Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Proper functioning of the cardiorespiratory system is imperative to achieve adequate oxygenation of maternal and fetal tissues. The maternal cardiorespiratory system undergoes significant changes during gestation to optimize oxygen delivery to the fetus and maternal tissues. Pulmonary disease is one of the most frequent maternal complications during pregnancy, and it may result in significant morbidity or mortality for the mother and her fetus. Depending on the specific diagnosis, other maternal complications of pregnancy may have an adverse or a positive effect on the pulmonary function of the gravida.
In this chapter, we briefly review the physiologic adaptations of the respiratory system that occur during gestation. Specific respiratory diseases that occur in pregnancy and the effects of the disease on pregnancy, and the effects of pregnancy on the disease, are discussed. The obstetrician should realize that most of the diagnostic tests that are useful in evaluating pulmonary function during gestation are not harmful to the fetus, and, if indicated, they should be performed. Most medications used to treat respiratory disease in pregnancy are also well tolerated by the fetus. With few exceptions, the diagnostic and treatment algorithms for respiratory disease in a pregnant woman closely resemble those used for a nonpregnant woman.
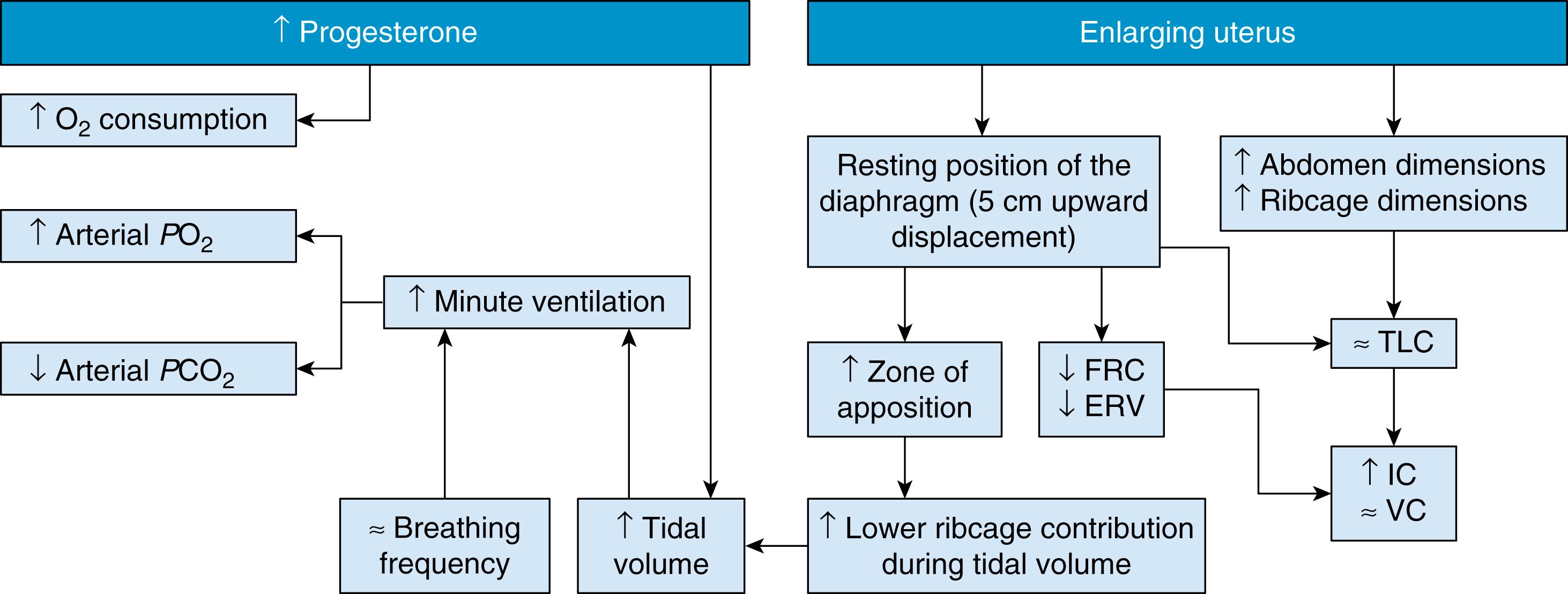
To accommodate both the increased metabolic demands of pregnancy and the physical changes from a gravid uterus, the respiratory system adapts with a higher minute ventilation to increase the availability of oxygen to tissues. There is no increase in respiratory rate; therefore the increase in maternal minute ventilation results from an increase in tidal volume. The almost 50% increase in tidal volume occurs at the expense of an 18% decrease in the functional residual capacity. The resulting hyperventilation of pregnancy results in a compensated respiratory alkalosis (i.e., arterial partial pressure of carbon dioxide [PaCO 2 ] ≤30 mm Hg) and a modest increase in arterial oxygenation tension (i.e., 101 to 104 mm Hg). The Pa co 2 decreases early in pregnancy in parallel with the change in ventilation; however, a further progressive decrease in Pa co 2 may occur. The decrease in Pa co 2 is even greater at higher altitudes, where the mother exhibits compensatory hyperventilation in an attempt to maintain the arterial partial pressure of oxygen as high as possible. The decrease in Pa co 2 is matched by an equivalent increase in renal excretion of bicarbonate with a resulting decrease in plasma bicarbonate concentration to maintain the arterial pH near the normal nonpregnant level of about 7.4.
It has been suggested that the hyperventilation of pregnancy results primarily from progesterone acting as a respiratory stimulant. Because hyperventilation has been observed during the luteal phase of the menstrual cycle and progesterone can produce similar changes in nonpregnant women, it is likely that this phenomenon results from progestational influences. , The Pa co 2 is linearly and inversely related to the log of the progesterone concentration. Wilbrand and colleagues reported that progesterone lowers the carbon dioxide threshold of the respiratory center. During pregnancy, the sensitivity of the respiratory center increases so that an increase in Pa co 2 of 1 mm Hg increases ventilation by 6 L/min in pregnancy, compared with 1.5 L/min in the nonpregnant state. , , It is possible that progesterone acts as a primary stimulant to the respiratory center independently of any change in carbon dioxide sensitivity or threshold. In addition to stimulating ventilation, progesterone may also increase levels of carbonic anhydrase B in the red blood cell. Schenker and associates reported that carbonic anhydrase levels increase in pregnant patients and in women taking oral contraceptives. An increase in the carbonic anhydrase level facilitates carbon dioxide transfer and tends to decrease Pa co 2 independently of any change in ventilation. This respiratory stimulant effect of progesterone has been used in the treatment of respiratory failure and emphysema. , ,
During gestation, ventilation is increased by the rise in tidal volume from approximately 500 to 700 mL in each breath. , Because there is no change in respiratory rate, minute ventilation rises from about 7.5 to 10.5 L/min. , , Minute ventilation increases in the first trimester and remains at that level throughout pregnancy. The physiologic dead space is increased by about 60 mL in pregnancy. This may result from dilation of the small airways. Residual volume is reduced by about 20%, from 1200 to 1000 mL. The vital capacity, which is the maximum volume of gas that can be expired after a maximum inspiration, does not change in pregnancy. ,
Observed changes in the configuration of the chest during pregnancy are in keeping with the findings of no change in vital capacity and a reduction in residual volume. The effect of pregnancy on pulmonary mechanics has been compared with the effect of a pneumoperitoneum. In both situations, the residual lung volume is decreased but ventilation remains unimpaired. Radiologic studies performed early in pregnancy have shown that the subcostal angle increases from 68 to 103 degrees before there is any mechanical pressure from the enlarging uterus. The level of the diaphragm rises by about 4 cm, and the transverse diameter of the chest increases by 2 cm. These changes account for the decrease in residual volume because the lungs are relatively compressed during forced expiration; however, the excursion of the diaphragm in respiration is increased by about 1.5 cm in pregnancy compared with the nonpregnant state. , The effect of pregnancy, via biochemical and mechanical pathways, on the pulmonary function, ventilatory pattern, and gas exchange is summarized in Fig. 58.1 .
All tissues require oxygen for the combustion of organic compounds to fuel cellular metabolism. The cardiopulmonary system delivers a continuous supply of oxygen and other essential substrates to tissues. Oxygen delivery depends on oxygenation of blood in the lungs, the oxygen-carrying capacity of the blood, and cardiac output. Under normal conditions, oxygen delivery exceeds oxygen consumption by about 75%. The amount of oxygen delivered is determined by the cardiac output (CO, in liters per minute) times the arterial oxygen content (Ca o 2 , in milliliters of O 2 per minute):
The arterial oxygen content is determined by the amount of oxygen that is bound to hemoglobin (i.e., arterial blood saturation with oxygen [Sa o 2 ]) and by the amount of oxygen that is dissolved in plasma (i.e., partial pressure of arterial oxygen [Pa o 2 × 0.0031]):
As can be seen in this formula, the amount of oxygen dissolved in plasma is negligible, and the arterial oxygen content therefore depends largely on hemoglobin concentration and arterial oxygen saturation. Oxygen delivery can be impaired by conditions that affect arterial oxygen content or cardiac output (flow), or both. Anemia leads to low arterial oxygen content because of a lack of hemoglobin binding sites for oxygen. Carbon monoxide poisoning likewise decreases oxyhemoglobin because of blockage of binding sites for oxygen. The patient with hypoxemic respiratory failure does not have sufficient oxygen available to saturate the hemoglobin molecule. Desaturated hemoglobin is altered structurally such that it has a diminished affinity for oxygen.
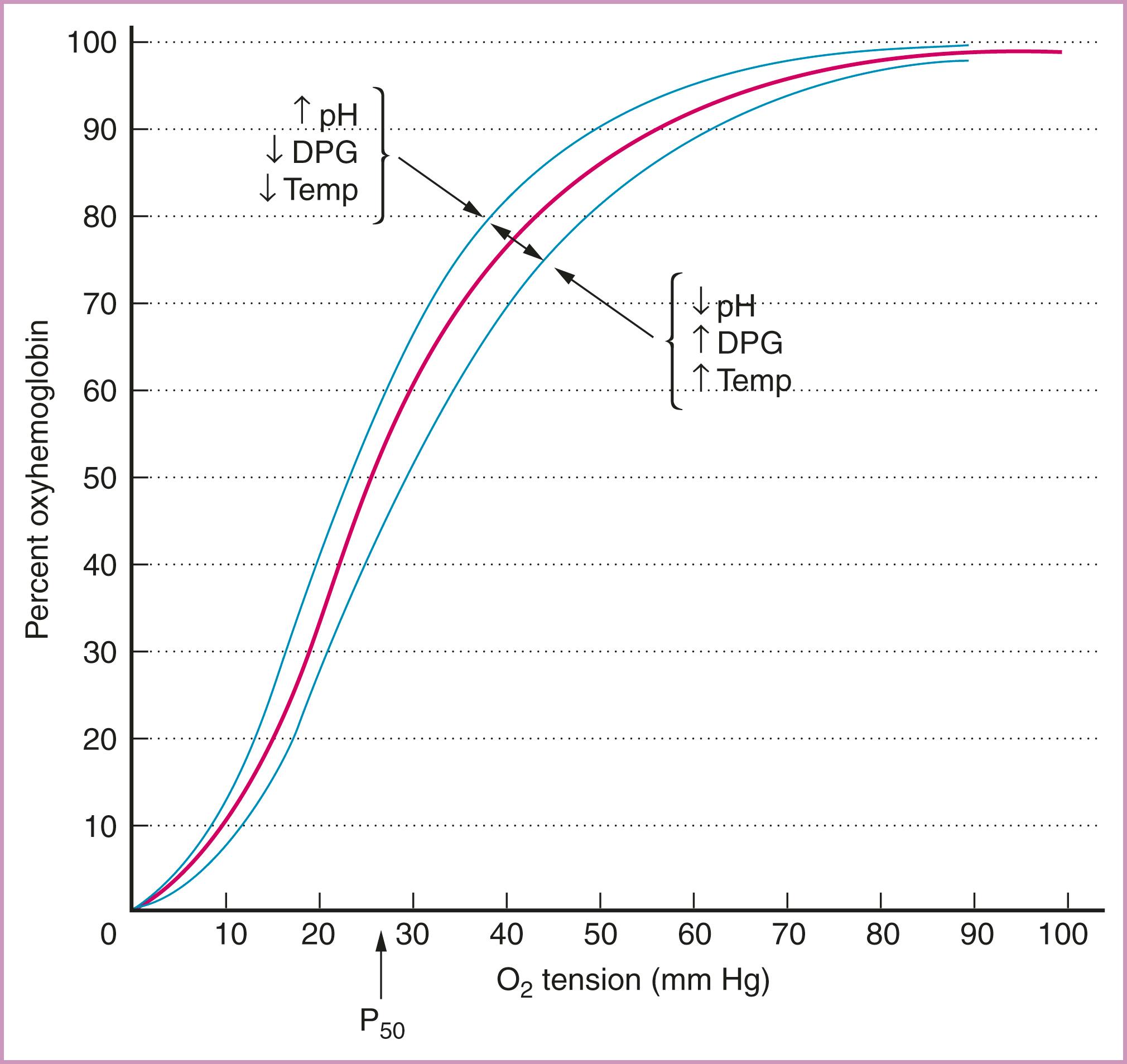
The amount of oxygen available to tissues also is affected by the affinity of the hemoglobin molecule for oxygen. The oxyhemoglobin dissociation curve ( Fig. 58.2 ) and the conditions that influence the binding of oxygen negatively or positively must be considered when attempts are made to maximize oxygen delivery. An increase in the plasma pH level or a decrease in temperature or the concentration of 2,3-diphosphoglycerate can increase hemoglobin affinity for oxygen. This Haldane effect shifts the curve to the left and results in uptake of oxygen by hemoglobin. If the plasma pH level decreases and the temperature or 2,3-diphosphoglycerate level increases, hemoglobin affinity for oxygen decreases, and more oxygen is available to tissues via the Bohr effect (see Fig. 58.2 ).
In certain clinical conditions, such as septic shock and adult respiratory distress syndrome (ARDS), there is maldistribution of flow relative to oxygen demand, leading to diminished delivery and consumption of oxygen. The release of vasoactive substances and microvascular thrombosis is hypothesized to result in the loss of normal mechanisms of vascular autoregulation, producing regional and microcirculatory imbalances in blood flow. This mismatching of blood flow with metabolic demand stems from tissue hypoperfusion and diminished oxygen delivery. The patient with diminished cardiac output resulting from hypovolemia or pump failure is unable to distribute oxygenated blood to tissues. Therapy directed at increasing the volume with normal saline, or with blood if the hemoglobin level is less than 10 g/dL, increases delivery of oxygen in the hypovolemic patient. The patient with cardiac failure may benefit from inotropic support and afterload reduction in addition to supplementation of intravascular volume.
Oxygen consumption is the product of the arteriovenous oxygen content difference (C (a−v) o 2 ) and cardiac output. Under normal conditions, oxygen consumption is a direct function of the metabolic rate :
The oxygen extraction ratio is the fraction of delivered oxygen that is actually consumed:
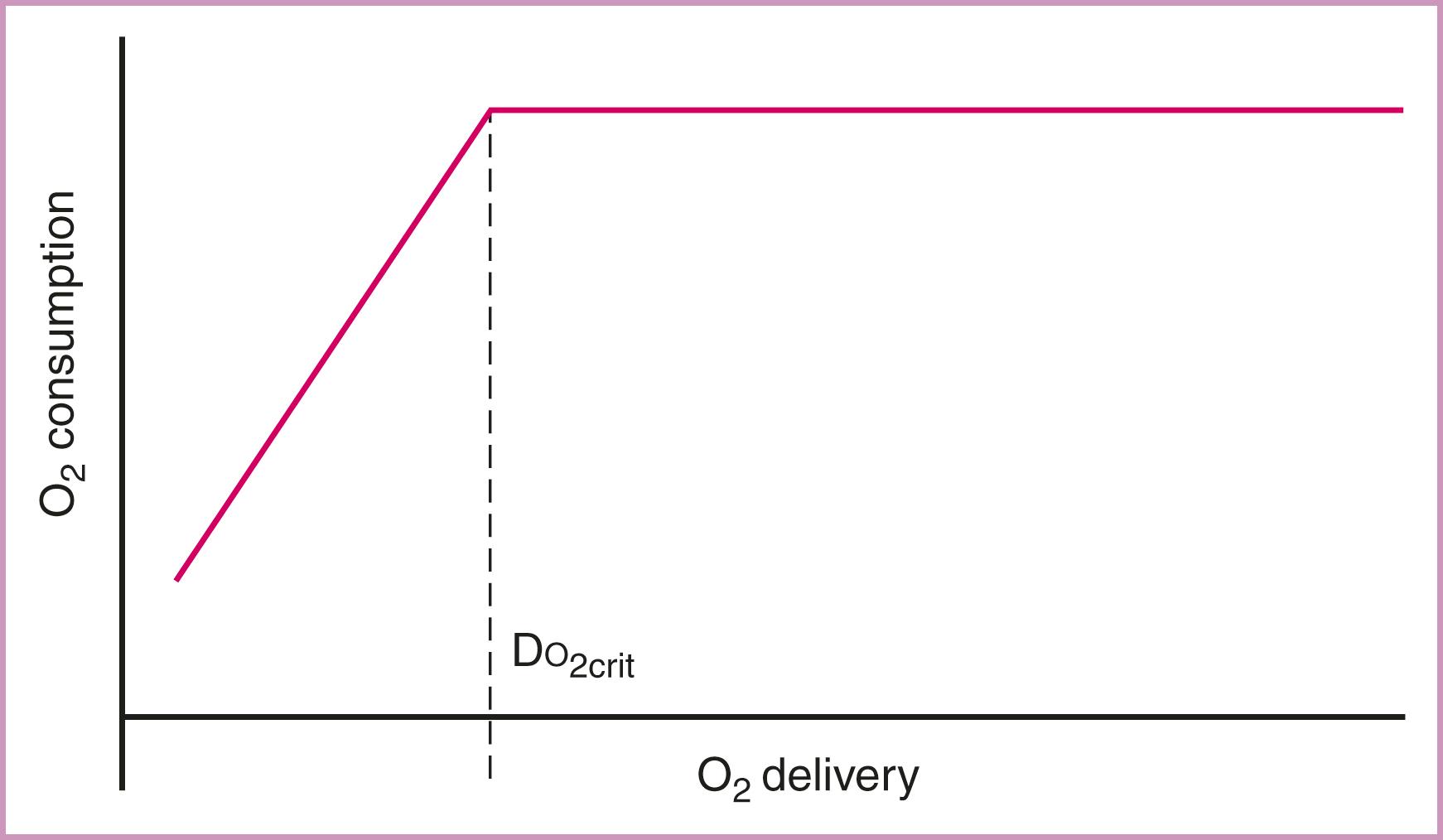
The normal oxygen extraction ratio is about 25%. A rise in the oxygen extraction ratio is a compensatory mechanism used when oxygen delivery is inadequate for the level of metabolic activity. A subnormal value suggests flow maldistribution, peripheral diffusion defects, or functional shunting. As the supply of oxygen is reduced, the fraction extracted from blood increases and oxygen consumption is maintained. If a severe reduction in oxygen delivery occurs, the limits of oxygen extraction are reached, tissues are unable to sustain aerobic energy production, and consumption decreases. The level of oxygen delivery at which oxygen consumption begins to decrease is called critical oxygen delivery ( Fig. 58.3 ). At the critical oxygen delivery level, tissues begin to use anaerobic glycolysis, with resultant lactate production and metabolic acidosis. If oxygen deprivation continues, irreversible tissue damage and death ensue.
The mixed venous oxygen tension and mixed venous oxygen saturation are parameters of tissue oxygenation. The
refers to the oxygen content of blood returning from the body to the heart after meeting tissue needs. The normal mixed venous oxygen tension is 40 mm Hg with a saturation of 73%. Saturations less than 60% are abnormally low. These parameters can be measured directly by obtaining a blood sample from the distal port of the pulmonary artery catheter. The
also can be measured continuously with special pulmonary artery catheters equipped with fiber optics. Mixed venous oxygenation is a reliable parameter in the patient with hypoxemia or low cardiac output, but findings must be interpreted with caution. When the
is low, oxygen delivery can be assumed to be low. However, normal or high
values do not guarantee that tissues are well oxygenated. In conditions such as septic shock and ARDS, the maldistribution of systemic flow may lead to an abnormally high
value in the face of severe tissue hypoxia. The oxygen dissociation curve must be considered when interpreting the
as an indicator of tissue oxygenation. Conditions that result in a left shift of the curve cause the venous oxygen saturation to be normal or high, even when the mixed venous oxygen content is low.
is useful for monitoring trends in a particular patient, because a significant decrease occurs when oxygen delivery has decreased because of hypoxemia or a decrease in cardiac output.
The physiologic anemia of pregnancy results in a reduction in the hemoglobin concentration and arterial oxygen-carrying capacity and content. Despite this hemodilution, oxygen delivery is maintained at or above normal because of the 50% increase in cardiac output. The pregnant woman therefore depends on cardiac output for maintenance of oxygen delivery more than the nonpregnant patient. Oxygen consumption increases steadily throughout pregnancy and is greatest at term, reaching an average of 331 mL/min at rest and 1167 mL/min with exercise. During labor, oxygen consumption increases by 40% to 60%, and cardiac output increases by about 22%. , Because oxygen delivery normally far exceeds consumption, the normal pregnant patient usually is able to maintain adequate delivery of oxygen to herself and her fetus, even during labor. When a pregnant patient has low oxygen delivery, she can very quickly reach the critical oxygen delivery level during labor, compromising herself and her fetus. The obstetrician therefore must make every effort to optimize oxygen delivery before allowing labor to begin in the compromised patient.
Pneumonia is fortunately a rare complication of pregnancy. , It is estimated to affect 0.2 to 8.5 per 1000 deliveries—impacting 1 to 6.7 per 1000 pregnancies. , Although rare, pneumonia contributes to considerable maternal mortality and is reportedly the most common nonobstetric infection to cause maternal mortality in the peripartum period. Maternal mortality was as high as 24% before the introduction of antibiotic therapy. Research reports have documented a dramatic decrease in maternal mortality to 0% to 4% with modern management and antibiotic therapy. , , Preterm delivery is a significant complication of pneumonia. Even with antibiotic therapy and modern management, preterm delivery continues to occur for 4% to 43% of gravidas who have pneumonia. , ,
There is an increasing incidence of pneumonia in pregnancy that may reflect the declining general health status of certain segments of the childbearing population (e.g., morbid obesity). The epidemic of human immunodeficiency virus (HIV) infection has increased the number of mothers who are potentially at risk for opportunistic lung infections. HIV infection is also associated with an increased risk for invasive pneumococcal disease (odds ratio [OR] = 41.8) and Legionnaires’ disease (odds ratio [OR] = 41.8). HIV infection further predisposes the pregnant woman to the infectious complications of acquired immunodeficiency syndrome (AIDS). , Reported incidence rates range from 97 to 290 cases per 1000 HIV-infected persons per year. HIV-infected persons are 7.8 times more likely to develop pneumonia than non–HIV-infected individuals with similar risk factors. Women with medical conditions that increase the risk for pulmonary infection, such as cystic fibrosis, are living to childbearing age more frequently than in the past. This disorder contributes to the increased incidence of pneumonia in pregnancy.
Pneumonia can complicate pregnancy at any time during gestation and may be associated with preterm birth, poor fetal growth, and perinatal loss. Madinger and associates reported 25 cases of pneumonia occurring among 32,179 deliveries and observed that fetal and obstetric complications were much more common than in earlier studies. Preterm labor complicated 11 of 21 gestations. Eleven patients had pneumonia at the time of delivery. Preterm delivery was more likely for women who had bacteremia, needed mechanical ventilation, and had a serious underlying maternal disease. In addition to the complication of preterm labor, there were three perinatal deaths in this series. Berkowitz and LaSala reported 25 patients with pneumonia complicating pregnancy; 14 women had term deliveries, one delivered preterm, three had a voluntary termination of pregnancy, three had term deliveries of growth-restricted infants, and four were lost to follow-up. Birth weight was significantly lower in the study group in this series (2770 ± 224 g versus 3173 ± 99 gm in the control group; P < .01). In this series, pneumonia complicated 1 of 367 deliveries. The investigators attributed the increase in the incidence of pneumonia in this population to a decline in general health status, including anemia, a significant incidence of cocaine use (52% versus 10% of the general population), and HIV positivity (24% versus 2% of the general population) in the study group.
Pneumonia has been stratified into mild and severe categories by the American Thoracic Society. Severe pneumonia is defined as need for mechanical ventilation or septic shock. Additional criteria include three or more of the following: respiratory rate≥30 breaths/min, PaO2/FiO2 ratio≤250, multilobar infiltrates, confusion/disorientation, uremia (blood urea nitrogen level≥20 mg/dL), leukopenia (white blood cell count<4,000 cells/μL), thrombocytopenia (platelet count<100,000/μL), hypothermia (core temperature<36°C), and hypotension requiring aggressive fluid resuscitation. It has been proposed that pregnancy outcomes vary by severity of illness. In a series evaluating pregnancy outcomes of severe pneumonia in 12 women, 2/12 (17%) resulted in maternal death, 4/12 (33%) liver failure, 4/12 (33%) renal failure, 5/12 (42%) ARDS, 2/12 (17%) fetal demise, 6/7 (86%) preterm delivery, 1/7 (14%) neonatal death. This series also showed concurrent development of preeclampsia with severe features in 3/12 (25%) women. Pneumonia, especially severe cases, must be promptly evaluated and treated to reduce maternal and neonatal morbidity and mortality.
Most series describing pneumonia complicating pregnancy have used incomplete methodologies to diagnose the etiologic pathogens for pneumonia, relying primarily on cultures of blood and sputum. In most cases, no pathogen was identified; however, pneumococcus and Haemophilus influenzae remain the most common identifiable causes of pneumonia in pregnancy. , , Because comprehensive serologic testing has rarely been done, the true incidences of viral, legionella , and mycoplasma pneumonia in pregnancy are difficult to estimate. The data presented by Madinger, Benedetti, Berkowitz, and their respective colleagues all support pneumococcus as the predominant pathogen causing pneumonia in pregnancy, and H. influenzae as the second most common organism. , , In the series of Berkowitz and LaSala, one patient was infected with Legionella species.
Pneumonia in pregnancy has several nonbacterial causes, including mumps, infectious mononucleosis, swine influenza, influenza A, varicella, coccidioidomycosis, and other fungi. Varicella pneumonia can complicate primary varicella infections in 5.2% to 9% of infections in pregnancy, compared with 0.3% to 1.8% in the nonpregnant population. Influenza A has a higher mortality rate among pregnant women than among nonpregnant patients. The increase in virulence of viral infections reported in pregnancy may result from the alterations in maternal immune status that characterize pregnancy, including reduced lymphocyte proliferative response, reduced cell-mediated cytotoxicity by lymphocytes, and a decrease in the number of helper T lymphocytes. , Viral pneumonias can also be complicated by superimposed bacterial infection, particularly pneumococcus. In the 2009 influenza A (H1N1) pandemic, it was reported that approximately 34% of intensive care admissions were complicated by bacterial coinfection and 16% of nonintensive-care cases were complicated by bacterial coinfection.
Mendelson syndrome describes chemical pneumonitis resulting from the aspiration of gastric contents in pregnancy. Chemical pneumonitis can be superinfected with pathogens present in the oropharynx and gastric juices, primarily anaerobes and gram-negative bacteria. Mendelson’s original report of aspiration consisted of 44,016 nonfasted obstetric patients between 1932 and 1945, and more than one-half had received “operative intervention” with ether by mask without endotracheal intubation. He described aspiration in 66 cases (rate of 1 case per 667 patients). Although several of the patients were critically ill from their aspirations, most recovered within 24 to 36 hours, and only two died from this complication (rate of 1 death per 22,008 patients). A review described 37,282 vaginal deliveries: 85% were performed with general anesthesia by mask and without intubation, and 65% to 75% had ingested liquids or solid food within 4 hours of onset of labor. The investigators found five mild cases of aspiration (1 per 7456 patients) with no sequelae. Another report described one occurrence of “mild aspiration” without adverse outcome among 1870 women undergoing nonintubated peripartum surgery with intravenous ketamine, benzodiazepines, barbiturates, fentanyl, or some combination of these drugs. Soreide and colleagues observed four episodes of aspiration each during 36,800 deliveries and 3600 cesarean deliveries with no mortality. In fact, in an analysis of serious complications related to obstetric anesthesia covering 2004 to 2009, 96,127 cesarean deliveries were reviewed with neuraxial anesthesia provided in 90,795 deliveries and general anesthesia provided in 5,332 deliveries, and there were no aspiration events in the groups. On the basis of these data, most hospitals permit free intake of clear liquids during labor. The risk for aspiration, pneumonia, and death from general anesthesia appears to be very low. This may reflect the use of modern techniques and therapy to reduce gastric pH.
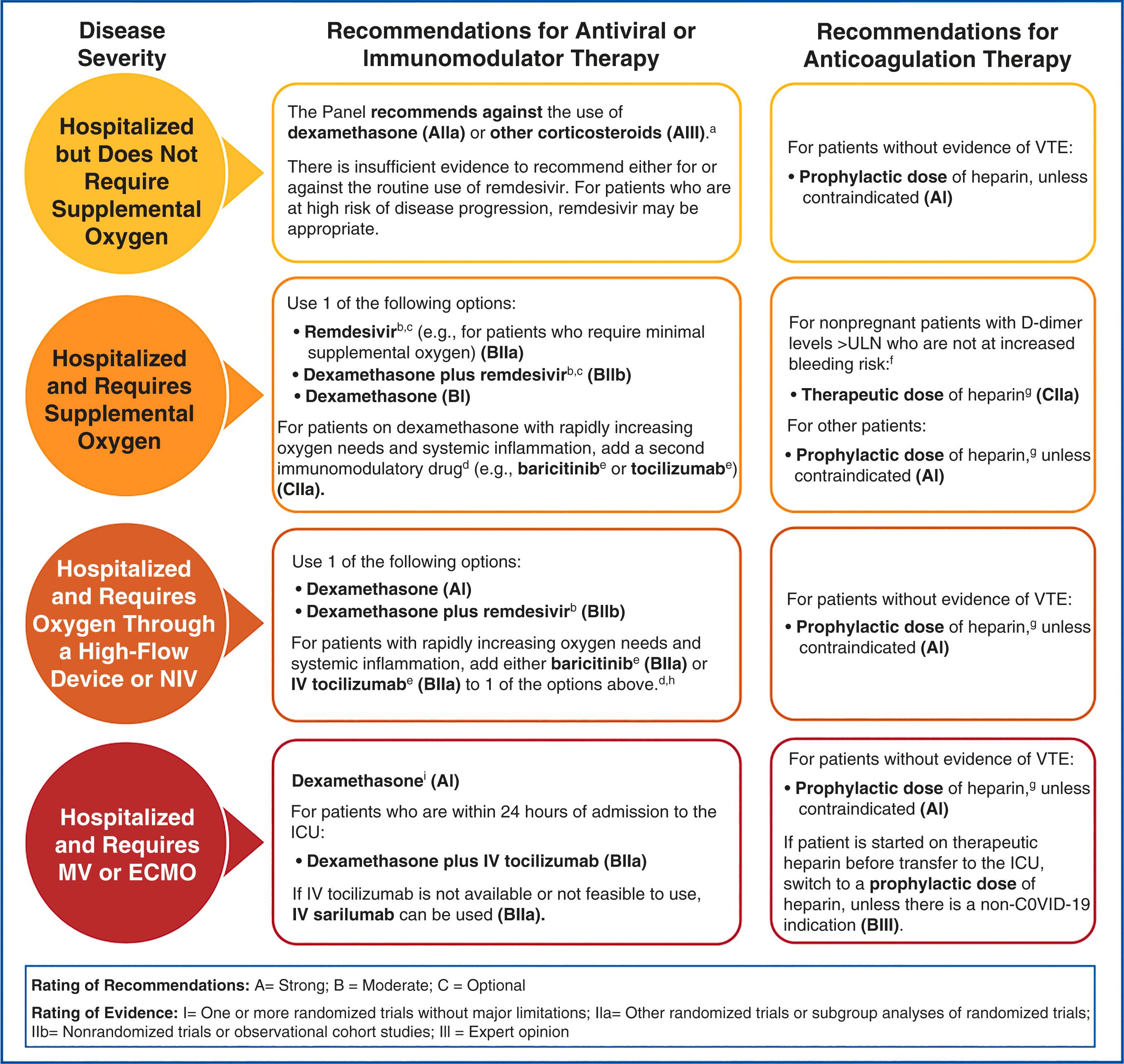
As noted, Streptococcus pneumoniae (pneumococcus) is the most common bacterial pathogen that causes pneumonia in pregnancy, and H. influenzae is the next most common. These pneumonias typically manifest as an acute illness accompanied by fever, chills, and a purulent, productive cough and are seen as a lobar pattern on the chest radiograph ( Fig. 58.3 ). Streptococcal pneumonia produces a “rusty” sputum, with gram-positive diplococci on Gram stain, and it demonstrates asymmetrical consolidation with air bronchograms on the chest radiograph. H. influenzae is a gram-negative coccobacillus that produces consolidation with air bronchograms, often in the upper lobes. Less common bacterial pathogens include Klebsiella pneumoniae, which is a gram-negative rod that causes extensive tissue destruction, with air bronchograms, pleural effusion, and cavitation seen on the chest radiograph. Patients with Staphylococcus aureus pneumonia present with pleuritis, chest pain, purulent sputum, and consolidation without air bronchograms identified on the chest radiograph.
Patients infected with atypical pneumonia pathogens, such as Mycoplasma pneumoniae, Legionella pneumophila, and Chlamydia pneumoniae , present with gradual onset of symptoms. They have a lower temperature, appear less ill, and have mucoid sputum, and a patchy or interstitial infiltrate is seen on the chest radiograph. The severity of the findings on the chest radiograph is usually out of proportion to the mild clinical symptoms. M. pneumoniae is the most common organism responsible for atypical pneumonia and is best detected by the presence of cold agglutinins, which appear in about 70% of cases.
The normal physiologic changes in the respiratory system associated with pregnancy result in a loss of ventilatory reserve. Coupled with the functionally altered immune response of pregnancy, this puts the mother and fetus at great risk from respiratory infection. Any gravida suspected of having pneumonia should be managed aggressively. The pregnant patient should be admitted to the hospital and a thorough investigation undertaken to determine the cause. One study examined 133 women admitted with pneumonia during pregnancy using protocols based on the British Thoracic Society and American Thoracic Society admission guidelines for management of nonpregnant individuals. The investigators reported that if the American Thoracic Society guidelines were used, 25% of the pregnant women with pneumonia could have avoided admission. Using the American criteria, none of the gravidas who would have been managed as an outpatient had any complications. If the British Thoracic Society guidelines had been used, 66% of the pregnant women in this group would have been assigned to outpatient therapy. However, 14% would have required readmission for complications. Most of the 133 women who were hospitalized with pneumonia in this study did not receive a chest radiograph for confirmation of the diagnosis. This limits the value of the study for guiding admission criteria for pneumonia in pregnancy. Until additional information is available, admission for all pregnant women with pneumonia is still recommended.
The workup should include a physical examination, arterial blood gas determinations, a chest radiograph, sputum Gram stain and culture, and blood cultures. The patient’s response to treatment and the clinician’s judgment should guide therapy. The success rates for identification of the bacterial cause with cultures range from 2.1% to approximately 50%, and the results can help tailor antibiotic choices. Urinary assays for pneumococcal antigen as well as Legionella antigen are also available and should be used in appropriate cases.
Percutaneous-transthoracic needle aspiration has been advocated as a valuable and safe method to increase the chance of establishing the causative agent for pneumonia. This test should be reserved for use in compromised individuals, suspected tuberculosis in the absence of a productive cough, selected cases of chronic pneumonia, pneumonia associated with neoplasm or a foreign body, suspected Pneumocystis jiroveci pneumonia, and suspected conditions that necessitate lung biopsy. Cold agglutinins and Legionella titers may also be useful. Empiric antibiotic coverage should be started, usually with a third-generation cephalosporin such as ceftriaxone or cefotaxime. Legionella pneumonia has a high mortality rate and sometimes manifests with consolidation, mimicking pneumococcal pneumonia. It is recommended that a macrolide, such as azithromycin, be added to the empiric therapy. Dual coverage has been demonstrated to improve response to therapy even for abbreviated macrolide regimens. This may reflect the added antiinflammatory effect of the macrolides. Azithromycin administration is an independent predictor of a positive outcome and reduced length of hospital stay for patients with mild to moderate community-acquired pneumonia. The use of macrolides to treat community-acquired pneumonia should be limited when possible, because their use has also been associated with increased penicillin resistance by S. pneumoniae .
When admission for pneumonia is required, there is evidence that inpatient and 30-day mortality rates have been reduced when antibiotics are administered in less than 8 hours. In fact, current evidence suggests that mortality at 1 year following emergency room presentation with clinical sepsis is reduced with administration of antibiotics in less than 1–3 hours. After the results of the sputum culture, blood cultures, Gram stain, and serum studies are obtained and a pathogen has been identified, the selection of antibiotic therapy can be narrowed and directed toward the identified cause. The third-generation cephalosporins are effective agents for most pathogens causing a community-acquired pneumonia. They are also effective against penicillin-resistant S. pneumoniae . The quinolones as a class have historically been avoided due to concerns for fetal cartilage damage in animal studies. However, with the emergence of highly resistant bacterial pneumonia, their use may be lifesaving and therefore justified in specific circumstances. The respiratory quinolones are effective against highly penicillin-resistant S. pneumoniae strains, and their use does not increase resistance. The respiratory quinolones include levofloxacin, gatifloxacin, and moxifloxacin. These agents are ideal for community-acquired pneumonia because they are highly active against penicillin-resistant strains of S. pneumoniae . They are also active against Legionella and the other atypical pulmonary pathogens. Another advantage is a favorable pharmacokinetic profile, such that blood or lung levels are the same whether the drug is administered orally or intravenously. Arguments against more extensive respiratory quinolone use are based on concerns about the potential for developing resistance, the variable incidence of Legionella, and cost. An additional caveat is that the respiratory quinolones are only partially effective against Mycobacterium tuberculosis . Evaluation for this infection should be done when considering the use of quinolones for pneumonia. If community-acquired methicillin-resistant S. aureus (CA-MRSA) pneumonia is suspected, vancomycin or linezolid should be added to empiric therapy. CA-MRSA is also susceptible to trimethoprim-sulfamethoxazole and is often resistant to β-lactam antibiotics. Additional therapy with clindamycin can be considered, as it has been shown to reduce production of staphylococcal exotoxins.
In addition to antibiotic therapy, oxygen supplementation should be given. Frequent arterial blood gas measurements should be obtained to maintain partial pressure of oxygen at 70 mm Hg, a level necessary to ensure adequate fetal oxygenation. Arterial saturation also can be monitored with pulse oximetry. When the gravida is afebrile for 48 hours and has signs of clinical improvement, an oral cephalosporin can be started, and intravenous therapy discontinued. A total of 10 to 14 days of treatment should be completed.
Pneumonia in pregnancy can be complicated by respiratory failure requiring mechanical ventilation. If this occurs, team management should include the obstetrician, a maternal-fetal medicine specialist, and an intensivist. In addition to meticulous management of the gravida’s respiratory status, she should be maintained in the left lateral recumbent position to improve uteroplacental perfusion. The viable fetus should be monitored with continuous fetal monitoring. If positive end-expiratory pressure greater than 10 cm H 2 O is required to maintain oxygenation, central monitoring with a pulmonary artery catheter should be instituted to adequately monitor volume status and maintain maternal and uteroplacental perfusion. There is no evidence that elective delivery results in overall improvement in respiratory function, so it should be reserved for the usual obstetric indications. However, if there is evidence of fetal compromise or profound maternal compromise and impending demise, delivery should be accomplished.
Pneumococcal polysaccharide vaccination prevents pneumococcal pneumonia in otherwise healthy populations with an efficacy of 65% to 84%. The vaccine is safe in pregnancy and should be administered to high-risk gravidas. Those at high risk include individuals with sickle cell disease with autosplenectomy, patients who have had a surgical splenectomy, and individuals who are immunosuppressed. Several studies have demonstrated an additional advantage to maternal immunization with the pneumococcal vaccine: a significant transplacental transmission of vaccine-specific antibodies in infants, measured at birth and at 2 months. Colostrum and breast milk antibodies are also significantly increased in women who have received the pneumococcal vaccine.
In the United States, influenza and influenza pneumonia are the ninth leading cause of death. In contrast to the general population, pregnant women seem to be at higher risk for influenza pneumonia. , Epidemiologic data from the 1918 to 1919 influenza A pandemic revealed a maternal mortality rate that approached 50% for pregnant women with influenza pneumonia. , Due to advances in medicine, the pregnancy-related mortality was much lower in the influenza A pandemic of 2009, confirmed as 12% of pregnancy-related deaths. Three types of influenza virus can cause human disease—A, B, and C—but most epidemic infections are caused by influenza A. Influenza A typically has an acute onset after a 1- to 4-day incubation period and first manifests as high fever, coryza, headache, malaise, and cough. In uncomplicated cases, results of the chest examination and chest radiograph are normal. If symptoms persist longer than 5 days, especially in a pregnant woman, complications should be suspected. Pneumonia may complicate influenza as the result of secondary bacterial infection or viral infection of the lung parenchyma. In the epidemic of 1957, autopsies demonstrated that pregnant women died most commonly of fulminant viral pneumonia, whereas nonpregnant patients died most often of secondary bacterial infection.
A large, nested, case-control study evaluated the rate of influenza-related complications over 17 influenza seasons among women enrolled in the Tennessee Medicaid system. This study demonstrated a high risk for hospitalization for influenza-related reasons in low-risk pregnant women during the last trimester of pregnancy. The study authors estimated that 25 of 10,000 women in the third trimester during the influenza season are hospitalized with influenza-related complications. A later, matched-cohort study using the administrative database of pregnant women enrolled in the Tennessee Medicaid system examined pregnant women between 25 and 44 years of age who were hospitalized with respiratory illness during the 1985 to 1993 influenza seasons. In this population of pregnant women, those with asthma accounted for one-half of all respiratory-related hospitalizations during the influenza season. Among pregnant women with diagnosis of asthma, 6% required respiratory hospitalization during the influenza season (OR = 10.63; 95% confidence interval [CI], 8.61 to 13.83) compared with women without a medical comorbidity. This study detected no significant increases in adverse perinatal outcome associated with respiratory hospitalization during flu season.
Data on pandemic 2009 influenza A (H1N1) suggest that pregnant women had an increased risk for hospitalization, ICU admission, and death. Furthermore, a study from the United Kingdom was performed comparing pregnancy outcomes in hospitalized women with H1N1 influenza with hospitalized women without infection. Of the 256 hospitalized women with H1N1 there was an increased risk of perinatal mortality rate (39 per 1000 births versus 7 per 10000 births, increased rate of stillbirth (27 versus 6 per 1000 births, P = .001), and preterm birth (aOR 4.0; 95% CI, 2.7 to 5.9). Pregnant women with initiation of treatment more than 4 days after symptom onset were more likely to be admitted to an ICU than those treated within 2 days after symptom onset. Therefore, a high index of suspicion, early diagnosis, and empiric treatment for suspected influenza are warranted in pregnancy.
Primary influenza pneumonia is characterized by rapid progression from a unilateral infiltrate to diffuse bilateral disease. The gravida may develop fulminant respiratory failure requiring mechanical ventilation and positive end-expiratory pressure. Aggressive therapy is indicated when pneumonia complicates influenza in pregnancy. Antibiotics should be started and directed at the likely pathogens that can cause secondary infection, including S. aureus, pneumococcus, H. influenzae, and certain enteric gram-negative bacteria. Antiviral agents, such as oseltamivir and zanamivir, should also be considered. It has been recommended that the influenza vaccine be given routinely to all gravidas in all trimesters of pregnancy to prevent the occurrence of influenza and the development of pneumonia. Women at high risk for pulmonary complications, such as those with asthma, chronic obstructive pulmonary disease, cystic fibrosis, and splenectomy, should be vaccinated early in pregnancy to prevent the occurrence of influenza and the development of secondary pneumonia. In addition to maternal protection, prospective studies have demonstrated higher cord blood levels of antibody to influenza in infants born to mothers immunized during pregnancy. There is a delay in the onset and decrease in severity of influenza in infants born with higher antibody levels.
In December 2019, a new coronavirus, known as severe acute respiratory syndrome coronavirus 2, emerged. Due to rapid worldwide spread of the virus, a pandemic of coronavirus disease, COVID-19, developed. This respiratory disease has variable presentation with asymptomatic, mild, severe, and critical disease. Symptoms typically include cough, fever, myalgia, headache, diarrhea, sore throat, and loss of taste or smell. In most cases, symptoms develop within 14 days of exposure with most developing within 4–5 days. Approximately 15% of symptomatic COVID-19 cases develop viral pneumonia. In a multicenter observational cohort study, the National Institute of Child Health and Human Development (NICHD) sought to investigate the association between disease severity and perinatal outcomes. Analysis of 1219 women across 33 US hospitals revealed a range of disease severity: 47% asymptomatic, 27% mild, 14% moderate, 8% severe, 4% critical. In addition, they determined that there is an increased risk for cesarean delivery (59.6% versus 34.0%; aRR = 1.57; 95% CI, 1.30 to 1.90), hypertensive diseases of pregnancy (40.0% versus 18.8%; aRR = 1.61; 95% CI, 1.18 to 2.20), and preterm birth (41.8% versus 11.9%; aRR = 3.53; 95% CI, 2.42 to 5.14).
Treatment options for COVID 19 are limited, and they are stratified by severity of disease according to the NIH ( https://www.covid19treatmentguidelines.nih.gov/therapeutic-management ). For patients with mild symptoms and within 5 days of onset, the oral antiviral Paxlovid (nirmatrelvir and ritonavir) may be considered. If Paxlovid is not available, a one-time IV course of sotrovimab or 3-day course of remdesivir have similar efficacy at preventing hospitalization and death. It is recommended that remdesivir and dexamethasone be administered to those hospitalized and requiring supplemental oxygen (noninvasive and high-flow). For patients with rapidly increased oxygen needs and systemic inflammation, a second immunomodulatory drug such as IV baricitinib or tocilizumab may be considered. Furthermore, it is recommended that those requiring invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO) be treated with dexamethasone plus IV tocilizumab or IV sarilumab ( Fig. 58.5 ). Treatment guidelines are constantly evolving to include the most evidence-based practices.
In 2020, the US Food and Drug Administration (FDA) authorized emergency release of Pfizer and Moderna messenger RNA (mRNA) vaccines for the prevention of symptomatic COVID 19. Pregnant women were not included in the original trials; however, it is recommended that the vaccines be offered to pregnant and postpartum women, especially those who are health care workers.
The varicella-zoster virus (VZV) is a DNA virus that usually causes a benign, self-limited illness in children, but it may infect up to 2% of all adults. VZV infection occurs in 0.7 of every 1000 pregnancies. Pregnancy may increase the likelihood of varicella pneumonia, complicating the primary infection. Varicella pneumonia typically develops several days after the rash onset and may be mild to severe. Radiographic evidence includes diffuse peribronchial infiltrates. Varicella pneumonia occurs most often in the third trimester, and the infection is likely to be severe. , , Prior to antiviral therapy the maternal mortality rate for varicella pneumonia was as high as 35% to 40%, compared with 11% to 17% for nonpregnant individuals. , With the use of acyclovir, data shows decreased mortality rates ranging from 11% to 14%. However, a later report documented 100% survival among 18 gravidas with varicella pneumonia who were treated with acyclovir. In this report, there was one intrauterine fetal death at 25 weeks’ gestation in a woman with varicella. In one report of 312 pregnancies, there was no increase in the number of birth defects and no consistent pattern of congenital abnormalities. In another report, 17 infants were delivered beyond 36 weeks’ gestation, and there was no evidence of neonatal varicella.
Zhang and colleagues carried out a recent large cohort study on all births using the United States Healthcare Cost and Utilization Project Nationwide Inpatient Sample database between 2003 and 2010. Descriptive statistics were used to measure baseline characteristics and outcomes of women with VZV infection. Multivariate logistic regression analyses were used to identify risk factors for the development of VZV-related morbidity and mortality. They identified 935 patients admitted for VZV infection among 7.7 million pregnancy admissions, representing an incidence of 1.21 cases/10,000 pregnancies (95% CI, 1.13 to 1.29). The incidence of VZV pneumonia was 2.5% (95% CI, 1.6 to 3.7). No maternal deaths were recorded during the 8-year study period. There were no significant risk factors identified for those who developed VZV pneumonia compared with those who had an uncomplicated VZV infection during pregnancy. They concluded that the incidence of VZV pneumonia and VZV infection associated with maternal death is significantly lower than previously estimated and may reflect better immunization and earlier interventions.
Varicella pneumonia usually manifests 2 to 5 days after the onset of fever, rash, and malaise and is heralded by the onset of pulmonary symptoms, including cough, dyspnea, pruritic chest pain, and hemoptysis. The severity of the illness varies from asymptomatic radiographic abnormalities to fulminant pneumonitis and respiratory failure ( Fig. 58.4 ).
All gravidas with varicella pneumonia should be aggressively treated with antiviral therapy and admitted to the ICU for close observation or intubation if indicated. Acyclovir, a DNA polymerase inhibitor, should be started. The early use of acyclovir was associated with an improved hospital course after the fifth day and a lower mean temperature, lower respiratory rate, and improved oxygenation. Treatment with acyclovir is safe in pregnancy. Among 312 pregnancies, there was no increase in the number of birth defects and no consistent pattern of congenital abnormalities. A dosage of 10 mg/kg given intravenously every 8 hours has been recommended.
Varicella vaccine is a live attenuated virus vaccine that was added to the universal childhood immunization schedule in the United States in 1995. The program of universal childhood vaccination against varicella in the United States has resulted in a sharp decline in the rate of death from varicella. However, varicella vaccine is not recommended for use in pregnancy. The overall decline in incidence of adult varicella infection because of childhood vaccination will probably result in a decreased incidence of varicella infection and varicella pneumonia during pregnancy.
A study assessed the risk for congenital varicella syndrome and other birth defects in offspring of women who inadvertently received varicella vaccine during pregnancy or within 3 months of conception. Fifty-eight women received their first dose of varicella vaccine during the first or second trimester. No cases (0%) of congenital varicella syndrome were identified among 56 live births (CI, 0 to 15.6). Among the prospective reports of live births, five congenital anomalies were identified in the susceptible cohort or the sample population as a whole. The investigator suggested that although the numbers in the study were small, the results should provide some reassurance to health care providers and women with inadvertent exposure before or during pregnancy.
Infection with the HIV virus significantly increases the risk for pulmonary infection. S. pneumoniae and H. influenzae are the most commonly isolated organisms. One report also identified Pseudomonas aeruginosa as a significant cause of bacterial pneumonia in HIV-infected individuals. Pneumocystis pneumonia, an AIDS-defining illness, occurs more frequently when the helper-T-cell count (CD4 + ) is less than 200 cells/mm 3 . Pneumocystis jiroveci pneumonia (PJP), formerly designated Pneumocystis carinii pneumonia (PCP), is the most common of the serious opportunistic infections in pregnant women infected with HIV. , P. jiroveci is the number one cause of pregnancy-associated AIDS deaths in the United States. Initial reports of PJP in pregnancy described a 100% maternal mortality rate. , However, in a 2001 review of 22 cases of PJP in pregnancy, the mortality rate was 50% (11 of 22 patients). This reported mortality rate is still higher than that reported for HIV-infected nonpregnant individuals. In that series, respiratory failure developed in 13 patients, and 59% required mechanical ventilation. The survival rate of gravidas requiring mechanical ventilation was 31%. In this series, maternal and fetal outcomes were better in cases of PJP that occurred during the third trimester of pregnancy.
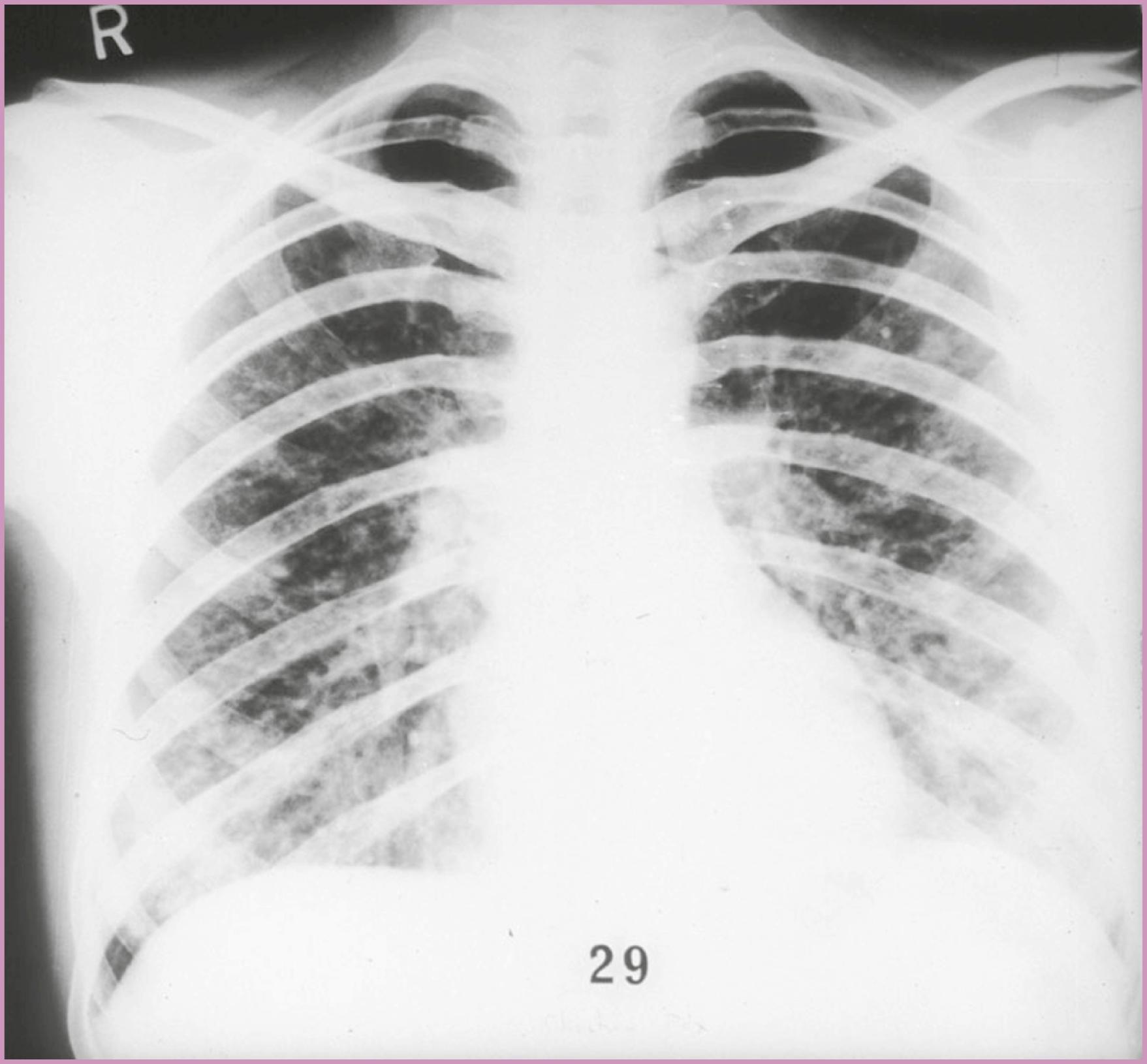
A high index of suspicion is necessary when gravidas at risk for HIV infection present with symptoms such as weight loss, fatigue, fever, tachypnea, dyspnea, and nonproductive cough. The onset of disease can be insidious, including normal radiographic findings, and it can then proceed to rapid deterioration. When the chest radiograph is positive, it typically exhibits bilateral alveolar disease in the perihilar regions and lower lung fields ( Fig. 58.6 ), which can progress to include the entire parenchyma. Diagnosis can be accomplished by means of sputum silver stains, bronchial aspiration, or bronchoscope-directed biopsy. Lung biopsy is recommended for definitive diagnosis.
Therapy for PJP in pregnancy includes trimethoprim-sulfamethoxazole (TMP-SMX). Gravidas with a history of PJP, a CD4 + lymphocyte count of less than 200/mm 3 , or oral pharyngeal candidiasis should receive prophylaxis. TMP-SMX is the drug of choice for prophylaxis and may provide cross-protection against toxoplasmosis and other bacterial infections. The usual dosage is one double-strength tablet (160 mg/m 2 of TMP and 800 mg/m 2 of SMX) given three times each week. Adverse reactions such as drug allergy, nausea, fever, neutropenia, anemia, thrombocytopenia, and elevated transaminase levels have been reported in 20% to 30% of nonpregnant individuals receiving TMP-SMX therapy. Complete blood cell count with a differential cell count and liver function tests should be obtained every 6 to 8 weeks to monitor for toxicity. Other regimens used for prophylaxis for individuals with intolerance to TMP-SMX include aerosolized pentamidine (300 mg every month by Respirgard II nebulizer) or dapsone (100 mg once daily). Hussain and colleagues found that the survival rate for patients treated with SMX alone was 71% (5 of 7 patients) and that the rate with SMX and steroids was 60% (3 of 5 patients); the overall survival rate for both groups was 66.6% (8 of 12 patients). The investigators concluded that PJP has a more aggressive course during pregnancy, with increased morbidity and mortality. However, treatment with SMX compared with other therapies may result in improved outcome. They also cautioned that withholding appropriate PJP prophylaxis may adversely affect maternal and fetal outcomes.
Initiation of therapy during the antepartum period can also prevent the rare occurrence of perinatally transmitted PJP. When a gravida is demonstrating symptoms consistent with a possible infection, a diligent search should be conducted to quickly identify PJP as the cause of pneumonia. When PJP is untreated, the maternal mortality rate can approach 100%. In summary, PJP pneumonia remains a dreaded complication of HIV infection and an AIDS-defining illness. There is a very high maternal and fetal mortality rate when PJP complicates pregnancy. Primary prophylaxis against Pneumocystis pneumonia with TMP-SMX in HIV-infected adults, including pregnant women and patients receiving highly active antiretroviral therapy, should begin when the CD4 + cell count is less than 200 cells/mm 3 or there is a history of oropharyngeal candidiasis. Prophylaxis should be discontinued when the CD4 + cell count increases to more than 200 cells/mm 3 for a period of 3 months.
Tuberculosis kills more than 1 million women per year worldwide, and it is estimated that 646 million women and girls are already infected with tuberculosis. In women between 15 and 44 years of age in developing countries, tuberculosis is the third most common cause of morbidity and mortality combined, and tuberculosis kills more women than any other infectious disease, including malaria and AIDS.
Epidemiologic information shows differences between men and women in prevalence of infection, rate of progression from infection to disease, incidence of clinical disease, and mortality resulting from tuberculosis. Case-notification rates from countries with a high prevalence of tuberculosis suggest that it may be less common among women. Seventy percent more smear-positive male than female tuberculosis patients are diagnosed every year and reported to the World Health Organization. Differences between men and women have also been shown in the development and outcome of active disease, with female cases having a higher rate of progression from infection to disease and a higher case-fatality rate. The conclusion of a research workshop on sex and tuberculosis was that a combination of biologic and social factors are responsible for these differences.
The incidence of tuberculosis in the United States began to decline in the early part of the 20th century and fell steadily until 1953, when the introduction of isoniazid (INH) led to a dramatic decrease in the number of cases, from 84,000 cases in 1953 to 22,255 cases in 1984. However, since 1984, there have been significant changes in tuberculosis morbidity trends. From 1985 to 1991, reported cases of tuberculosis increased by 18%, representing approximately 39,000 more cases than expected had the previous downward trend continued. This increase results from many factors, including the HIV epidemic, deterioration in the health care infrastructure, and more cases among immigrants. , Between 1985 and 1992, the number of tuberculosis cases among women of childbearing age increased by 40%. ,
A retrospective cohort study using National Inpatient Sample data from 2002–14 was performed to compare perinatal morbidity in those women with tuberculosis and those without. Using information from the 57,393,459 pregnancy admissions identified, a tuberculosis infection incidence of 7.1 per 100,000 pregnancy-related hospitalizations was determined. Hispanic women (12.9 per 100,000) and women born outside of the United States had the highest incidence of infection. The incidence of disease decreased across the study time frame with an annual percent decrease 3.6%, 2.7%, and 1.9% among Hispanic, Black, and White mothers, respectively. This is consistent with public health and CDC data that suggests a decrease in rates of TB over time with 2019 reports showing the lowest incidence rate recorded of 2.7 per 100,000 persons. Furthermore, the study demonstrated an increased risk of severe preeclampsia, eclampsia, placenta previa, postpartum hemorrhage, sepsis, and anemia in those women with active TB compared to those without. When comparing a composite of pregnancy complications in TB-positive and TB-negative women, TB-positive women had an 80% increased rate of pregnancy complications.
The emergence of drug-resistant tuberculosis has become a serious concern, with some strains showing resistant to multiple drugs (multidrug-resistant tuberculosis, MDR-TB), including the two most commonly used drugs, isoniazid (INH) and rifampin (RIF). in 2019, of the estimated 10 million new TB cases, an estimated 3.3% of new TB cases and 18% of previously treated cases had MDR-TB. Many centers advocate directly observed therapy in the treatment of multidrug-resistant disease. Pregnancy complicates treatment of multidrug-resistant tuberculosis for the following reasons:
Several antimycobacterial drugs are contraindicated during gestation.
Patients and physicians may fear the effects of chest radiography on the fetus.
Untreated, infectious, multidrug-resistant tuberculosis may be vertically and laterally transmitted.
Despite concern about teratogenicity of the second-line antituberculosis medications, careful timing of treatment initiation can result in clinical cure.
Most gravidas with tuberculosis in pregnancy are asymptomatic. All gravidas at high risk for tuberculosis ( Box 58.1 ) should be screened with either a tuberculin skin test (TST), such as the subcutaneous administration of intermediate-strength purified protein derivative (PPD), or with an interferon-gamma release assay (IGRA). IGRAs are in vitro blood tests of cell-mediated immune response. The sensitivity of the PPD is 90% to 99% for exposure to tuberculosis while IGRAs have a specificity greater than 95% for the diagnosis of latent tuberculosis infection. IGRA results can be available in 24 to 48 hours, no follow-up visit is required for diagnosis, and they are not affected by bacille Clamette-Guérin (BCG) vaccination status.
Human immunodeficiency virus infection
Immunosuppression
Close contact with persons known or suspected to have tuberculosis
Medical risk factors known to increase risk for disease if infected
Birth in a country with high tuberculosis prevalence
Medically underserved status
Low income
Alcohol addiction
Intravenous drug use
Residency in a long-term care facility (e.g., correctional institution, mental institution, nursing home or facility)
Health professionals working in high-risk health care facilities
If pulmonary TB infection is suspected, acid-fast bacilli (AFB) smear microscopy should be performed. Two direct amplification tests using molecular biology methods to detect M. tuberculosis in clinical specimens are also available: the Mycobacterium tuberculosis Direct (MTD) Test (Gen-Probe, San Diego, CA) and the Amplicor Mycobacterium tuberculosis (MTB) Test (Roche Diagnostic Systems, Branchburg, NJ). Both tests amplify and detect M. tuberculosis 16S ribosomal DNA. When testing acid-fast, stained smear-positive respiratory specimens, each test has a sensitivity and specificity of 95% and 100%, with a similar specificity if the acid-fast is negative, but a decreased sensitivity of 40%–77%
Immigrants from areas where tuberculosis is endemic may have received the BCG vaccine, and they are likely to have a positive response to the PPD. However, this reactivity should wane over time. The PPD should be used to screen these patients for tuberculosis unless their skin tests are known to be positive. If BCG vaccine was given 10 years earlier and the PPD is positive with a skin test reaction of 10 mm or more, the individual should be considered infected with tuberculosis and managed accordingly.
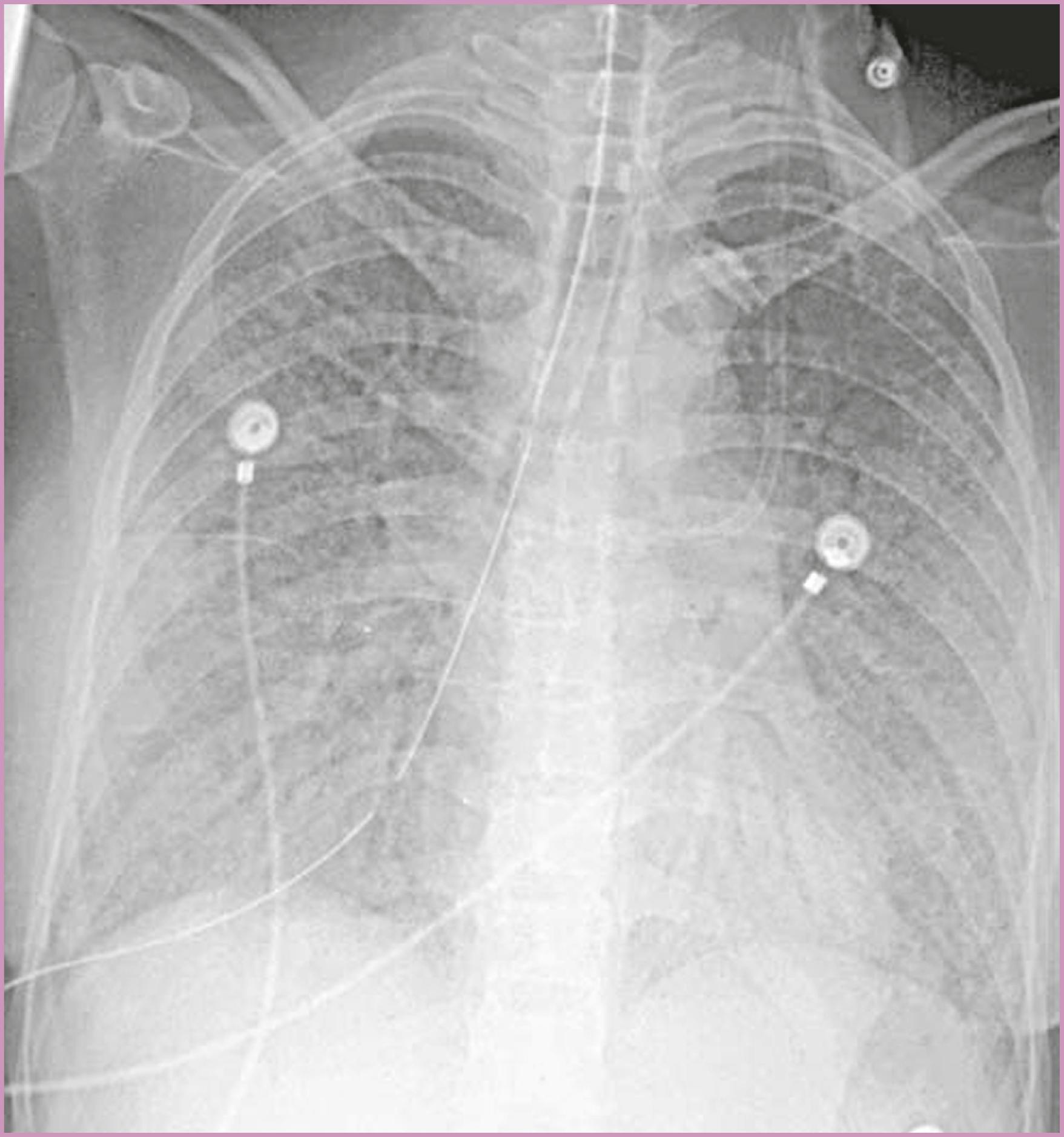
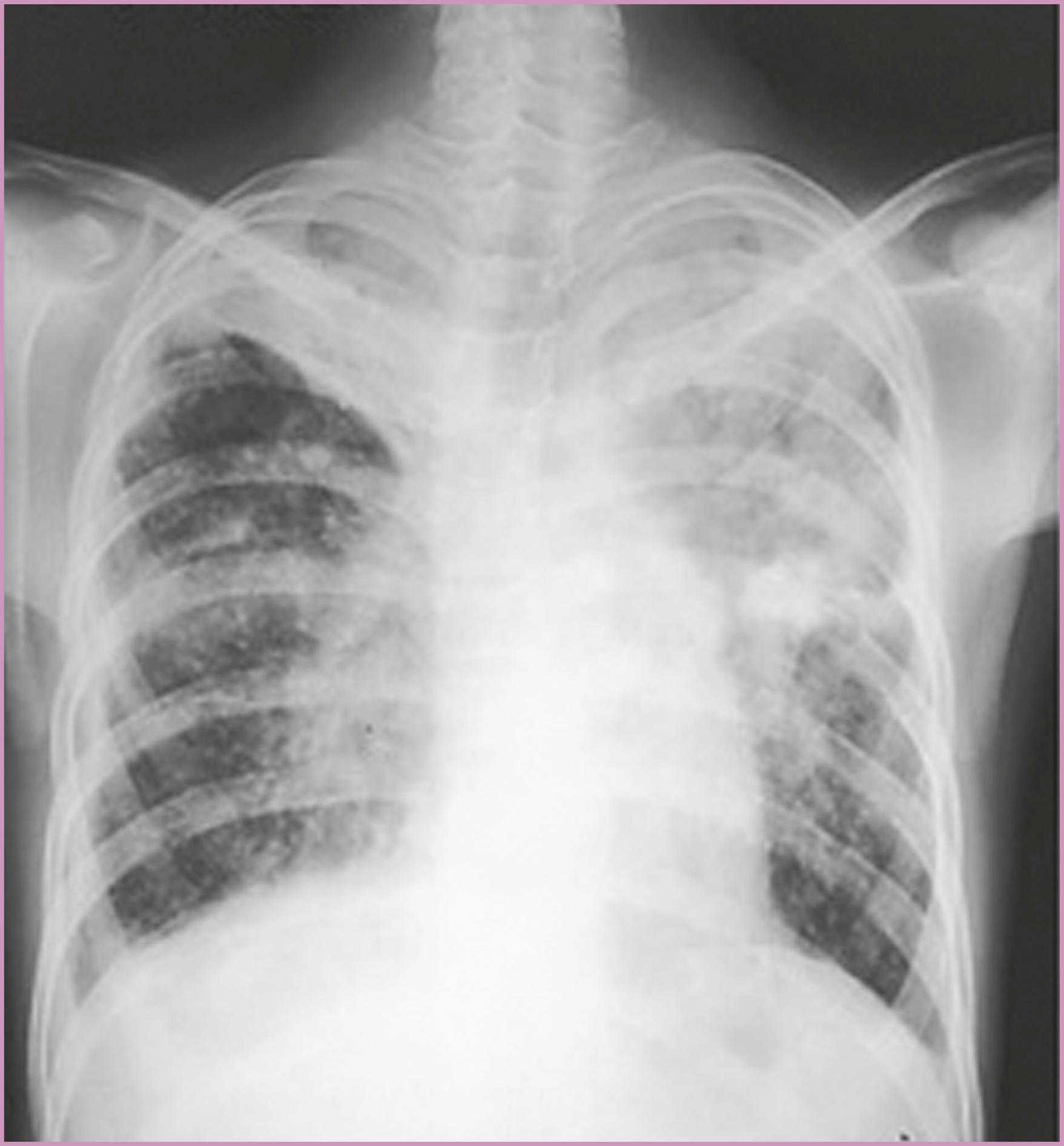
Asymptomatic women with a positive PPD skin test result must be evaluated for active tuberculosis with a thorough physical examination for extrapulmonary disease, and a chest radiograph after they are beyond the first trimester. Symptoms of active tuberculosis should prompt an immediate chest radiograph. The symptoms include cough (74%), weight loss (41%), fever (30%), malaise and fatigue (30%), and hemoptysis (19%). Individuals with active pulmonary tuberculosis may have radiographic findings, including adenopathy, multinodular infiltrates, cavitation, loss of volume in the upper lobes, and upper medial retraction of hilar markings ( Fig. 58.7 ). The finding of AFB in early-morning sputum specimens confirms the diagnosis of pulmonary tuberculosis. At least three early-morning sputum samples should be examined for the presence of AFB. If sputum cannot be produced, sputum induction, gastric washings, or diagnostic bronchoscopy may be indicated.
Extrapulmonary tuberculosis occurs in up to 20% of cases in the United States; however, the pattern may occur in 60% to 70% of all patients with AIDS. Extrapulmonary sites include lymph nodes, bone, kidneys, intestine, meninges, breasts, and endometrium. Extrapulmonary tuberculosis appears to be rare in pregnancy. When it is confined to the lymph nodes, it has no effect on obstetric outcomes, but tuberculosis at other extrapulmonary sites does adversely affect the outcome of pregnancy. Jana and colleagues documented that tuberculosis lymphadenitis did not affect the course of pregnancy, labor, or perinatal outcome. However, compared with control women, the 21 women with tubercular involvement of other extrapulmonary sites had higher rates of antenatal hospitalization (24% versus 2%; P < .0001), infants with low Apgar scores (≤6) soon after birth (19% versus 3%; P = .01), and low-birth-weight (<2500 g) infants (33% versus 11%; P = .01). Rarely, mycobacteria invade the uteroplacental circulation, and congenital tuberculosis results. , The diagnosis of congenital tuberculosis is based on one of the following factors :
Demonstration of primary hepatic complex or cavitating hepatic granuloma by percutaneous liver biopsy at birth
Infection of the maternal genital tract or placenta
Lesions seen in the first week of life
Exclusion of the possibility of postnatal transmission by a thorough investigation of all contacts, including attendants
Most gravidas with a positive PPD result are asymptomatic during pregnancy and have no evidence of active disease; they are classified as infected without active disease (latent tuberculosis). The risk for progression to active disease is highest in the first 2 years of conversion. It is important to prevent the onset of active disease while minimizing maternal and fetal risk. An algorithm for management of the positive PPD is presented in Fig. 58.9 . , In women with latent TB, the recommended treatment is a 4-month daily regimen of RIF (4R). , The alternatives include a 3-month daily regimen of INH and RIF (3HR), or a 6- or 9-month daily regimen of INH (6H or 9H), with pyridoxine (vitamin B6) supplementation. Isoniazid should be accompanied by pyridoxine (vitamin B 6 ) supplementation (50 mg/d) to prevent the peripheral neuropathy that is associated with INH treatment. Women with an unknown or prolonged duration of PPD positivity (>2 years) have a low risk of progression during pregnancy and should receive treatment 2–3 months postpartum. Isoniazid is associated with hepatitis in pregnant and nonpregnant adults. However, monthly monitoring of liver function tests may prevent this adverse outcome. Among individuals receiving INH, 10% to 20% will develop mildly elevated values detected on liver function tests. These changes resolve after the drug is discontinued.
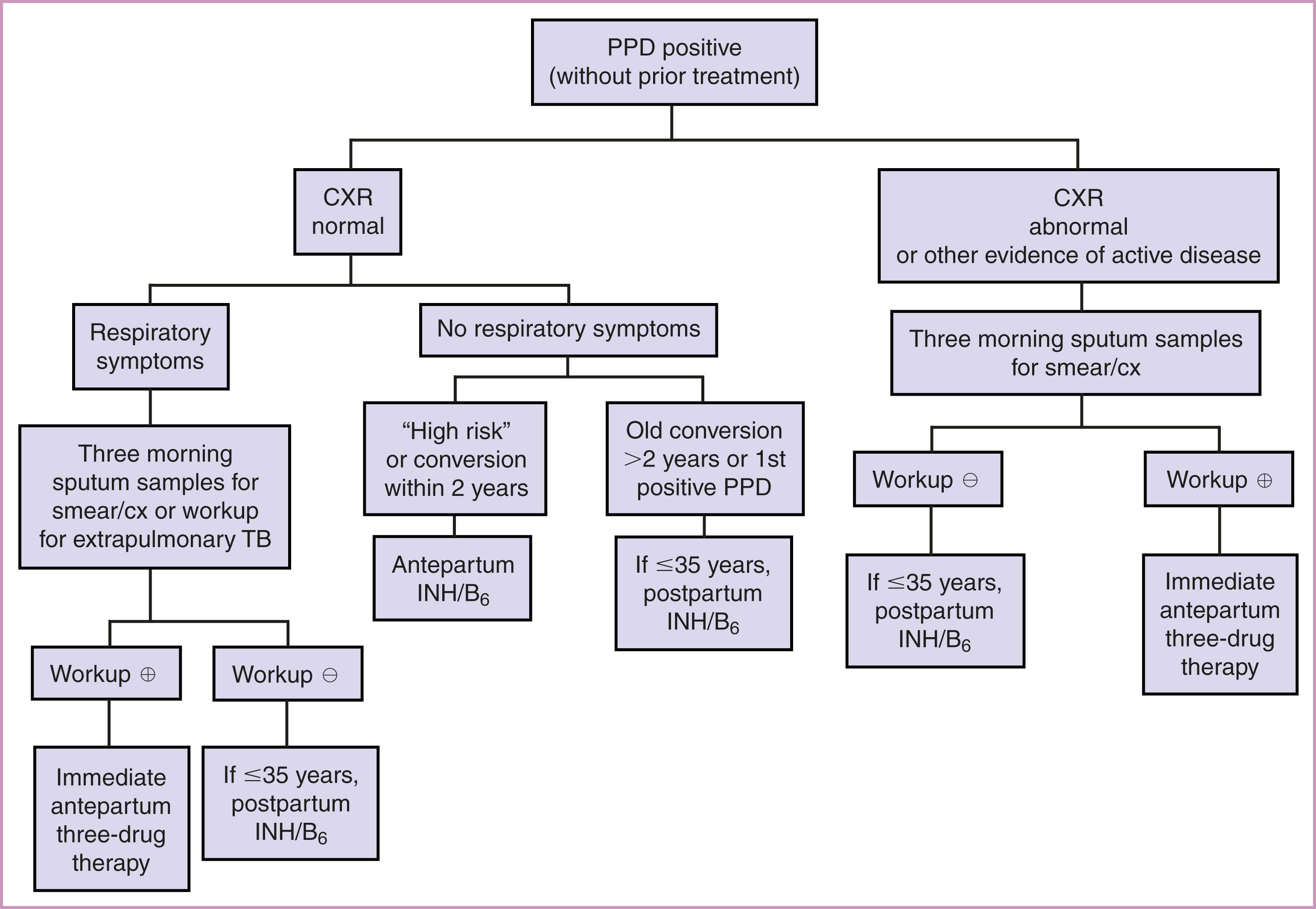
The gravida with active tuberculosis should be treated with INH, RIF, and ethambutol (EMB) daily for 2 months, followed by INH and RIF daily, or twice weekly for 7 months (for a total of 9 months of treatment). ( Table 58.1 and 58.2 ). Resistant disease may begin with the initial infection with resistant strains (33%), or it can develop during therapy. The development of resistance is more likely in individuals who are nonadherent with therapy. Ethambutol is teratogenic in animals, but this effect has not been seen in humans.
| Drug | Dosage Route | Daily Dose | Weekly Dose | Major Adverse Reactions |
|---|---|---|---|---|
| First-Line Drugs (for Initial Treatment) | ||||
| Isoniazid | PO, IM | 5 mg/kg, up to 300 mg | 15 mg/kg, up to 900 mg | Hepatic enzyme elevation, peripheral neuropathy, hepatitis, hypersensitivity |
| Rifampin | PO | 10 mg/kg, up to 600 mg | 10 mg/kg, up to 600 mg | Orange discoloration of secretions and urine, nausea, vomiting, hepatitis, febrile reaction, purpura (rare) |
| Rifabutin | PO | 5 mg/kg, up to 300 mg | NA | Rash, leukopenia, neutropenia, thrombocytopenia |
| Rifapentine a | PO | NA | 10–20 mg/kg | Rash, anemia, lymphocytopenia, GI disturbance |
| Pyrazinamide a | PO | 40–55 kg, 1000 mg 56–75 kg, 1500 mg 76–90 kg, 2000 mg |
40–55 kg, 2000 mg 56–75 kg, 3000 mg 76–90 kg, 4000 mg twice weekly |
Hepatotoxicity, hyperuricemia, arthralgias, rash, GI upset |
| Ethambutol | PO | 40–55 kg, 800 mg 56–75 kg, 1200 mg 76–90 kg, 1600 mg |
40–55 kg, 2000 mg 56–75 kg, 2800 mg 76–90 kg, 4000 mg |
Optic neuritis (decreased red-green color discrimination, decreased visual acuity), rash |
| Streptomycin a | IM | 15 mg/kg, | 25–30 mg/kg, thrice weekly | Ototoxicity, nephrotoxicity |
| Second-Line Drugs (Daily Therapy) | ||||
| Capreomycin a | IM | 15 mg/kg | 25 mg/kg, thrice weekly | Auditory, vestibular, and renal toxicity |
| Kanamycin a | IM | 15 mg/kg | 25 mg/kg, thrice weekly | Auditory and renal toxicity, rare vestibular toxicity |
| Ethionamide a | PO | 15–20 mg/kg, 500 mg daily | — | GI disturbance, hepatotoxicity, hypersensitivity |
| p -Amino-salicylic acid | PO | 4000 mg BID or TID | — | GI disturbance, hypersensitivity, hepatotoxicity, sodium load |
| Cycloserine a | PO | 10–25 mg/kg, 250–500 mg daily or BID | — | Psychosis, convulsions, rash |
| Levofloxacin a | PO, IV | 750–1000 mg daily | — | Hepatotoxicity, sleep disturbance, |
| Moxifloxacin a | PO, IV | 400 mg daily | — | GI disturbance, headache |
a Use is contraindicated in pregnancy, although may be considered in drug-resistant TB cases.
| Diagnosis | Treatment |
|---|---|
| Latent |
|
| Active Disease |
|
| HIV-Related |
|
The most common side effect of ethambutol therapy is optic neuritis. Streptomycin should be avoided during pregnancy because it is associated with cranial nerve VIII damage in neonates. Antituberculosis agents not recommended for use in pregnancy include ethionamide, streptomycin, capreomycin, kanamycin, cycloserine, and pyrazinamide. However, case reports documenting the use of these antituberculosis agents in pregnancy revealed no adverse fetal or neonatal effects. There were no congenital abnormalities, and pregnancy outcomes for the treated individuals were good. Untreated tuberculosis has been associated with higher morbidity and mortality rates among pregnant women. The management of the gravida with multidrug-resistant tuberculosis should be individualized. The patient should be counseled about the small risk for teratogenicity and should understand that the risk for postpartum transmission of tuberculosis to the infant may be higher among those born to patients with drug-resistant tuberculosis. In patients with untreated active disease at the time of delivery, separation of the mother and newborn should be accomplished to prevent infection of the newborn. The strategy can be revisited after initiation of treatment, resolution of symptoms, or demonstration of negative sputum cultures.
Congenital tuberculosis through vertical transmission is described by transplacental transmission through umbilical veins to the fetal liver and lungs; or aspiration and swallowing of infected amniotic fluid in utero or intrapartum causing primary infection of fetal lungs and gut. Transplacental infection typically occurs late in pregnancy. In newborns diagnosed with TB, a horizontal spread in the postpartum period by droplet or ingestion from a mother or undiagnosed family member with active disease is most commonly suggested. Transmission of tuberculosis through breast milk does not occur. Women who are being treated with antituberculosis drugs may breastfeed. Only 0.75% to 2.3% of INH and 0.05% of RIF is excreted into breast milk. Ethambutol excretion into breast milk is also minimal. However, if the infant is concurrently taking oral antituberculosis therapy, excessive drug levels may be reached in the neonate, and breastfeeding should be avoided. Breastfed infants of women taking INH should receive a multivitamin supplement that includes pyridoxine. Neonates of women taking antituberculosis therapy should have a tuberculin skin test at birth and again when 3–4 months of age. If congenital TB is suspected the neonate/infant should have an evaluation that includes a tuberculin skin test and IGRA, chest radiograph, lumbar puncture, cultures (blood and respiratory specimens), and evaluation of the placenta with histologic examination (including AFB staining culture). The TST in newborns is usually negative, but an IGRA test may be positive in some cases. Infants born to women with active tuberculosis who do not have evidence of congenital tuberculosis at the time of delivery should receive INH chemoprophylaxis and be reevaluated in 3 months. If maternal disease has been inactive for 3 months as evidenced by negative maternal sputum cultures, INH may be discontinued. Infants of women with multidrug-resistant tuberculosis should probably be placed with an alternative caregiver until there is no evidence of active disease in the mother. The newborn should also receive BCG vaccine and INH prophylaxis. Active tuberculosis in the neonate should be treated appropriately with INH and RIF immediately on diagnosis, or with multiagent therapy if drug-resistant organisms are identified. Infants and children who are at high risk for intimate and prolonged exposure to untreated or ineffectively treated persons or drug-resistant TB should receive the BCG vaccine.
In summary, high-risk gravidas should be screened for tuberculosis and treated appropriately for latent tuberculosis infection without overt disease and with antituberculosis therapy (isoniazid, rifampin, ethambutol) for active disease. The newborn also should be screened for evidence of tuberculosis. Proper screening and therapy will lead to a good outcome for the mother and infant in most cases.
Asthma may be the most common potentially serious medical condition to complicate pregnancy. It is characterized by chronic airway inflammation with increased airway responsiveness to a variety of stimuli, and airway obstruction that is partially or completely reversible. Factors that may lead to worsening asthma during pregnancy include pregnancy-associated changes in levels of sex hormones, psychosocial stress, nonadherence, and respiratory infections. Approximately 5%–8% of pregnancies are complicated by asthma, , and the prevalence and morbidity rates are increasing. Insight into the pathogenesis of asthma has changed with the recognition that airway inflammation occurs in almost all cases. The medical management for asthma emphasizes treatment of airway inflammation to decrease airway responsiveness and prevent asthma symptoms.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here