Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The role of the cardiac surgeon in the catheterization laboratory has traditionally been surgical backup for the interventional cardiologist. Such service is losing necessity as the development and mastery of new interventional procedures progress and correlate with the advancement of medical device technology.
New minimally invasive interventional procedures are founded upon the historical open surgical approach and take a defined pathway from conception to clinical acceptance. This pathway requires collaboration among surgeons, interventionalists, medical device manufacturers, and regulatory bodies such as the U.S. Food and Drug Administration.
The heart team model, with face-to-face communication between surgeon and cardiologist, should be considered the standard of care in all cardiac centers for approaching complex coronary lesions and structural heart defects.
Because the future of interventional cardiology and cardiac surgery will require close collaboration between surgeons and cardiologists, a clinical training pathway that incorporates certain fundamental principles and skills from both surgery and cardiology training programs will be necessary.
The role of the cardiac surgeon is first to do surgery with respect to invasive techniques involving structures of the heart. In regard to interventional cardiologists and in the catheterization (cath) lab, the cardiac surgeon can be broadly described in three categories. The first and most historical undertaking is providing backup for the interventional cardiologist in case of an untoward complication or surgical emergency. This role has lost relevancy with the advent and subsequent mastery of new procedures. Formal surgical standby for routine percutaneous coronary intervention (PCI), in which a surgeon is preemptively notified and available, is no longer required or routine because complications that require surgical intervention have become quite uncommon, reaching 0.56%, in established PCI centers. However, current published guidelines for elective PCI still recommend the availability of an experienced surgical team that can be activated quickly in the event of an emergency in the cath lab.
The second role is a hybrid between a surgeon and an interventionalist. The hybrid cardiac surgeon takes an active role in interventional procedures and, in some cases, has formal cross-training in interventional cardiology. Hybrid cardiac surgeons serve an important function in today’s more complex cardiac interventional procedures such as transcatheter aortic valve replacement (TAVR), percutaneous mitral valvuloplasty, perivalvular leak intervention, percutaneous mitral valve repair, transcatheter mitral valve replacement (TMVR), and combined coronary artery bypass grafting (CABG)/PCI or valve/PCI procedures. With the heart team model becoming widely adopted, hybrid surgeons will help to shape the cath lab of the future. As complex surgical procedures are combined with a catheter-based approach, there is a need for skill sets from both a surgeon and interventionalist simultaneously at the interventional table.
Lastly, the cardiac surgeon serves as an innovation consultant. The cardiovascular surgeon’s intraoperative experience and technical development are deeply rooted in the surgical correction of structural heart disease. From years of performing open-heart procedures, cardiac surgeons gain mastery and develop unique perspectives on the three-dimensional (3D) anatomy of the heart and great vessels. The cardiac surgeon continues to contribute valuable insight to the development and refinement of new techniques and procedures, which will ultimately become the standard of care for the cardiovascular interventionalist of tomorrow.
This chapter explores the roles and close collaboration between cardiac surgeons and interventional cardiologists. As the two fields merge, respective knowledge is combined for innovation to advance science of complex cardiovascular diseases and to create unique plans for patients from a repertoire of therapeutic options that reduce risk and provide better outcomes.
During the early years of percutaneous transluminal coronary angioplasty (PTCA) and PCI, surgeons were, by necessity, to remain in house on surgical standby for urgent consultations and surgical emergencies that resulted from early interventional procedures. Fraught with technical pitfalls and clinical unknowns, early complications in the cath lab required an emergent trip to the operating room (OR), often with a patient in extreme cardiogenic shock. These PCI complications are life threatening, with hemodynamic instability and associated prolonged periods of ischemia. As a result, indications for emergency CABG after failed PCI have become well established. The most common indications include abrupt vessel closure, dissection, incomplete revascularization, coronary perforation, and unsuccessful dilation or PCI, in addition to other miscellaneous clinical scenarios. Such emergencies require the availability of a cardiac surgery team experienced in emergency aortocoronary operations and on-site cardiac surgical backup to provide all aspects of readily available cardiac surgical support.
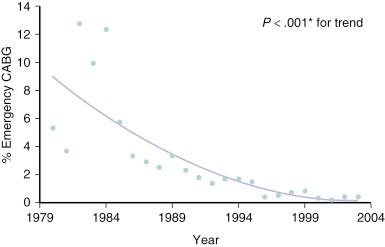
As angiography, PTCA, and surgical techniques improved and medical equipment became more advanced, surgeons were no longer required to remain in such close proximity to the cath lab. This was particularly true after introduction of the Gianturco–Roubin stent and its rapid adoption as a “bailout” device by the interventional cardiology community. Almost uniformly, and for decades, active PCI centers have enjoyed a steady reduction in the percentage of cases that require emergency CABG after failed PCI ( Fig. 33.1 ). However, despite near universal trends that demonstrate decreased morbidity and mortality from PCI, the consequences of complications remain potentially catastrophic. Thus it is still common practice and is recommended by the American College of Cardiology (ACC)/American Heart Association (AHA)/Society of Cardiovascular and Angiography and Interventions (SCAI) practice guidelines that the majority of elective PCI be performed at centers with an active open heart surgery program and that certain interventional decisions be discussed with a cardiac surgeon before proceeding with PCI.
In the event of emergent need for aortocoronary bypass, outcomes have been shown to be better in centers with an open heart surgery program ( Table 33.1 ). According to current guidelines, on-site surgical backup for elective PCI at centers that have acceptable annual volume of at least 75 procedures or more should provide emergency hemodynamic support and expeditious revascularization to manage complications that cannot be addressed in the cath lab.
| Patients (%) | |||||
|---|---|---|---|---|---|
| Outcomes | Without On-Site CABG | With On-Site CABG | Unadjusted P Value | Adjusted OR (95% CI) a | Adjusted P Value |
| All PCIs | |||||
| No. of patients | 8168 | 617,686 | 621,530 b | ||
| Mortality | 492 (6) | 20,393 (3.3) | < .001 | 1.29 (1.14–1.47) | < .001 |
| CABG | 160 (2) | 8321 (1.4) | < .001 | 1.05 (0.87–1.27) | .59 |
| Primary/rescue PCI | |||||
| No. of patients | 1795 | 34,537 | 36,235 b | ||
| Mortality | 202 (11.3) | 4209 (12.2) | 0.24 | 0.93 (0.80–1.08) | .34 |
| CABG | 82 (4.6) | 1772 (5.1) | 0.29 | 0.95 (0.73–1.22) | .67 |
| Nonprimary/rescue PCI | |||||
| No. of patients | 6373 | 583,149 | 585,295 b | ||
| Mortality | 290 (4.6) | 16,184 (2.8) | < .001 | 1.38 (1.14–1.67) | .001 |
| CABG | 78 (1.2) | 6549 (1.1) | .45 | 0.92 (0.73–1.17) | .52 |
a Adjusted for age, gender, race, year, Charison comorbidity score, primary diagnosis of acute myocardial infarction, acuity, multivessel PCI, and stent use.
b Adjusted models excluded 4324 patients (0.7%) missing on patient covariates; 45 (4 among primary/rescue PCI) among patients without on-site CABG and 4279 (93 among primary/rescue PCI) among patients with on-site CABG.
The ACC/AHA/SCAI 2014 Expert Consensus Document provides recommendations for PCI without on-site surgery that are a composite of recommendations that include requirements for both facilities and personnel. The issues of cardiac surgical backup and the level of committed resources needed are frequently revisited, and most centers use the first-available OR model to remain efficient and maximize resources. Some centers rely on off-site surgical backup, but this is the exception rather than the rule because such practice exposes the patient to a finite but potentially fatal risk and is deemed unnecessary in most areas. Recommendations for primary PCI for ST-elevation myocardial infarction (STEMI) without on-site cardiac surgery require: (1) a highly experienced physician and catheterization team, (2) a proven plan for rapid transport to a nearby hospital with appropriate support and resources, and (3) that it be executed in a timely fashion. The apparent success of centers that perform PCI without on-site cardiac surgery programs depends on operator experience and volume and can also be attributed to fairly strict patient selection and criteria defined by Wharton et al.
It should be noted that, in low-volume centers (<200 PCIs per year), adverse event estimates lose stability and may therefore be unreliable. The 2013 PCI competency document identified a signal that suggested that these low-volume centers were associated with worse outcomes. Thus laboratories that perform fewer than 200 procedures annually and that are not serving isolated or underserved populations should be questioned. Multiple low-volume and partial-service PCI centers within a geographic area diffuse the PCI expertise, increase costs for the overall health care system, and have not been shown to improve access. If the transfer time is 30 minutes or less, it is reasonable to assume that transfer to the nearest PCI center will provide reperfusion as rapidly as if it were available at the first hospital. For this reason, development of PCI facilities within a 30-minute emergency transfer time to an established facility is strongly discouraged.
Many procedures that were historically performed by surgeons as open surgeries are currently routinely performed percutaneously or with a cutdown in the cath lab or interventional suite such as hybrid OR by interventionalists of various disciplines, including cardiovascular surgeons, cardiologists, vascular surgeons, and interventional radiologists. These procedures are growing in number and variety and include pacemaker insertion, mechanical ablation of atrial fibrillation (Maze procedure), closure of patent foramen ovale, and repair of atrial septal defect, in addition to endovascular alternatives to open carotid endarterectomy, repair and resection of abdominal and thoracic aortic aneurysms, and peripheral artery bypass. The most recent surgical procedures performed with a small cutdown or percutaneous interventional approach include TAVR, TMVR, and mitral valvuloplasty or clipping. Although not as prevalent as vascular surgeons performing many of their traditionally open surgeries percutaneously in the interventional suite (i.e., endovascular abdominal aneurysm repair [EVAR]), analogous procedures such as thoracic endovascular aortic repair (TEVAR) are performed more and more in the hybrid OR by cardiac surgeons. In addition, a few hybrid cardiac surgeons have acquired formal coronary angiography and PCI training and possess skills sufficient for hospital privileging.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here