Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Diagnosis and treatment of most respiratory disorders depend heavily on understanding the basic physiological principles of respiration and gas exchange. Some respiratory diseases result from inadequate ventilation. Others are caused by abnormalities of diffusion through the pulmonary membrane or abnormal blood transport of gases between the lungs and tissues. Therapy is often entirely different for these diseases, so it is not satisfactory simply to make a diagnosis of “respiratory insufficiency.”
In the previous few chapters, we discussed several methods for studying respiratory abnormalities, including measuring vital capacity, tidal air, functional residual capacity, dead space, physiologic shunt, and physiological dead space. This array of measurements is only part of the armamentarium of the clinical pulmonary physiologist. Some other tools are described here.
Among the most fundamental of all tests of pulmonary performance are determinations of the blood partial pressure of oxygen (P o 2 ), carbon dioxide (CO 2 ), and pH. It is often important to make these measurements rapidly as an aid in determining appropriate therapy for acute respiratory distress or acute abnormalities of acid–base balance. The following simple and rapid methods have been developed to make these measurements within minutes, using no more than a few drops of blood.
Blood pH is measured using a glass pH electrode of the type commonly used in chemical laboratories. However, the electrodes used for this purpose are miniaturized. The voltage generated by the glass electrode is a direct measure of pH and is generally read directly from a voltmeter scale, or it is recorded on a chart.
A glass electrode pH meter can also be used to determine blood CO 2 . When a weak solution of sodium bicarbonate is exposed to CO 2 gas, the CO 2 dissolves in the solution until an equilibrium state is reached. In this equilibrium state, the pH of the solution is a function of the CO 2 and bicarbonate ion (HCO 3 − ) concentrations in accordance with the Henderson-Hasselbalch equation explained in Chapter 31 :
When the glass electrode is used to measure CO 2 in blood, a miniature glass electrode is surrounded by a thin plastic membrane. A solution of sodium bicarbonate of known concentration is in the space between the electrode and plastic membrane. Blood is then superfused onto the outer surface of the plastic membrane, allowing CO 2 to diffuse from the blood into the bicarbonate solution. Only a drop or so of blood is required. Next, the pH is measured by the glass electrode, and the CO 2 is calculated using the formula that was previously provided.
The concentration of O 2 in a fluid can be measured by a technique called polarography . Electric current is made to flow between a small negative electrode and the solution. If the voltage of the electrode is more than −0.6 volt different from the voltage of the solution, O 2 will deposit on the electrode. Furthermore, the rate of current flow through the electrode will be directly proportional to the concentration of O 2 (and therefore to PO 2 as well). In practice, a negative platinum electrode with a surface area of about 1 square millimeter is used, and this electrode is separated from the blood by a thin plastic membrane that allows diffusion of O 2 but not diffusion of proteins or other substances that will “poison” the electrode.
Often, all three of the measuring devices for pH, CO 2 , and P o 2 are built into the same apparatus, and all these measurements can be made within a minute or so using a single droplet-sized sample of blood. Thus, changes in the blood gas levels and pH can be followed almost moment by moment at the bedside.
In many respiratory diseases, particularly in asthma, the resistance to airflow becomes especially great during expiration, sometimes causing tremendous difficulty in breathing. This condition has led to the concept called maximum expiratory flow , which can be defined as follows. When a person expires with great force, the expiratory airflow reaches a maximum flow beyond which the flow cannot be increased any more, even with greatly increased additional force. This is the maximum expiratory flow. The maximum expiratory flow is much greater when the lungs are filled with a large volume of air than when they are almost empty. These principles can be understood by referring to Figure 43-1 .
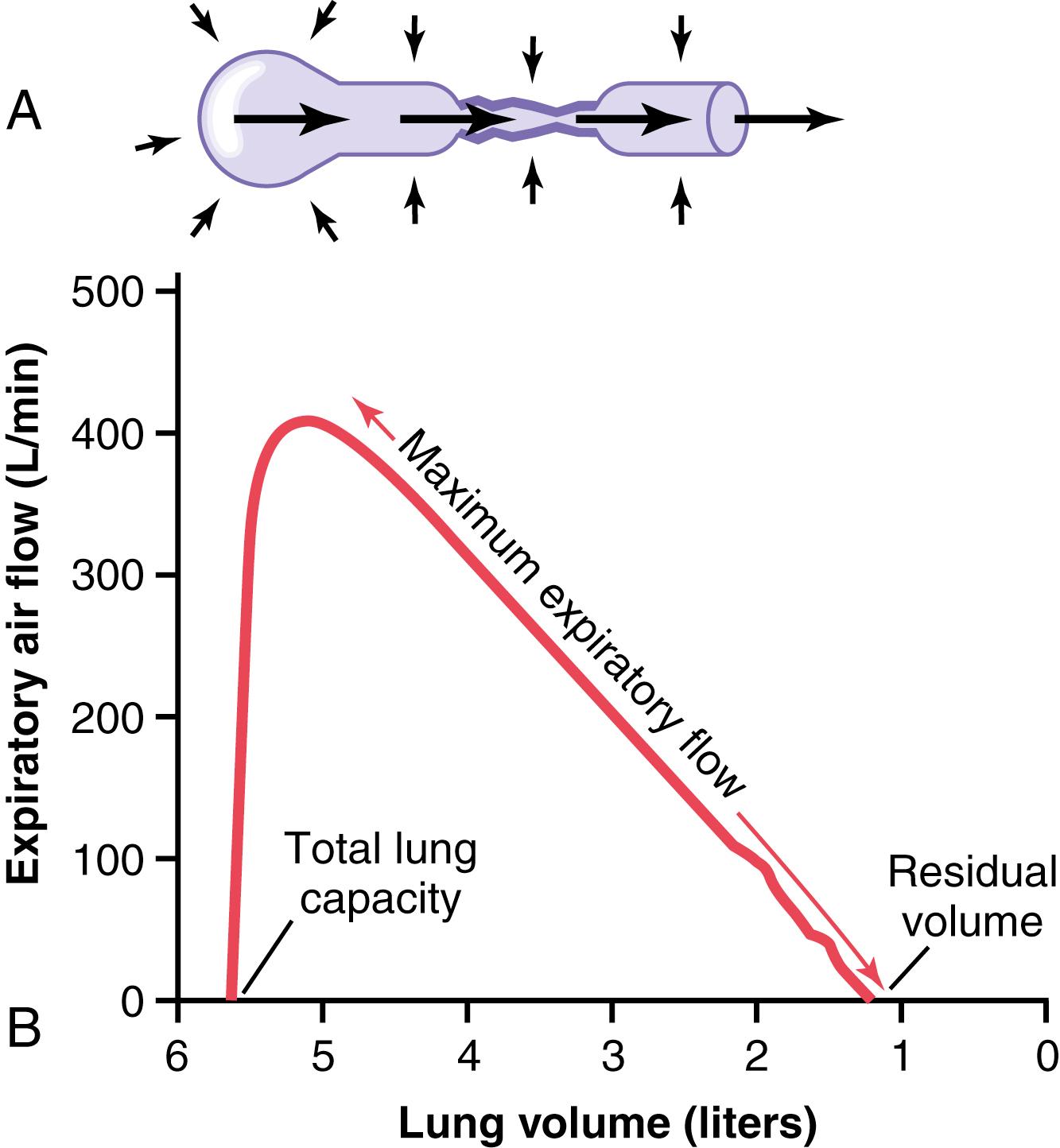
Figure 43-1A shows the effect of increased pressure applied to the outsides of the alveoli and air passageways caused by compressing the chest cage. The arrows indicate that the same pressure compresses the outsides of the alveoli and bronchioles. Therefore, not only does this pressure force air from the alveoli toward the bronchioles, but it also tends to collapse the bronchioles at the same time, which will oppose movement of air to the exterior. Once the bronchioles have almost completely collapsed, further expiratory force can still increase the alveolar pressure greatly, but it also increases the degree of bronchiolar collapse and airway resistance by an equal amount, thus preventing further increase in flow. Therefore, beyond a critical degree of expiratory force, a maximum expiratory flow has been reached.
Figure 43-1B shows the effect of different degrees of lung collapse (and therefore also of bronchiolar collapse) on the maximum expiratory flow. The curve recorded in this section shows the maximum expiratory flow at all levels of lung volume after a healthy person first inhales as much air as possible and then expires with maximum expiratory effort until he or she can expire at no greater rate. Note that the person quickly reaches a maximum expiratory airflow of more than 400 L/min. However, regardless of how much additional expiratory effort the person exerts, this is still the maximum flow rate that he or she can achieve.
Note also that as the lung volume becomes smaller, the maximum expiratory flow rate also becomes less. The main reason for this phenomenon is that in the enlarged lung, the bronchi and bronchioles are held open partially by way of elastic pull on their outsides by lung structural elements. However, as the lung becomes smaller, these structures are relaxed so that the bronchi and bronchioles are collapsed more easily by external chest pressure, thus progressively reducing the maximum expiratory flow rate as well.
Figure 43-2 shows the normal maximum expiratory flow-volume curve, along with two additional flow-volume curves recorded in two types of lung diseases: constricted lungs and partial airway obstruction. Note that the constricted lungs have both reduced total lung capacity (TLC) and reduced residual volume (RV). Furthermore, because the lung cannot expand to a normal maximum volume, even with the greatest possible expiratory effort, the maximal expiratory flow cannot rise to equal that of the normal curve. Constricted lung diseases include fibrotic diseases of the lung, such as tuberculosis and silicosis , and diseases that constrict the chest cage, such as kyphosis, scoliosis , and fibrotic pleurisy .
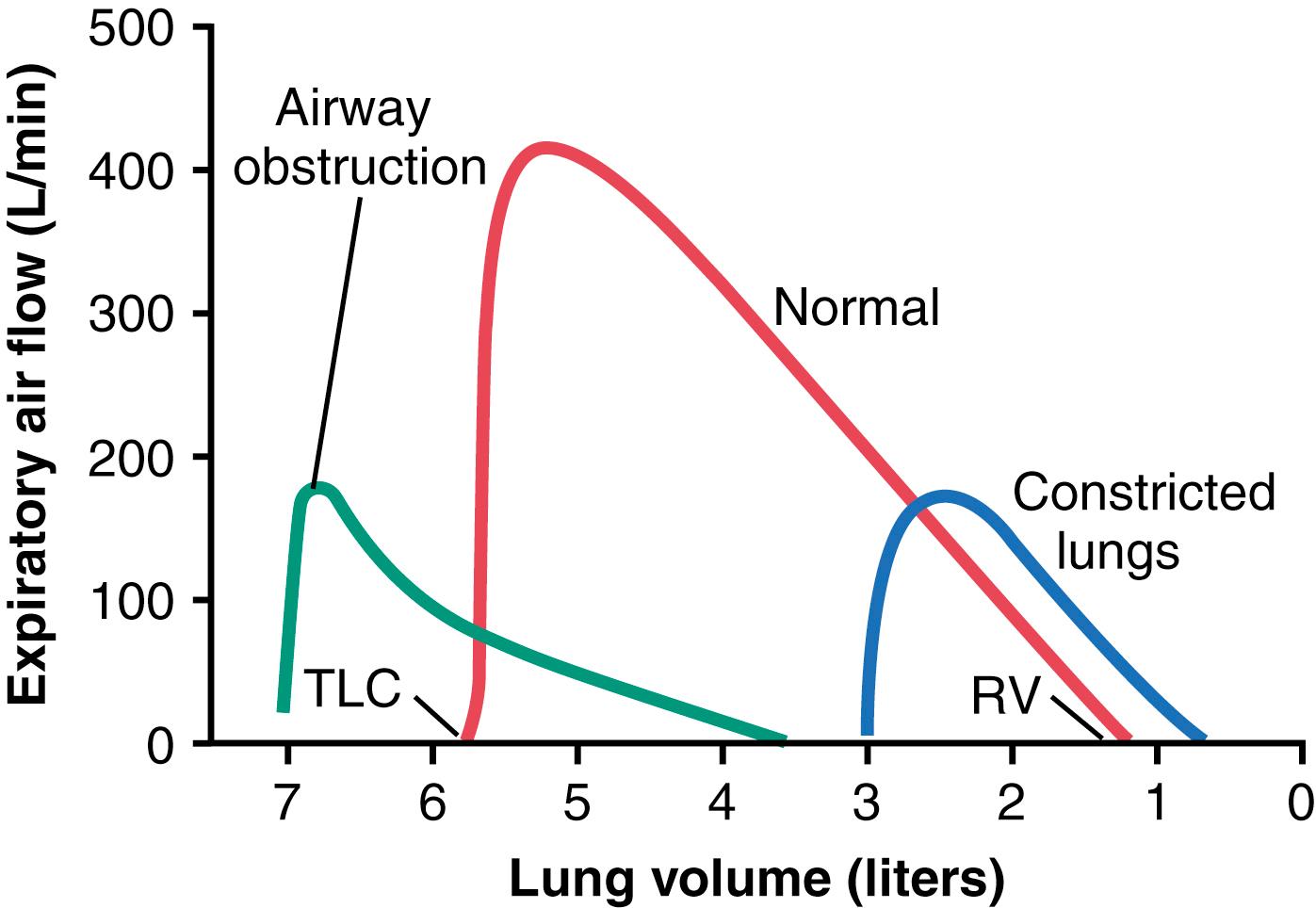
In diseases with airway obstruction , it is usually much more difficult to expire than to inspire because the closing tendency of the airways is greatly increased by the extra positive pressure required in the chest to cause expiration. By contrast, the extra negative pleural pressure that occurs during inspiration actually “pulls” the airways open at the same time that it expands the alveoli. Therefore, air tends to enter the lung easily but then becomes trapped in the lungs. Over a period of months or years, this effect increases both the TLC and RV, as shown by the green curve in Figure 43-2 . Also, because of the obstruction of the airways, and because they collapse more easily than normal airways, the maximum expiratory flow rate is greatly reduced.
The classic disease that causes severe airway obstruction is asthma . Serious airway obstruction also occurs in some stages of emphysema .
Another useful clinical pulmonary test, and one that is also easy to perform, is to record the forced expiratory vital capacity (FVC) on a spirometer. Such a recording is shown in Figure 43-3A for a person with normal lungs and in Figure 43-3B for a person with partial airway obstruction. In performing the FVC maneuver, the person first inspires maximally to the TLC and then exhales into the spirometer with maximum expiratory effort as rapidly and as completely as possible. The total distance of the downslope of the lung volume record represents the FVC, as shown in the figure.
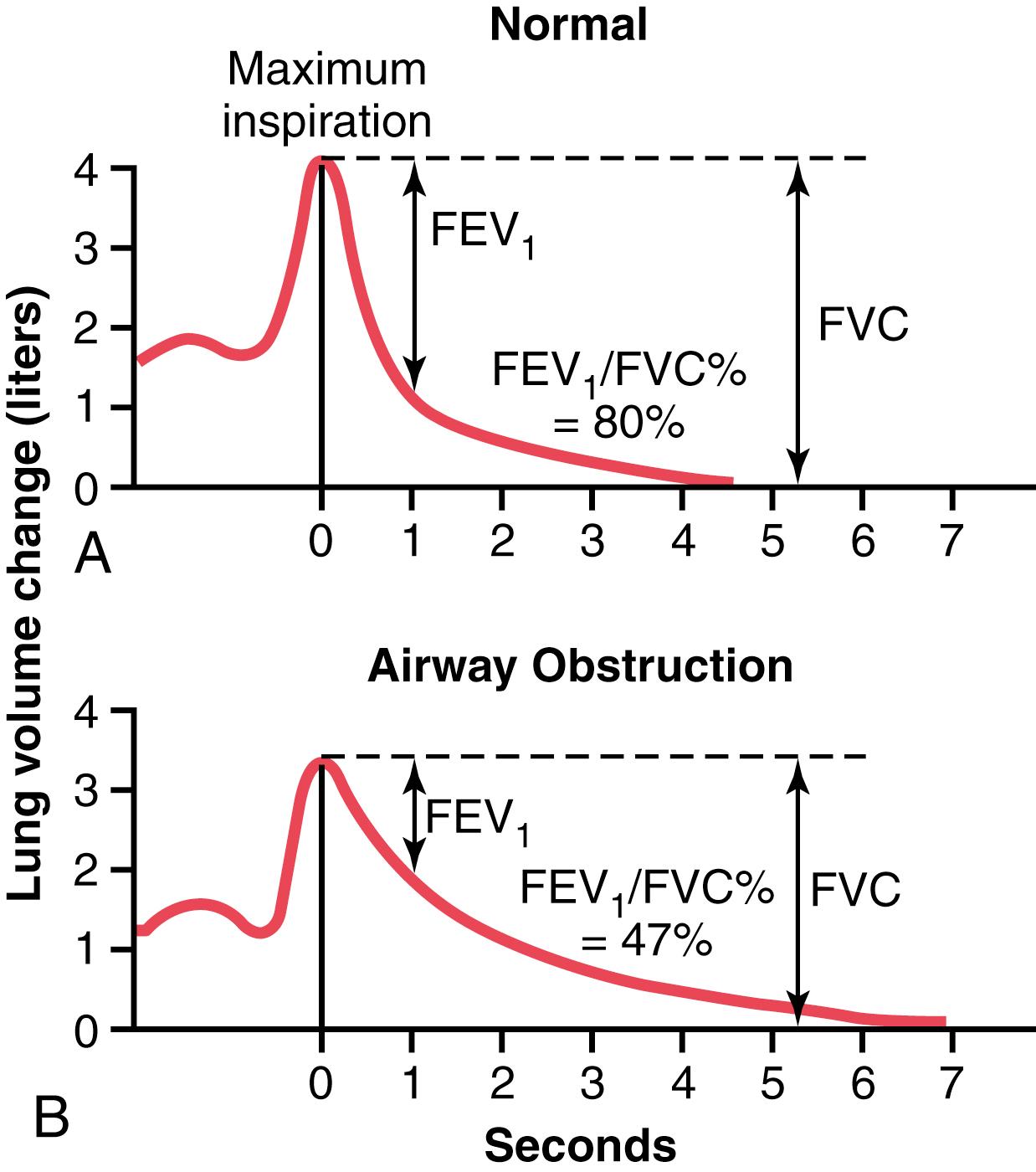
Now, study the difference between the two records for (1) normal lungs and (2) partial airway obstruction. The total volume changes of the FVCs are not greatly different, indicating only a moderate difference in basic lung volumes in the two persons. There is, however, a major difference in the amounts of air that these persons can expire each second , especially during the first second. Therefore, it is customary to compare the recorded forced expiratory volume during the first second (FEV 1 ) with the normal value. In the normal person (see Figure 43-3A ), the percentage of the FVC that is expired in the first second divided by the total FVC (FEV 1 /FVC%) is 80%. However, note in Figure 43-3B that with airway obstruction, this value decreases to only 47%. In persons with serious airway obstruction, as often occurs with acute asthma, this value can decrease to less than 20%.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here