas the ACS recommends against CBE, the NCCN recommends a clinical encounter noting that it provides the opportunity to perform a number of important clinical activities that may not otherwise be done if a woman only obtains a screening mammogram ( Table 23.3 ). In addition to performing breast cancer risk assessment, advising on risk reduction strategies, including healthy lifestyle interventions and risk-based screening recommendations, CBE is a part of this encounter.
|
a As recommended by the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Breast Cancer Screening and Diagnosis (Version 2.2016).
b Not approved by the US Food and Drug Administration for this indication.
The primary benefit of screening mammography is a reduction in death from breast cancer. Early detection with screening mammography may also result in lower morbidity because surgery is often less extensive and toxic systemic chemotherapy may be less frequently needed.
Systematic reviews of RCTs of screening mammography have demonstrated an approximate 20% mortality reduction in women ages 40 to -74 years. However, all but the Age Trial were initiated in the early 1980s or earlier. Although RCTs have demonstrated a mortality reduction, they are limited by the quality of mammography available at the time the study was conducted, with many cancers having to be 1 cm or greater to be detected. A meta-analysis of observational case-control studies showed a significant 48% mortality reduction with modern screening mammography. Although observational data are limited by bias, these data are believed by many to better reflect contemporary screening practices in which the detection of subcentimeter breast cancers is commonplace. Modeling studies have demonstrated a 29% to 54% mortality reduction with annual mammographic screening, providing further support for a larger mortality reduction than seen in the RCTs.
Various guidelines from the ACS, USPSTF, and NCCN and recommendations from professional organizations, including the American College of Radiology, American College of Obstetrics and Gynecology, American College of Physicians, and American Academy of Family Physicians, are currently available to guide breast cancer screening in women at average risk ( Table 23.4 ). A number of organizations recommend annual mammographic screening beginning in the 40s, whereas others recommend biennial screening beginning at age 50.
| Organization | When to Initiate Screening | Frequency of Screening | When to Stop Screening |
|---|---|---|---|
| American Academy of Family Physicians (AAFP) | Follow US Preventive Services Task Force (USPSTF) recommendations | Follow USPSTF recommendations | Follow USPSTF recommendations |
| American Cancer Society (ACS) | Opportunity to begin screening at ages 40–44 Regular screening starting at age 45 |
Annually from age 45–54 Biennially starting at age 55 with opportunity to continue annually |
Continue screening mammography as long as overall health is good and life expectancy is 10 yr or longer |
| American College of Obstetricians and Gynecologists (ACOG) | Annual screening starting at age 40 | Annually | Not specified |
| American College of Physicians (ACP) | Individualized for women ages 40–49 Regular screening starting at age 50 |
Biennially | Age 75 yr or older Women of any age with life expectancy <10 yr |
| American College of Radiology (ACR) | annual screening starting at age 40 | Annually | Should be considered as long as the patient is in good health and is willing to undergo additional testing if an abnormality is detected |
| National Comprehensive Cancer Network (NCCN) | Annual screening starting at age 40 | Annually | Upper age limit is not yet established Consider comorbid conditions limiting life expectancy (e.g., ≤10 yr) and whether therapeutic interventions are planned |
| US Preventive Services Task Force (USPSTF) | Individualized for women ages 40–49 Regular screening beginning at age 50 |
Biennially | Insufficient evidence to recommend for or against screening at age 75 or older |
All recommending organizations agree that screening beginning at age 40 results in a mortality reduction and that annual screening leads to fewer breast cancer deaths than does biennial screening. Among women ages 40 to 49 years, the reduction in mortality ranges from 15% in RCTs to 47% in observational studies. Early detection and successful treatment results in significantly more life-years gained for this decade than for any other decade. Organizations that recommend against annual mammographic screening and delaying initiation of mammographic screening to the 50s cite concerns that the false positives and overdiagnosis of screening may not balance the benefits of screening for women in their 40s. However, the USPSTF analysis shows that the false-positive rate for the same screening interval (annual or biennial) is equivalent for women in their 40s and 50s and that the biopsy rate is actually higher for women in their 50s. In addition, it is recognized that women undergoing their first screening mammogram have a higher recall rate owing to a lack of prior mammograms for comparison. Moving the initiation of screening to age 50 would further increase the incidence of false-positive mammograms at this age.
Most clinicians recognize overdiagnosis of breast cancer as less an issue of screening and more of a problem related to overtreatment. It is clear that we need to understand which “breast cancers” do not require treatment. Clinical trials are opening to begin to investigate this question. Having said this, it is important to recognize that the number of overdiagnosed breast cancers is not necessarily influenced by the age of initiation of screening mammography or the interval in which screening occurs. It has been shown that breast cancers that do not progress (i.e., an overdiagnosed breast cancer) also do not spontaneously resolve. These cancers will be diagnosed at the first screening after their development, whether that is at age 40 or 50 (or somewhere in between) and whether the screening occurs annually or biennially.
Full-field digital mammography (FFDM) is the current standard of care for breast cancer screening. Early data have shown that tomosynthesis increases the detection of breast cancer and reduces the recall rate. This suggests that this modality has the potential to provide a more favorable balance of benefits and risks of screening mammography.
Women who have a high risk of breast cancer because of a known inherited mutation, a strong family history with a greater than 20% lifetime risk of breast cancer, or prior exposure to therapeutic radiation between the ages 10 and 30 years should be more aggressively screened with supplemental breast magnetic resonance imaging (MRI) in addition to annual screening mammography. It is suggested that women with high-risk lesions such as atypical ductal or lobular hyperplasia or LCIS should also be considered for supplemental screening with breast MRI based on emerging evidence. NCCN outlines screening strategies for these high-risk women, including age of initiation and frequency of screening. Although the optimal frequency and timing of breast MRI scans is not well established, it is frequent practice to perform annual screening mammogram and annual screening breast MRI scans staggered every 6 months. This paradigm provides the opportunity for the detection of “interval” cancers (defined as cancers detected between scheduled screening examinations), which may occur more frequently in high-risk women. Breast MRI scanning is more sensitive in detecting breast masses in dense breasts (such as in young women), whereas mammograms are more sensitive in detecting breast calcifications. Therefore both are used to obtain optimal breast screening in high-risk women. Other screening tests, such as breast ultrasonography, are not routinely recommended for breast cancer screening. Over half of US states now mandate that women be informed about the increased risk of breast cancer associated with dense breasts and the option of supplemental screening, but specific tests to recommend in these women remains an area of investigation.
Research is being done to identify even more effective screening technologies and approaches. In addition to enhanced imaging modalities, these involve the evaluation of biomarkers in serum, breast duct fluid, saliva, and tears. These strategies offer great hope for improving breast cancer early detection. It is anticipated that in the future, blood- or biofluid-based early detection tests will be used in conjunction with or in place of more standard imaging screening tests.
Despite being largely preventable through lifestyle modifications, an estimated 140,250 new cases of CRC will be diagnosed in the United States during 2018, and an estimated 50,630 lives will be lost to CRC. Modifiable risk factors for CRC are obesity, smoking, alcohol, and red or processed meat consumption. The World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) has estimated that 47% of CRC cases, or 63,652 cases, could be prevented each year through a healthy diet, weight management, and physical activity. Nonmodifiable risk factors include a personal or family history of CRC or adenomatous polyps, including the inherited conditions of familial adenomatous polyposis (FAP) and hereditary nonpolyposis colorectal cancer (HNPCC), and a personal history of chronic inflammatory bowel disease. In addition, the presence of adenomas at screening is a strong predictor of CRC risk.
Globally, the incidence of CRC varies greatly, with as much as a 10-fold variation in both sexes, with the highest rates found in Australia/New Zealand and the lowest in Western Africa.
Mortality rates vary somewhat less than do incidence rates, with mortality being the highest in Central and Eastern Europe, and the lowest in Western Africa. Although more than half of new CRC cases occur in more developed regions of the world, more than half of the deaths due to CRC occur in less developed regions.
Despite the development of numerous risk prediction models for different populations, there is no validated tool with high discriminatory power that can be easily applied in various settings to estimate risk of colorectal neoplasia. Nevertheless, the Freedman risk model/NCI's Colorectal Cancer Risk Assessment tool, developed in 2009, has been validated in an independent cohort (unlike many of the other proposed models) and is relevant to a large proportion of the US population that is eligible for CRC screening. Although the model was developed and validated based on non-Hispanic white men and women, and its generalizability to other racial and ethnic groups is uncertain, it is believed that the variables that contribute to the model have deep penetration into the US population, regardless of race and ethnicity, and that it generalizes to nonwhite populations within the United States better than do non-US models, of which there are many. The Freedman model originally predicted the future 10- and 20-year absolute risk of CRC, with an area under the curve (AUC) of 0.61 (95% CI, 0.60–0.62) for men and 0.61 (95% CI, 0.59–0.62) for women, comparable to other cancer risk prediction models. In its current form, the model now predicts 5-year, 10-year, and lifetime absolute CRC risk. Imperiale and colleagues tested the ability of the model to predict current risk of advanced neoplasia, reasoning that knowledge of current risk may more directly affect short-term decisions regarding screening than knowledge of future risk, and that this could lead to increased adoption of CRC screening and to tailored screening strategies wherein those at higher risk for advanced neoplasia would be directed toward colonoscopy as the preferred method of screening and those with a lower risk of such a finding toward less invasive screening methods. Results of the study demonstrated that the model's discriminatory power for predicting current advanced neoplasia was in the moderate-to-good range, with an AUC of 0.71 (95% CI, 0.68–0.73) in the 5-year analysis, which is better than the AUC of the model in its original validation cohort as well as the Gail model when applied to an independent cohort to estimate 5-year breast cancer risk. The authors concluded that the tool can be used to estimate current risk of advanced neoplasia, making it potentially useful for tailoring and improving CRC screening efficiency among those at average-risk of CRC. The Cleveland Clinic Score Against Colon Cancer risk model is another tool that is available online and is similar to the Freedman/NCI model in terms of the variables it incorporates. However, it is unclear if it has been evaluated for publication in a peer-reviewed journal.
The biology of CRC makes it an ideal candidate for early detection and preventive interventions. The colon is a relatively accessible organ, and the vast majority of CRCs develop from adenomas, a precancerous stage that can last as long as 20 to 30 years, providing ample time for its detection and removal before it evolves into cancer.
The USPSTF currently recommends that average-risk individuals be screened for CRC beginning at age 50 and continuing until age 75. For average-risk adults between 76 and 85 years of age, the decision to screen should be an individual one, considering a patient's past screening history and overall health. The USPSTF does not recommend a particular screening test. Multiple CRC screening examinations exist, although they differ with respect to the level of evidence supporting their use, their test performance to detect cancer and adenomas, and their risk of harms. There has been no assessment of the relative benefits and harms among the tests, because comparative studies are currently limited in their study design and power to detect cancers, any mortality benefits, and associated harms. No screening test has been shown to reduce all-cause mortality.
Screening options include stool tests, direct visualization tests, and a US Food and Drug Administration (FDA)–approved blood test (Epi proColon). However, because the blood test has very limited data on its performance, and because the data that are available suggest that its ability to detect CRC is worse than that of other noninvasive tests, this section will discuss only the stool and direct visualization tests.
Guaiac-based fecal occult blood tests (gFOBTs), fecal immunohistochemical tests (FITs), and a FIT combined wit stool DNA testing (Cologuard) are the three options for stool-based testing. Because stool-based tests do not reliably predict adenomas, these tests are early detection tests and cannot prevent CRC from occurring. The gFOBTs have the strongest direct evidence supporting their use as a CRC screening modality, with biennial screening reducing CRC mortality by 9% to 22% and annual screening reducing CRC mortality by 32% after 11 to 30 years of follow-up compared with no screening. The gFOBTs are noninvasive and inexpensive, although they can have a high false-positive rate and patient compliance is often low because of the test's dietary restrictions.
FITs are rapidly replacing gFOBTs because they do not have dietary restrictions and because they offer at least equal and likely better detection of CRC. Different FITs use different assay methods, so FIT sensitivity has varied widely across studies. In the largest studies of the USPSTF evidence review for its 2016 recommendation, the sensitivity was 73.8% (95% CI, 62.3–83.3) for the quantitative OC FIT-CHEK and 78.6% (95% CI, 61.0–90.5) for the qualitative OC-Light, based on single stool samples. Sensitivity could be increased by using three stool samples or by lowering the assay cutoff value; however, specificity decreased with increasing sensitivity. Data are lacking regarding the impact of FITs on CRC mortality. Finally, FITs are generally inexpensive, with a Centers for Medicare and Medicaid Services (CMS) reimbursement of $23 and a mean commercial reimbursement of $21.
The newest stool-based screen combines a FIT with DNA marker analysis. Sensitivity of one-time FIT-DNA testing for CRC was shown to be 92.3% in one large study, significantly better than the sensitivity of one-time FIT alone (73.8%); yet, specificity was lower (86.6% versus 94.9). In other studies, the sensitivity of FIT was superior when multiple samples or lower assay cutoff values were used; and specificity for the FIT-DNA test is lower than for all FIT assays, resulting in this test having the highest false-positive rate among FIT screens. Data regarding a possible CRC mortality benefit for the FIT-DNA test are lacking. Finally, this is the most expensive stool test, with a CMS reimbursement rate of $493.
There are few adverse outcomes associated with stool-based tests directly, aside from the risk of missed cancers. However, serious adverse events can occur during follow-up colonoscopy after positive stool test results, and some data suggest that the rate of perforations in these colonoscopies may be higher than for those performed in average-risk screening populations. The pooled estimate from the USPSTF evidence review was 8 (95% CI, 2–32) perforations per 10,000 diagnostic colonoscopies. A modeling study from the Cancer Intervention and Surveillance Modeling Network (CISNET) Colorectal Cancer Working Group estimated the number of complications (defined as perforations, gastrointestinal bleeding, nausea and vomiting, ileus, dehydration, abdominal pain, myocardial infarction, angina, arrhythmias, congestive heart failure, respiratory arrest, syncope, hypotension, or shock) in a population of 1000 screened individuals to be 10 to 11 with annual FIT and 9 to 10 with FIT-DNA at 3-year intervals.
Direct visualization tests offer not only the ability to detect CRC early, but also to prevent it through detection and subsequent removal of precancerous lesions. These tests include flexible sigmoidoscopy (FS), colonoscopy, and computed tomography (CT) colonography (CTC). In the case of CTC, follow-up endoscopy is required for removal of polyps detected during the screen.
Meta-analysis of four RCTs of FS in over 450,000 individuals demonstrated a 27% reduction (incidence rate ratio [IRR], 0.73; 95% CI, 0.66–0.82) in CRC mortality at 11 to 12 years of follow-up (findings limited to distal CRC). A single RCT from Norway demonstrated a 38% reduction in CRC mortality when FS was combined with FIT. Although use of FS in the United States is uncommon, the 2016 USPSTF guideline recommends a 5-year screening interval for FS alone and a 10-year interval if combined with annual FIT testing.
Despite a lack of RCT evidence supporting the use of colonoscopy, it remains the gold standard of screening because of its high sensitivity and specificity for the identification of polyps and CRC and it ability to concomitantly diagnose and reduce risks associated with biopsied precancerous lesions. Observational evidence supporting the use of colonoscopy comes from a large prospective analysis of the Nurses' Health Study and the Health Professionals Follow-Up Study, which demonstrated a 68% (hazard ratio [HR], 0.32 [95% CI, 0.24–0.45]) reduction in CRC mortality in those who self-reported screening colonoscopy compared with those who never underwent screening endoscopy. In this study, statistically significant reductions were seen for both distal and proximal CRC, although the mortality reduction was greater in the distal colon. There are currently a number of RCTs underway for colonoscopy. At least two of them are comparing colonoscopy and FIT (NCT01239082 and NCT00906997); the Nordic-European Initiative on Colorectal Cancer (NordICC) is comparing colonoscopy and usual care (NCT00883792) ; and one trial in Sweden is comparing colonoscopy with FIT or no screening (NCT02078804).
Drawbacks of lower endoscopy include its invasiveness, risk of serious complications, expense, and operator dependence. The estimated number of complications from the aforementioned CISNET modeling study is 14 to 15 with colonoscopy at 10-year intervals and 9 to 12 with FS at 5-year intervals.
As with colonoscopy, there is currently no direct evidence establishing the effectiveness of CTC in reducing CRC incidence and mortality. However, a number of studies have examined the diagnostic accuracy of CTCs and are summarized in the 2016 USPSTF evidence review. In studies in which bowel preparation was performed before the CTC, per-person sensitivity and specificity to detect adenomas 10 mm or larger ranged from 66.7% to 93.5% and 86.0% to 97.9%, respectively. For detection of adenomas 6 mm or larger, sensitivity and specificity were 72.7% to 98.0% and 79.6% to 93.1%, respectively. The sensitivity to detect advanced adenomas ranged from 87.5% to 100%. Limited data suggest that CTC without bowel preparation results in lower sensitivity to detect adenomas 10 mm or larger and 6 mm or larger in addition to advanced adenomas.
Serious harms of CTC in asymptomatic individuals appear to be uncommon. The risk of perforation is less than 2 per 100,000 examinations. Data are lacking to estimate any serious adverse events from follow-up colonoscopy after CTC. Exposure to radiation is perhaps the most important potential harm from CTC; however, the evidence to date suggests that the benefits of CTC screening every 5 years far outweigh the potential radiation risks. Finally, although this test is relatively noninvasive, it can result in the identification of extracolonic findings that may or may not be clinically important. Estimates are that extracolonic findings occur in 41% to 69% of CTC procedures, but only 5% to 37% of CTC procedures have extracolonic findings that require actual diagnostic follow-up, and an even smaller percentage result in findings that require any definitive treatment.
Cervical cancer provides the “perfect” paradigm for cancer prevention, having a vaccine to reduce the incidence of infection from the sole causative agent, human papillomavirus (HPV), and screening tests to identify women infected with HPV or cytologic findings that indicate precancerous conditions that can be successfully managed to prevent the development of invasive disease. The incidence and mortality of cervical cancer in the United States has decreased by over 75% since widespread screening with the Papanicolaou (Pap) test and treatment of precancerous lesions began decades ago.
Infection with high-risk HPV is a necessary, although not sufficient, cause of cervical cancer. Most HPV is transient and poses little risk of persistent infection. The natural history of cervical cancer involves (1) HPV infection; (2) persistent HPV infection; (3) development of a precancerous cervical lesion; and (4) progression to invasive cervical cancer. Opportunities for interventions exist throughout the continuum, including education about HPV transmission, HPV vaccination, screening with HPV and Pap tests, and treatment of precancerous or cancerous lesions of the cervix. Interventions at steps 1 to 3 provide the greatest opportunity to reduce the incidence and mortality from cervical cancer.
HPV is the most common sexually transmitted infection in the United States, with over 80% of sexually active individuals acquiring an HPV infection by age 45. A number of other cancers have been associated with HPV infection, including oropharyngeal (largely in men), vaginal, vulvar, penile, and anal (mostly in men who have sex with men). Although most HPV is transient in nature, factors that determine which HPV infections will persist and progress are not completely understood. The HPV genotype appears to be the most important determinant. HPV-16 has the highest carcinogenic potential, causing approximately 55% to 60% of all cases of cervical cancer worldwide. HPV-18 is the next most carcinogenic genotype, accounting for 10% to 15% of cervical cancers. Approximately 12 other genotypes are associated with the remainder of cases of cervical cancer.
The risk of HPV infection is greatest among teens and women in their early 20s and decreases with increasing age. Most infections with high-risk HPV do not result in persistent infections or cancer and are very likely to regress among women with both normal and abnormal cytology results. However, persistence of HPV infection appears to increase with age, with HPV infections detected in women older than 30 years being more likely to reflect a persistent infection. Identifying women with a persistent HPV infection is one of the best ways to distinguish women at increased risk of developing cervical dysplasia who require closer monitoring.
With the recognition of HPV as the primary etiologic factor in cervical cancer, variables previously identified as risk factors are now better classified as cofactors that either increase the risk of HPV infection or increase the likelihood that an infection will be persistent, thus increasing the risk of cervical neoplasia. These include age at first coitus, number of sexual partners for a woman or her partner, history of sexually transmitted disease including HPV infection, absence of HPV vaccination, immunosuppression (human immunodeficiency virus [HIV]–positive individuals, transplant recipients), younger age at first pregnancy, high parity, long-term oral contraceptive (OC) use, poor nutritional status, tobacco use, race, or ethnicity [Hispanic, especially if not English speaking, or African American women), socioeconomic status, and infrequent Pap smear screening.
Diethylstilbestrol (DES) exposure in utero is largely a historical risk factor for the development of cervical cancer. DES is a synthetic form of the hormone estrogen that was prescribed to pregnant women between 1940 and 1971 to prevent miscarriage and premature labor. It has been well defined that the daughters of women who took DES while pregnant (so-called “DES daughters”) have a 40-fold increased risk of developing clear cell adenocarcinoma of the vagina and cervix compared with unexposed women. However, this type of cancer remains rare, with only 1 in 1000 DES daughters developing it. Risk of cervical cancer related to DES exposure in utero has been shown to persist in DES daughters above the age of 40.
The Pap test is considered to be one of medicine's most successful cancer screening tests. Although there are no RCTs that have evaluated the efficacy of the Pap test, there is overwhelming observational evidence that cervical cancer incidence and mortality have decreased since Pap testing was integrated into routine clinical practice in the United States.
Cervical cancer is characterized by a long sojourn time, with precursor lesions identifiable long before the development of invasive disease. Cytologic screening for these lesions (e.g., cervical intraepithelial neoplasia [CIN]) with the Pap test is effective in reducing both the incidence and mortality from cervical cancer because these premalignant lesions are generally amenable to interventions that prevent the progression to invasive disease. HPV testing shows whether a person has a current HPV infection. It does not provide information on previous HPV infections. Genotype testing is now available for HPV types 16 and 18 and can be used to aid in the clinical management of precursor lesions, but is not currently used in cervical cancer screening.
Studies of HPV cervical cancer screening have found that HPV testing alone was more sensitive but less specific than cytologic evaluation alone. For CIN3+ outcomes, sensitivity ranged from 86% to 97% for HPV testing versus 46% to 50% for cytologic evaluation at a colposcopy referral threshold of “atypical squamous cells of undetermined significance” (ASCUS). For CIN2+ outcomes, sensitivity ranged from 63% to 98% for HPV testing versus 38% to 65% for cytology. However, specificity for CIN2+ and CIN3+ was consistently 3% to 5% lower for HPV testing than for cytology.
A 2012 study identified that a negative HPV test result provided greater reassurance against CIN3+ over an 18-year follow-up than did a normal Pap result. Although baseline Pap and HPV tests equally predicted who would develop CIN3+ within the first 2 years of follow-up, only HPV testing predicted who would develop CIN3+ 10 to 18 years later. These data support extending the screening interval in women who have negative cotesting results.
Updated screening guidelines were released in 2012 jointly by the ACS, the American Society for Colposcopy and Cervical Pathology (ASCCP) and the American Society for Cervical Pathology (ASCP) ( Table 23.5 ) and separately by the USPSTF. These guidelines apply to women at average risk of cervical cancer who have a cervix.
| Age | Screening Recommendation b |
|---|---|
| Aged <21 yr | No screening c |
| Aged 21–29 yr | Cytology alone every 3 yr c |
| Aged 30–65 yr | Human papillomavirus (HPV) and cytology “cotesting” every 5 yr (preferred) |
| OR | |
| Cytology alone every 3 yr (acceptable) | |
| Age >65 yr | No screening following adequate negative prior screening |
a The following are not considered to be at average risk: women with a history of CIN2+ or cervical cancer in the past 20 years, diethylstilbestrol (DES) daughters, and those with human immunodeficiency virus (HIV) infection or immunosuppression due to other factors (e.g., transplant patients).
b Average-risk women should not be screened annually at any age by any method.
c HPV testing should not be used for screening in this age group.
Increasing evidence and an improved understanding of the natural history of cervical cancer led to the recognition that previous recommendations for annual screening were excessive and resulted in an increased rate of false positives. Annual screening leads to a very small increment in cancers prevented, at the cost of a very large excess of unnecessary procedures and treatments due to the high prevalence of transient, benign HPV infections and associated lesions, most of which will regress within a year or two. Of those that do not regress, on average, they are many years from causing cancer. Women at average risk at any age should not be screened annually by any screening method. Recommended screening intervals for women at average risk are based on age and clinical history.
Women ages 21 through 30 should be screened every 3 years with the Pap test. HPV testing is not recommended in women under age 30 because the risk of HPV infection is high, but the likelihood of a persistent infection is extremely low. Cotesting every 5 years with cytologic evaluation and HPV testing provides benefits similar to a Pap test alone done every 3 years for women 30 to 65 years of age. Therefore, in women age 30 to 65 years who want to lengthen the screening interval, cotesting every 5 years with Pap and HPV is recommended. ACS, ASCCP, and ASCP preferred cotesting over the Pap test alone, whereas the USPSTF deemed both cotesting and Pap test alone as acceptable.
Certain low-risk groups should no longer be screened. These include women under age 21, low-risk women over age 65, and women who have undergone a hysterectomy. Evidence has shown that screening women age 21 and younger does not reduce cervical cancer incidence and mortality but is associated with significant harms. Treatment of CIN in women younger than age 21 has been shown to increase the risk of adverse pregnancy outcomes, including cervical incompetence and preterm delivery. Women age 65 and older who have had the recommended screening in the past 10 years and are considered to be at low risk of cervical cancer do not need to continue screening beyond age 65. Women who are not low risk include those with immunosuppression (e.g., HIV or transplant patients), DES daughters, and those with a prior history of a high-grade precancerous lesion of the cervix or cervical cancer within the past 20 years. It is important to note that older women who have not had recent screening are a population at risk for the development of cervical cancer. Although cervical cancer incidence rates are on the decline, 50% of women diagnosed with cervical cancer have not been screened 3 to 5 years prior to diagnosis. Women who have had hysterectomy do not need to have cervical cancer screening unless the hysterectomy was done for the management of CIN2+ or cervical cancer, in which case they should follow the recommended surveillance guidelines. When a supracervical hysterectomy is performed (i.e., leaving the cervix intact), screening recommendations should be based on cervical cancer risk.
At this time, women who receive the HPV vaccination should continue to follow current cervical cancer screening recommendations, because HPV vaccination does not protect against all types of oncogenic HPV. In addition, the HPV vaccination rate is far from optimal at this time, and immunization registries do not identify who has received the recommended series of vaccinations. Although HPV vaccination is an important step toward cervical cancer prevention, it does not remove the need for routine cervical cancer screening.
As previously noted, studies have found that HPV testing alone was more sensitive than cytologic evaluation alone. In 2014, the FDA approved a currently marketed HPV test to include the additional indication of primary cervical cancer screening.
Although HPV testing alone is more sensitive than cytologic evaluation alone, it is also less specific. Strategies that maximize detection of CIN3+ by immediate referral to colposcopy with follow-up testing and triage of women at intermediate risk of CIN maximizes the benefits of cervical cancer screening while decreasing the potential harm. The Addressing the Need for Advanced HPV Diagnostics (ATHENA HPV) Study evaluated the role of primary HPV screening in women 25 years of age and older and validated an effective triage algorithm. In this trial, HPV-positive specimens underwent genotyping for HPV-16 and HPV-18. If a specimen was positive for HPV-16 or HPV-18, colposcopy was performed. If a specimen was negative for HPV-16 or HPV-18, cytologic evaluation was performed on the specimen. If the cytologic evaluation results were abnormal, colposcopy was performed. If both HPV genotyping and cytologic findings were normal, repeat cotesting was performed in 1 year. The trial concluded that primary HPV screening in women 25 years of age or older is as effective as a screening strategy that uses cytologic evaluation in those 25 to 29 years old and cotesting in those 30 years of age or older. In addition, this paradigm requires fewer screening tests.
In 2015, the ASCCP and the Society of Gynecologic Oncology (SGO) provided interim guidance for the use of the FDA-approved HPV test for primary cervical cancer screening. They concluded that, because of its equivalent or superior effectiveness in women 25 years of age or older, the FDA-approved primary HPV screening test can be considered an alternative to current cytology-based cervical cancer screening methods. However, primary HPV testing is not currently considered a standard of care.
Prevention of cervical cancer through education about HPV transmission, HPV vaccination, and screening with cytologic evaluation with or without HPV testing has the potential to eliminate invasive cervical cancer as a disease of concern for women. Primary HPV screening is emerging as a new screening modality.
Lung cancer is the most common cause of cancer-related death worldwide and the second most diagnosed cancer in the United States for both men and women. Estimated figures for 2016 in the United States exceed 224,000 new cases and 158,000 deaths as reported by the ACS. In China alone, estimated figures for 2015 are 733,000 new cases and 610,000 deaths. Lung cancer incidence and death rates in the United States have been declining slightly for men and women, with the magnitude of the decrease varying by race and ethnicity.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here