Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In recent years, there has been an explosion of discovery about proteins that function to organize components of the signal transduction machinery with their effectors at specific subcellular locales. Many of these molecular scaffolds control the assembly, trafficking, subcellular location and activity of epithelial transport proteins and their regulators, and, thus, are critical determinants of epithelial transport modulation. Here, the review the present state of knowledge about the two major classes of molecular scaffolds, PDZ-proteins and AKAPs, and discuss their role in renal epithelial transport.
Keywords
PDZ protein; AKAP; NHERF; Shank; Dystrophin; Lin-7; CASK
PDZ domains (also known as DHR domains or GLGF repeats) are ~90 amino acid, protein–protein interaction modules that bind short amino-acid motifs (4–5 residues) generally found at the extreme COOH-terminus of target proteins. More rarely, PDZ domains recognize internal sequences that mimic the COOH-terminal binding motif. The term PDZ is derived from the names of the three proteins that the structure was originally identified from (PSD 95, a post synaptic density protein), Dlg (Dropsophila Disc large tumor suppressor), and ZO-1 (zona occludens, the tight junction protein). Since its discovery as a region of sequence homology in these few proteins, the PDZ domain has become recognized as one of the most common interaction modules. The human genome contains over 250 PDZ domains in nearly 100 human proteins. The structure is evolutionarily conserved, emerging largely in metazoans, perhaps to accommodate the increased signaling needs of multicellular organisms.
PDZ domain containing proteins usually possess multiple protein–protein recognition modules. Because the domains act independently and allow concurrent recruitment of different binding targets, PDZ proteins function as molecular scaffolds. Indeed, PDZ proteins facilitate multi-protein complex formation, and organize expression of target proteins on specific membrane domains for a wide range of physiological processes. A growing body of work has strongly implicated PDZ proteins in targeting and clustering various receptors, channels, transporters, and signal transduction elements at specific plasma membrane domains in different cell types, including neurons, muscle, and the visual system. PDZ proteins play especially important roles in epithelial transport processes.
PDZ domains have been traditionally divided into three different classes, categorized by the nature of their ligands. The different ligand classes are distinguished by differences in the binding residues found at the extreme COOH of target proteins ( Figure 14.1 ). Type I domains recognize the sequence, X-S/T-X-Φ* (where X=any amino acid; Φ=hydrophobic amino acid; *=COOH terminus). Type II domains bind to ligands with the sequence X-Φ-X-Φ*. Type III domains interact with X-D/E-X-Φ* sequences. Binding specificity within each domain class can be conferred by the variant (X) residues, as well as residues outside the canonical binding motif, especially at the -3 and -4 positions (where 0 position is the C-terminal residue). Moreover, a few PDZ domains do not fall into any of these specific classes.
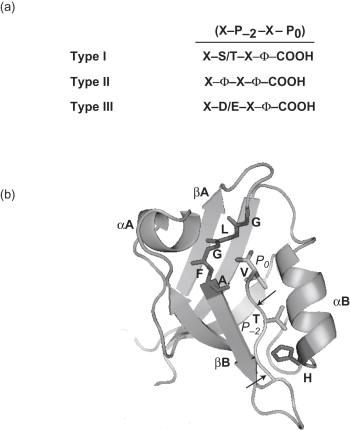
Based on large-scale proteomic analysis of PDZ-ligand interactions, it has been suggested the traditional three-class definition be extended to include 16 distinct binding classes. Such a classification has been proposed to predict specific interaction partners of known PDZ domains with greater fidelity than the traditional scheme.
In recent years, the structures of over 20 different PDZ domains have been solved at atomic resolution. Like many protein–protein recognition modules, PDZ domains are small globular structures. Comprised of six β-strands (βA-βF) and two alpha helices (αA and αB), PDZ domains fold into a six stranded beta sandwich ( Figure 14.1 ). The peptide ligand inserts into a binding cleft, created by the βB strand and the αB helix, effectively forming an additional antiparallel beta strand. An extensive network of hydrogen bonds and hydrophobic interactions stabilizes binding of the peptide. For instance, the conserved glycine-leucine-glycine-phenylalanine-alanine (GLGF) motif contained within a βA-βB linker provides a cradle of main chain amides, and confers recognition of the terminal carboxylate group of the peptide. A hydrophobic pocket accommodates the hydrophobic COOH-terminal residue, thereby accounting for preferential interaction with proteins ending with a hydrophobic residue (the so-called P0 position).
Binding specificity among the different binding classes is determined partly by an interaction between the P-2 residue of the target protein and the first residue of the PDZ domain αB-helix. In Class I PDZ domains, a conserved histidine residue forms hydrogen bonds with the invariant P-2 serine or threonine residue in the target protein. In class II PDZ domains, this position of the PDZ domain and the P-2 residue of the target protein are usually occupied by a hydrophobic amino acid.
Binding specificity within each domain class is also observed. At least three factors account for this. First, unique residues within or adjacent to the peptide-binding groove in the PDZ domain can interact with the target at sites other than the P-2 and P0 residues. For example, the side chain of the P-1 target protein residue usually points away from the invariant interaction surface but, in some cases, it can bond with residues that are distinct to a particular PDZ domain. Likewise, the P-3 side chain can make contact with unique residues in the interaction groove. Sites proximal to the archetypal, four amino acid-binding motif can also interact with regions outside the canonical-binding site, and thereby also contribute to binding specificity and affinity. Second, because interacting residues in PDZ domains can undergo large ligand-dependent conformational changes, variations in binding pocket flexibility may contribute to binding specificity. Such a mechanism has been proposed to explain the different binding specificity of the two highly homologous PDZ domains in NHERF1. Finally, genome-wide analysis of PDZ domain binding suggests that PDZ domain selectivity is also achieved by the cellular and subcellular context of the interaction, and this may actually play a more important role than inherent binding specificity.
PDZ interactions can be dynamically regulated to control the composition and stoichiometry of different multimeric complexes. Phosphorylation of the binding target is the most common mechanism. This is explained by the fact the P-2 serine or threonine in canonical type I PDZ targets can be a substrate for phosphorylation. In these cases, phosphorylation of the residue creates an energetically unfavorable PDZ ligand. For example, phosphorylation of the COOH-terminal site in the Kir 2.3 channel by Protein Kinase A inhibits its interaction with the synaptic PDZ protein, PSD-95, to regulate the channel. Likewise, phosphorylation of the P-2 serine in the β2 adrenergic receptor uncouples the receptor from the NHERF1 PDZ protein, and disrupts receptor recycling in the post-endocytic pathway.
Phosphorylation of sites within PDZ proteins is emerging as an additional mechanism for modulating PDZ binding. Evidence for this was first provided by observations that the interaction of a PDZ protein, NHERF1 (see below), with CFTR is negatively regulated by phosphorylation of a residue in the second PDZ domain. Phosphorylation of sites in or near the first PDZ domain of NHERF1 also disrupt interaction with the Na-phosphate co-transporter, Npt2a. Phosphorylation of sites that are involved in PDZ–PDZ protein oligomerization has also been observed. This is believed to modulate the extent to which some PDZ proteins can form higher order scaffolding complexes.
Finally, switching interactions with different PDZ proteins can differently regulate the activity and localization of target proteins. This occurs when the target has the capacity to bind to several PDZ proteins that have different properties. For example, TIP-1, a protein that consists of a single PDZ domain and lacks other protein–protein interaction modules, binds to certain target proteins to antagonize the scaffolding functions of canonical PDZ proteins.
A number of PDZ proteins are preferentially expressed at polarized membrane domains or within critical sorting compartments ( Figure 14.2 ), where they perform retention/sorting operations and organize local signaling complexes at polarized locales. Examples of PDZ proteins that predominately reside at the basolateral membrane of certain intestinal and renal epithelia include syntrophin (see “Dystrophin-Associated Protein Complex,” below), Lin-7 (see “Lin-7/CASK/SAP97,” below), the ErbB interacting protein, ERBIN, and certain members of the membrane associated guanylate kinase family of PDZ proteins, such as CASK, PSD-93, and SAP97 (aka Discs large homolog 1 ). Other PDZ proteins, including the sodium hydrogen exchange regulator factors (see “NHERF, “below), Shank2E, and PSD-95, are chiefly expressed on or near the apical membrane. Some PDZ proteins, such as zonula occludens, PALS1 (Stardust), and PATJ (Disc lost), play important roles in the generation and maintenance of the tight junction. Still others, like CAL, which is primarily located in the Golgi or SNX27, and syntenin, which are found in endosomes, reside in biosynthetic or endocytotic sorting compartments.
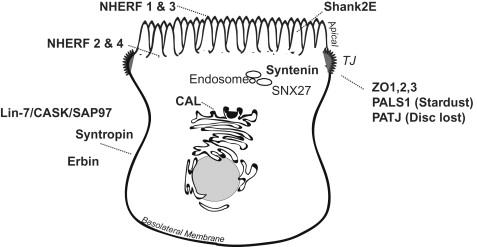
A PDZ-binding motif can serve as a polarized sorting or retention signal. One of the first examples evolved from studies with the GABA transporters or GATs ; deletion of the PDZ-binding motif from the apical isoform GAT-3 caused the transporter to localize randomly to both apical and basolateral membranes. Basolateral membrane expression of several membrane proteins has also been found to require a PDZ-binding motif. For instance ERBB receptors, which play crucial roles in morphogenesis and oncogenesis, interact with a basolateral PDZ protein, called ERBIN, and require a PDZ-binding motif for basolateral membrane expression. ERBIN is targeted to the basolateral membrane by its leucine-rich repeat domain. Efficient basolateral membrane expression of a number of transporters that interact with the basolateral PDZ protein Lin-7 also require an intact PDZ-binding site (see below).
Members of the MAGUK (membrane associated guanylate kinase) family of PDZ proteins are the archetypal PDZ scaffolds. MAGUK proteins are equipped to assemble large molecular complexes, having one to three PDZ domains, a SRC homology 3 domain (SH3), and a catalytically inactive guanylate kinase-like (GK) domain. In addition to the PDZ domains, the GK and the SH3 domains function as independent protein–protein interaction modules; GK domains recruit scaffold adaptor molecules called guanylate kinase-associated proteins or GKAPs, while SH3 domains have been shown to coordinate interaction with at least one non-receptor tyrosine kinase. The SH3 and GK domains can also interact with one another, forming a composite SH3–GK structure that acts as an additional intermolecular protein–protein interaction domain with a binding specificity that is distinct from either SH3 or GK domains.
The PSD-95 family, encoded by four genes (PSD-95/SAP90, PSD-93/Chapsyn-110, SAP102, and SAP97), exemplifies MAGUK proteins. Two of these, PSD-93 and SAP97 (see below), are expressed in renal epithelial cells. However, the best characterized member, PSD-95, is largely expressed in excitable tissues, and plays central roles in maintaining and modulating the strength and structure of glutamatergic synapses. Generally, its properties and functions are likely to be applicable to the other MAGUKs, including those expressed in the kidney.
Like many scaffolds, PSD-95 not only contains multiple protein–protein interaction modules, it also assembles into multimers, creating an extended platform for efficient scaffolding. These qualities, combined with palmitoylation-dependent membrane tethering and synaptic localization signals, make PSD-95 ideally designed to cluster ion channels, receptors, trafficking proteins, and signal transduction machinery at the post-synaptic membrane. In doing so, PSD-95 influences trafficking, endocytosis, and activities of target proteins at the synapse. Organizing local signaling complexes is one of the most important clustering functions of PSD-95. For example, the PDZ domains in PSD-95 independently interact with the calcium/calmodulin-activated nitric oxide synthase, nNOS, and NMDA (N-methyl-D-aspartate) receptors to form a ternary complex. The organization is thought to be important for regulated synthesis of nitric oxide. Because NMDA receptors are permeable to calcium, the physical linkage of nNOS with the excitatory receptors is believed to allow nitric oxide production to be efficiently coupled to receptor activation, calcium influx, and local changes in intracellular calcium. Significantly, disruption of NMDAR interaction with PSD-95 dissociates the receptors from downstream neurotoxic signaling, without blocking synaptic activity or calcium influx.
Local signaling complexes that control the production of NOS in the kidney have been proposed. One may involve PSD-93, the predominate MAGUK in renal epithelial cells. Similar to PSD-95, PSD-93 associates with the plasmalemma via palmitoylation-dependent tethering signals, where it recruits and clusters various target proteins, including nNOS. In the kidney, PSD-93 is largely expressed along the basolateral membrane of the thick ascending limb, macula densa cells and the distal nephron. In the macula densa, PSD-93 colocalizes with the pool of nNOS that is associated with intracellular vesicles and the basolateral membrane. It remains to be tested if PSD-93 interaction with nNOS in the macula densa coordinates regulated NO production in the manner that is observed with PSD-95 at the excitatory synapse.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here