Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Restenosis is a pathologic response to injury that leads to narrowing of the vessel segment as a result of negative vascular remodeling and neointimal proliferation of vascular smooth muscle cells.
Clinical and angiographic predictors of stent restenosis include diabetes, nonsmoking status, female sex, acute coronary syndrome, previous percutaneous coronary intervention (PCI), saphenous vein graft disease, small vessel diameter, long lesions, high angiographic complexity, ostial location, and chronic total occlusions.
The lumen size achieved at the end of the procedure is an important modifiable predictor of restenosis.
The central roles of cellular proliferation and growth factor signaling within the restenotic lesion provide the basis for contemporary drug-eluting stent (DES) technology.
DES use has drastically reduced angiographic and clinical restenosis across broad lesion and patient subsets, but those with restenotic lesions, diabetes mellitus, bypass graft disease, and bifurcations continue to be problematic.
In contemporary trials using newer-generation DESs, adverse events are driven as much by progression of coronary artery disease (CAD) in untreated sites (de novo disease) as by target-lesion failure, with similar predictors.
Nature does nothing without purpose or uselessly. Aristoteles (384–322 BC)
Restenosis, the arterial wall healing response to mechanical injury, has plagued cardiologists since the introduction of balloon angioplasty by Gruntzig and collaborators. This chapter will describe our current understanding of the mechanisms, clinical features, impact, and treatment of restenosis in contemporary percutaneous coronary intervention (PCI).
The initial consequences of balloon angioplasty or coronary stenting are de-endothelialization, mechanical disruption of atherosclerotic plaque, often with dissection into the tunica media and occasionally adventitia, and stretch of the entire artery. Endothelial injury, platelet aggregation, inflammatory cell infiltration, release of growth factors, medial smooth muscle cell (SMC) modulation and proliferation, proteoglycan deposition, and extracellular matrix remodeling are the major milestones in the temporal sequence of the response to this trauma. In the majority of patients, the healing response includes both stabilization of the SMC structures and reendothelialization of the artery without significant reduction in vessel diameter. In contrast, restenosis is a pathophysiologic response to injury, which leads to narrowing of the vessel segment, due to negative vascular remodeling and/or neointimal proliferation ( Fig. 34.1 ). Neointimal hyperplasia (NIH) ultimately is a result of inappropriate migration and proliferation of vascular SMC, and is driven by multiple factors ( Fig. 34.2 ). Bare-metal stents (BMS) were developed to mitigate elastic recoil and negative remodeling, but they remain prone to NIH. Drug-eluting stents (DES) were developed to prevent NIH, and these devices (especially first-generation DES) can be accompanied by delayed reendothelialization, which has been associated with stent thrombosis. Thus, the durability of coronary interventions largely depends on a healthy healing response to arterial injury. While a great deal is known about the general response to injury, what remains less clear are the particular mechanisms that differentiate an optimal “healthy” injury pattern leading to reendothelialization without NIH from a pathologic one resulting in either NIH or incomplete reendothelialization.
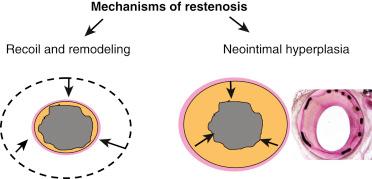
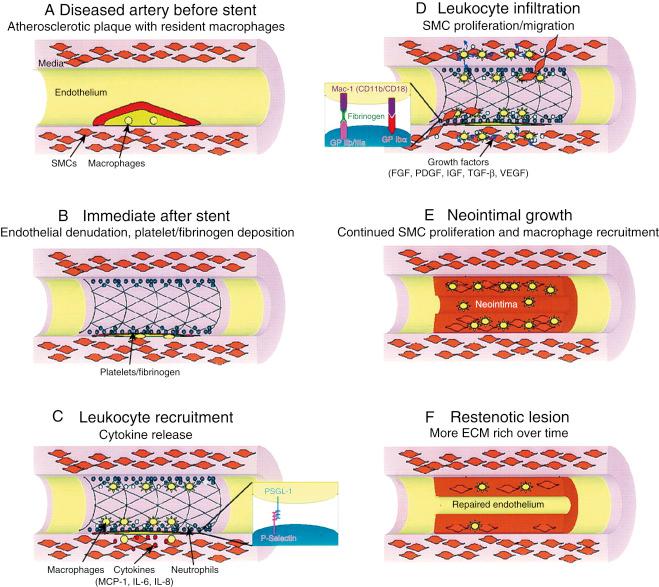
In animal models, denuding the endothelium is sufficient for the development of NIH. Several mechanisms appear to directly link endothelial activation to restenosis. First, nitric oxide-mediated responses to flow and shear stress provide a protective response. Therefore, the loss of a functional endothelium likely contributes to pathological healing. Second, endothelial injury leads to the production of cytokines such as platelet derived growth factor (PDGF) and transforming growth factor β (TGF-β) which can induce migration and proliferation of vascular smooth muscle cell (VSMC). Endothelial response to injury also promotes platelet adhesion. Platelet activation and deposition has been shown to occur almost immediately after endothelial injury in vivo, and results in the platelet production of cytokines and growth factors including PDGF. Elevated platelet reactivity measured at the time of PCI has been associated with increased restenosis rates after balloon angioplasty.
The central role that inflammatory pathways play in the pathophysiology of restenosis is also generally well accepted. After a layer of platelets and fibrin are deposited at the injured site, adhesion molecules such as P-selectin on platelets attach to circulating leukocytes via receptors such as P-selectin glycoprotein ligand (PSGL-1) to promote leukocyte rolling along the injured surface. Leukocytes then bind tightly to platelets through the interaction of leukocyte Mac-1 with platelet glycoprotein (GP) Ibα and through leukocyte integrin cross linking with fibrinogen which is bound to the platelet GP IIb/IIIa receptor. Migration of leukocytes occurs across the platelet-fibrin layer as these cells diapedese through tissue, driven by chemical gradients of chemokines released from SMCs and resident macrophages.
The precise cellular and molecular mechanisms of inflammation following arterial injury are dependent on the specific type of injury (i.e., atherogenic vs. balloon mechanical vs. stent). For example, experimental stent deployment in animal arteries causes sustained elevation of monocyte chemotactic protein-1 (MCP-1) postinjury (∼14 days) compared to balloon-injured arteries (<24 hours). Antibody-mediated blockade of C-C chemokine receptor 2 (CCR2), a primary leukocyte receptor for MCP-1, markedly diminished neointimal thickening after stent-induced, but not balloon-induced, injury in nonhuman primates.
Inflammatory responses to the stent itself have also been proposed. Innate immune responses, which have a predominance of monocyte/macrophage infiltrates, have been described. Antigen-specific adaptive immune hypersensitivity responses typified by infiltration of T-cells and B-cells in conjunction with eosinophils may also play a role in restenosis (reviewed by Byrne et al. ). The corrosion of stent material, leading to the activation of thrombospondin-1-dependent inflammatory pathways may also play a role in the restenotic processes following stenting.
Investigators have pursued antirestenosis strategies using systemic antiinflammatory therapies, including liposome-encapsulated bisphosphonates, prednisone, anti-CD18 or anti-CCR2 blockade, and peroxisome proliferator-activated receptor (PPAR-γ) activator rosiglitazone. Experimental observations support a causal relationship between inflammation and experimental restenosis. Antibody-mediated blockade or selective absence of Mac-1 diminished leukocyte accumulation and limited neointimal thickening after experimental angioplasty or stent implantation. Corticosteroids have been shown to reduce the influx of mononuclear cells, to inhibit monocyte and macrophage function, and to influence SMC proliferation. However, clinical trials with systemic corticosteroids to prevent restenosis have shown disappointing results.
The SMC is vital to the healing process after arterial injury due to its ability to migrate, proliferate, and synthesize extracellular matrix (ECM) upon stimulation. Growth factors released from platelets, leukocytes, and SMC stimulate a granulation or cellular proliferation phase. SMCs from the media and adventitia migrate into the intima layer. Aided by fracture of the internal elastic membrane, these cells migrate into the intima, where they shift from a contractile to the synthetic phenotype. SMCs may proliferate from 24 hours to 2 to 3 months after vascular injury, returning to a contractile phenotype after this period. The PDGF is a potent promoter of this SMC migration. Adventitial myofibroblasts (an α-actin staining cell) also proliferate and migrate into the neointimal and appear to play an important role in supplying the intima layer with proliferative cellular elements for new lesion formation. Assessment of cellular proliferation status in atherectomy suggests that cells of the monocyte/macrophage lineage also proliferate within human in-stent restenotic tissue.
While cellular division is essential for the subsequent development of restenosis, so too is the synthesis of various collagen sub-types and proteoglycans. Over a longer period of time, the artery enters a phase of remodeling involving ECM protein degradation and resynthesis. Accompanying this phase is a shift to less cellular elements and greater production of ECM. This ECM actually constitutes the major component of the restenotic lesion; NIH has been shown to be predominately a low cellular tissue.
The central role of cellular proliferation and growth factor signaling within the restenotic lesion provided the basis for anti-restenosis pharmacological strategies targeting cell cycle division early after stent implantation. The clinical success targeting cellular division pathways illustrate the central role these pathways play in the formation of NIH.
In the absence of injury, SMCs are quiescent and exhibit very low levels of proliferative activity. However, during restenosis, SMCs that migrate to the intima progress through the G1/S transition of the cell cycle. The different phases of the cell cycle of eukaryotic cells are regulated by a series of protein complexes composed of cyclins (D, E, A, B), cyclin-dependent kinases (CDKs; CDK4, CDK2, p34 cdc2 ) and their cyclin-dependent kinase inhibitors (CKIs; p27 Kip1 , p70, p16 INK4 ). The function of CKIs is regulated by changes in their concentration as well as in their localization in the cell. SMC p27 Kip1 is downregulated after arterial injury when cell proliferation increases. p21 Cip1 is not observed in normal arteries, but is upregulated along with p27 Kip1 in later phases of arterial healing response and is associated with a significant decline in cell proliferation and an increase in procollagen and TGF-β synthesis. These findings suggest that p27 Kip1 and p21 Cip1 are endogenous regulators of G1 transit in vascular SMC and inhibit cell proliferation after arterial injury. p27 Kip1 and p21 Cip1 bind and alter the activities of cyclin D–, cyclin E–, and cyclin A–dependent kinases (CDK2) in quiescent cells, leading to failure of G 1 /S transition and cell cycle arrest. Overexpression of p27 Kip1 results in cell cycle arrest in the G1 phase. Conversely, inhibition of p27 Kip1 increases the number of cells in S phase.
The level of p27 Kip1 is also regulated by constituents of the ECM. Mature collagen (polymerized type 1 collagen) suppresses p70 S6k and has been shown to increase the levels of p27 Kip1 , while monomeric collagen, which is present during degradation of ECM in the synthesis phase of restenosis, downregulates p27 Kip1 . Consistent with its regulatory role in SMC proliferation and the pathobiology of restenosis, polymorphisms in P27 kip1 that enhance the production of this cell cycle inhibitor are associated with reduced SMC proliferation and decreased risk of restenosis following PCI.
Sirolimus (Rapamycin) was initially discovered in 1975 as an antifungal produced by Streptomyces hygroscopicus isolated from an Easter Island soil sample. Sirolimus binds the ubiquitously expressed FK506-binding protein 12 (FBP12) protein and blocks activation of the serine/threonine kinase named mammalian target of rapamycin (mTOR). Blockade of mTOR in this manner leads to inhibitory effects on cell-cycle kinases including p27, cyclins, and pRb phosphorylation resulting in cell cycle arrest at G1/S phase ( Fig. 34.3 ). In cellular models and animal experiments, sirolimus inhibits VSMC migration and proliferation and has important antiinflammatory effects.
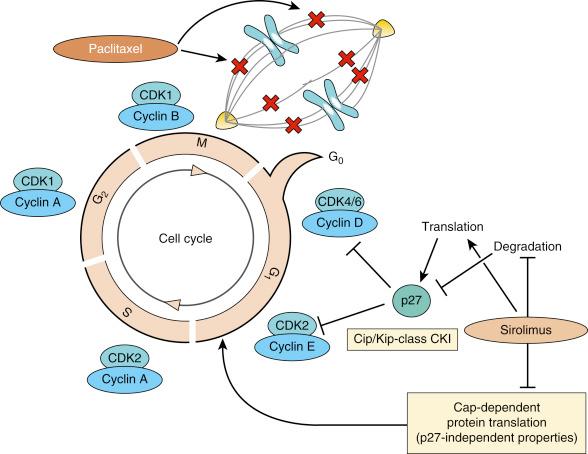
Newer generation DES have employed sirolimus analogues with only minor pharmacokinetic differences. For example, the chemical structure of zotarolimus differs from sirolimus at the carbon 40 position in which a tetrazole substitution is made in place of the hydroxyl group of sirolimus. This modification does not affect FKBP12 binding, nor in vitro T cell and VSMC effects. However, it does lead to more lipophilicity and a shorter half-life compared to sirolimus. Everolimus is another sirolimus analogue with a 2-hydroxyethyl substitution at position 40. It results in decreased in vitro potency but similar in vivo efficacy in immunosuppression and transplant models.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here