Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Prevalence: (a) the overall prevalence of diabetic retinopathy (DR) in diabetic patients is approximately 40%; (b) DR is more common in type 1 than in type 2 diabetes, (c) sight-threatening disease is present in up to 10%, and (d) proliferative disease affects 5–10% of the diabetic population (60% after 30 years in type 1).
Risk factors: (a) duration of diabetes (50% after 10 years if diagnosed before age 30 years; 5% of type 2 diabetics have DR at presentation), (b) poor control of diabetes, (c) pregnancy, (d) hypertension, (e) nephropathy, (f) hyperlipidaemia, (g) smoking, (h) obesity, and (i) anaemia.
The classification proposed in the Early Treatment Diabetic Retinopathy Study is widely used internationally. An abbreviated version is set out in Table 13.1 ; other commonly used clinical categories are as follows:
Background diabetic retinopathy: (a) microaneurysms, (b) dot and blot haemorrhages, and (c) hard exudates.
Diabetic maculopathy: vision-threatening macular oedema or ischaemia.
Preproliferative diabetic retinopathy: (a) cotton wool spots, (b) venous changes, (c) intraretinal microvascular anomalies (IRMA), and (d) deep retinal haemorrhages.
Proliferative diabetic retinopathy (PDR): (a) neovascularization on or within one disc diameter of the disc (NVD) and/or (b) new vessels elsewhere (NVE) in the fundus.
Advanced diabetic eye disease: (a) tractional retinal detachment, (b) persistent vitreous haemorrhage, and (c) neovascular glaucoma.
| Category/description | Management |
|---|---|
| Nonproliferative diabetic retinopathy | |
| No DR | Review in 12 months. |
| Very mild Microaneurysms only. | Review most patients in 12 months. |
| Mild Any or all of: microaneurysms, retinal haemorrhages, exudates, cotton wool spots, up to the level of moderate NPDR. No IRMA or significant beading. | Review range 6–12 months, depending on severity of signs, stability, systemic factors, and patient's personal circumstances. |
Moderate Severe retinal haemorrhages (more than ETDRS standard photograph 2A: approximately 20 medium–large per quadrant) in 1–3 quadrants or mild IRMA.
|
Review in approximately 6 months. PDR occurs in up to 26%, high-risk PDR in up to 8% within 1 year. |
Severe The 4-2-1 rule; one or more of:
|
Review in 4 months. PDR in up to 50%, high-risk PDR in up to 15% within 1 year. |
| Very severe Two or more of the criteria for severe NPDR. | Review in 2 or 3 months. High-risk PDR in up to 45% within 1 year. |
| Proliferative diabetic retinopathy | |
| Mild/moderate NVD or NVE, but extent insufficient to meet the high-risk criteria. | Treatment considered according to severity of signs, stability, systemic factors, and patient's personal circumstances such as reliability of attendance for review. If not treated, review in up to 2 months. |
High-risk
|
Treatment advised—see text. Should be performed immediately when possible, and certainly same day if symptomatic presentation with good retinal view. |
Microaneurysms: tiny red dots ( Fig. 13.1 ) that tend to be the earliest signs.
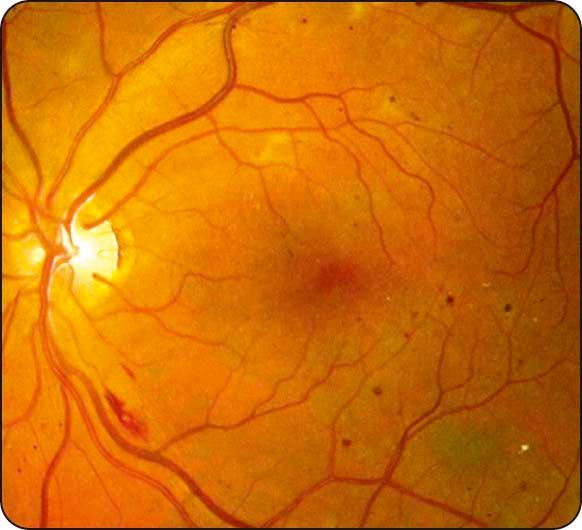
Retinal nerve fibre layer haemorrhages: superficial and flame-shaped ( Fig. 13.2 ).
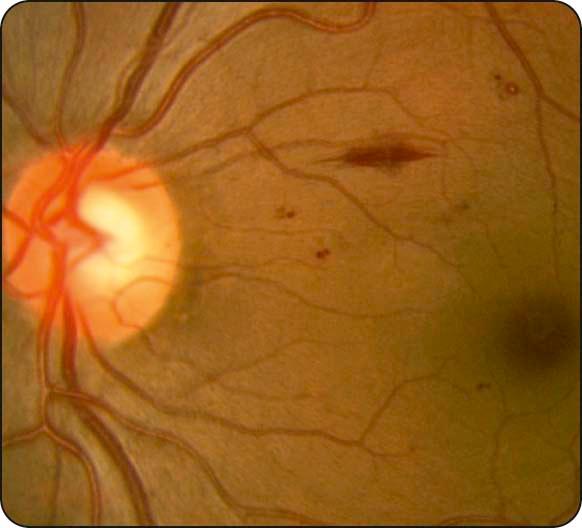
Intraretinal haemorrhages: located in the compact middle layers of the retina and have a red ‘dot/blot’ configuration ( Fig. 13.3 ).
Deeper dark round haemorrhages: haemorrhagic infarcts located within the middle retinal layers; risk marker for progression to retinal neovascularization.
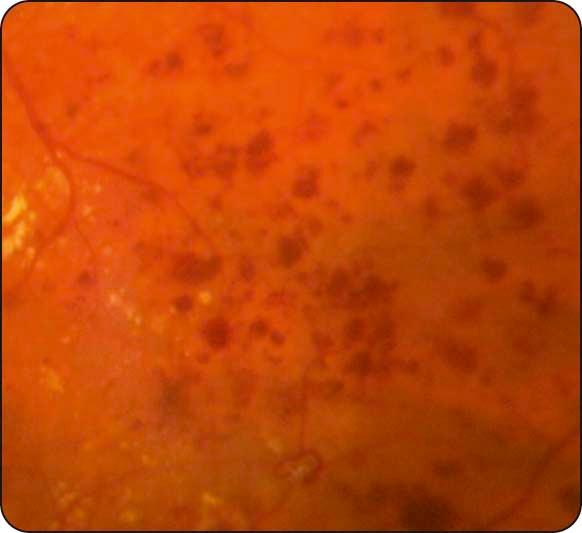
Hard exudates: waxy yellow lesions with distinct margins, often arranged in clumps and/or rings surrounding leaking microaneurysms ( Fig. 13.4 ).
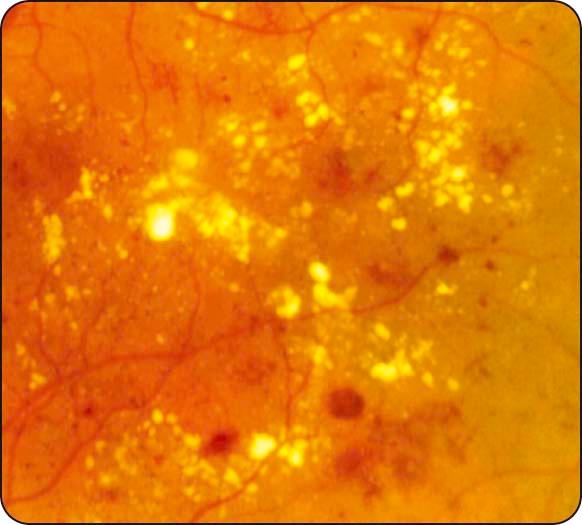
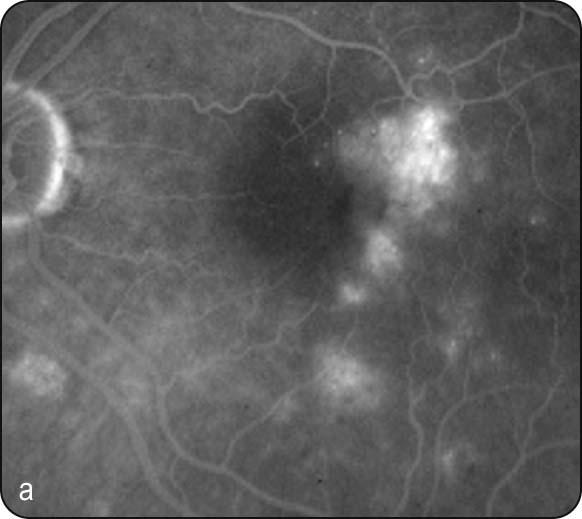
Diabetic maculopathy: the most common cause of visual impairment in diabetics; (a) localized macular oedema is caused by focal leakage ( Fig. 13.5a ), (b) diffuse oedema is caused by leakage, often with accumulation of fluid at the fovea in cystoid configuration (CMO); FA shows diffuse late hyperfluorescence with a flower-petal pattern ( Fig. 13.5b ); (c) in ischaemic maculopathy, the macula may appear relatively normal despite reduced VA; FA shows capillary nonperfusion and an enlarged foveal avascular zone (FAZ) ( Fig. 13.5c ).
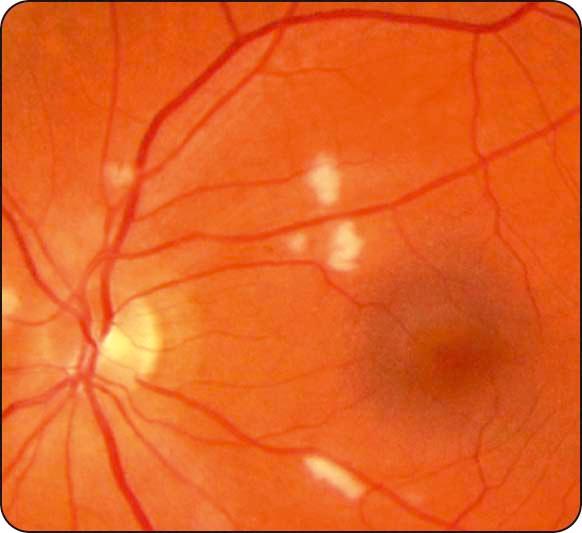
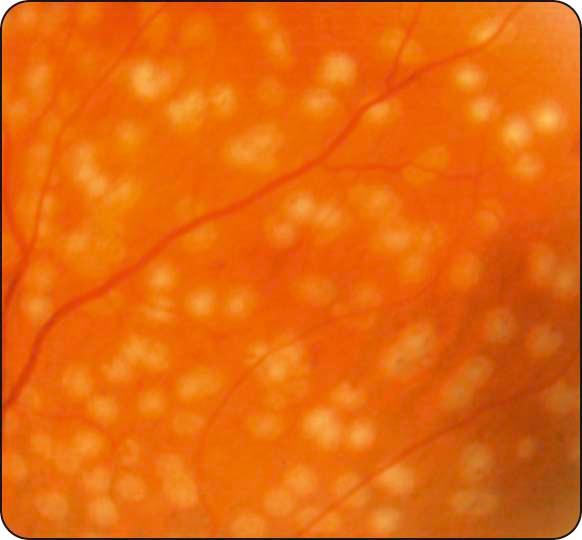
Cotton wool spots: small whitish fluffy lesions consisting of focal accumulations of neuronal debris within the nerve fibre layer ( Fig. 13.6 ).
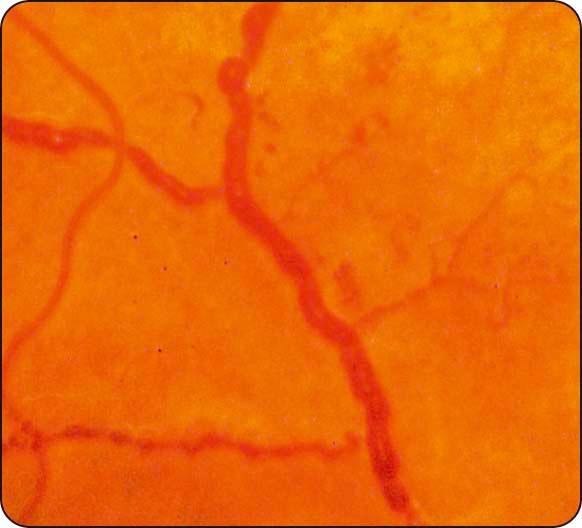
Venous anomalies ( Fig. 13.7 ) : seen in ischaemia, consist of (a) generalized dilatation and tortuosity, (b) looping, (c) ‘beading’ (focal narrowing and dilatation), and (d) segmentation; the extent of the involved retinal area correlates with the risk of developing PDR.
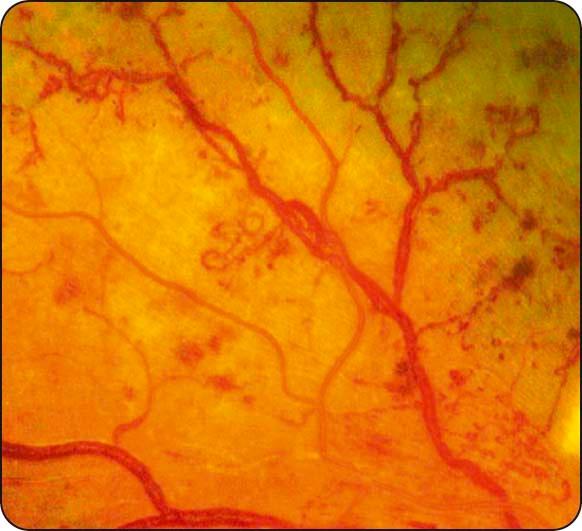
IRMA: fine irregular intraretinal arteriovenous shunts often seen adjacent to areas of capillary nonperfusion ( Fig. 13.8 ); FA shows absence of leakage.
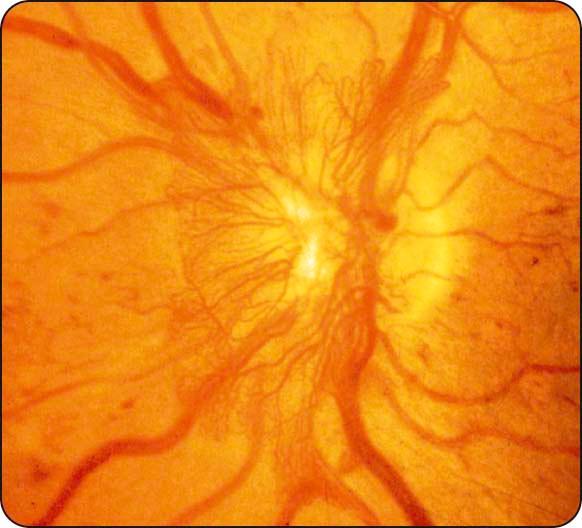
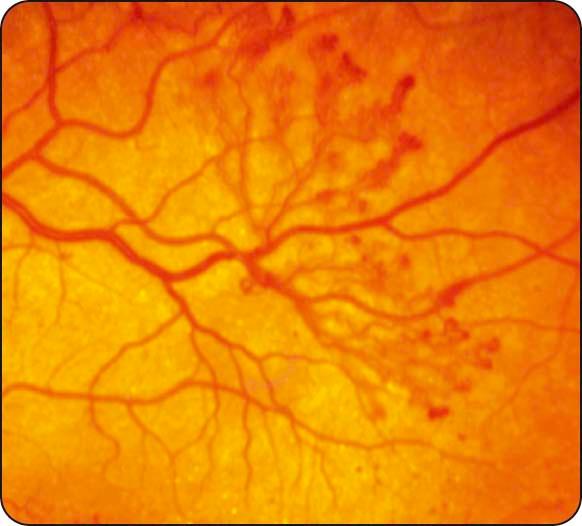
Neovascularization: (a) NVD describes neovascularization on or within one disc diameter of the optic nerve head ( Fig. 13.9 ), (b) NVE describes neovascularization further away from the disc ( Fig. 13.10 ), and (c) new vessels on the iris (rubeosis iridis) carry a high risk of progression to neovascular glaucoma (see Chapter 10 ).
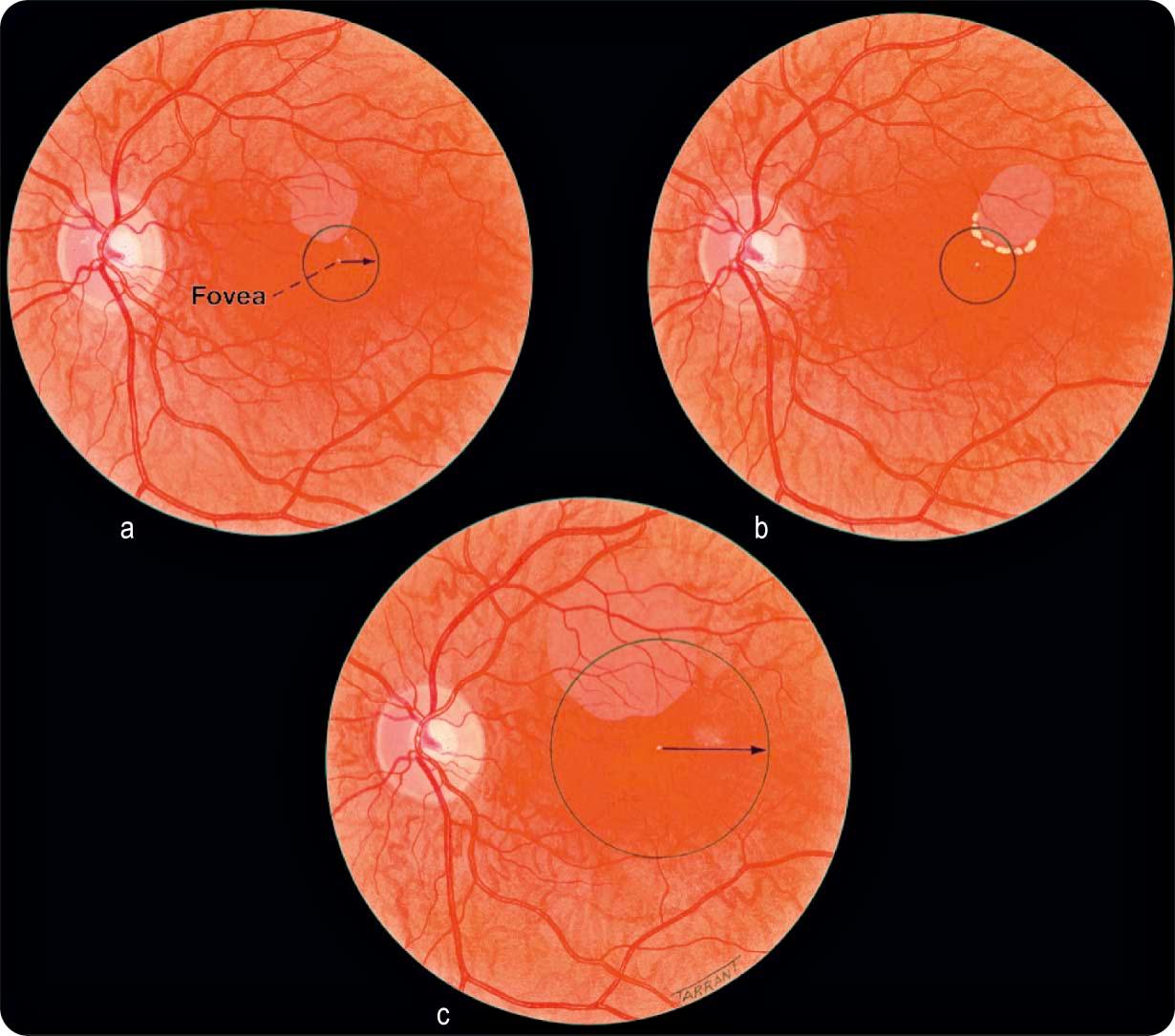
Definition: retinal thickening within 500 µm of the centre of the macula ( Fig. 13.11a ), exudates within 500 µm of the centre of the macula, if associated with retinal thickening ( Fig. 13.11b ), or retinal thickening one disc area (1500 µm) or larger, any part of which is within one disc diameter of the centre of the macula ( Fig. 13.11c ); eyes with CSMO should be considered for laser because this reduces the risk of visual loss by 50%.
Pretreatment FA: delineates the area and extent of leakage, and detects ischaemic maculopathy (poor prognosis; see Fig. 13.5c ).
Focal treatment: light/moderate intensity burns (50–100 µm, 0.1 sec) applied to microaneurysms associated with CSMO; treatment as close as 300 µm from the centre of the macula may be considered in some cases, with a lighter intensity reaction closer to the centre.
Grid treatment: burns (100 µm, 0.1 sec) applied to areas of diffuse retinal thickening more than 500 µm from the centre of the macula to give a very light intensity reaction; treatment should be particularly light if significant macular ischaemia is present.
Results: 70% of eyes achieve stable VA, 15% show improvement, and 15% subsequently deteriorate; it may take up to 4 months for oedema to respond.
Poor prognostic factors: (a) significant macular ischaemia, (b) foveal exudates, (c) diffuse macular oedema, (d) CMO, (e) severe retinopathy at presentation, (f) uncontrolled systemic hypertension, (g) renal disease, (h) poorly controlled blood glucose, and (i) smoking.
Frequency-doubled micropulse Nd : YAG laser: (e.g. ‘pattern scan laser’–PASCAL); offers the potential for a less destructive retinal effect than argon, with barely visible burns at the level of the RPE.
Micropulse diode laser: short duration (microseconds) burns to the RPE without significantly affecting the outer retina and choriocapillaris.
Intravitreal anti-VEGF agents: show promise and are playing an increasingly prominent role.
Intravitreal triamcinolone: research suggests that pseudophakic eyes with CSMO may benefit from intravitreal steroid injection followed by prompt laser.
Pars plana vitrectomy: when macular oedema is associated with tangential traction from a thickened and taut posterior hyaloid, and possibly in other circumstances.
Lipid-lowering drugs: may reduce the requirement for laser treatment.
Risk characteristics and benefits: The landmark Diabetic Retinopathy Study established the characteristics of high-risk proliferative disease and the effect of PRP, showing that (a) mild NVD with haemorrhage carries a 26% risk of visual loss, reduced to 4% with PRP, (b) severe NVD without haemorrhage has a 26% risk of visual loss, reduced to 9%, (c) severe NVD with haemorrhage carries a 37% risk of visual loss, reduced to 20%, and (d) severe NVE with haemorrhage carries a 30% risk of visual loss, reduced to 7% with treatment.
Complications: visual field defects of sufficient severity to legally preclude driving; patients should also be made aware that there is some risk to central vision, and that night and colour vision may be affected.
Laser settings: (a) spot size depends on the contact lens used (e.g. Goldmann 200–500 µm, panfundoscopic lens 100–300 µm), (b) duration 0.05–0.1 sec, and (c) power sufficient to produce only a light intensity burn ( Fig. 13.12 ).
PRP technique: 1500–2000 burns in a scatter pattern extending from the posterior fundus to cover the peripheral retina in one or more sessions. In eyes with severe NVD, 3000 or more burns may be required. Many practitioners leave two disc diameters untreated at the nasal side of the disc to preserve paracentral field. Topical anaesthesia is adequate in most patients, although a local block is sometimes necessary. Follow-up is after 4–6 weeks.
Intravitreal anti-VEGF therapy: likely to have an increasing role, probably as an adjunct to PRP (e.g. to encourage resolution of persistent vitreous haemorrhage).
Definition: serious vision-threatening complication of DR that occurs in patients in whom treatment has been inadequate or unsuccessful.
Diagnosis
Haemorrhage–may be preretinal (retrohyaloid; Fig. 13.13 ), intragel, or both.
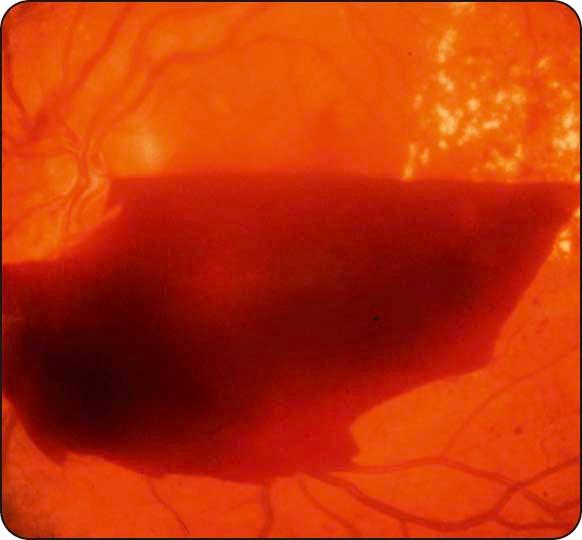
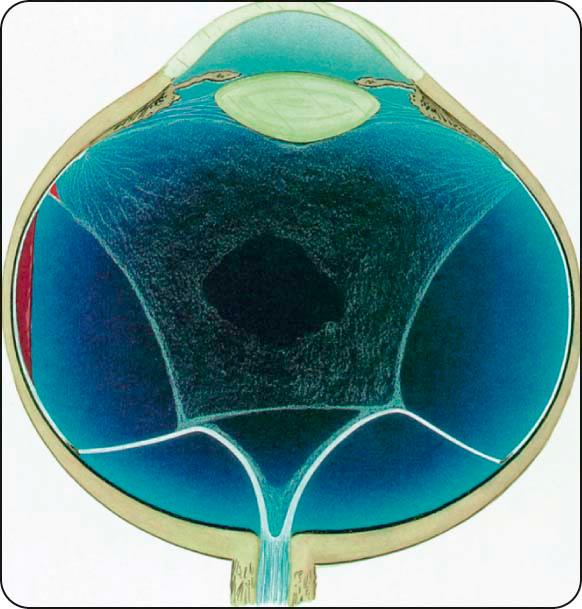
Tractional retinal detachment is caused by progressive contraction of fibrovascular membranes at areas of vitreoretinal attachment ( Fig. 13.14 ).
Rubeosis iridis; may lead to neovascular glaucoma.
Pars plana vitrectomy (PPV)
Indications ( Fig. 13.15 ): (a) severe persistent unilateral vitreous haemorrhage precluding adequate PRP, (b) severe bilateral vitreous haemorrhage, (c) progressive tractional retinal detachment (RD) threatening or involving the macula, (d) combined tractional and rhegmatogenous RD (urgent), and (e) dense persistent premacular subhyaloid haemorrhage.
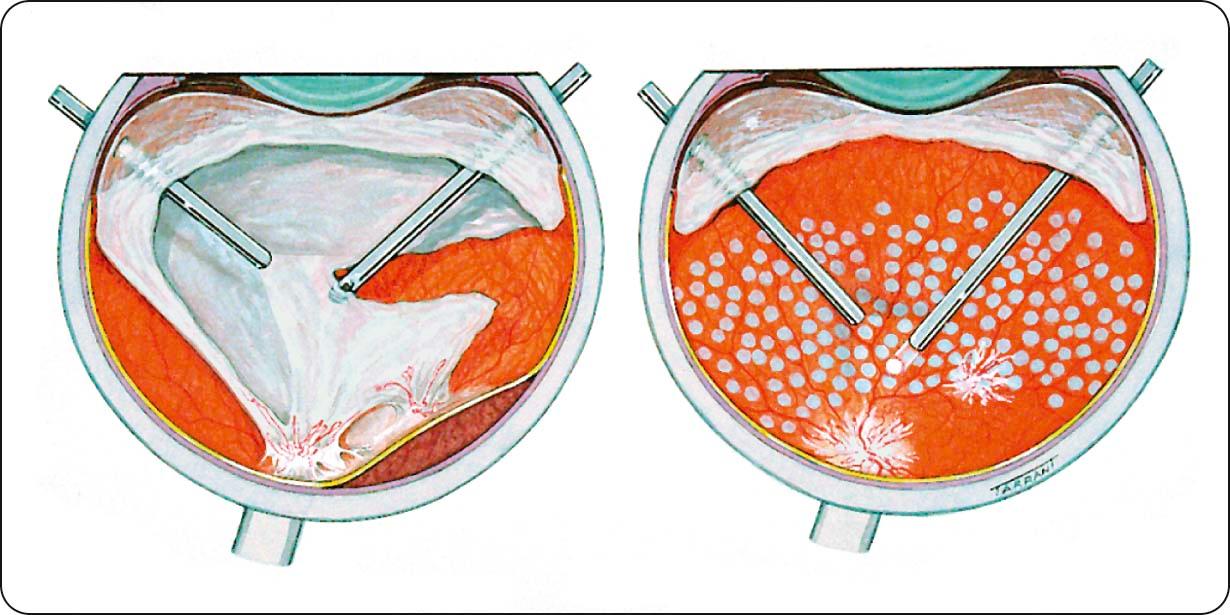
Visual results: 70% achieve visual improvement, 10% worsen, and the rest are unchanged.
Age: more than 50% of patients are older than 65 years.
Hypertension, particularly in branch retinal vein occlusion (BRVO).
Hyperlipidaemia.
Diabetes mellitus.
Oral contraceptive pill: the most common association in younger females.
Raised IOP in central retinal vein occlusion ( CRVO).
Smoking is a likely risk factor.
Uncommon predispositions may be more important under the age of 50 years; (a) myeloproliferative disorders, (b) acquired hypercoagulable states (e.g. hyperhomocysteinaemia, lupus anticoagulant, antiphospholipid antibodies), (c) inherited hypercoagulable states (e.g. activated protein C resistance, and protein C and protein S deficiencies), (d) inflammatory disease associated with occlusive periphlebitis (e.g. Behçet syndrome, sarcoidosis), and (e) miscellaneous causes (e.g. chronic renal failure, orbital disease, dehydration).
All patients: (a) blood pressure, (b) erythrocyte sedimentation rate (ESR), (c) full blood count (FBC), (d) blood glucose, (e) serum lipids, (f) plasma protein electrophoresis, (g) urea, electrolytes, and creatinine, (h) thyroid function tests (dysfunction associated), and (i) ECG (left ventricular hypertrophy associated).
Selected patients: (e.g. younger than 50 years, bilateral RVO, previous thromboses, family history of thrombosis); (a) chest X-ray, (b) C-reactive protein (CRP), (c) thrombophilia screen, (d) anticardiolipin antibody (IgG and IgM), lupus anticoagulant), (e) autoantibodies, (f) serum angiotensin-converting enzyme, (g) fasting plasma homocysteine, and (h) treponemal serology.
Diagnosis
Presentation: macular involvement may cause sudden onset of blurred vision, metamorphopsia, and a relative field defect; peripheral occlusions may be asymptomatic.
Acute signs: (a) dilatation and tortuosity of the affected venous segment, (b) flame-shaped and dot/blot haemorrhages, (c) retinal oedema, and (d) sometimes cotton wool spots affecting the drained retinal sector ( Fig. 13.16a ); site of occlusion is often identifiable at an arteriovenous crossing point.
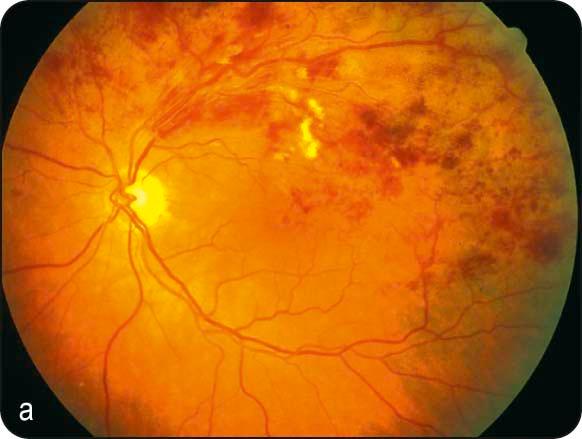
FA: variable delayed venous filling, masking by blood, vessel wall staining, hypofluorescence due to capillary nonperfusion, and ‘pruning’ of vessels in the ischaemic areas ( Fig. 13.16b ).
OCT: demonstrates and quantifies macular oedema.
Chronic signs: (a) hard exudates, (b) venous sheathing and sclerosis, (c) residual haemorrhage ( Fig. 13.17 ), and (d) collaterals that appear as slender tortuous venules developing locally or across the horizontal raphe between the inferior and superior vascular arcades.
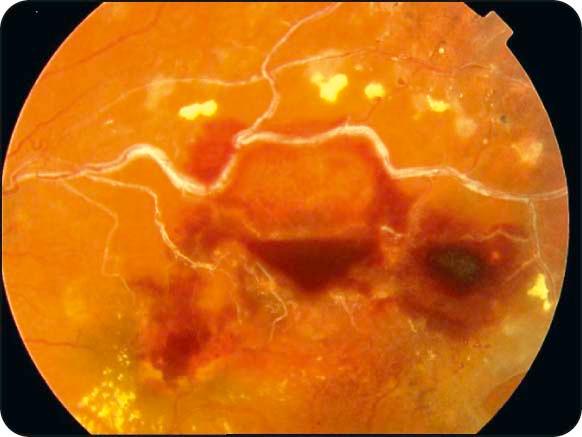
Treatment
Prognosis: 50% of untreated eyes retain 6/12 or better; 25% will be <6/60.
Complications: (a) chronic macular oedema—patients with visual acuity of 6/12 or worse may benefit from laser photocoagulation, provided the macula is not significantly ischaemic; (b) retinal neovascularization (usually NVE) develops in approximately 40%, often within 6–12 months, and can lead to recurrent vitreous and preretinal haemorrhage.
Follow-up: at 3 months with FA if vision is compromised, and provided retinal haemorrhages have cleared sufficiently.
Indications for laser: macular oedema associated with good macular perfusion and VA 6/12 or worse after 3–6 months.
Relative contraindications to laser: (a) VA less than 6/60, (b) symptoms for more than 1 year, and (c) macular ischaemia.
Subsequent follow-up: 3- to 6-monthly intervals for up to 2 years to detect neovascularization.
Treatment of macular oedema: (a) grid laser photocoagulation: spots of 50–100 µm, 0.1 s duration one burn width apart, to produce a gentle reaction in the area of leakage as identified on FA, up to the edge of the FAZ; (b) other options include intravitreal triamcinolone, intravitreal anti-VEGF agents, periocular steroid injection, and arteriovenous sheathotomy.
Neovascularization: not normally treated unless vitreous haemorrhage occurs because early treatment does not appear to affect the visual prognosis; if appropriate, involved area is treated with scatter laser photocoagulation (200–500 µm, 0.05–0.1 s, one burn width apart, medium reaction).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here