Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Renovascular disease (RVD) encompasses a range of disorders that affect renal artery structure and kidney blood flow. The primary importance of RVD relates to renal hypoperfusion causing hypertension (HTN) and loss of renal excretory function. Both in turn contribute to cardiovascular morbidity, dialysis dependence, and death. The most common pathology and major focus of the present chapter is atherosclerotic renal artery stenosis (RAS), found in 7% of elderly individuals in the United States ( Fig. 127.1 ). Less common etiologies include renal artery fibromuscular dysplasia (FMD); dissection, aneurysms, and trauma; arteritis; and developmental abnormalities of the middle aorta and its branches.
Most patients with RVD have atherosclerotic lesions, including more than 90% of patients referred for renal artery stenting. , In 840 patients undergoing open renal revascularization at our institution, 81% had atherosclerotic lesions, 14% FMD, and 1% dissection. The other 3% were children with hypoplastic renal arteries, mid-aortic syndrome, or dissection.
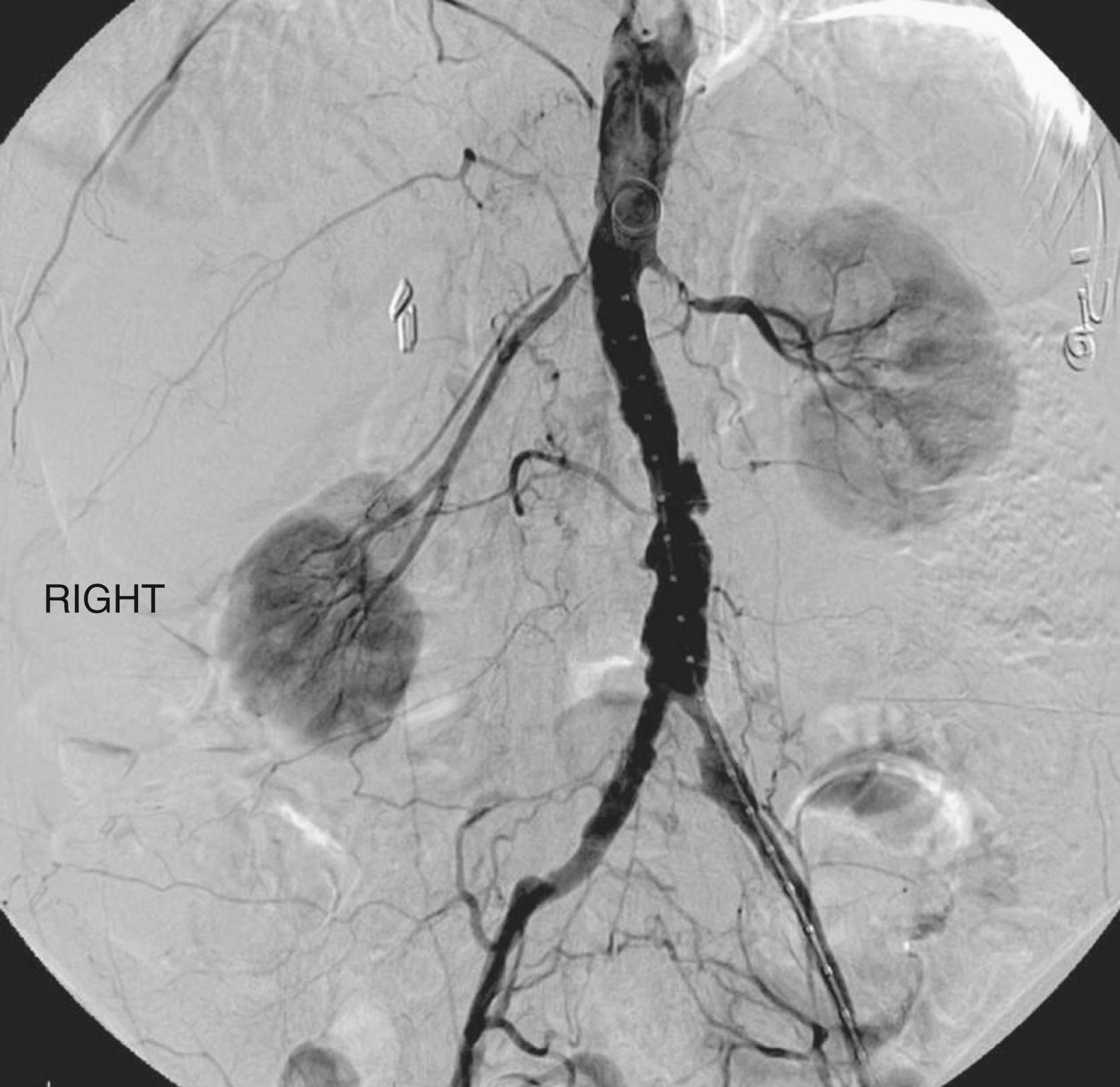
Atherosclerotic RVD is typically most severe at the renal artery ostia, but stenosis may occur at any level, including small intraparenchymal branches. , Ostial lesions are typically contiguous with a sheet of aortic plaque perpendicular to the renal artery ( Fig. 127.2 ). This sheet is often heavily calcified and resistant to simple balloon dilation and more effectively overcome with balloon-mounted stents. ,
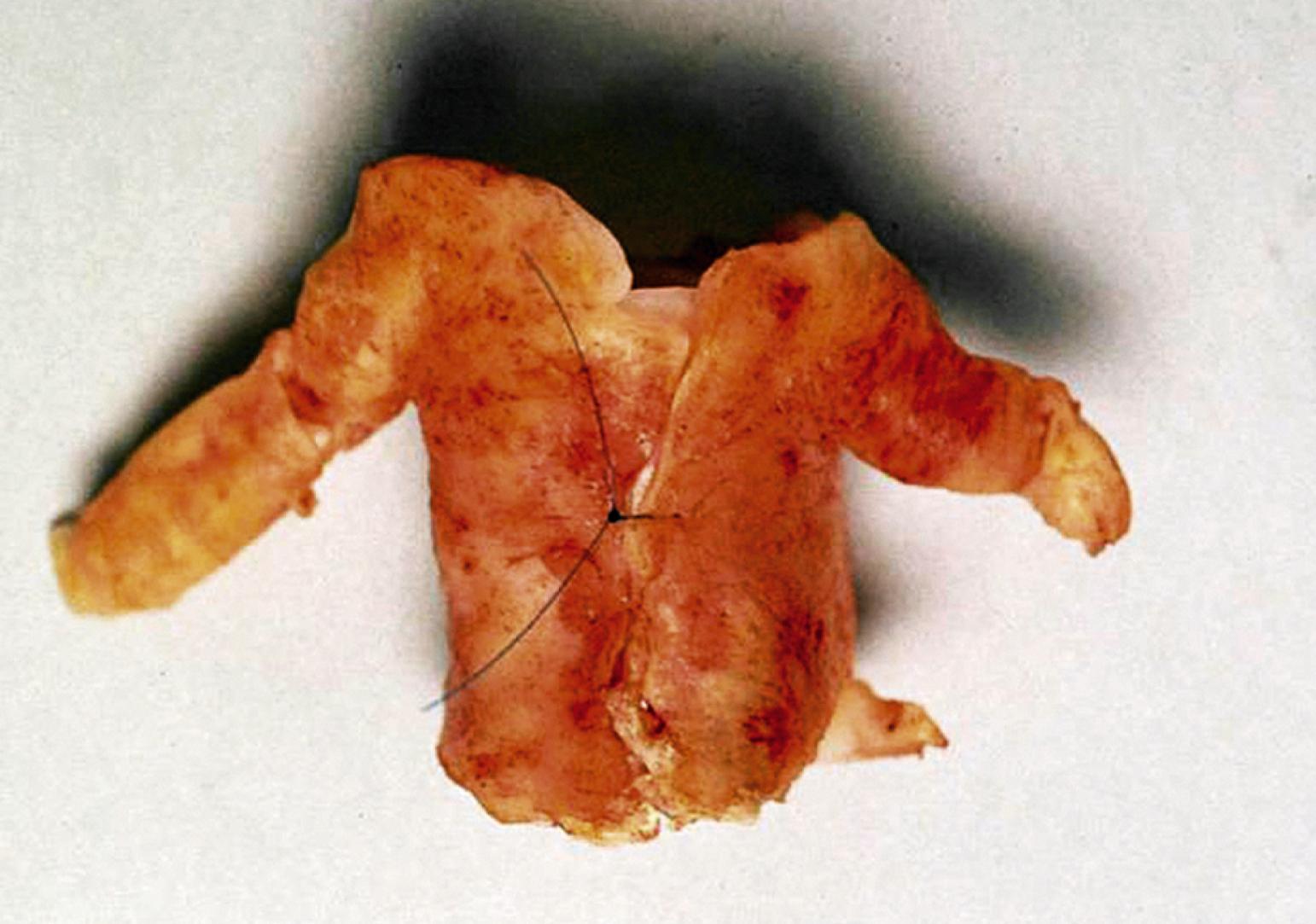
FMD in contrast typically affects the main renal artery and its branches away from the ostia (see Ch. 143 , Fibromuscular Disease). In adults, FMD often creates a series of web-like stenoses, alternating with segments of normal or dilated lumen caliber, giving rise to the classic “string of beads” morphology typical of the medial fibroplasia subtype ( Fig. 127.3 ). Renal FMD in children is more often the intimal fibroplasia subtype and less likely to cause this stereotypical angiographic appearance.
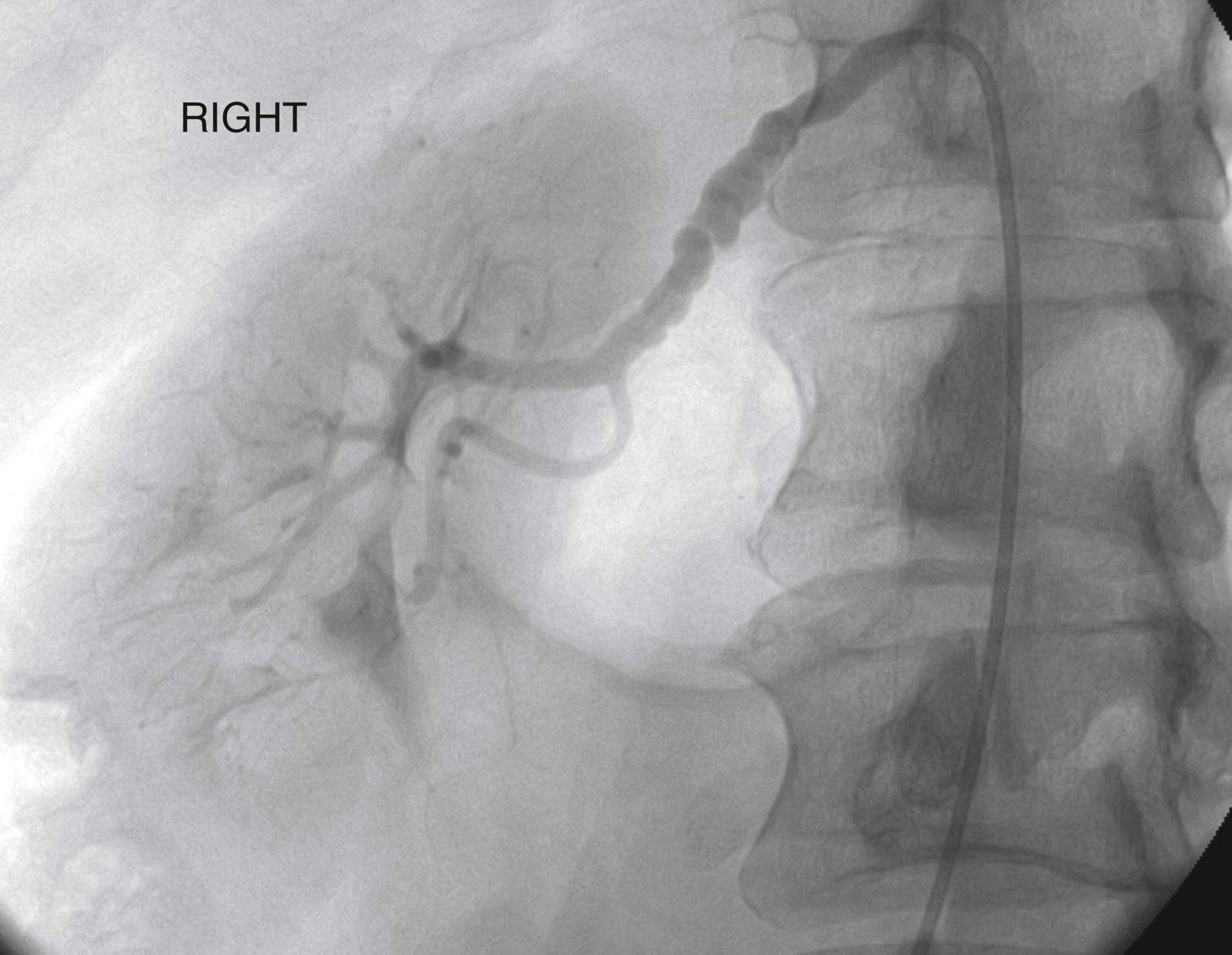
Dissection of the renal arteries can be spontaneous or caused by blunt or penetrating trauma, endovascular manipulation (e.g., guide wires and catheters), severe HTN, or by renal artery wall pathology such as FMD. Renal dissection often originates from aortic dissection that can obstruct the ostia with flaps or extend out the renal artery and branches. Dissection can result in acute occlusion or thrombosis of the renal artery with infarction of the kidney. Dissections may extend into the kidney parenchyma, limiting options for revascularization, and as they heal, dissections may create stenosis or aneurysmal degeneration (see Ch. 130 , Renovascular Disease: Acute Occlusive and Ischemic Events). Less common renal artery pathologies include the various arteritides and congenital syndromes (see Ch. 132 , Renovascular and Aortic Developmental Disorders and Ch. 138 , Vasculitis and Other Uncommon Arteriopathies).
Late in the 19th century, Tigerstedt and Bergman isolated a substance from rabbit kidneys they called “renin” that elevated the blood pressure (BP) of control animals after infusion. Reduced kidney blood flow would later be linked to elevated BP, and Goldblatt’s landmark experiments in the 1930s demonstrated that HTN from RAS could be reversed by correcting renal artery stenosis or by removing the ipsilateral kidney. This work laid the foundation for research that would define components of the renin–angiotensin–aldosterone system (RAAS) as biochemical mediators of BP regulation.
Hypertension in response to RVD results from a complex cascade of molecular and cellular events precipitated by hypoperfusion of one or both kidneys. A flow-limiting RAS creates a pressure drop sensed distal to the stenosis by the juxtaglomerular apparatus that prompts granular cell release of renin into the systemic circulation in an attempt to normalize the BP. Renin catalyzes cleavage of angiotensinogen to form the decapeptide angiotensin I, which is then cleaved primarily in the pulmonary circulation by angiotensin converting enzyme (ACE) to form the octapeptide angiotensin II (Ang II). Circulating Ang II is then the primary effector of renin-mediated increases in systemic BP, promoting peripheral vasoconstriction and blood volume expansion. , Ang II acts on a variety of cell types through specific G protein–coupled receptors designated ATR1 and ATR2. ATR1 activates intracellular signaling pathways that promote vasoconstriction and mitogenic responses within the vessel wall and myocardium. Ang II also indirectly promotes vasoconstriction by upregulating expression of norepinephrine and endothelin-1. ATR1 activation also can also promote formation of reactive oxygen species that inactivate nitric oxide. Crosstalk between ATR1 and other receptors (e.g., growth factor-activated receptor tyrosine kinases) amplifies smooth muscle cell vasoconstrictor and proliferative signaling pathways (see Ch. 46 , Systemic Complications: Renal).
Fluid retention also contributes to renovascular HTN. Ang II acts directly on renal tubules to promote sodium and water reabsorption but also stimulates adrenal release of aldosterone, a potent mediator of renal tubular sodium reabsorption. Adaptive responses can limit volume expansion in patients with unilateral RAS as the contralateral normal kidney mounts a compensatory natriuresis. , , Patients with bilateral RAS or RAS in a solitary kidney cannot compensate, resulting in so-called “Goldblatt volume-dependent” renovascular HTN, which can be severe. Ang II and its downstream product, Angiotensin III (Ang III) also contributes to HTN via acting on specific regions of the brain responsible for autonomic control of BP, water retention, blunting of the baroreceptor reflex, and by stimulating the release of ACTH and vasopressin from the pituitary to promote synthesis of cortisol by the adrenal glands and the thirst response, respectively. ,
More recent research has illustrated a far more complex RAAS with independently functioning tissue-specific RAAS, the most important of which is the renal RAAS. Overactivation of the intrarenal RAAS in the setting of hypertension or renal malperfusion can autoamplify Ang II production independent of the systemic RAAS. This provides a continuous source of the Ang II to potentiate vasoconstriction, attenuate natriuresis and maintain hypertension. Additionally, intracellular renin has been described in the brain but it is unclear if this indicates local synthesis. The biochemical and functional complexity of the central RAS remains to be fully defined but likely contributes to BP regulation. , , Angiotensin-(1–6) (Ang 1–6), an alternative product of angiotensinogen processing, has been shown to counteract some of the deleterious effects of Ang II excess, attenuating hypertension, inflammation and fibrosis.
While renovascular HTN begins with activation of the RAAS, chronic structural changes sustain and amplify the problem. The structural changes in RVD are distinct from those observed in essential HTN, in which resistance vessels exhibit eutrophic remodeling, with little change in wall mass. However with RAS, chronic Ang II excess promotes cell proliferation, fibrosis, inflammation and oxidative stress within the vasculature, which subsequently contributes to hypertrophic remodeling of the wall of resistance vessels and myocardium. , Wall thickening and stiffening amplifies mechanical leverage in wall constrictor function to promote cardiovascular embarrassment. Hypertrophic changes in wall structure persists after RAS is corrected, which may limit clinical improvement in BP. Hypertrophic remodeling in RVD may be reversed over time with inhibition of the RAAS (e.g., ACE inhibition) by blunting Ang II production and potentiating the formation of Ang 1–7.
Ischemic nephropathy refers to loss of renal excretory function attributed to RVD, but its molecular and cellular basis are incompletely understood. The kidney lacks significant collateral arterial flow, so acute renal artery occlusion generally leads to infarction within minutes to hours. In contrast, the kidney can adapt to a broad range of systemic blood pressures, preserving parenchymal oxygenation and glomerular filtration. Chronic hypoperfusion, however, eventually impairs kidney function through a number of mechanisms, some of which are irreversible. Severe hypoperfusion drops glomerular filtration pressure and leads to ischemic damage. Chronic excess Ang II and aldosterone promote expression of profibrotic cytokines and growth factors, including transforming growth factor-β, a master regulator of extracellular matrix production, and platelet-derived growth factor, a potent mitogen for smooth muscle cells, renal fibroblasts, and glomerular mesangial cells. , , Ang II also induces nuclear factor-κB, a potent master switch for proinflammatory pathways. Each of these contributes to glomerulosclerosis and tubulointerstitial injury. Atherosclerotic lesions themselves may contribute to loss of excretory function by chronically releasing microembolic debris. If perfusion is severely compromised, the renal parenchyma will become hypoxic, with resulting ischemic injury and inflammatory infiltration associated proinflammatory cytokine release, but the contribution of frank ischemic necrosis to renal parenchymal atrophy is less well-established.
Severe systemic HTN resulting from unilateral RAS may directly injure the opposite kidney. The damage to excretory function may be irreversible despite subsequent renal revascularization or medical control of HTN.
The etiology of chronic kidney disease is often complex and multifactorial, particularly in patients with established atherosclerosis where risk factors for medical renal disease include diabetes, hyperlipidemia, essential HTN, smoking, and others. Thus patients with atherosclerotic RAS typically have other risk factors that may contribute to renal insufficiency independent of reduced parenchymal perfusion; patients with intrinsic parenchymal disease and those with advanced parenchymal atrophy from chronic ischemia benefit little from revascularization.
While animal experiments and clinical experience have proven that reduced kidney blood flow can cause renin-mediated HTN, loss of excretory function, kidney atrophy or even infarction, many individuals found to have RAS on imaging will be entirely asymptomatic. , These individuals have “anatomic” disease that is “functionally” silent, because their lesions are hemodynamically inconsequential, or intrinsic adaptive responses are robust enough to maintain BP and glomerular filtration, or the ipsilateral kidney may be so damaged that it is incapable of significant renin release.
The clinical significance of a stenotic renal artery may be confounded by the high prevalence of essential HTN and intrinsic kidney disease among older individuals with atherosclerosis , and increased circulating Ang II among patients with obesity and insulin resistance. Thus the prevalence of RVD defined by imaging alone may overestimate the prevalence of disease that will benefit from intervention. Provocative tests and chemical assays have been of limited value in helping prove a particular renal lesion is driving HTN or ischemic nephropathy. This creates an awkward dilemma: to prove a particular RAS will respond to reconstruction with improved BP and renal function, one often has to first perform the reconstruction.
Historically, estimates of the prevalence and natural history of RAS in the general population was extrapolated from high risk cohorts (see below) ( Table 127.1 ). To address this, Hansen and colleagues used renal duplex ultrasonography (RDU) to screen for RAS among healthy individuals aged 65 and older who were living independently in the community, without regard to BP or renal function (Cardiovascular Health Study- CHS). Subjects were screened at enrollment and then prospectively monitored for incident disease over time. The prevalence of a hemodynamically significant RAS at baseline was 7%, of which one in eight had bilateral disease, illustrating the significant number of relatively healthy older individuals in the United States with undiagnosed RAS. Additionally, 50% of subjects without RAS had elevated BP versus 72% of those with RAS, and 28% of those with RAS were normotensive. Therefore, many individuals with RVD likely have essential HTN unrelated to their stenosis. , The majority of subjects in both groups had normal renal function, but elevated creatinine was twice as likely in those with RAS (16% vs. 8%).
| Series | Year | Number of Patients | Risk Factor | Patients with RAS (%) | Comments |
|---|---|---|---|---|---|
| Hansen et al. | 2002 | 834 | Age >65 years | 6.8 | Elderly living independently |
| Jean et al. | 1994 | 196 | CAD | 22 | Suspected CAD, undergoing angiography |
| Miralles et al. | 1998 | 168 | Peripheral arterial disease | 40 | Aortoiliac occlusive disease |
| Appel et al. | 1995 | 45 | Dialysis | 22 | New-onset dialysis |
| Davis et al. | 2009 | 434 | Hypertension (adult) | 32 | — |
| Lawson et al. | 1977 | 74 | Hypertension (children) | 78 | — |
| Wu et al. | 2007 | 163 | Carotid stenosis | 20 | — |
The prevalence of RAS has also been estimated from autopsy series. Schwartz and White reported RAS in 6% of individuals <55 years of age and 40% of those >75, with bilateral lesions found in approximately half of these patients. In another autopsy series, Holley reported 22% had significant RAS. The prevalence of atherosclerotic RVD in autopsy series is clearly impacted by atherosclerosis being the leading cause of death in developed countries.
Angiographic series have screened for RVD in patients with coronary, carotid, and peripheral artery disease (PAD) and found rates of RAS ranging from 22% to 40% of patients ( Table 127.1 ).
Given the impact of RAS on BP and renal function, its prevalence will be higher in cohorts prescreened for HTN or renal failure. We used RDU to evaluate 629 adults with newly diagnosed HTN and found 25% had significant RAS. This doubled in patients over 60 years old with severe HTN. RAS has been reported in 9% to 41% of patients requiring dialysis, but prevalence varies significantly by age, gender, and ethnicity. It is highest in the elderly and surprisingly infrequent among African Americans with end-stage renal disease.
Understanding the natural history of RAS is critical in order to properly balance risks and benefits of various treatment options. Older studies used serial arteriography to monitor severity of stenosis and reported progression in 11% to 35% of lesions managed medically. Arteries with severe baseline stenosis went on to occlude in 10% of individuals. , Zierler employed serial RDU to follow 80 patients with established renovascular HTN after classifying baseline stenosis for each artery as >60%, <60%, or normal. After 3 years, 8% of normal arteries and 43% with <60% stenosis had progressed to a high-grade stenosis, and 7% with >60% stenosis at baseline went on to occlude. Factors associated with progression included age, systolic BP, smoking, female gender, and poorly controlled HTN.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here