Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Rubeola (i.e., measles) is an acute, highly contagious, vaccine-preventable viral infection. , In the prevaccination era, measles was an almost universal experience during childhood. The incidence of measles declined markedly in the US after the licensure of a live measles vaccine in 1963. In 2000 the US declared elimination of endemic measles (i.e., absence of sustained virus transmission for >12 months) due to high rates of 2-dose measles-mumps-rubella (MMR) vaccine coverage. The recent measles outbreaks have been linked to international travel-related virus introduction by primarily unvaccinated US residents visiting measles-endemic countries, with subsequent spread through underimmunized communities.
Despite substantial progress made in global measles control, large outbreaks continue to occur worldwide due to low rates of vaccine coverage. , Measles is a major cause of child mortality in low-income and middle-income countries located in the African and South East Asia region. Due to the highly contagious nature of measles, maintaining high levels of population immunity by vaccination is crucial to prevent resurgence.
Measles virus (MV) is an enveloped, nonsegmented, negative-strand RNA virus belonging to the Morbillivirus genus in the Paramyxoviridae family. MV is closely related to rinderpest virus, a devastating cattle pathogen that was eradicated in 2011. Sequencing data indicate that MV diverged from rinderpest virus in the sixth century BCE. MV genome consists of 15,894 nucleotides and encodes eight proteins. The two surface envelope glycoproteins and the hemagglutinin (H) and fusion (F) proteins are the main targets for development of neutralizing antibodies. The matrix (M) protein is important in virus assembly. The internal proteins, nucleoprotein (N), polymerase phosphoprotein (P), and large protein (L), form the nucleocapsid. The multiple N and P protein interactions during the MV replication cycle form viral factories in the form of membraneless organelles that promote nucleocapsid structure assembly. Measles virus replication occurs in the cytoplasm in association with discrete bodies. Two nonstructural proteins (C and V) regulate the cellular response to infection.
The H protein binds to cellular receptors for MV located on the surface of target cells. The receptors are signaling lymphocytic activation molecule (SLAM or CD150) on immune cells and nectin-4 on epithelial cells. , The receptor binding H protein interacts with the F glycoprotein and mediates virus attachment and host cell entry. The F protein plays a role in membrane fusion and enhances cell-to-cell spread of the virus. Neutralizing antibodies are primarily directed against the H protein and confer lifelong immunity.
Wild-type MV infects only primates. Although MV is a monotypic virus, minor genetic and antigenic variation occur in some virus strains without compromise of long-term innumity. The World Health Organization (WHO) has recognized 24 MV genotypes. Genetic characterization of MV is crucial to strengthen case-based surveillance, differentiate imported from endemic strains, track transmission patterns, differentiate vaccine from wild-type MV strains, and monitor measles elimination targets by documenting absence of circulating endemic strains. , Between 2016–2018, the WHO Global Measles and Rubella Laboratory Network detected only 6 genotypes (B3, D4, D8, D9, G3, and H1). Two genotypes (B3 and D8) accounted for 95% of reported sequences. MV is inactivated by lipid solvents, heat, and light.
Measles is transmitted by direct contact with respiratory droplets or, less frequently, by airborne inhalation. The incubation period is about 10–14 days from exposure to onset of symptoms. People with measles are contagious from 4 days before to 4 days after rash onset. Measles is highly contagious, with a basic reproductive number (R 0 ) (i.e., the average number of secondary cases generated by a single case of measles in a 100% susceptible population) of 12–18. Symptomatic infection develops in 90% of susceptible contacts.
The pathogenesis of measles infection is complex. The virus infects a variety of cells using multiple receptors including entry receptors (CD150 + and nectin-4), binding receptor, and dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN + ). , , The receptors are expressed on multiple types of target cells in many organs. Measles virus enters the respiratory tract by infecting CD150 + or binding to DC-SIGN + dendritic cells or alveolar macrophages. , The infected cells cross the respiratory epithelium and migrate to bronchus-associated lymphatic tissues and draining lymph nodes. Memory T lymphocytes and B lymphocytes in the lymphatic tissue that express CD150 + are preferentially infected by MV. , Infected lymphocytes then spread the virus to respiratory epithelial cells through the basolateral surface via the alternative epithelial cell receptor nectin-4. , Virus particle budding and shedding from the apical cell surface of infected respiratory epithelial cells facilitates droplet or aerosol transmission of MV to susceptible contacts.
Following initial local spread to the respiratory tract, systemic dissemination of MV and spread to various organs occurs rapidly via infected lymphocytes and DC-SIGN + dendritic cells. Pathologic changes associated with cell-associated viremia include lymphoid hyperplasia of the adenoids, tonsils, lymph nodes, spleen, and intestinal tract. Target-infected cells include the epithelial or endothelial cells, B and T lymphocytes, monocytes or macrophages, and dendritic cells. Multinucleated giant cells are found most commonly during the early stage of measles in the nasopharynx, tonsils, and bronchial mucosa. Intranuclear inclusions and syncytial epithelial cells can be seen. Mononuclear infiltration with peribronchial inflammation occurs in the respiratory tract. Histopathology of measles skin rash demonstrates perivascular, lymphocytic infiltrates. In experimental studies, infection of dermal myeloid and lymphoid cells is followed by viral spread to keratinocytes in the epidermis.
Adaptive immune responses are important in developing and maintaining lifelong immunity to the measles virus. The onset of rash coincides with development of measles-specific humoral and cellular immune responses and rapid clearance of infectious virus. In contrast, clearance of MV RNA is slow; viral RNA can persist in peripheral blood mononuclear cells for several months, despite development of humoral immunity. , Studies in infected rhesus macaques have shown the persistence of MV RNA in lymph nodes and detection of distinct populations of MV-specific T cells for up to 6 months after resolution of rash. Persistence of MV RNA for weeks to months after infection may be associated with a period of immunosuppression. , , In contrast, persistence of MV RNA can result in development of lifelong immunity to MV via sustained B-cell stimulation and ongoing antibody maturation in secondary lymphoid tissues. ,
Neutralizing immunoglobulin G (IgG) antibodies confer lifelong immunity to measles. Measles-specific serum immunoglobulin M (IgM) appears with the onset of rash and persists for 6–8 weeks, followed by a sustained production of measles-specific serum IgG. , Neutralizing antibodies to the H and F proteins are necessary for protection. , , People vaccinated with killed virus in whom atypical measles developed after exposure to MV had neutralizing antibodies to H protein but lacked neutralizing antibody to F protein. In contrast, recipients of live, attenuated vaccine demonstrate neutralizing antibody to the H and F proteins and are immune to infection.
Whereas humoral immunity is key to prevent measles, the cellular immune response appears to be important in aborting symptoms during acute infection, in viral clearance, and in recovery. , Children with agammaglobulinemia fully recover from measles in the absence of a specific antibody response, whereas deficiency in cellular immunity predisposes children and adults to severe or fatal infection. During acute illness in macaque models, the cellular immune response mediated by CD8 + T lymphocytes is most important for MV clearance. , Production of cytokines, such as interferon γ, contribute to the generation of a predominant helper T lymphocyte subtype 1 (Th1) response during acute illness. , During recovery, as viral RNA persists, a shift to the Th2 response influenced by interleukin (IL)-4, IL-10, and IL-13 promotes the development of MV-specific antibodies but can suppress Th1 responses and macrophage activation in response to intracellular microbes. , , Ongoing viral protein production due to MV RNA persistence in secondary lymphoid tissue may promote continued T-cell production, B-cell selection, production of high avidity neutralizing antibodies, and development of lifelong immunity. , ,
A striking feature of the immune response to MV is the induction of transient cellular immunosuppression after infection. The Th2 response during recovery may suppress Th1 responses and macrophage activation in response to intracellular microbes. , Reduced responsiveness to non-MV antigens can last for several weeks to months after acute infection, resulting in increased susceptibility to secondary infections leading to mortality. , Studies have documented an increased susceptibility to secondary infections for several years after recovery from measles accompanied by a decrease in antibody to other microbes. , Other abnormalities include transient lymphopenia, suppression of delayed-type hypersensitivity responses, and decreased lymphocyte proliferation. , The mechanisms of MV-induced immune suppression remain unclear. Based on tropism of MV to memory lymphocytes, immune amnesia from direct infection and depletion of memory immune cells has been proposed. , ,
Measles affects only humans; there is no known animal reservoir. Despite the availability of a safe and efficacious vaccine, the epidemiology of measles has changed globally with the occurrence of multiple, large outbreaks in 2018–2019, undermining elimination goals. , However, the incidence and mortality rates have dramatically declined since the introduction of the measles vaccine.
In the decade before the licensure of measles vaccine in 1963, annually an average of 549,000 measles cases and nearly 500 measles deaths occurred in the US. In the prevaccine era, an estimated 3–4 million cases of measles occurred annually. More than 95% of children contracted measles by 15 years of age (highest attack rate at 5–9 years of age), and most adults therefore were not susceptible during cyclic epidemics. Epidemics of measles occurred in 2- to 5-year cycles, reflecting the changing pool of susceptible people in the community. In temperate climates, measles epidemics typically occurred in late winter through early spring, related in part to social contact patterns (e.g., children gatherings at schools) and environmental factors facilitating MV transmission.
In the vaccine era, measles is uncommon in highly immunized populations. In high-income settings with high rates of measles vaccine coverage and levels of population immunity, adolescents and adults are most susceptible to measles. , Protective transplacental immunity to measles is conferred to infants whose mothers had measles infection or received measles vaccine. Infants whose mothers had measles infection have passive measles antibody for longer periods than infants whose mothers received measles vaccine. The morbidity and mortality rates for measles are highest among infants, the immunocompromised (e.g., HIV infection, leukemia), and severely malnourished children, including those with vitamin A deficiency. , Measles can be severe in secondary household cases.
Following the implementation of a single-dose measles vaccine program in the US in 1963, a dramatic decline in measles incidence was seen through the 1980s until 1989–1991, when about 55,685 cases and 123 deaths were reported. , This major resurgence resulted from decreased measles vaccination rates among preschool-aged children, who often resided in crowded, urban areas, and susceptibility of adolescents because of primary vaccine failure (i.e., failure to respond to a first dose). , In 1989 the Advisory Committee on Immunization Practices (ACIP) recommended a second dose of measles-containing vaccine as a combined MMR vaccine.
After high 2-dose vaccine coverage was attained, reported measles cases declined rapidly to 309 cases in 1995. In 2000 the US declared elimination of endemic measles (i.e., absence of continued, year-round transmission). Elimination of endemic measles was sustained in the US through 2011. However, imported cases and limited spread continued to occur each year. During the post-elimination period (2001–2011), a total of 911 measles cases were reported (median, 61 cases/year; range, 37–220 cases), with a low annual population incidence (<1 case per million people). ,
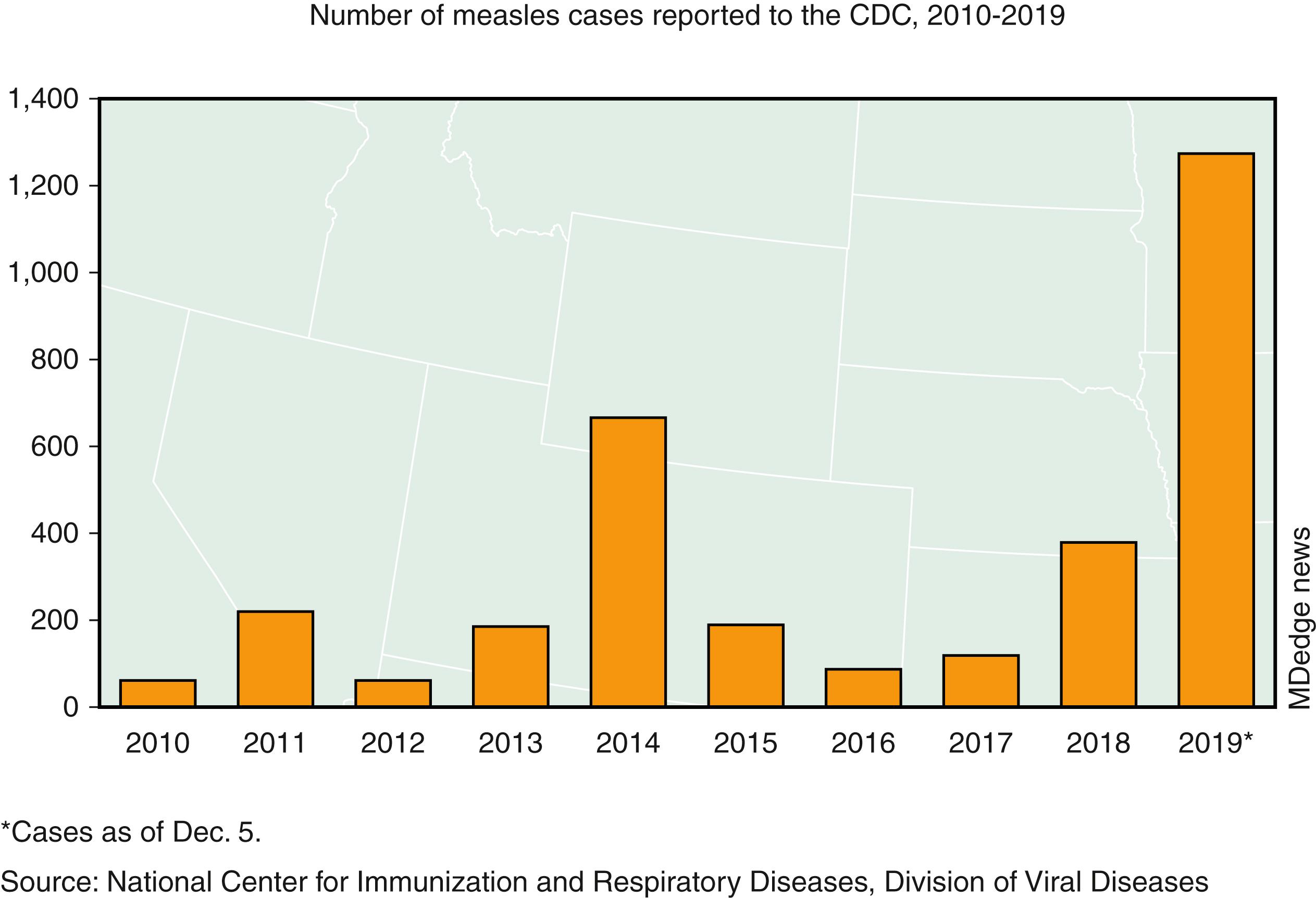
The epidemiology of measles in the US in the past decade has changed significantly. , , , Although childhood and adolescent immunization coverage for at least 1 MMR vaccine dose exceeds 90% in the post-elimination era, measles cases and outbreaks have increased in unimmunized and underimmunized vulnerable subpopulations. , , Following a steady rise in the number of reported measles cases in 2008 (140 cases), 2011 (220 cases), and 2013 (187 cases), there was a 3-fold increase in measles cases in 2014 (667 cases and 23 outbreaks) than any previous year since 2000. In 2014 one large outbreak of 383 cases occurred in Ohio, primarily among unimmunized Amish missionaries returning from the Philippines, which was experiencing a large measles outbreak. , In 2014–2015, a multistate outbreak that began in a Disney theme park in California resulted in 159 measles cases. After 2010, 10 of 13 outbreaks have reported 20 or more cases. ,
From 2001–2015, the incidence of measles has remained low (<1 case per million people) in the US. However, the increasing number of international importations of measles has resulted in resurgence of measles, especially among unvaccinated people. From 2001–2016, a median of 28 imported measles cases (range, 18–80) were reported to the Centers for Disease Control and Prevention (CDC) annually; of the import cases, 62% were US residents and 87% of reported cases occurred among individuals who were unvaccinated or whose vaccination status was not verified.
Vaccine refusal is an important contributor to the changing epidemiology of measles. In 2017 the Somali-American community residing in the State of Minnesota experienced a 75-case outbreak due to low vaccination coverage related to misinformation about MMR vaccine. In 2018 a total of 17 outbreaks occurred in the US. Large outbreaks in Washington state, New York, and New Jersey affecting close-knit, undervaccinated communities contributed to most of the cases. , Orthodox Jewish communities were primarily affected in the New York outbreaks. Outbreaks are precipitated by introduction of the virus into individual communities by unvaccinated US residents, who contracted measles after travel to countries experiencing measles outbreaks, including Israel, Ukraine, and Philippines. Subsequent spread through unimmunized or underimmunized subpopulations results in outbreaks. ,
In 2019, 1282 cases of measles were reported in the US, representing the greatest number annually since 1992 ( Fig. 227.1 ). An analysis of confirmed cases showed that approximately 90% were unvaccinated or had an unverified vaccination status. In the New York outbreaks, safety concerns about the MMR vaccine among affected communities was an important contributory factor. A review of measles outbreaks showed a steady decline in the proportion of imported measles cases, suggesting that sustained domestic measles transmission may pose a threat to measles elimination in the US.
Efforts to contain measles outbreaks carry a substantial economic burden. In the 2018–2019 New York outbreak involving 649 confirmed cases, approximately 6% developed complications, and 41% of those patients were managed in the intensive care unit. The overall cost of containment efforts (i.e., MMR vaccine, immuneglobulin administration, quarantine) during the 2018–2019 outbreak in New York exceeded 8 million dollars. Given the high level of contagiousness, measles outbreaks can occur in communities where <10% are susceptible. In the 2014–2015 outbreak, MMR vaccination rates declined below the necessary threshold to sustain community immunity, leading to secondary cases. Maintaining high targets for 2-dose MMR vaccine coverage, especially among school-aged children, is crucial. Approximately 94%–95% immunization coverage is required to maintain herd immunity and interrupt MV transmission. ,
Vaccine hesitancy is a major risk factor for resurgence of measles in the US. , Modeling studies have associated measles outbreaks with international travel and low childhood vaccination rates due to nonmedical exemptions (NMEs). In addition, the 2019 coronavirus disease pandemic has resulted in decreased ordering and administration of MMR routine pediatric vaccine. Combating vaccine hesitancy and antivaccine movement on a national level is crucial to curb the resurgence of measles. , , Innovative strategies are required to counter the ongoing threat of vaccine resistance, such as: (1) providing evidence-based communication about vaccine preventable diseases, MMR, and other vaccine safety and efficacy; (2) countering the antivaccine movement on social media and e-commerce platforms; (3) legally mandating healthcare provider counseling of parents seeking vaccine exemptions; (4) discontinuing NMEs; (5) requiring the MMR vaccine for school attendance; and (5) pursuing advocacy and concerted efforts on vaccine diplomacy.
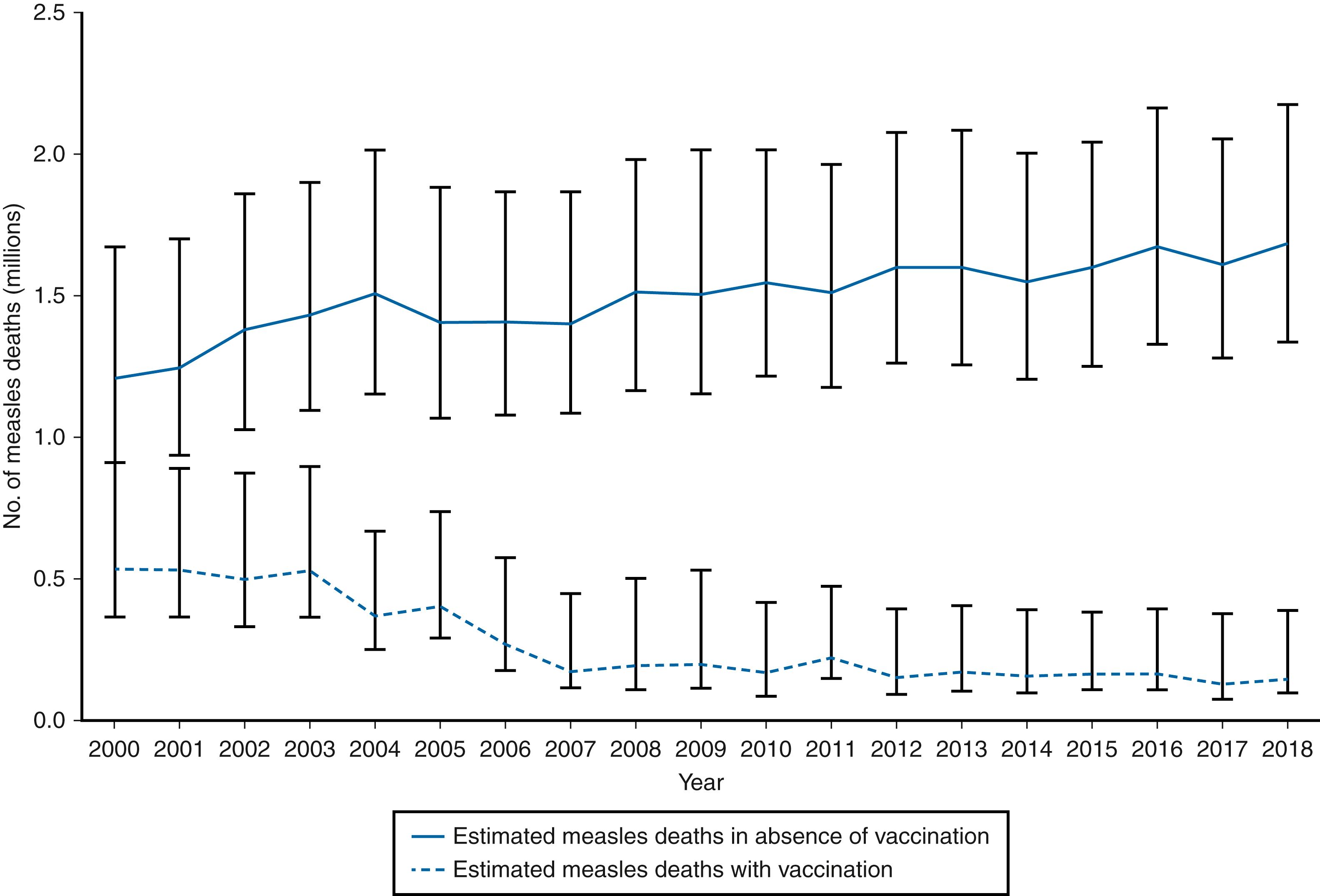
Substantial progress has been made in global measles control and elimination since the introduction of measles-containing vaccine (MCV). , , In 1980, before widespread vaccination, measles accounted for an estimated 2.6 million deaths annually. With improved measles-control strategies, estimated measles deaths worldwide decreased by 73%, from 535,600 in 2000 to 142,300 in 2018. Between 2000–2018, there was a 59% decline in the number of reported cases from 853,479 in 2000 to 353,236 in 2018. Between 2000–2018, there was a 66% decline in the annual reported measles incidence from 145–49 cases per million population. During the 2000–2018 period, measles vaccination prevented an estimated 23.2 million deaths worldwide ( Fig. 227.2 )
Measles eradication is biologically and technically feasible, and nations in every WHO region have achieved elimination. , In 2016 measles elimination was verified in the WHO Region of the Americas (AMR). The WHO Global Vaccine Action Plan endorsed by the World Health Assembly in 2012 established measles elimination targets in five of the six WHO regions by 2020. However, elimination progress has stalled due to a massive global resurgence of measles in 2018 and 2019. , ,
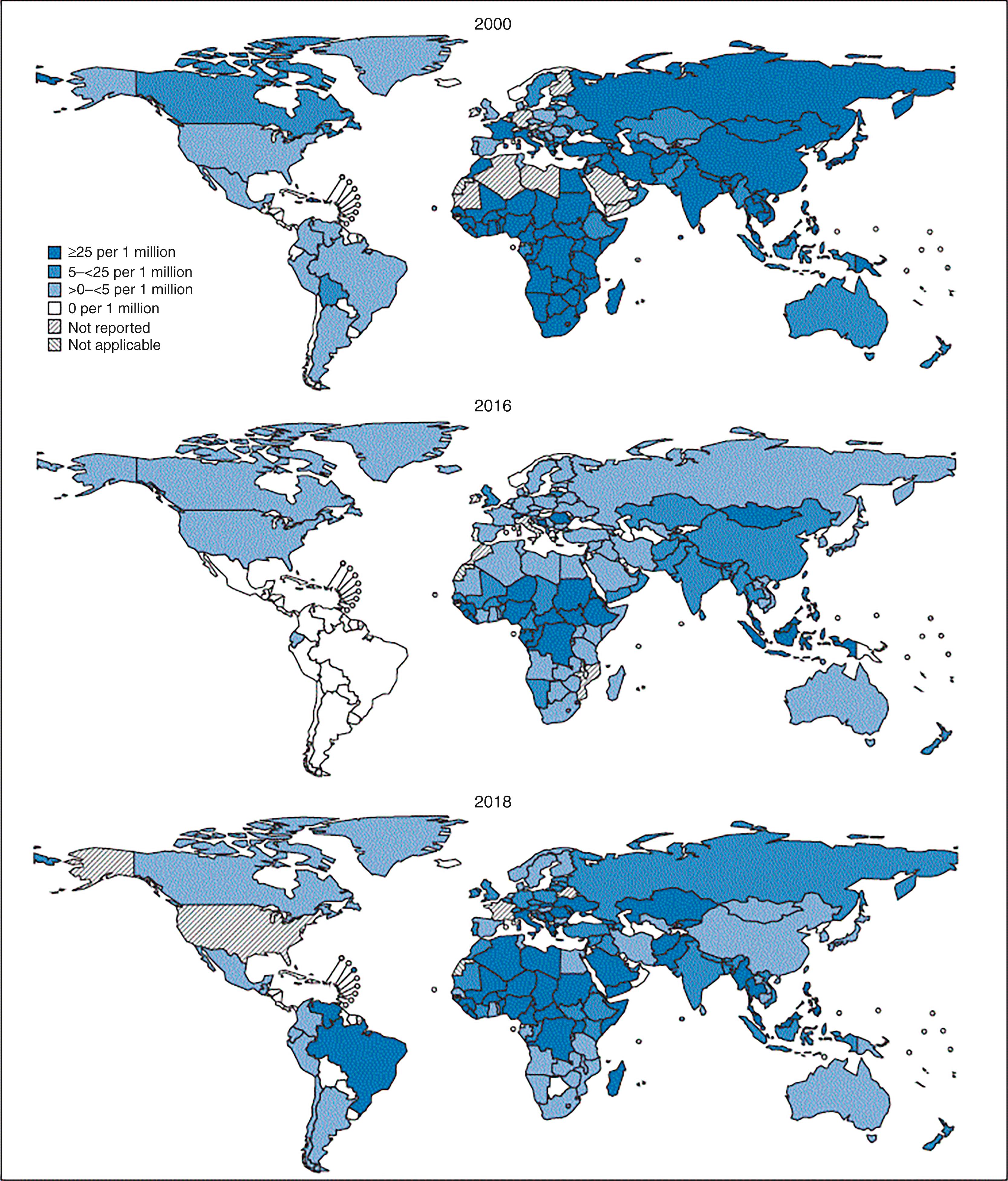
In 2018 measles case counts increased by 167% compared with 2016. An increase in the incidence of measles was reported in five WHO regions during 2016–2018 ( Fig. 227.3 ). In 2018 the incidence of measles was >600 per million in 5 (3%) of 179 reporting countries (Democratic Republic of the Congo [DRC], Liberia, Madagascar, Somalia, and Ukraine) and accounted for 45% (157,239 cases) of all reported cases worldwide. In 2019–2020, the DRC experienced the world’s largest measles outbreak, resulting in the deaths of >7000 children. In Samoa, the measles outbreak was associated with a population attack rate of approximately 3%. Major outbreaks in Venezuela (2018) and Brazil (2019) have resulted in reestablishment of endemic measles transmission in the AMR region. Large measles outbreaks occurred in the European Union in 2017–2019 primarily due to immunity gaps and consequences of undervaccination in most countries. In 2018 transmission was reestablished in countries that had previously achieved measles elimination, including Albania, Czech Republic, Greece, and the UK. ,
In tropical settings, measles outbreaks have a variable seasonal pattern and may be highly irregular, especially in low-income countries with high fertility rates. Infants and young children are most susceptible to measles, especially in densely crowded, urban, low-income settings with suboptimal vaccine coverage. , However, in high-income countries with increasing vaccination coverage and levels of population immunity, large outbreaks have shifted to adolescents and young adults, especially in the European and Western Pacific regions. , , Measles outbreaks in Europe pose a risk to unvaccinated, US residents returning from international travel with subsequent community spread. ,
The global resurgence of measles varies from country to country and is caused by many factors, including weak or conflict-affected health systems, inadequate surveillance of immunization campaigns, financial barriers, inadequate country ownership, and increasing vaccine hesitancy. , , , , In 2018 global coverage for one dose of MCV was 86%, while coverage for 2 doses of MCV was only 69%. In post-elimination countries, waning immunity may also be responsible for measles outbreaks involving adults who had previously received 2 doses of MCV and were protected during prior outbreaks. Vaccine-derived immunity may wane over a decade due to absence of boosting by regular wild-type MV following interruption of endemic circulation. The role of waning immunity in the changing epidemiology of measles needs further investigation.
To achieve measles elimination goals, financial investments and political will are needed to strengthen routine immunization systems, increase 2-dose MCV coverage and supplemental immunization activities, close immunity gaps, and improve case-based surveillance. , , Innovative, affordable point-of-care measles diagnostic tests and alternative vaccine delivery strategies, including microneedle patches, are being developed for low-income countries. The microneedle patches could be administered in low-income countries by community-based healthcare workers with minimal training.
After an 8- to 12-day incubation period, a 2- to 4-day prodromal illness characterized by fever and usually one of the “three Cs” develops including cough, conjunctivitis (nonpurulent), and coryza. , During the 2- to 4-day prodrome, a pathognomonic enanthem called Koplik spots , which are bluish white, 1-mm lesions on a red base, may be visible on the buccal mucosa. Koplik spots initially are discrete and appear anywhere on the buccal mucosa, including the hard and soft palate, but they characteristically are located opposite the lower premolars. Lesions eventually coalesce and spread rapidly throughout the buccal mucosa. After the enanthem has been present for 12–72 hours, it starts to slough with the appearance of the rash.
The rash usually begins at the peak of the respiratory symptoms, typically about 14 days after exposure and 2–3 days after appearance of the Koplik spots. The rash is erythematous and initially maculopapular, starts on the forehead or posterior occipital area, and spreads within 3 days to the trunk and extremities ( Fig. 227.4 ). The rash is more likely to be confluent over the head and upper extremities than over the trunk and lower extremities ( Fig. 227.5 ). The rash usually lasts for 3–5 days and fades from red to copper to brown in a cephalocaudal direction; fine desquamation follows. In children with vaccine-induced immunity or vaccine-modified measles, rash may be mild without cough, coryza, or conjunctivitis. In malnourished children, a deeply pigmented rash with more extensive desquamation may occur. The rash can become mildly hemorrhagic, and petechiae or purpura occasionally occurs.
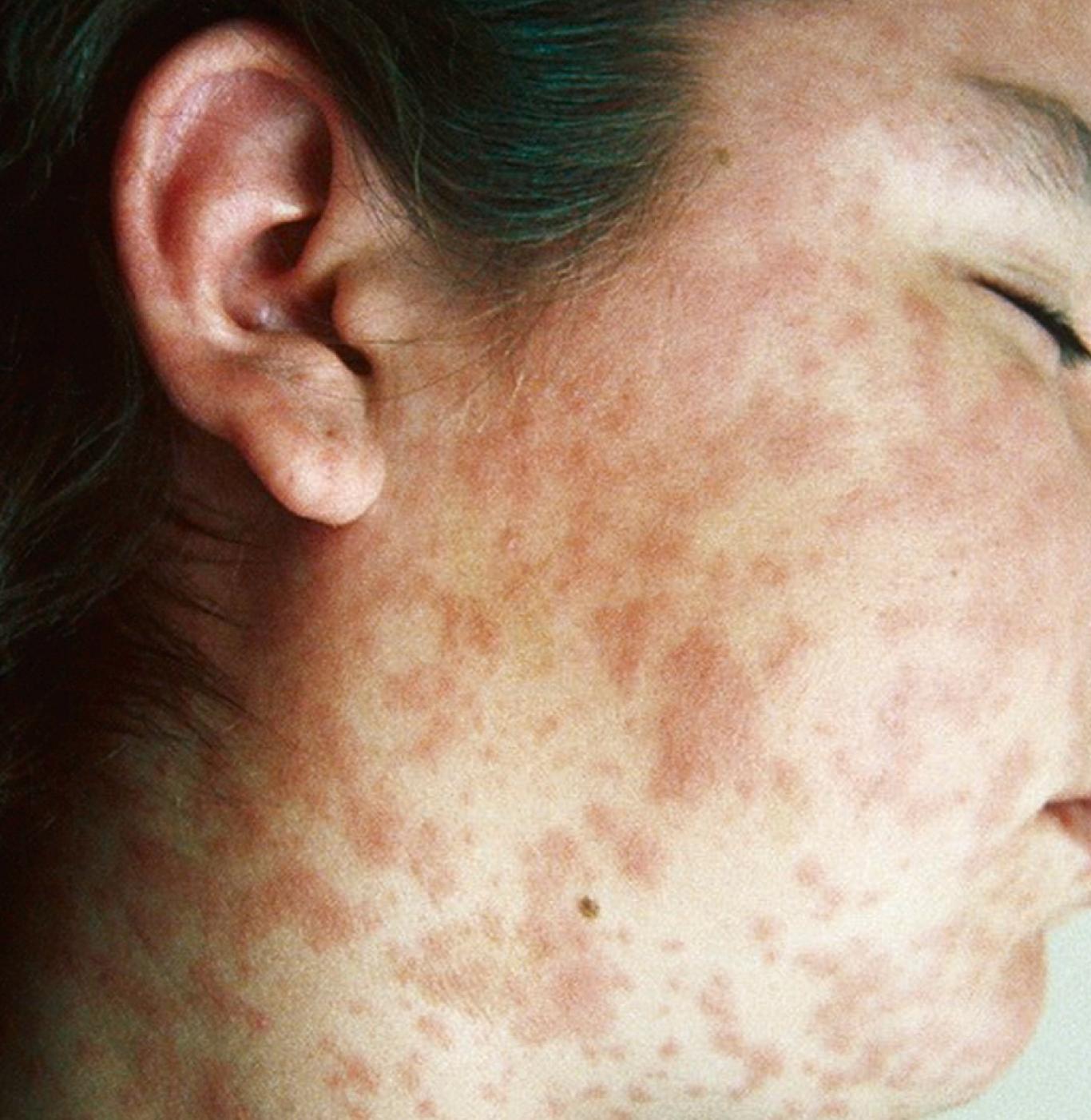
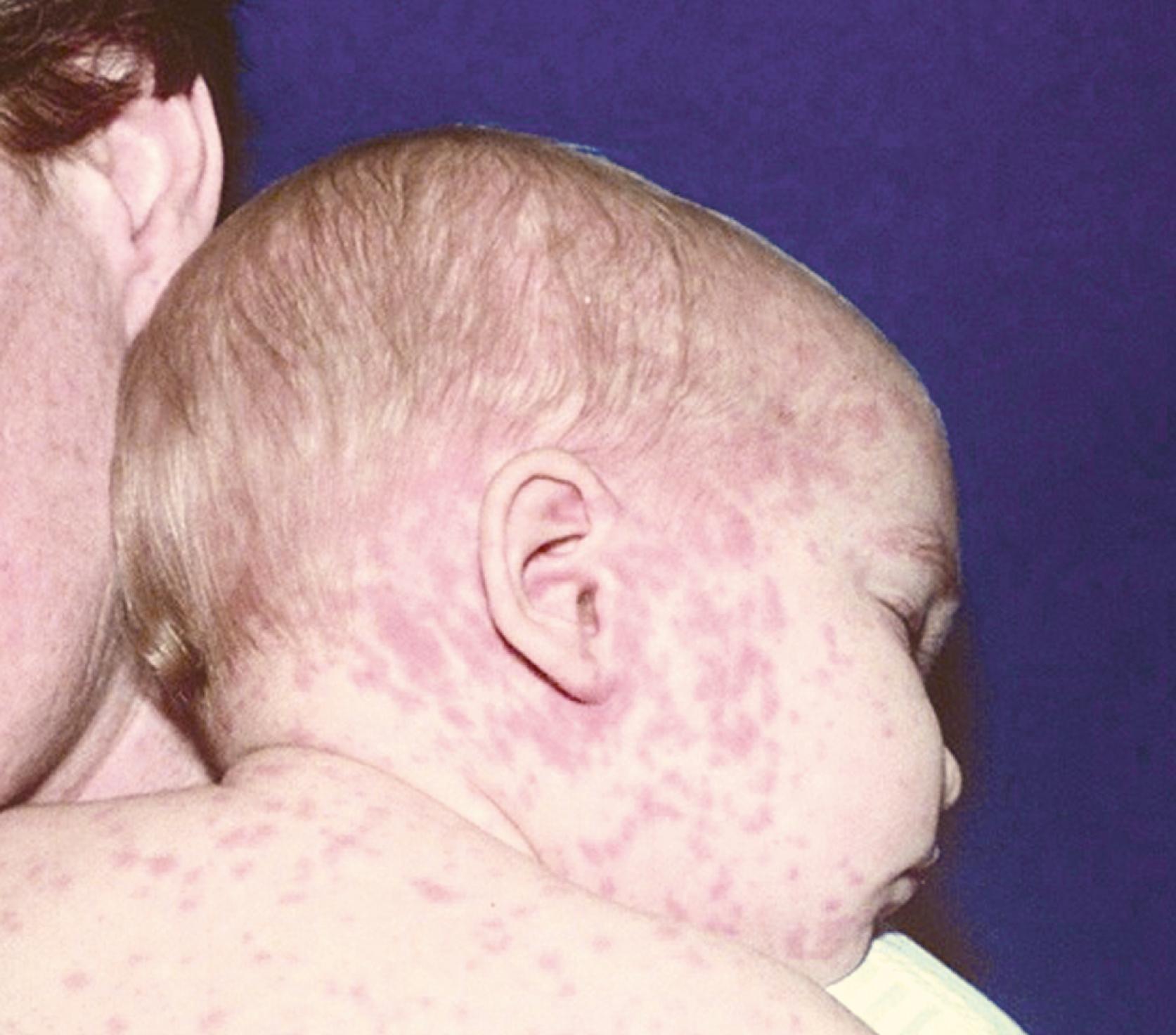
Symptoms during the exanthematous phase include high fever (up to 40°C) that peaks 2–3 days after rash onset, continued respiratory symptoms, nonpurulent conjunctivitis, pharyngitis, and mild generalized lymphadenopathy. After the fever peaks, it usually resolves rapidly, followed by clinical recovery in uncomplicated measles. Persistent fever is a possible indication of secondary bacterial infection. Occasionally, diarrhea, vomiting, and abdominal pain may occur. In low-income countries, diarrhea is a major complication of measles.
Transient anicteric hepatitis has been reported in young adults with measles. An atypical form of measles occurred in people infected with wild-type MV who had previously received formalin-inactivated (i.e., killed) measles vaccine in the US between 1963–1968. The rash of atypical measles was nonconfluent with caudocephalad progression, which sometimes had a petechial and vesicular component. Pneumonia, hilar adenopathy, pleural effusion, and prolonged recovery (≥2 weeks) were common.
Severe hemorrhagic measles (i.e., black measles) is most common among infants in developing countries and is characterized by a sudden onset of high fever accompanied by seizures or altered mental status. Other manifestations include pneumonia; an extensive hemorrhagic exanthem and enanthem; bleeding from the mouth, nose, and gastrointestinal tract; and disseminated intravascular coagulation. The prognosis is poor, and fatalities are common.
Modified measles is an attenuated form of infection that can occur in people who have received immuneglobulin after exposure to measles. The clinical manifestations of modified measles are milder than those of typical infection, and the incubation period is prolonged (14–20 days). Complications are rare.
Laboratory abnormalities include leukopenia and lymphopenia. The diagnosis of measles is confirmed by serology, virus isolation, or detection of measles virus RNA by reverse transcriptase polymerase chain reaction (RT-PCR) on clinical samples. , , , Serology (often by enzyme immunoassay) is the most widely used method to confirm the diagnosis. A presumptive diagnosis can be made by detection of MV-specific IgM antibody on a single serum sample. MV-specific IgM has a sensitivity of 83%–89% and specificity of 95%–99%. A fourfold or greater rise in the IgG antibody titer from paired acute and convalescent sera obtained at least 10 days apart also indicates recent infection.
MV-specific IgM antibody usually is detectable 12 days after rash onset, peaks within 1–3 weeks after rash onset, and persists for 6–8 weeks in unimmunized people. , , Timing of specimen collection, vaccination history, and the type of assay can influence the sensitivity of measles IgM assays. False-negative IgM results occur in approximately 20%–25% of patients during the first 72 hours after rash onset; repeat testing is recommended if the generalized rash persists for >72 hours. , , IgM antibody can be present transiently or absent in infected people who previously received 1 or 2 doses of measles vaccine. MV-specific IgM antibody testing therefore is not recommended in vaccinated people.
In the US and other countries with high MMR vaccine coverage, serology and RT-PCR assay are recommended to confirm the diagnosis. Specimens from the throat or nasopharynx are preferred for RT-PCR assay, and sampling more than one site may increase detection. RT-PCR can detect MV before measles-specific IgM antibodies are detectable, and has a sensitivity of 94% and specificity of 99%. , Suspected measles cases must be reported immediately to the local or state health department. In the US, specimens for diagnostic testing is submitted to state public health laboratories or the CDC. MV sometimes can be isolated from blood, urine, and secretions of the throat and nasopharynx, but isolation is difficult and not recommended routinely. MV genotyping may be beneficial for differentiating wild-type from vaccine virus strains and in the performance of molecular epidemiologic studies to identify sources and transmission patterns of imported disease.
In countries with a low incidence of measles, clinical diagnosis may be difficult for physicians who are unfamiliar with the disease; in addition, the positive predictive value is low for the CDC recommended measles case definition, warranting the need for laboratory confirmation. , The differential diagnosis of measles includes rubella, other viral illness (such as enterovirus, human herpes virus 6, infection, adenovirus, Epstein-Barr virus, parvovirus B19, dengue), and streptococcal or staphylococcal infections. , Drug hypersensitivity, Rocky Mountain spotted fever, leptospirosis, and Kawasaki disease also can be confused with measles.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here