Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
We extend our deepest thanks to the original author of this chapter—Dr. Aldo V. Londino. Dr. Londino, until the time of his passing in 2000, was the sole pediatric rheumatologist in western Pennsylvania and was widely recognized for his superb teaching and clinical skills. We are also grateful for the contributions of the previous authors, Drs. Andrew H. Urbach, Sara C. McIntire and Paul Rosen; as well as photograph contributors: Bernard Cohen, John Zitelli, A’Delbert Bowen, Basil Zitelli, J. Carlton Gartner Jr., J. Jeffrey Malatack, Joseph McGuire, Holly Davis, Kathryn Torok, Virginia Steen, Joseph Warnicki, Albert Biglan, Cora Lennox, Sang Park, Chester Oddis, Amisha Shah, David Witte, and Susan Gelnett.
The rheumatic diseases of childhood are a heterogeneous group of disorders usually manifested by signs and symptoms of inflammation. Although significant progress has been made in the understanding of the pathophysiology of these disorders, much remains to be discovered about their etiologies. Furthermore, despite available laboratory markers, the cornerstones of diagnosis remain the history and physical examination. Knowledge of the natural history of these disorders is also helpful for diagnosis and management.
Most of the common rheumatic diseases that occur during childhood are classified as inflammatory arthritis, connective tissue disorders, or vasculitides, although overlap does occur. Noninflammatory disorders that cause musculoskeletal pain include joint hypermobility syndromes and pain amplification syndromes. This chapter illustrates the more distinctive clinical features of these unique disorders.
A meticulous rheumatologic history is the foundation of accurate diagnosis ( Box 7.1 ). Careful attention should be given to the exact location of pain reported by the patient. Muscle, bone, and tendon or ligament insertion pain (enthesitis) may be interpreted as joint pain, unless the clinician asks specifically for the parent or child to describe the symptoms. Often it is helpful for the clinician to ask the child to point with one finger to the site of maximal discomfort.
Musculoskeletal complaints
Joint swelling with warmth and/or erythema
Morning stiffness lasting >20 min that improves with activity
Gelling phenomenon after prolonged inactivity
Decreased muscle strength or endurance
Constitutional symptoms
Prolonged or recurrent fevers (periodic or episodic)
Fatigue without clear triggers
Weight loss, anorexia
Linear growth disturbance
Ocular symptoms
Eye redness and/or pain
Vision changes
Reduced ocular movements or proptosis
Cardiopulmonary symptoms
Dyspnea (resting or exertional)
Hemoptysis
Chest pain
Gastrointestinal symptoms
Dysphagia (solids or liquids)
Early satiety or reflux
Melena or hematochezia
Abdominal pain
Neurologic symptoms
Headache
Seizure
Altered mental status
Focal weakness
Cutaneous and mucous membrane symptoms—See Box 7.2
Psychosocial history
Family stressors
Personal stressors (school, development, etc.)
Altered sleep patterns
Depression or anxiety
Family history
Autoimmune disorders
Autoinflammatory disorders
Chronic pain and psychiatric disorders
The patient’s age and gender serve as initial guides to a possible etiology. The clinician must then try to discern whether musculoskeletal symptoms are inflammatory or mechanical. Pain that involves swelling, morning stiffness, warmth, redness, and improvement with movement is often indicative of inflammation. Pain that is worse at the end of the day, worse with activity, and lacking persistent swelling is usually more mechanical in nature. Pain that is constant and unremitting or worsened by light touch is characteristic of nerve pain.
A history of prior illnesses, medications, immunizations, trauma, bites, and the acuteness of symptoms can be a clue to diagnosis. Joint pain (arthralgia) is a common symptom of childhood. However, the symptoms associated with inflammatory joint pain (arthritis) are uncommon in the pediatric population. In children with arthritis, the duration and pattern of the symptoms can be telling. Acute migratory arthritis affecting large and small joints is seen in acute rheumatic fever (ARF). Arthritis resolving within a few weeks is consistent with a reactive arthritis (i.e., Streptococcus, Epstein-Barr virus, parvovirus B19). An additive arthritis persisting for more than 6 weeks is consistent with a chronic form of arthritis (i.e., juvenile idiopathic arthritis [JIA]). The chronic arthritides cause indolent and persistent joint changes. Joint stiffness, or gel phenomenon, can be seen not only in the morning but also after a child has napped or been immobile in a vehicle. As the day progresses, the child with chronic arthritis may become more limber and may even appear normal.
Differences in the quality and duration of arthritis exist among the various rheumatoid diseases. The arthritis in patients with systemic lupus erythematosus (SLE) may feature less swelling with more intense pain, whereas the arthritis of children with JIA is characterized by more stiffness and swelling and less pain. The joint pain of the patient with SLE may be intermittent in nature. The joint stiffness of JIA is usually a daily occurrence without treatment. The joints of ARF can also be distinguished from those of JIA by the presence of exquisite pain that is out of proportion to physical findings. A rapid response to nonsteroidal antiinflammatory drugs (NSAIDs) further supports a clinical impression of ARF.
A careful family history can be helpful. Children with an extensive family history of autoimmune diseases in their first-degree relatives are at slightly greater risk for developing a rheumatologic condition. Because these diseases are complex genetic traits, there is often little direct genetic linkage. Some diseases such as psoriasis, SLE, ARF, and autoimmune thyroiditis have a stronger genetic penetrance than other rheumatic diseases. Other conditions have little or no penetrance. A child with first-degree relatives with adult rheumatoid arthritis is not at increased risk for developing JIA.
A rheumatologic diagnosis requires a thorough joint examination and meticulous general physical examination with special attention to the skin, mucous membranes, nail beds, and muscles. The elucidation of joint inflammation by examination may be the only indication of a rheumatic disease. Because joints are near the surface of the body, the examiner has an excellent opportunity to obtain significant information about many diseases.
The physical examination begins with observation of the child and parents walking from the waiting area to the examination room. The physician notes the general appearance of the patient and interactions among family members. Nutritional status and an incremental graph of height and weight must be carefully documented. Certain skin and mucous membrane changes provide valuable information ( Box 7.2 ). Muscle strength can be evaluated by testing resistance capacity of individual muscle groups and grading them on a standard scale ( Table 7.1 ).
Malar rash—fixed erythematous eruption
Discoid rash—rare in childhood; often heals with atrophy and scarring
Periungual erythema
Telangiectasias
Digital pits/ulcers and/or Raynaud’s phenomenon
Alopecia and fracturing of frontal hair
Heliotrope rash—violaceous eyelid edema
Gottron papules—scaly, symmetrical, erythematous papules over MCP and PIP joints
Skin changes: thickening, contractures, calcinosis
Palpable purpura
Livedo reticularis—lacy, fishnet appearance of skin
Evanescent salmon-pink rash
Erythema nodosum—panniculitis with septal inflammation
Rheumatoid extensor nodules
Psoriasis
Onycholysis (lifting of the distal portion of the nail), nail pits
Balanitis circinata—small, shallow, painless ulcers of the glans penis and urethral meatus
Keratoderma blennorrhagicum—clear vesicles on erythematous bases that progress to a papulosquamous eruption, often on palms and soles
| Muscle Grade | Description |
|---|---|
| 5 | Complete ROM against gravity with full resistance |
| 4 | Complete ROM against gravity with some resistance |
| 3 | Complete ROM against gravity |
| 2 | Complete ROM with gravity eliminated |
| 1 | Evidence of slight contractility; no joint motion |
| 0 | No evidence of contractility |
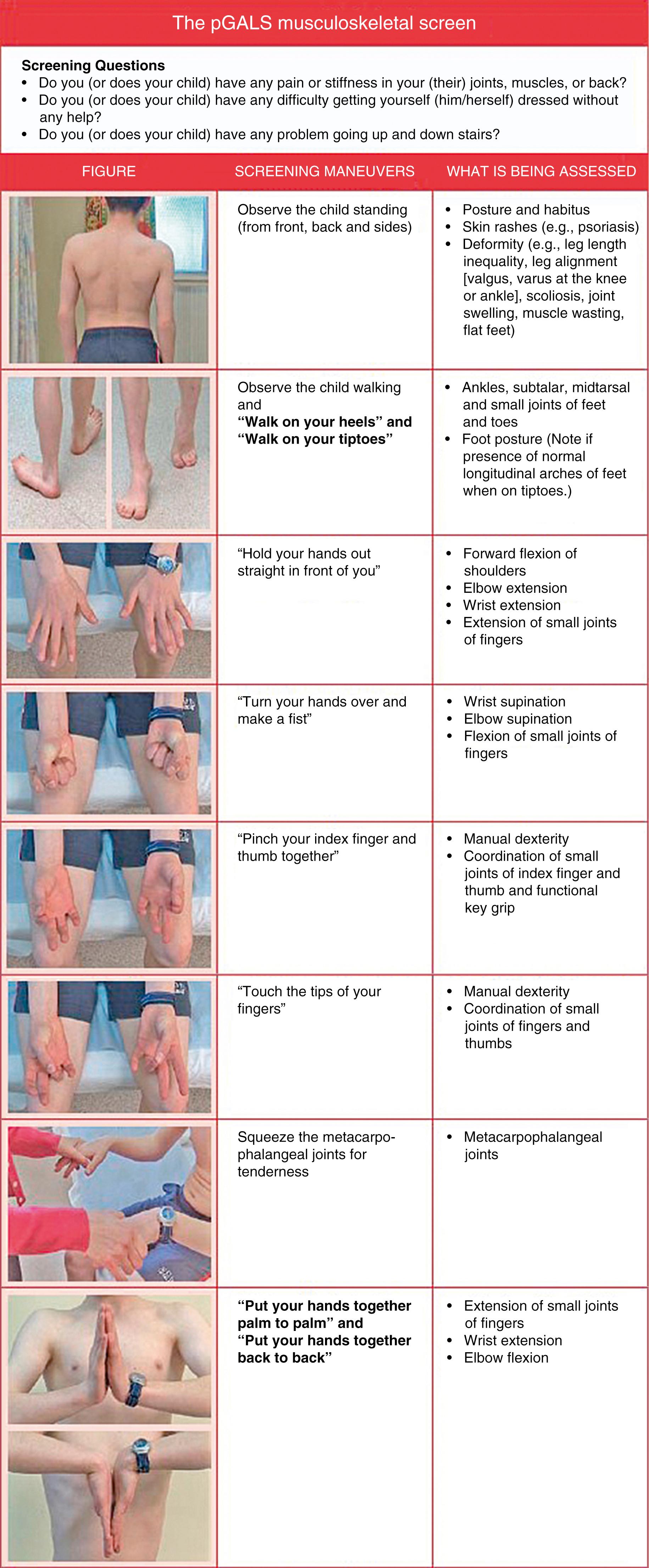
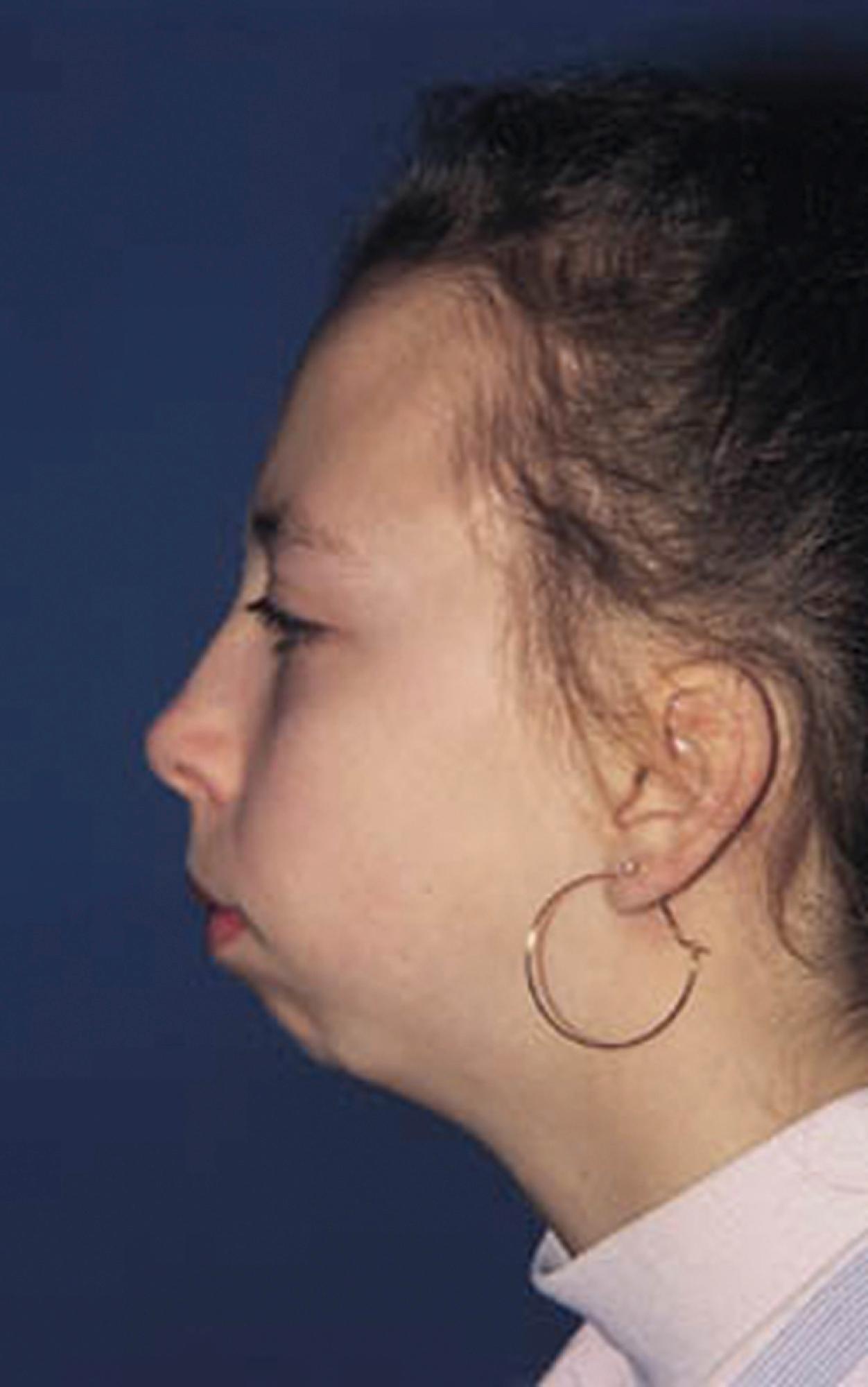
The hallmark of a good physical examination of the musculoskeletal system is a careful examination of the joints, consisting of inspection, palpation, and measurement of each joint’s range of motion (ROM). A validated, evidence-based tool to systematically perform a screening musculoskeletal exam in children suited for general practitioners has been developed and is known as pGALS (pediatric G ait, A rms, L egs, S pine) ( Fig. 7.1 ). The instrument is sensitive for the detection of musculoskeletal abnormality and with routine use can be completed in a few minutes. If concerning findings are detected with pGALS, more focused physical exam maneuvers should be completed to better define the abnormality. Large effusions are easily felt and often ballotable; synovial hypertrophy may be more subtle and has a doughy, spongy, or boggy feel. Synovial outpouchings are common in children with arthritis and can resemble ganglion cysts or Baker’s cysts. Arthritis in children may be subtle and often appreciated only because of pain or decreased ROM. For example, careful observation of the temporomandibular joint (TMJ) may reveal micrognathia ( Fig. 7.2 ) or assessment of leg lengths may demonstrate a significant discrepancy; both are clues to the diagnosis of JIA.
JIA is a collection of diseases that share the cardinal feature of chronic arthritis without other known etiology. JIA is one of the most common rheumatic diseases of childhood with an incidence of 10 cases per 100,000 population per year. The prevalence of JIA is approximately 100 per 100,000 population. JIA in the United States is estimated to affect more than 300,000 children. Seven subtypes are included under the rubric of JIA: (1) oligoarthritis (persistent or extended), (2) polyarthritis (rheumatoid factor [RF] negative), (3) polyarthritis (RF positive), (4) psoriatic arthritis, (5) enthesitis-related arthritis (ERA), (6) systemic arthritis, and (7) undifferentiated arthritis ( Table 7.2 ). The JIA onset type is based on the disease presentation during the first 6 months of illness. Of note, no laboratory tests, such as a positive anti-nuclear antibody (ANA) or RF, are required to make a diagnosis of JIA.
Adapted from table in Gowdie PJ, Tse SML. Juvenile idiopathic arthritis. Pediatr Clin N Am . 2012;59:301–327.
Saurenmann RK, Rose JB, Tyrrell P, et al. Epidemiology of juvenile idiopathic arthritis in a multiethnic cohort: Ethnicity as a risk factor. Arthritis Rheum . 2007;56(6):1974–1984.
Thierry S, Fautrel B, Lemelle I, Guillemin F. Prevalence and incidence of juvenile idiopathic arthritis: A systematic review. Joint Bone Spine . 2014;81(2):112–117.
| Age of Onset | Percentage of Total Cases | Joint Symptoms | Other | |
|---|---|---|---|---|
| Oligoarthritis | Early childhood | 35%–50% | Four or less joints—typically knees, ankles, wrists | Many are ANA+ |
| Polyarthritis RF negative |
2–4 years old and 6–12 years old | 17%–25% | Five or more joints | May have C-spine, TMJ arthritis |
| Polyarthritis RF positive |
Early adolescence | 3%–5% | Five or more joints symmetric, erosive | May have rheumatoid nodules |
| Enthesitis-related | Late childhood or early adolescence | 5%–25% | Lower extremity predominant; may affect hips, sacroiliac joints | Enthesitis, HLA-B27+, axial involvement, family history of HLA-B27–associated disease |
| Psoriatic | 2–4 years old and 9–11 years old | 5%–15% | Small or large joints | Nail pits, dactylitis, psoriasis, family history of psoriasis |
| Systemic | Any age | 5%–15% | Oligoarticular or polyarticular | Fever, rash, serositis, lymphadenopathy, organomegaly |
| Undifferentiated | 10% | Fulfills criteria for more than one category or does not fulfill criteria for any category |
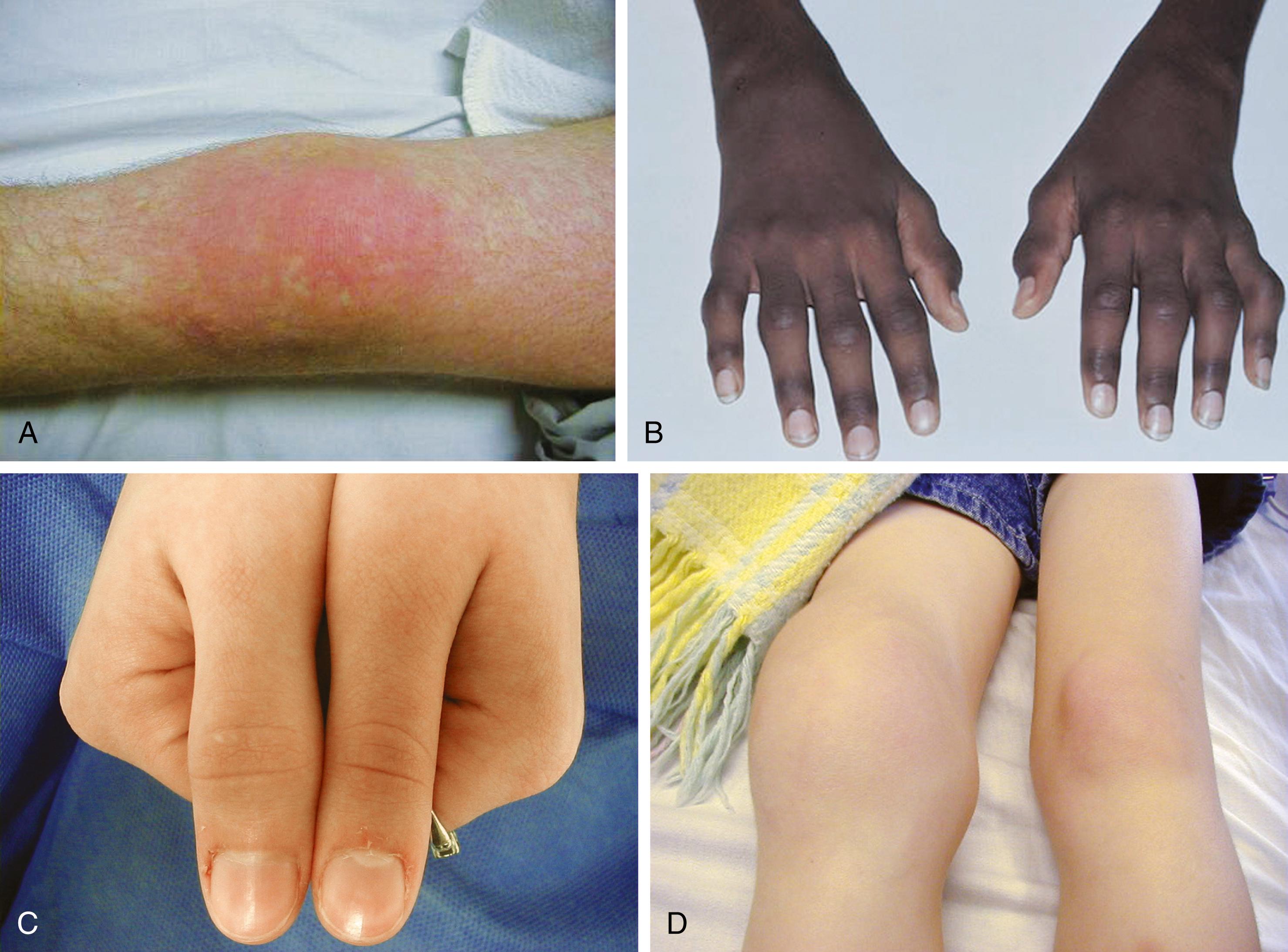
All forms of JIA feature inflammation of the synovial tissue as the key feature. Synovium is usually hypertrophied, and joint effusions may occur. On physical examination ( Fig. 7.3 ), joint swelling, loss of normal anatomical landmarks, tenderness, decreased joint mobility ( Fig. 7.4 ), warmth, erythema, and joint deformity may be noted. It is typical for the child with JIA to have more joint stiffness than pain. Symptoms often develop gradually over a period of weeks or months before evaluation. Morning stiffness is often reported and the duration of morning stiffness correlates well with the degree of inflammation in children with JIA. Immobility and weather changes may exacerbate symptoms, although they have no impact on the underlying inflammatory component of the disease. Although arthralgia alone can be the initial presentation of JIA, the diagnosis cannot be confirmed without the presence of arthritis on physical examination.
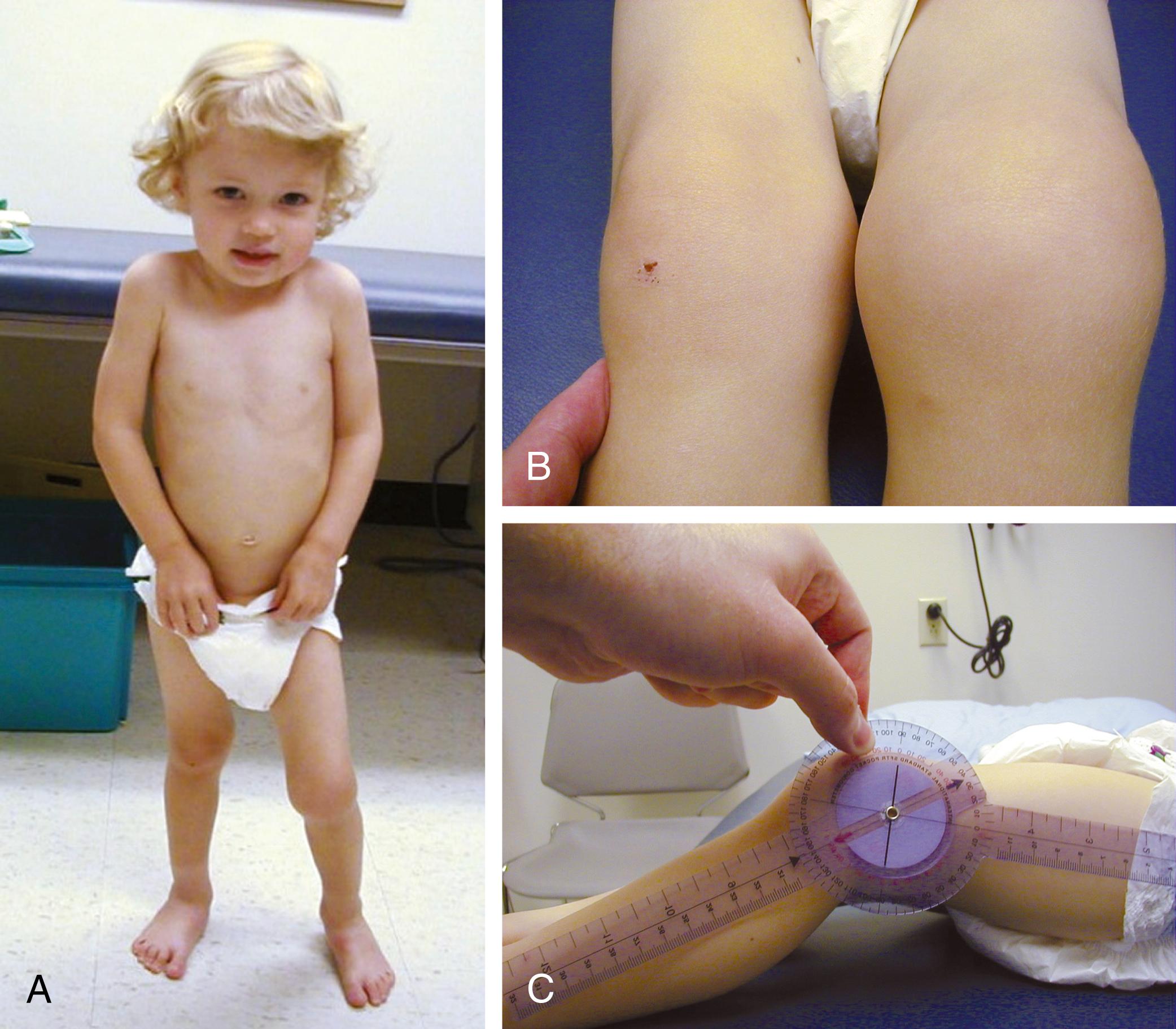
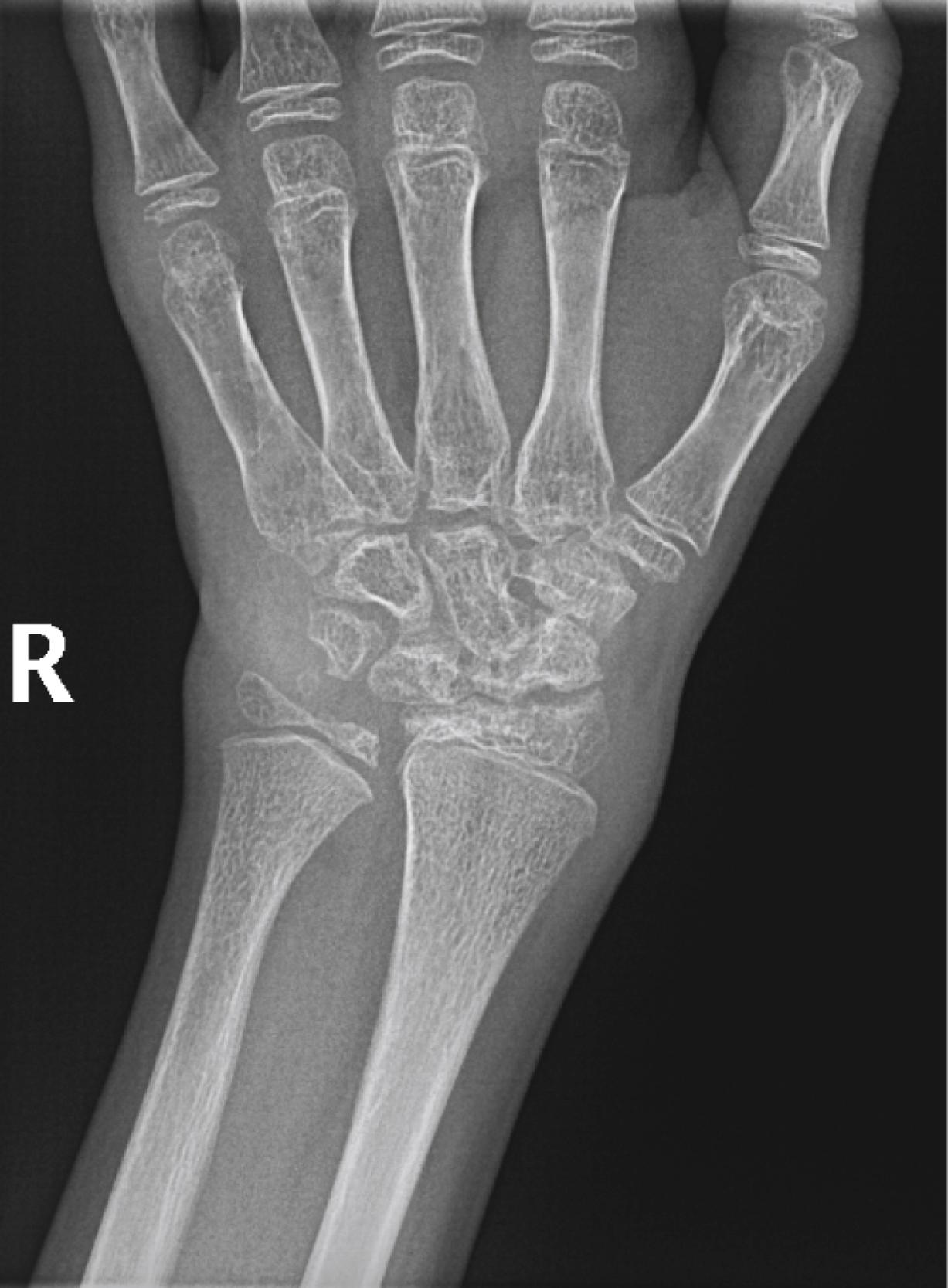
Despite objective signs of arthritis, the patient with JIA may not experience pain. When inflammation persists for a long enough period, destruction of the articular surfaces and bony structures may occur ( Fig. 7.5 ). Because of the poor regenerative properties of articular cartilage, these deformities are usually permanent. Fortunately, most cases of JIA are not associated with permanent joint deformity.
Oligoarticular JIA is defined as arthritis in one to four joints and accounts for 40% to 50% of cases of JIA. The large joints (knees, ankles, and elbows) are often asymmetrically involved. Systemic symptoms do not dominate the clinical picture. Persistent disease affects no more than four joints throughout the disease. Extended disease affects more than four joints after the first 6 months.
Polyarticular JIA (RF-negative or seronegative) accounts for approximately 20% to 25% of all children with JIA. To make the diagnosis, five or more joints must be involved in the absence of prominent systemic signs and symptoms. RF negative polyarthritis can occur as young as 1 year old, with a peak incidence at 2 years old.
The onset of polyarthritis may be insidious or acute. Children with seronegative disease generally have a better prognosis, but a subset can progress to joint destruction and flexion contractures. Any synovial joint may be involved in the inflammatory process, including the knees, wrists, elbows, and ankles, small joints of the feet, and proximal interphalangeal (PIP) and metacarpophalangeal (MCP) joints. The lumbosacral spine is usually spared.
The seropositive group is believed to be nearly identical to the adult entity of rheumatoid arthritis. Although onset of RF positive polyarthritis can occur as early as 8 years old, it usually occurs in the early teens and girls predominate. Whereas 80% of all adult patients are seropositive, only 5% of children with JIA are positive for RF. This seropositive subgroup tends to progress to destructive synovitis with prolonged chronic course.
Physical findings provide some additional clues to a diagnosis of seropositive disease. The subcutaneous nodules that occur in seropositive disease are firm, nontender nodules on the skin surface with a predilection for pressure points or extensor areas. The most common location is the elbow, but the nodules also can occur on the heels, hands, knees, ears, scapula, sacrum, and buttocks. Other features of seropositive disease may include cutaneous vasculitis, Felty syndrome (leukopenia and splenomegaly), and Sjögren syndrome (keratoconjunctivitis sicca and xerostomia with or without parotid swelling).
Psoriatic arthritis is diagnosed in a child with arthritis and psoriasis. Criteria are also met when a child with arthritis has two of the three findings: dactylitis (“sausage digit”), nail pitting (or onycholysis), or psoriasis in a first-degree relative. Whereas the forms of arthritis previously discussed have weak genetic penetrance, psoriasis and its related disorders are often found through a given family’s pedigree. A patient may often present to their physician with just dactylitis of one or multiple toes. In such a situation, in addition to a detailed joint examination, a detailed skin and nail examination should be performed ( Fig. 7.6 ). Special attention should be paid to the scalp, the umbilicus, posterior surface of the external ears, gluteal cleft, and shins for the detection of psoriasiform lesions.
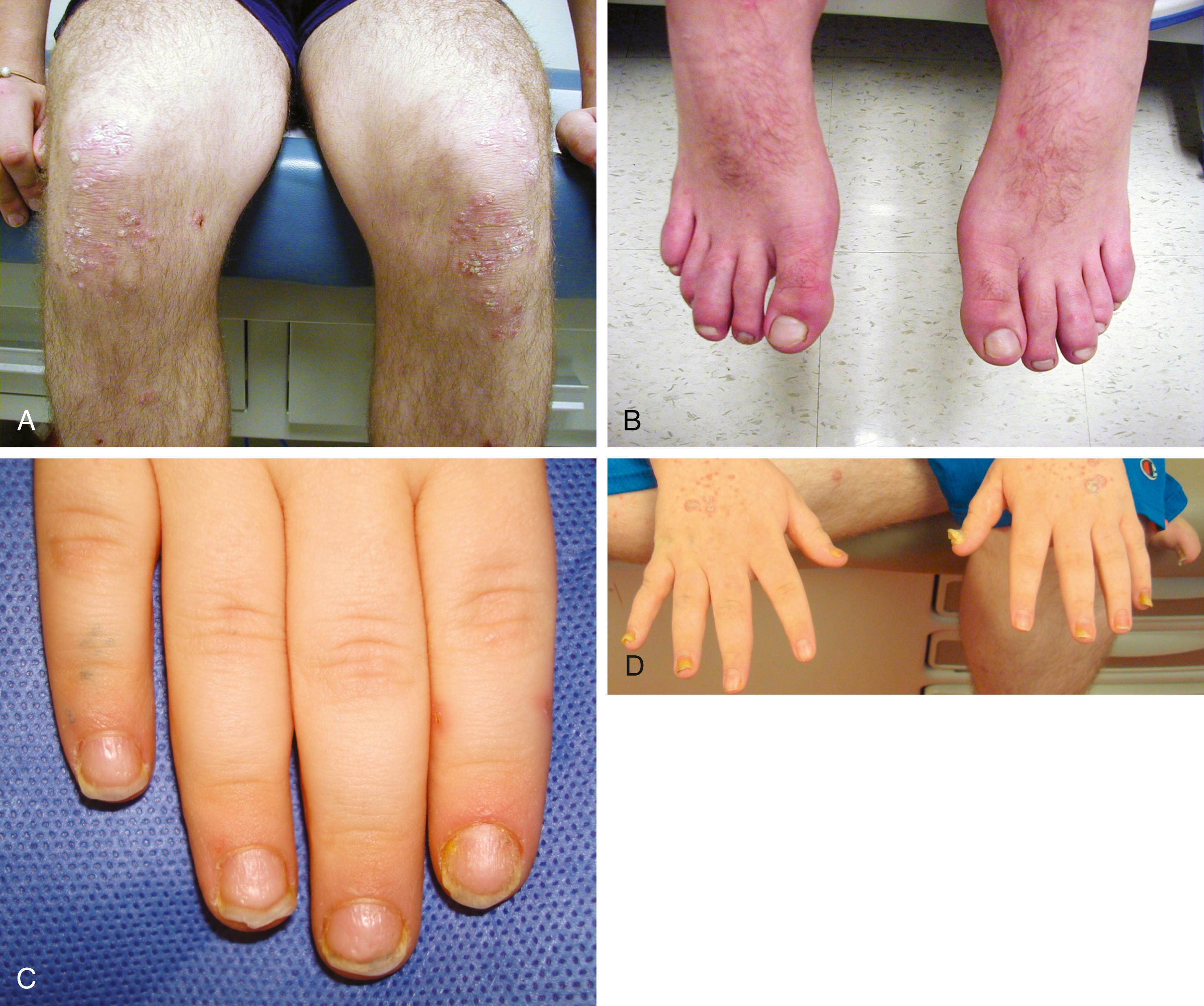
Arthritis with enthesitis features inflammation of the tendon and tendon insertion site (i.e., Achilles). The other features that distinguish this disease group include sacroiliitis, HLA-B27 positivity, male predominance, and acute uveitis (presenting with eye pain and redness). This group also has a strong family predominance with first-degree relatives with ankylosing spondylitis, inflammatory bowel disease, and reactive arthritis. A male patient older than 6 years old presenting with arthritis should raise suspicion for ERA. Younger patients present with peripheral arthritis. Axial arthritis affecting the spine often does not present until the late teen years or second decade.
Systemic juvenile idiopathic arthritis (sJIA), also known as Still’s disease, accounts for approximately 10% of all children with JIA. Fever, rash, irritability, arthritis, and visceral involvement dominate the clinical presentation. The patient’s temperature usually rises to greater than 102.2°F (39°C), and this often occurs twice daily in a double quotidian pattern. Although the late afternoon is a typical time for a temperature rise, many other patterns may occur. Chills are associated with fever, but rigors rarely occur. Other manifestations of sJIA, such as rash and joint symptoms, may wax and wane during febrile periods. A helpful clinical feature during the febrile phase is one subnormal temperature during every 24-hour period, which suggests sJIA.
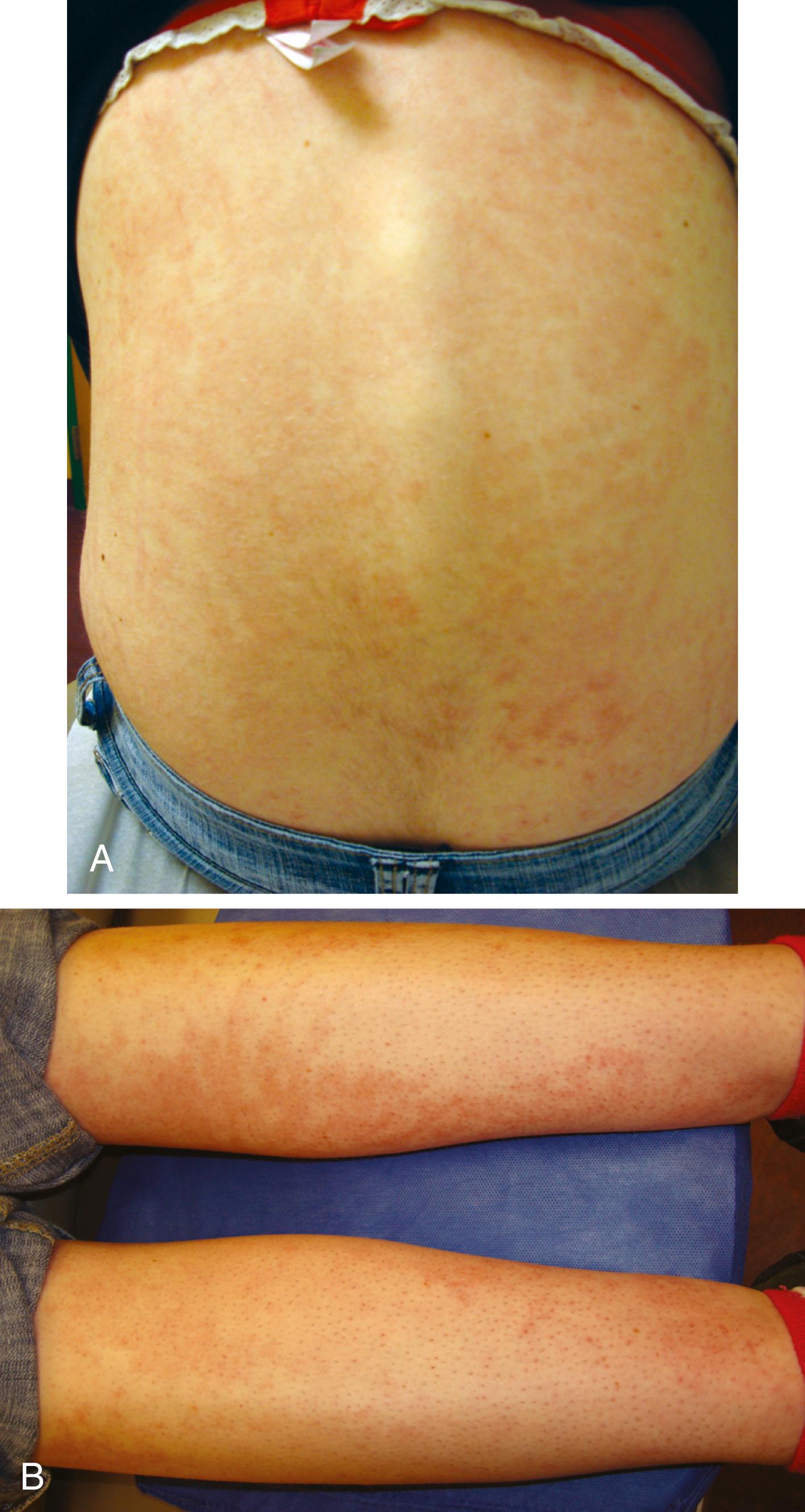
The rash of sJIA is macular, 2 to 6 mm in diameter, evanescent with fever, and salmon or red in color, with slightly irregular margins ( Fig. 7.7 ). An area of central clearing often exists. The rash usually occurs on the trunk and proximal extremities, but it may also be distal in distribution, with palms and soles affected. Although the rash generally does not produce discomfort, some older patients report pruritus. Superficial mild trauma to the skin, exposure to warmth, and emotional upset may precipitate the rash (Koebner phenomenon). Arthritis does not occur invariably at the onset of sJIA, though approximately 50% of patients with sJIA progress to a chronic, destructive inflammatory arthritis.
When fever of unknown origin is the sole initial presentation of systemic arthritis, it must remain a diagnosis of exclusion until the clinician observes other features during the physical and laboratory examination. Nonspecific arthralgia and myalgia can be prominent early, as can hepatosplenomegaly and lymphadenopathy. Serositis, pleuritis, pericarditis, hyperbilirubinemia, liver enzyme elevation, leukocytosis, and anemia are supporting clinical features.
Patients with sJIA are at risk for developing a potentially fatal disorder called macrophage activation syndrome (MAS). Patients present with a toxic appearance, fever, hepatosplenomegaly, and lymphadenopathy. If not recognized early, MAS can progress to hepatic failure, encephalopathy, and disseminated intravascular coagulation. Development of MAS is not unique to sJIA, and a diligent search for other etiologies should be undertaken ( Box 7.3 ). Laboratory testing that supports a diagnosis of MAS includes evidence of hepatitis, coagulopathy, and hyperferritinemia. In addition, the white blood cell (WBC) count, hemoglobin, and platelet counts are depressed, with a normal or low sedimentation rate associated with hypofibrinogenemia ( Table 7.3 ). Diagnosis can be aided by bone marrow aspiration, demonstrating activated macrophages engulfing surrounding cells, but this is not required for diagnosis.
Systemic JIA
Systemic lupus erythematosus
Kawasaki disease
Mixed connective tissue disease
Juvenile dermatomyositis
Vasculitis
Systemic sclerosis
Autoinflammatory disorders
| Systemic Juvenile Idiopathic Arthritis | Macrophage Activation Syndrome | |
|---|---|---|
| Fever | + (intermittent or quotidian) | + (unremitting) |
| Hepatosplenomegaly | +/− | + |
| Arthritis | + | +/− |
| Rash | + (maculopapular/evanescent) | + (petechiae/purpura) |
| Lymphadenopathy | +/− | + |
| Encephalopathy | − | +/− |
| Sedimentation rate | ↑ | Normal or ↓ |
| White blood cell (WBC) count | ↑ | ↓ |
| Hemoglobin | Normal or ↓ | ↓ |
| Platelets | ↑ | ↓ |
| Transaminases | Normal | ↑↑ |
| Ferritin | Normal or ↑ | ↑↑ |
| Fibrinogen | Normal or ↑ | ↓ |
| Prothrombin time (PT)/partial thromboplastin time (PTT) | Normal | ↑ |
| Lactate dehydrogenase | Normal or ↑ | ↑↑ |
| Triglycerides | Normal | ↑ |
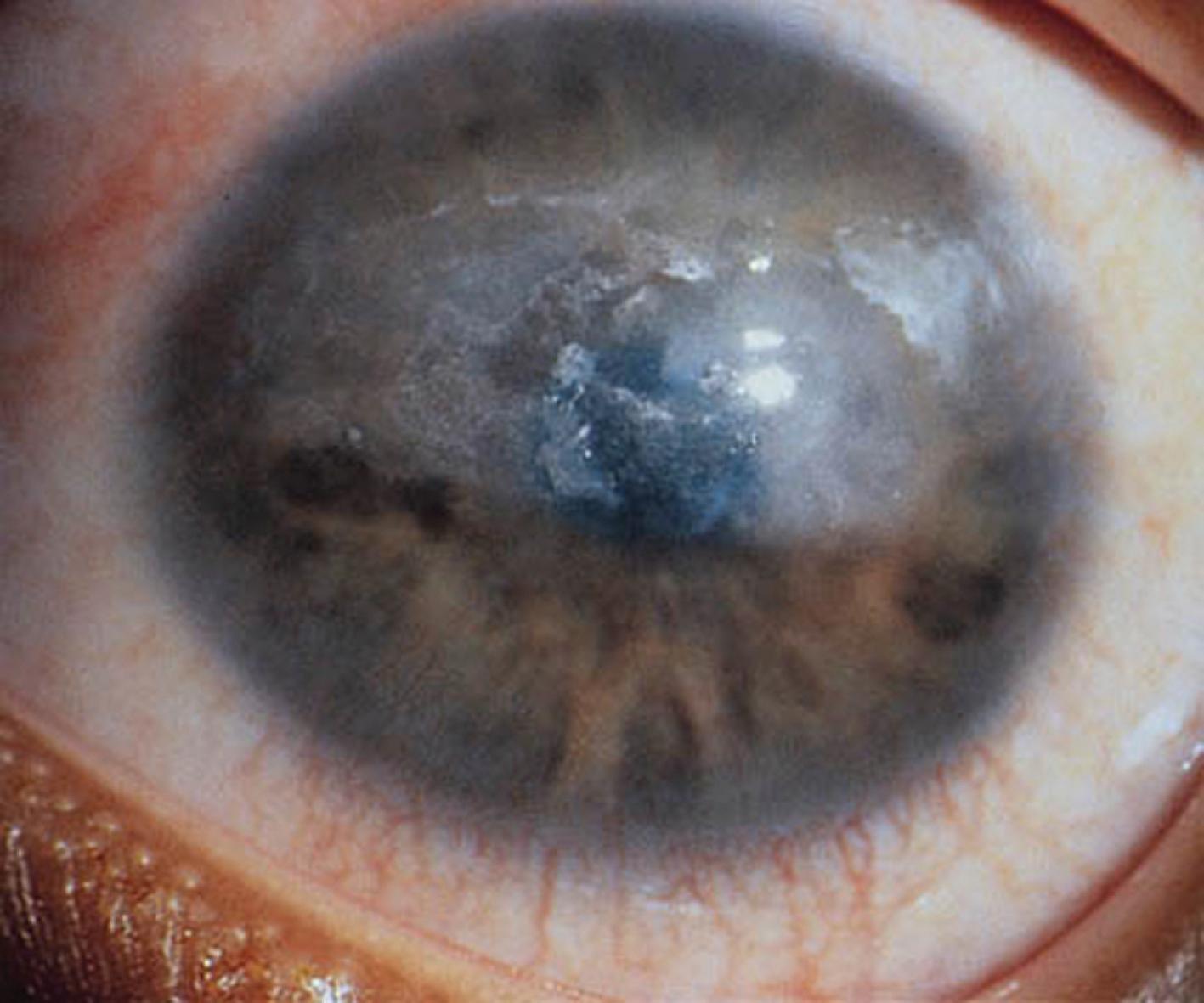
Patients with JIA are at risk of developing iridocyclitis (or uveitis). Although photophobia, eye pain, and erythema can occur, uveitis is often asymptomatic. For that reason, children with oligoarticular, psoriatic, and polyarticular JIA must receive slit-lamp examinations frequently.
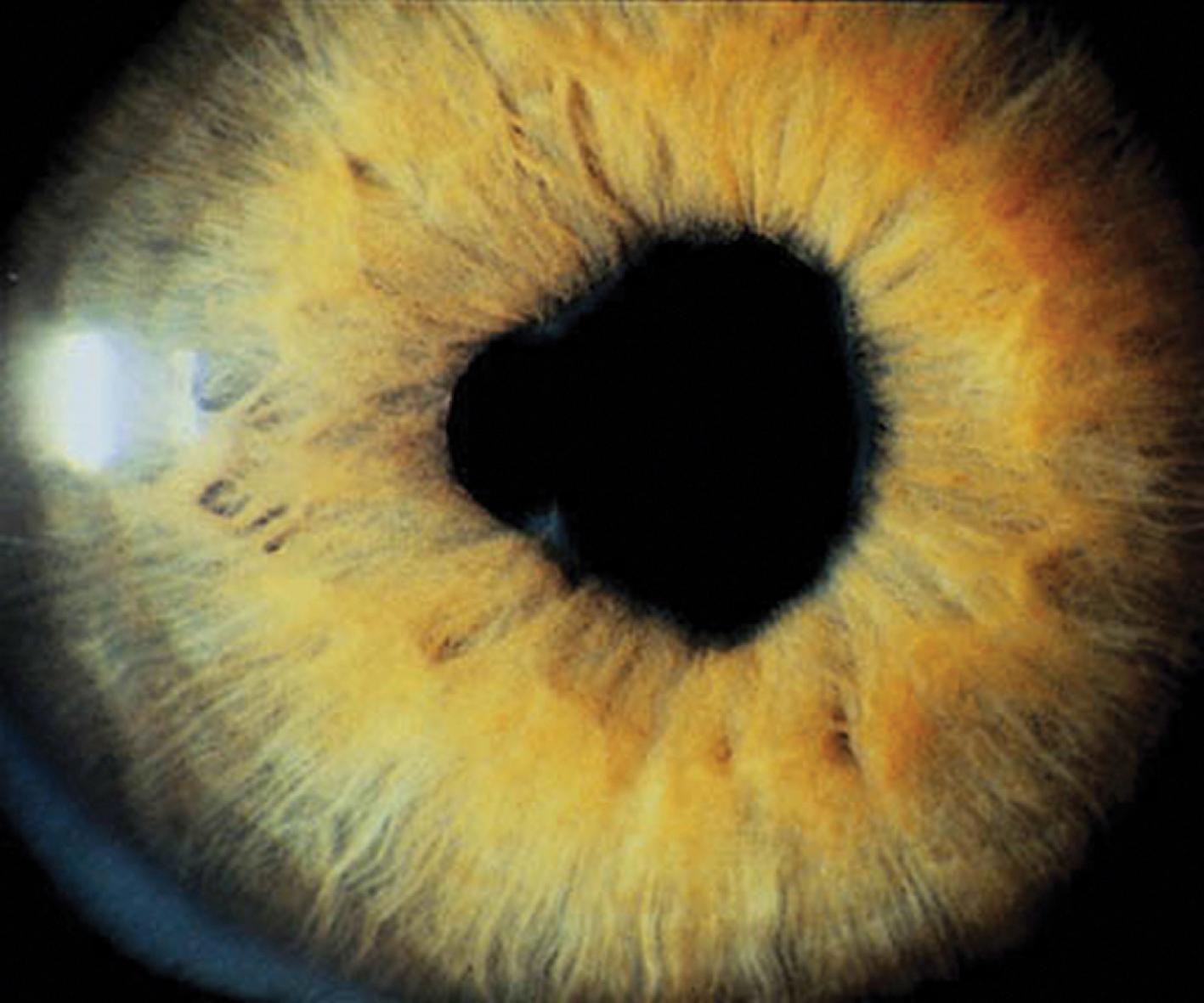
The first clinical sign of uveitis is cellular exudate in the anterior chamber. If the uveitis is left untreated, synechiae (adhesions) between the iris and lens may develop, leading to an irregular and poorly functioning pupil ( Fig. 7.8 ). Further along in the clinical course, band keratopathy (calcium deposits in the cornea; Fig. 7.9 ) may occur, as well as cataracts or glaucoma. Ophthalmologic complications do not parallel the activity of the arthritis, so strict adherence to the recommendations for eye examination outlined in Table 7.4 is necessary to help prevent visual loss.
Adapted from Cassidy J, Kivlin J, Lindsley C, et al. Ophthalmologic examinations in children with juvenile rheumatoid arthritis. Pediatrics . 2006;117:1843–1845.
Angeles-Han ST, Ringold S, Beukelman T, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Screening, Monitoring, and Treatment of Juvenile Idiopathic Arthritis-Associated Uveitis. Arthritis Care Res . 2019;71(6):703–716.
| Subtype | Anti-Nuclear Antibody Status | Age at Onset | Disease Duration | Frequency of Eye Examinations |
|---|---|---|---|---|
| Oligoarthritis, RF-negative polyarthritis, psoriatic and undifferentiated arthritis | Positive
Negative |
<7 years old | ≤4 years | 3 months |
| >4 years | 6–12 months | |||
| ≥7 years oldN/A | AnyN/A | 6–12 months6–12 months | ||
| RF-positive polyarthritis, systemic, and enthesitis related arthritis | N/A | N/A | N/A | 6–12 months |
Linear growth retardation may occur in the child with active JIA, especially with systemic or polyarticular disease. Oligoarticular arthritis can present with growth abnormalities as well, usually confined to leg length discrepancy or an enlarged hand or foot related to refractory ankle or wrist involvement. During early illness, bony development may be advanced with premature epiphyseal fusion; later in the course of the illness the opposite may be true. The overall degree of growth retardation and the ultimate prognosis for reaching adult height are related to the severity and duration of inflammation as well as the use of systemic corticosteroids. Treatment with steroid-sparing agents such as NSAIDs, disease-modifying antirheumatic drugs (DMARDs) (i.e., methotrexate), biologic agents (i.e., rituximab, tociluzumab, infliximab), or small molecules (i.e., tofacitinib) is currently preferred.
Cardiac involvement occurs in more than one-third of patients with sJIA. Pericarditis, myocarditis, and endocarditis occur, with pericarditis being the most common. Chest pain, a friction rub, tachycardia, and dyspnea, may occur, with supportive chest radiograph, electrocardiogram, and echocardiographic findings. These episodes may last for weeks to months and are usually associated with a generalized flare of disease. Various other extraarticular manifestations, including hepatosplenomegaly and lymphadenopathy, are particularly common in sJIA. A more recently described complication of sJIA is high-fatality parenchymal lung disease and pulmonary hypertension that occurs in a small subset of patients. Children may exhibit cough, tachypnea, erythematous clubbing of the fingers or hypoxia at diagnosis of lung disease, but many patients have no respiratory symptoms until late in the disease course. Diagnosis is made by findings of septal thickening, crazy paving, peripheral/peribronchovascular consolidation, or ground-glass opacities on computed tomography (CT) of the chest. Associations with the development of parenchymal lung disease include younger age of sJIA onset, trisomy 21, atypical pruritic rash, diagnosis of MAS, and history of anaphylaxis to tocilizumab.
Because JIA is a clinical diagnosis, strict clinical criteria have been established to make the diagnosis. Children should have objective joint findings (arthritis) for a minimum of 6 consecutive weeks coupled with the exclusion of other causes of arthritis in children ( Box 7.4 ).
Systemic lupus erythematosus (SLE)
Kawasaki disease
Acute rheumatic fever (ARF)
Henoch-Schönlein purpura
Polyarteritis nodosa
Dermatomyositis
Inflammatory bowel disease
Malignancy (leukemia, lymphoma, neuroblastoma)
Lyme disease
Viral syndrome
Malaria
Septic arthritis/infectious osteomyelitis
Periodic fever syndromes
Serum sickness-like reaction
SLE
Polyarticular JIA
Fibromyalgia
Hypermobility Syndrome
Enthesitis-related arthritis
Reactive arthritis ( Yersinia, Salmonella, Shigella, Campylobacter, & Chlamydia trachomatis )
Post-infectious arthritis (parvovirus B19, hepatitis B, rubella, enteroviruses, alphaviruses)
Blau syndrome
Inflammatory bowel disease
Multiple epiphyseal dysplasia
Cystic fibrosis
Septic arthritis
Reactive arthritis ( Yersinia, Salmonella, Shigella, Campylobacter, & C. trachomatis )
Post-infectious arthritis (alphaviruses, Epstein-Barr virus [EBV], varicella)
Acute rheumatic fever
Amplified musculoskeletal pain syndrome
Juvenile spondyloarthropathy
Pigmented villonodular synovitis
Psoriatic arthritis
Lyme disease
Crystalline arthropathy
Celiac disease
Because of its destructive nature, pyogenic arthritis (e.g., Staphylococci , Streptococci , Haemophilus influenzae ) must be ruled out in any child with active joint disease, especially monoarthritis. Any intensely red and tender joint should raise suspicion of a bacterial pathogen. This combined with systemic symptoms of infection (fever, chills, malaise, rigors) should prompt the clinician to perform diagnostic arthrocentesis early in the course of the illness.
Lyme arthritis can mimic oligoarticular JIA. This tick-borne illness is caused by the spirochete Borrelia burgdorferi with acute manifestations, including a distinctive rash, known as erythema migrans (see Chapter 13 , Fig. 13.50 ). A child may develop arthritis as a late manifestation months or years after exposure, often without having recalled a tick bite or the acute manifestations. The arthritis seen in Lyme usually affects the knee but may affect other large joints in a monoarticular pattern with spontaneous exacerbations and remissions ( Fig. 7.10 ).
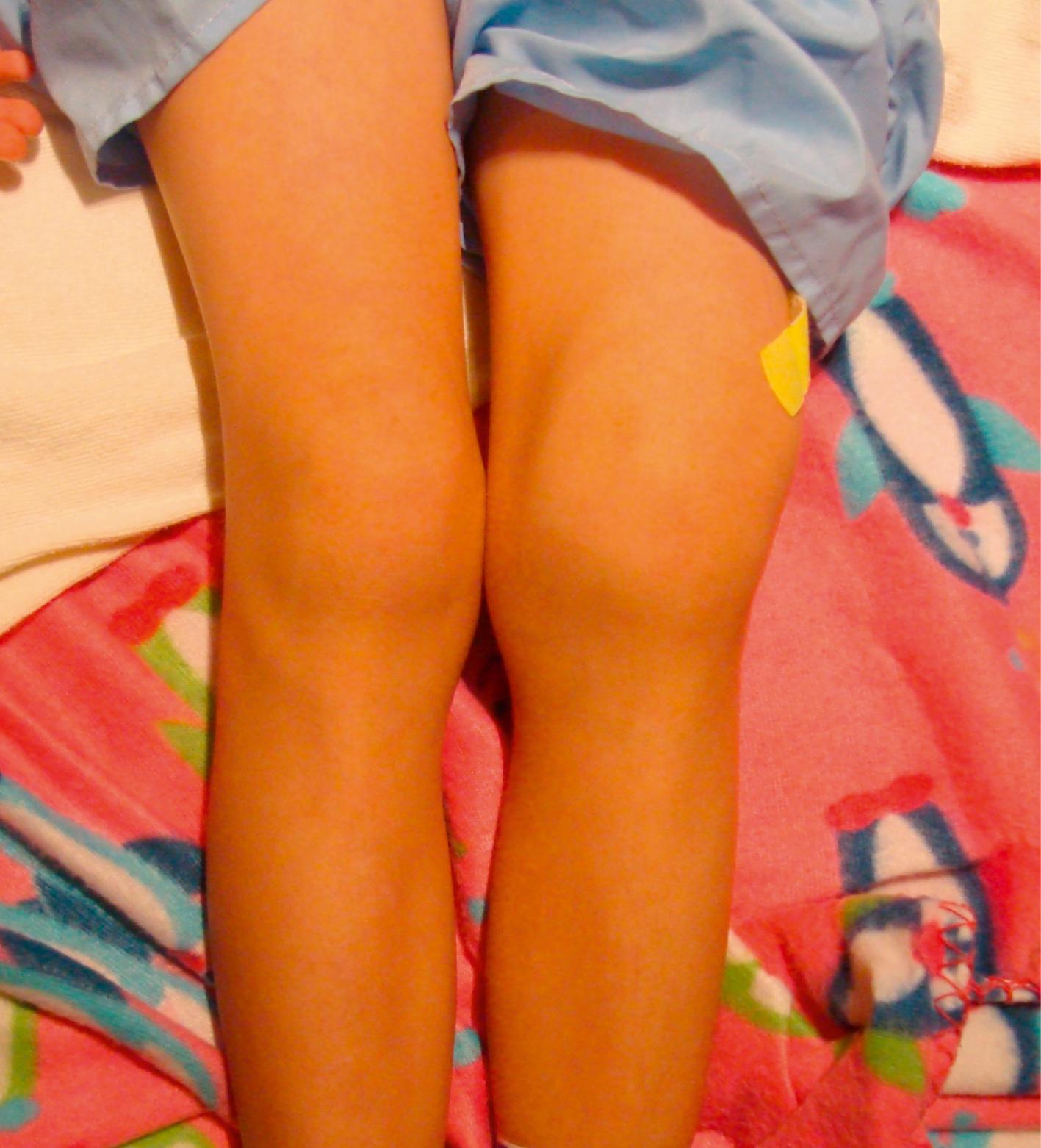
Other infections cause a reactive arthritis that dissipates in less than 6 weeks. Recent infection with Salmonella, Shigella, Yersinia, and Campylobacter organisms should also be considered. Many viruses also cause arthritis. These include rubella, hepatitis B, adenovirus, and herpesviruses, including Epstein-Barr virus, cytomegalovirus, varicella zoster, and herpes simplex. Parvoviruses, mumps, and enteroviruses, including echovirus and coxsackievirus, are associated with acute polyarthritis and occasionally have been recovered from joints. Other viruses result in reactive arthritis and may not infect the joint directly.
Poststreptococcal phenomena include ARF and poststreptococcal reactive arthritis. In ARF, Jones criteria are used for diagnosis. Arthritis presents in migratory fashion and pain may be out of proportion to examination. Use of NSAIDs alters the course of the arthritis. The child must be monitored for cardiac sequelae, such as valvulitis and congestive heart failure. In poststreptococcal reactive arthritis, Jones criteria are not met, but evidence of preceding streptococcal infection is present. Arthritis will resolve but arthralgia can last for 6 months. The joint pain may be axial and affect the spine. In both cases, children are given prophylactic antibiotics to prevent future streptococcal infections.
Malignancies, such as neuroblastoma and leukemia, may present with musculoskeletal pain. More careful evaluation generally reveals bone pain. Sickle cell disease can have prominent digital involvement or deep bone pain related to infarction. Hemophilia, tuberculosis, and gonorrhea infection must be considered in the patient with oligoarthritis. Differential diagnoses of joint pain are proposed in Box 7.4 .
SLE is a multisystem autoimmune disease with a myriad of clinical presentations. SLE accounts for 10% of adult patients with rheumatic diseases but less than 5% of children seen in pediatric rheumatology practices. The incidence is estimated at 0.5 per 100,000 children per year. From prevalence data, it has been inferred that there are between 5000 and 10,000 children with SLE in the United States. The disease is rare in children younger than 5 years old. Before menarche, the male-to-female ratio is equal. After menarche, the ratio of affected females to males approaches 8:1. Native American, Hispanic, African American, and Asian populations are more commonly affected than Caucasian populations, and often have more severe disease. The incidence of other connective tissue diseases is higher among family members of patients with SLE. Hematologic malignancies and immunodeficiencies are also reported in increased frequency among the relatives of patients with SLE. These well-described phenomena may reflect a genetic alteration of immunity or, as some researchers suggest, the effects of a transmissible agent. The high incidence of the disease in females supports the role of hormonal factors as contributing or modulating agents in the pathogenesis of SLE. Other investigators suggest the influence of viruses, sunlight, and emotional stress on the development of lupus.
SLE may present in an insidious fashion and hence escape early diagnosis, or it may present acutely and progress rapidly, leading to the patient’s demise. All organ systems have the potential to become involved, but the most common are skin, musculoskeletal, and renal systems in pediatric systemic lupus erythematosus (pSLE). The etiology of SLE is unknown. Because of the large number of serologic markers known to occur in SLE, it is considered by many to be the prototype of autoimmune diseases. To increase diagnostic accuracy, the American College of Rheumatology (ACR) revised its classification criteria of lupus ( Table 7.5 ). This classification, which combines clinical and serologic markers, is highly sensitive and specific for the diagnosis of this disease, reaching almost 100% sensitivity and specificity when four of the 11 criteria are present; however, the criteria are not meant for the clinical application of diagnosis and should be used as a study guide rather than applied to the clinical arena.
Modified from Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum . 1997;40:1725.
From Sag E, Tartaglione A, Batu ED, et al. Performance of the new SLICC classification criteria in childhood systemic lupus erythematosus: a multicentre study. Clin. Exp. Rheumatol . 2014;32:440–444.
| Criteria | Comments |
|---|---|
| Malar (butterfly) rash | Fixed erythema, flat or raised over the malar eminences, sparing the nasolabial folds |
| Discoid-lupus rash | Erythematous raised patches with adherent scaling and follicular plugging; atrophic scarring can be seen in older lesions |
| Photosensitivity | By patient history or physician observation |
| Oral ulcerations | Oral or nasopharyngeal, usually painless, observed by physician |
| Arthritis | Nonerosive; two or more peripheral joints affected by tenderness, swelling or an effusion |
| Serositis | Pleuritis defined as history of pleuritic chest pain or rub heard by physician or a pleural effusion; pericarditis documented on ECG, rub, or pericardial effusion |
| Renal disorder b | Cellular casts or persistent proteinuria >0.5 g/day or urine dipstick results of >3+ if quantification not performed |
| Neurological disorder b | Seizures or psychosis (without other offending drug or metabolic cause) |
| Hematological disorder | Hemolytic anemia or leukopenia (leukocyte count <4000/µL) or lymphopenia (lymphocyte count <1500/µL) or thrombocytopenia (platelet count <100,000/µL) |
| Immunological disorder b | Anti-dsDNA: Antibody native DNA or anti-Smith; or positive finding of antiphospholipid antibodies based on (1) abnormal level of IgG or IgM anticardiolipin antibodies, (2) a positive test result for LAC, and (3) false positive test for syphilis known to be positive for 6 months and confirmed by Treponema pallidum testing |
| ANA test | An abnormal titer of ANA by immunofluorescence or an equivalent assay |
a Four of 11 criteria provide a sensitivity of 96% and a specificity of 96%. In a pediatric population these criteria had a sensitivity of 76.6% and a specificity of 93.4%.
The early symptoms are often nonspecific and sometimes go unrecognized as harbingers of serious disease. Fever, fatigue, malaise, anorexia, and weight loss may be the only symptoms. In the adolescent population, these symptoms may be all the more difficult to interpret. Conversely, this multisystem disease may present with a plethora of physical findings, and the presentation may be so dramatic that the diagnosis is readily apparent. The most common organ manifestations at disease presentation in pSLE are musculoskeletal, cutaneous, renal, and hematologic.
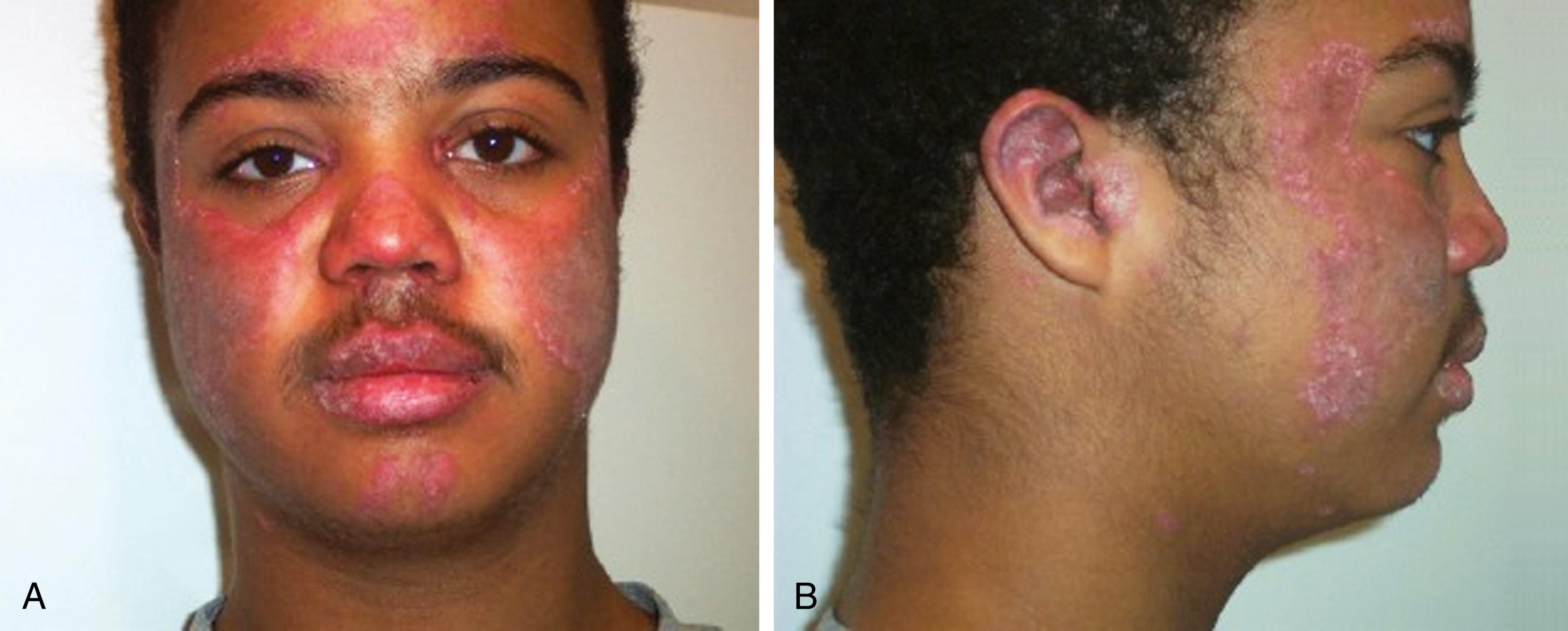
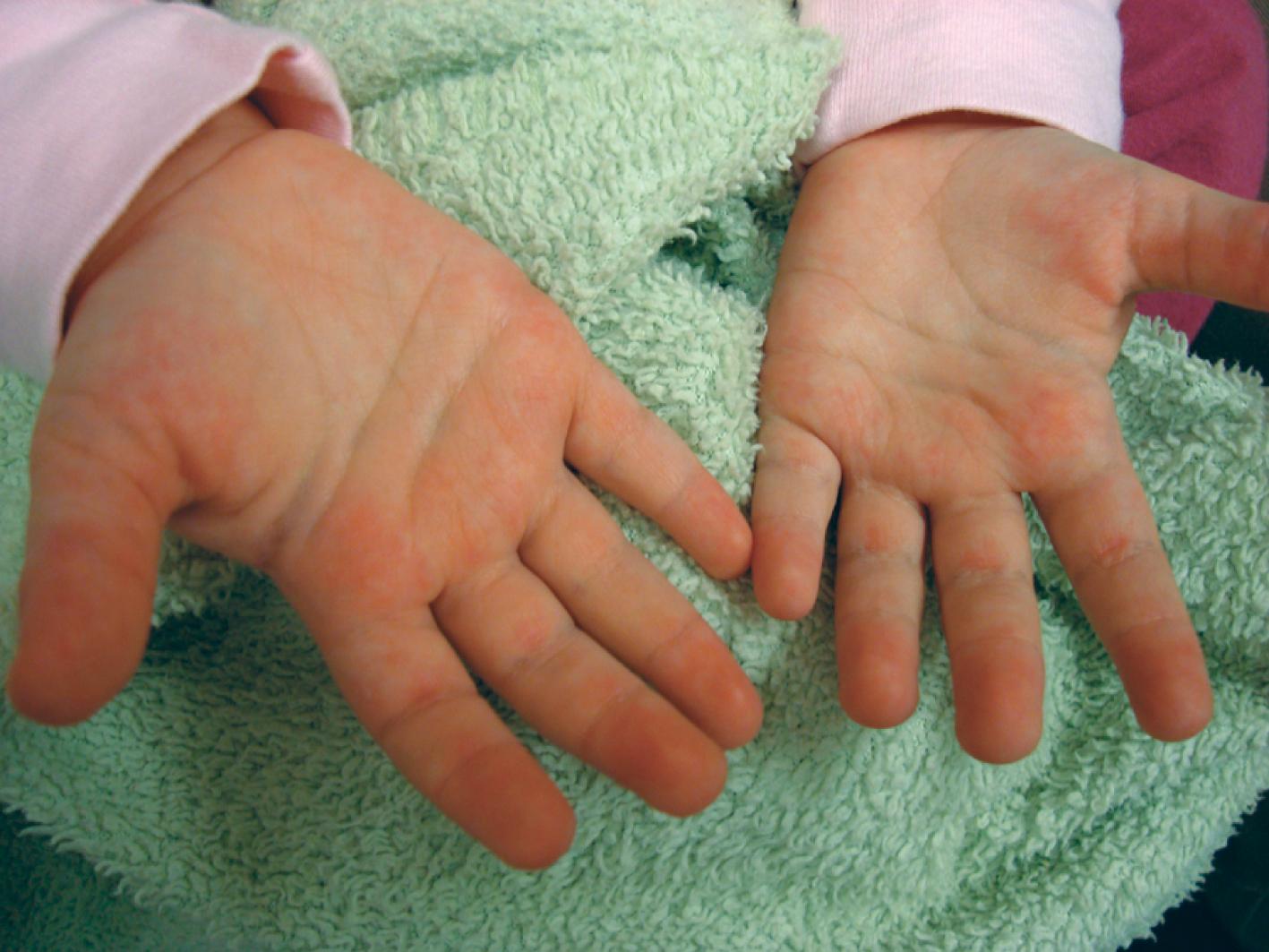
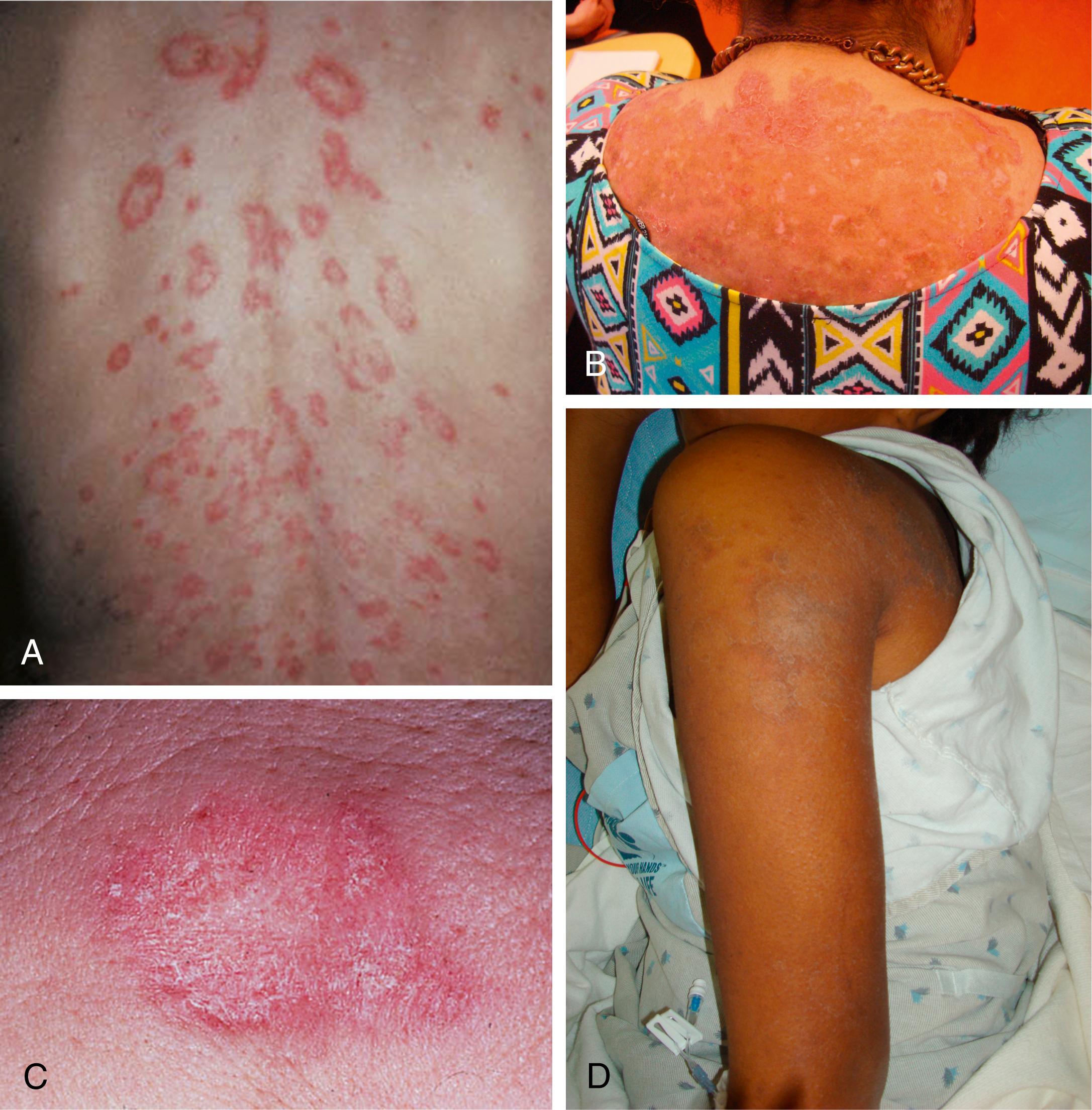
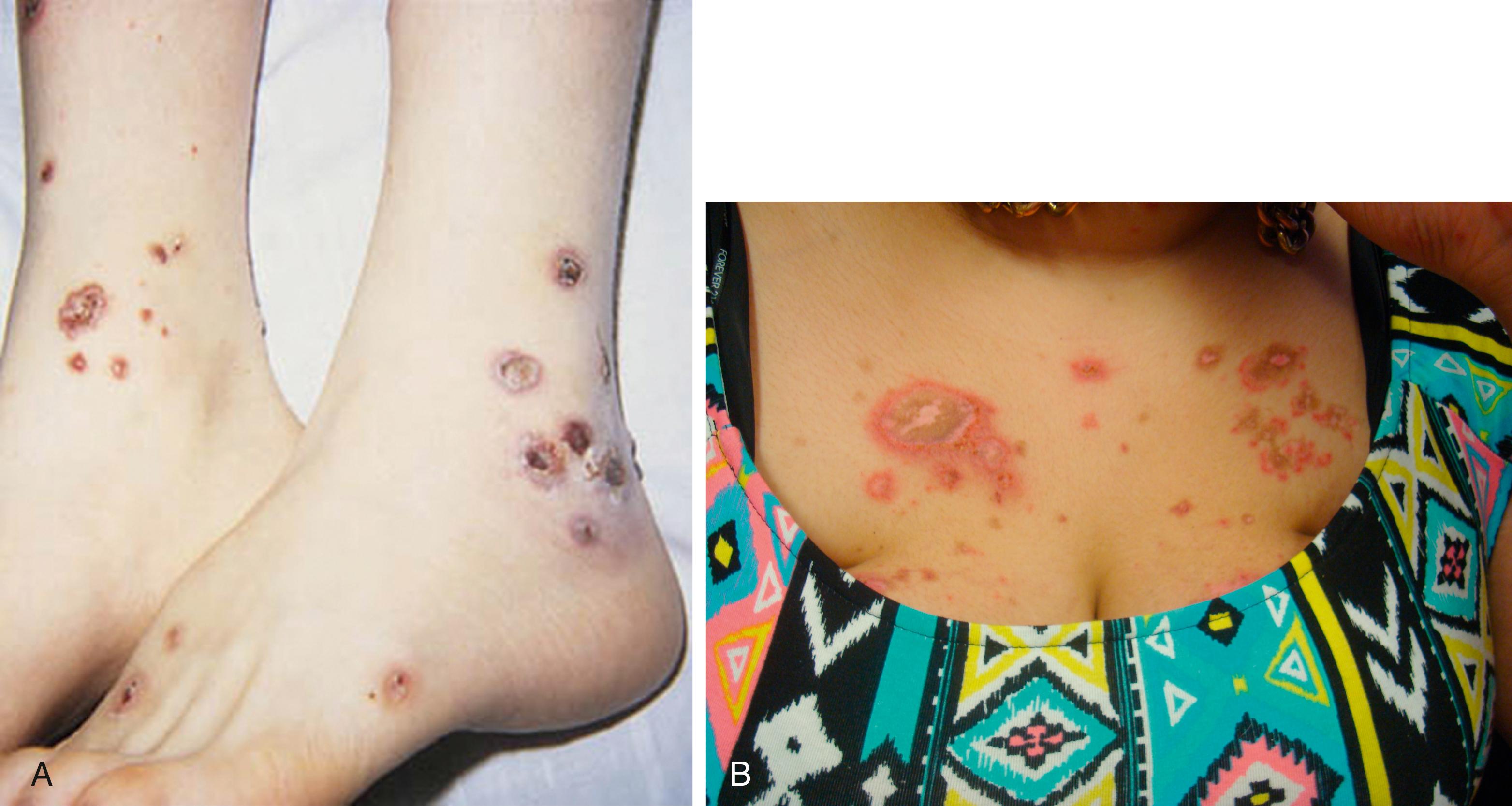
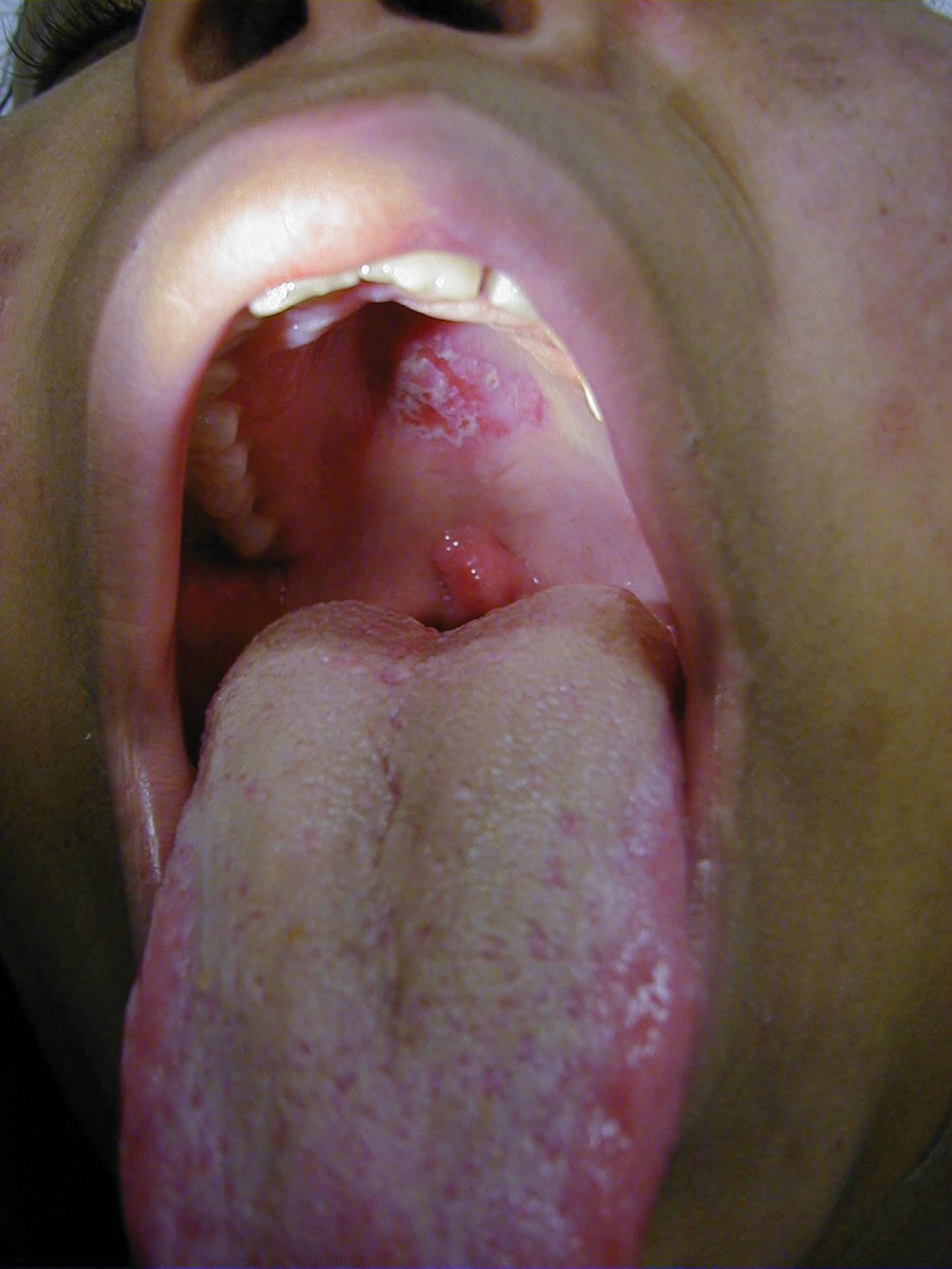
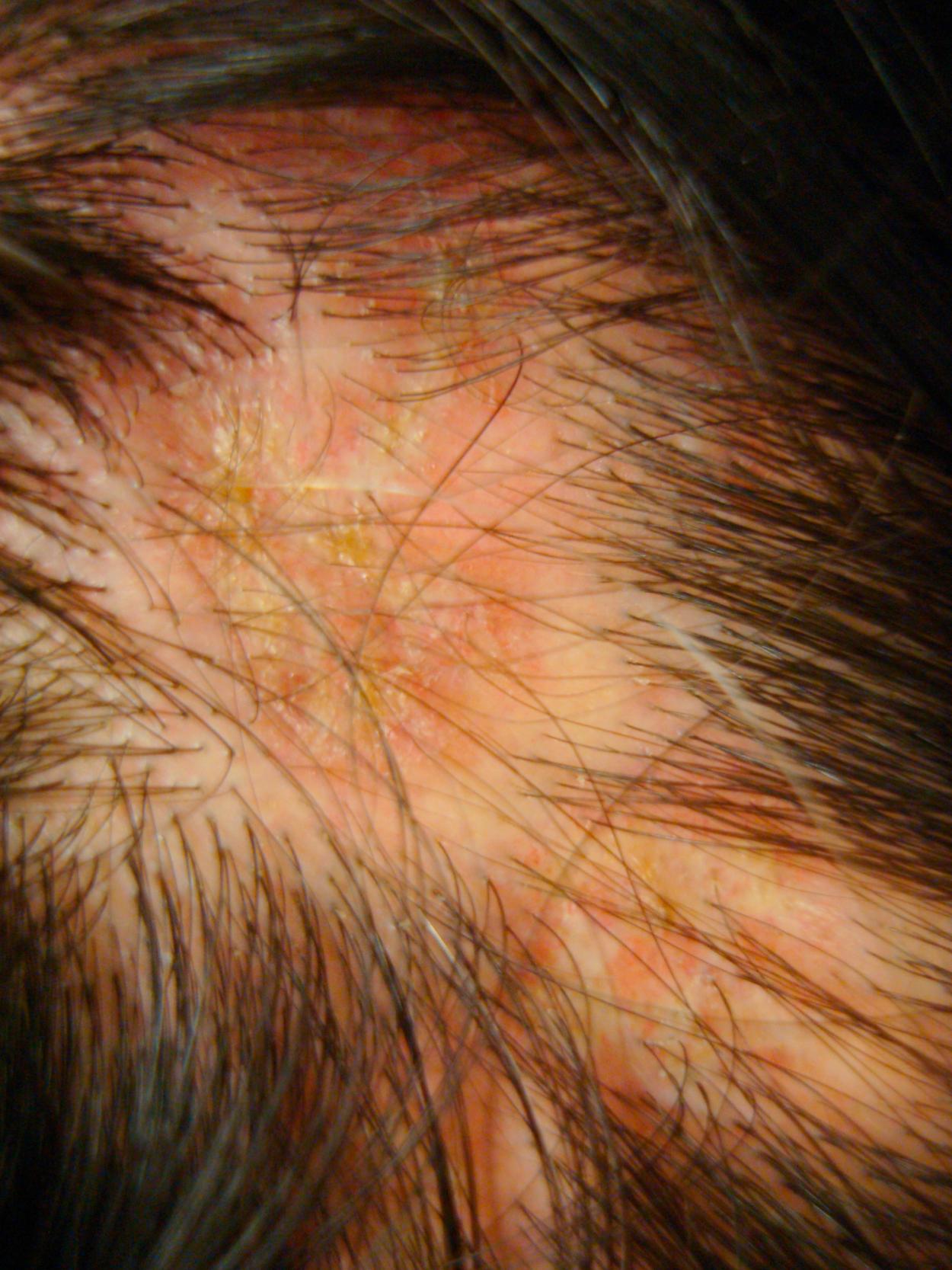
Cutaneous manifestations of SLE occur at some time during the course of the disease in 80% of affected individuals. There are a variety of cutaneous findings, including a malar rash ( Fig. 7.11 ), photosensitive rash, palmar erythema ( Fig. 7.12 ), annular erythema, vasculitic skin lesions with nodules or ulcerations, Raynaud phenomenon ( Table 7.6 ), and associated ulcerations, alopecia, and discoid lupus. The most frequent skin manifestations of SLE are malar rash and photosensitive rash. The classic malar “butterfly” rash is a maculopapular rash distributed over the cheeks (malar eminences) and extending over the bridge of the nose, while sparing the nasolabial folds. The malar rash is photosensitive in 30% of patients. In general, sun exposure may not only exacerbate the skin disease but also cause a systemic flare of disease. Appropriate sun protection and avoidance is strongly encouraged. An annular photosensitive rash is frequently associated with the “Sjögren syndrome antibodies” anti-Ro (SS-A) and anti-La (SS-B). The typical morphology of the rash of lupus is defined as reddish purple and raised with a whitish scale ( Fig. 7.13 ). Vasculitic rashes are more violaceous and may be associated with nodules, ulceration, and palpable purpura ( Fig. 7.14 ). These lesions are commonly found in an acral distribution, and typically result in postinflammatory hyperpigmentation. Discoid lupus, although uncommon (5% to 10% of patients with pSLE), leaves the most damage, with follicular plugging and scarring. Isolated discoid lupus is rarely seen in children; but when it occurs, it is usually without systemic manifestations. The same holds true for subacute cutaneous lupus, which is uncommon, and presents as a serpiginous-like photosensitive erythematous rash associated with positive anti-Ro (SS-A) antibody. Mucosal erosions and ulcers of the oral cavity and nasal mucosa are part of lupus as well; the most well-recognized manifestation is the “silent” large violaceous ulceration of the hard palate ( Fig. 7.15 ). Alopecia occurs in 20% of patients and may present as broken hair shafts or patchy, red, scaling areas on the scalp, which may eventually scar and cause permanent hair loss ( Fig. 7.16 ). Other reported mucocutaneous findings are livedo reticularis (lacy, fishnet appearance of the skin), urticaria, and telangiectasia. The presence of a livedoid rash may be the clinician’s only clue to an associated hypercoagulable state manifested by anti-phospholipid antibodies.
Fleck DE, Hoeltzel MF. Hand and foot color change: diagnosis and management. Pediatr Rev . 2017;38;511.
Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc . 2014;89(2):207.
| Disease | Epidemiology | Triggers | Duration | Clinical Exam | Associated Disorders | Comments |
|---|---|---|---|---|---|---|
| Raynaud phenomenon, primary | Common, 10%–15% prevalence, F>M, | Cold exposure, emotion, stimulants, smoking | Minutes to hours | Triphasic (white/blue/red) color change of fingers/toes with clear demarcation; Numb when ischemic, aching/painful with reperfusion | None | May be limited to single digit |
| Raynaud phenomenon, secondary | Uncommon, variable prevalence in underlying conditions | Cold exposure, disease activity | Hours to Continuous | Triphasic changes; chronic digital ulcers or skin pits; poor nail health; abnormal nailfold capillaries | SLE SSc MCTD aPLS Vasculitis |
Often requires vasodilators |
| Acrocyanosis | Common, 10%–15% prevalence, F>M, onset in adolescence, low BMI is a risk factor | Cold exposure, limb in dependent position, medications (stimulants, TCAs) | Minutes | Symmetric, painless blue/purple discoloration of hands/feet without clear demarcation; blanching with pressure; affected skin/nails appear healthy | Anorexia nervosa POTS Genetic CTDs |
May be accompanied by cutis marmorata on affected limbs |
| Perniosis | Uncommon, F>M, peak 2nd–5th decades of life, low BMI is a risk factor | Cold and damp conditions | Continuous for 1–3 weeks | Edematous, erythematous or violaceous papules/plaques/nodules on the dorsum of fingers or toes; blisters or ulcers may develop; onset 12–24 h after cold exposure | None | Symptoms usually limited to winter months |
| Erythromelalgia | Rare, F>M, onset in early adolescence | Exercise, heat exposure | Minutes to continuous | Symmetrical, diffuse, painful erythema of hands, feet, or face; affected areas feel hot | Myeloproliferative disorder (adults-only); rarely inherited | Pain is often severe and disabling |
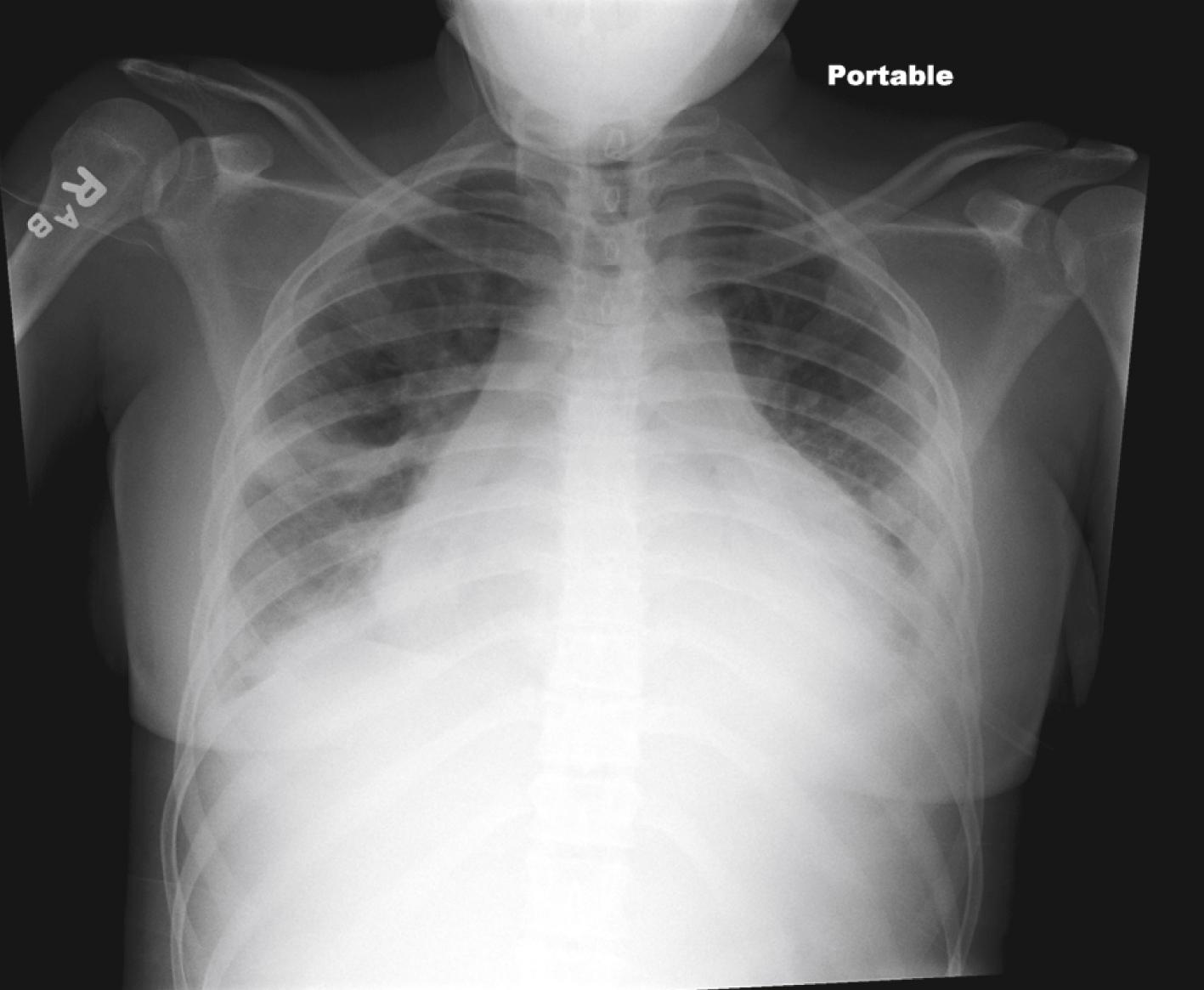
The heart is often significantly involved in patients with lupus. Although the pericardium is involved most commonly, the myocardium and the endocardium may also be of clinical importance. Pericarditis with associated pericardial effusion can be painless and may present only as cardiomegaly on a chest radiograph ( Fig. 7.17 ) or as pericardial effusion on an echocardiogram. Although pericarditis is usually mild, it can progress to life-threatening cardiac tamponade. If the myocardium is affected, serious complications including dysrhythmias, heart failure, and infarction can result. Libman-Sacks endocarditis is the term given to the verrucous projections of fibrinoid necrosis in the endocardium. These lesions rarely cause clinical symptoms, although the presence of a murmur raises suspicion of endocardial disease. The mitral valve is most commonly involved, although aortic and tricuspid valves may be similarly affected. The presence of Libman-Sacks endocarditis should also alert the clinician to the possibility of an underlying anti-phospholipid antibody syndrome. The major cardiac morbidity associated with SLE, which is gaining more recognition in the adolescent pSLE population, is premature atherosclerosis. Several factors contribute to the risk of atherosclerosis, including chronic inflammatory processes, altered endothelial function, lipid abnormalities, nephritis, and proteinuria. Monitoring and controlling classic Framingham risk factors in addition to factors attributable to lupus are critical to address long-term morbidity.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here