Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Renal tumors are the second most common abdominal tumor seen in infants and children behind neuroblastoma. They represent a wide spectrum from benign to extremely malignant tumors ( Box 64.1 ). These tumors include Wilms tumor (WT) (also referred to as nephroblastoma or renal embryoma), renal cell carcinoma (RCC), clear cell sarcoma of the kidney (CCSK), rhabdoid tumor of the kidney (RTK), congenital mesoblastic nephroma (CMN), cystic renal tumor, and angiomyolipoma. Advances in the management of these tumors have been significant over the past six decades since Sidney Farber first administered dactinomycin (actinomycin D) for advanced-stage WTs. Multidisciplinary and multi-institutional prospective randomized cooperative group trials in both North America and Europe by the Children’s Oncology Group (COG-formerly the National Wilms Tumor Study Group [NWTSG]) and the Société Internationale d’Oncologie Pédiatrique (SIOP) have produced a large body of evidence-based knowledge to establish the optimal risk-based treatments. A summary of the important information gained from both previous COG and SIOP studies is seen in Table 64.1 . The goal of this chapter is to describe the early history of WT, followed by a discussion of the etiologic factors in renal tumor formation, molecular genetics, pathologic subtypes and premalignant syndromes, and current treatment algorithms for children.
Wilms tumor (favorable, unfavorable)
Renal cell carcinoma
Renal tumors associated with TFE3 or TFEB translocations
Clear cell sarcoma
Malignant rhabdoid tumor of the kidney
Renal cell sarcoma
Renal adenocarcinoma
Ossifying renal tumor of infancy
Renal medullary carcinoma
Renal neurogenic tumor
Renal teratoma
Metanephric tumor (adenoma, stromal tumor, adenofibroma)
Mesoblastic nephroma
Angiolipoma
Cystic nephroma or partially cystic nephroma
Diffuse hyperblastic perilobar nephroblastomatosis
Nephrogenic rest
| Study | Key Study Conclusions |
|---|---|
| NWTS-1 | Anaplasia is established as the most important factor in patient outcome. Vincristine and dactinomycin in combination are more effective than alone for stage II and III tumors. RT provided no survival advantage in children younger than 24 months with stage I FH tumors who also received 15 months of dactinomycin. Stage III tumors with a confined spill did not require whole abdominal RT, but had comparable outcomes with flank radiation. |
| NWTS-2 | Six months of vincristine and dactinomycin is equal to 15 months in children with FH. In children with stage II, III, or IV tumors, doxorubicin improved relapse-free survival. Confirmed that RT could be avoided in all children with stage I WT if they received vincristine and dactinomycin. Established that delaying RT longer than 10–14 days after resection was associated with a higher incidence of abdominal relapse, particularly among patients with unfavorable-histology tumors. |
| NWTS-3 | First study to stratify children by histology and grade. Children with stage I tumors with an 11-week regimen composed of vincristine and dactinomycin only had a 4-year RFS and OS of 89.0% and 95.6%. The addition of doxorubicin to the treatment of children with stage III tumors improved survival. No benefit was found with the addition of doxorubicin or RT for children with stage II tumors. No benefit from the addition of cyclophosphamide to the treatment of children with stage IV tumors. In stages II–IV, unfavorable histology, the addition of cyclophosphamide to a three-drug regimen improved survival for diffuse anaplasia but not focal. 10 Gy abdominal radiation was equal to 20 Gy. This was an important finding, because it eliminated the need for an age-adjusted dose schedule and significantly reduced the recommended dose of radiation and its subsequent early and late toxicity. Anaplastic tumors tend to be more resistant to chemotherapy and are also resistant to RT. |
| NWTS-4 | Analysis from early NWTS 1–4 studies identified a group of patients whose excellent outcomes were unaffected by chemotherapy. These children were younger than 2 years of age, had tumors that weighed less than 550 g, and were stage I with favorable histology. Addressed dose intensification and also evaluated the use of two time intervals for the administration of chemotherapy: a short course (18–26 weeks, depending on the regimen and stage) vs a longer course (54–66 weeks). Key findings were that the pulse-intensive regimens produced less hematologic toxicity than the standard regimens and 6 months of chemotherapy is as good 15 months. Patients with CCSK were treated with vincristine, dactinomycin, and doxorubicin, and OS improved on NWTS-4 compared with NWTS-3 (OS 83% vs 66.9% at 8 years, P <0.01). An increased incidence of local recurrence was seen in NWTS-4 enrolled children in whom biopsy of lymph nodes was not performed, particularly in stage I cases. Children with intravascular extension into the intrahepatic vena cava have reduced morbidity if treated with preoperative chemotherapy. |
| NWTS-5 | Outcomes for patients with LOH at 1p and 16q were at least 10% worse than those without LOH. First study with specific relapse protocols. EFS and OS of patients with stage I and II relapse at 4 years were 71.1% and 81.8%, respectively. EFS and OS of patients with stage III and IV relapse at 4 years were 42.3% and 48.0%, respectively. Therapy for stage I focal and diffuse anaplasia with only EE-4A is inadequate. 10 Gy abdominal radiation for stage II and III patients with AH histology is inadequate. Regimen I improved survival for all patients with CCSK. |
| SIOP-1 | Preoperative RT can reduce intraoperative rupture. |
| SIOP-6 | Pretreatment with chemotherapy almost always reduces the bulk of the tumor. |
| SIOP-9 | Pretreatment of chemotherapy results in a different pattern of histology compared with those who do not undergo neoadjuvant therapy. Randomization between 4 and 8 weeks of preoperative therapy with dactinomycin and vincristine did not decrease the incidence of tumor rupture, nor did it result in more tumors being downstaged. For all cases, tumor size decreased by more than half in 52% of the cases. During the second 4 weeks of therapy, there was another 50% reduction in 33% of the cases. In SIOP-9, patients were spared whole-lung RT if they rapidly responded to three-drug chemotherapy by 6 weeks. The 5-year RFS for patients with stage IV disease receiving preoperative chemotherapy alone was 62.5%. |
| SIOP 2001 | First study allowed NSS for unilateral polar or peripherally noninfiltrating tumors. NSS was able to be performed in 3% of patients, and 5-year EFS and OS were equivalent to those in patients who did not undergo NSS, although some patients were upstaged due to positive margins. After NSS, 65% had positive margins, mandating intensified therapy. The 5-year OS and EFS survival after NSS was 98.4 (95% CI, 95.3–100.0%) and 92.5 (95% CI, 86.9–98%). Omission of doxorubicin in the treatment of stage II–III, intermediate-risk Wilms tumor did not affect EFS or OS (excluding blastemal patients). |
The first descriptions of WT have been variably attributed to either Rance in 1814 or Wilms in 1899. The first known specimen of this tumor was preserved by the British surgeon John Hunter between 1763 and 1793. This specimen of a bilateral tumor in a young infant remains in the Hunterian Museum of the Royal College of Surgeons in London. Carl Max Wilhelm Wilms was a German pathologist and surgeon. Wilms’ name became indelibly linked to this tumor in children after publication of his comprehensive monograph in 1899 titled “Die Mischgeschwülste der Niere,” which described seven children with nephroblastoma as part of a monograph on “mixed tumors of the kidney.” In this monograph, he proposed that the tumor cells originate during the development of the embryo. The first successful nephrectomy was probably performed by Thomas Jessop at the General Infirmary in Leeds, England on the 7th of June, 1877 on a 2-year-old child with hematuria and a tumor of the kidney.
Resection was recognized early as an effective treatment for WT and remains the cornerstone of all therapies. However, at the beginning of the 20th century, survival was approximately 5% and operative mortality was high. In 1916, radiation therapy (RT) was added as an adjuvant therapy by Friedlander. In the 1930s, Ladd and Gross described the principles of operative therapy for WT, including transperitoneal exposure and early ligation of the renal pedicle. They stressed the need to remove the perirenal fat in order to resect lymphatic extensions and avoid rupture of the renal capsule, principles we continue to follow today. From 1931–1939, survival from resection alone, involving ligation of the renal pedicle before removal, was 32% at the Children’s Hospital in Boston. Gross and Neuhauser later proposed the routine addition of abdominal radiation to WT therapy and reported an estimated 47% cure. Thus, by1940, most patients began to receive postoperative irradiation to the renal fossa. This decreased the local recurrence rate but did not significantly affect the incidence of pulmonary metastases or improve the long-term survival. Under the tutelage of Gross, pediatric surgeons in North America generally performed primary resection of WT, and in Europe, the Paris school led by Schweisguth and Bamberger reported early success with preoperative irradiation, establishing a precedent for initial adjuvant therapy.
WT was the first malignancy in which the importance of adjuvant treatment was recognized. This principle was espoused by Sidney Farber decades before it would be applied to other pediatric and adult solid tumors. The concept of adjuvant therapy “was based upon the supposition that in the children with WT who died, the tumor must have metastasized already at the time of discovery of the primary tumor,” although evidence of spread might not have been available. Dactinomycin was the first active agent identified for the treatment of WT. In an early study, from 1957–1964, of the 53 patients who had no demonstrable metastases on admission and who were treated with a combined regimen of operation, local radiation, and dactinomycin, an 89% 2-year disease-free survival was reported, a very reasonable rate of survival even today. In patients with metastases identified at presentation, 18 of 31 (58%) were alive and free of disease more than 2 years later. In the early 1960s, vincristine sulfate was identified as an active agent against WT and was added to the standard therapy.
WT is the most frequent tumor of the kidney in infants and children. Its incidence is 7.6 cases for every million children younger than 15 years, or 1 case per 10,000 infants. This translates into 600–650 cases a year in North America. It is less common in East Asian populations than in white children, but is more frequent in black children. The mean age at diagnosis is 36 months, with most children presenting between the ages of 12 and 48 months. Tumors tend to occur earlier in boys than girls. WT incidence decreases over the age of 10 and is less common under 6 months of age. However, it still comprises 20% of all renal tumors in children younger than 6 months. Bilateral Wilms tumors (BWT) occur in 4–13% of patients.
WT occurs in several well-described syndromes. These make up about 10% of all WT cases and include sporadic aniridia, isolated hemihypertrophy, the Denys–Drash syndrome (nephropathy, renal failure, male pseudohermaphroditism, and WT), genital anomalies, Beckwith–Wiedemann syndrome (BWS; visceromegaly, macroglossia, omphalocele, and hyperinsulinemic hypoglycemia in infancy), and the WAGR complex (WT with aniridia, genitourinary malformations, and mental retardation). These congenital syndromes have helped identify the genetic and etiologic mechanisms inherent in developing a WT. For example, children with WAGR syndrome, which is associated with a chromosomal defect in 11p13, are at a 30% higher risk of developing WT than a normal child. Aniridia is usually diagnosed at birth and helps identify these children. BWS is a second common syndrome associated with WT. BWS affects 1 in 14,000 children. These children are at an increased risk of several types of embryonal tumors, including WT. The most frequently observed tumors in BWS are WT and hepatoblastoma, which comprise 43% and 12% of reported associated cancers, respectively. The risk for malignant development is greatest in the first decade of life. Three large studies of children with BWS reported tumor frequencies of 7.1% (13/183), 7.5% (29/388), and 14% (22/159).
WT is an embryonal tumor that was originally thought to fit the model proposed by Knudson’s “two-hit” hypothesis for cancer development. However, the genesis of WT has been shown to be far more complex. Multiple mutated WT genes have been identified, as well as areas of loss of genetic material and allelic uniqueness (loss of heterozygosity [LOH]) that are important to tumor development. A few of these genetic anomalies have been evaluated in clinical trials to assess their prognostic significance and are now being used in conjunction with traditional factors, such as stage, to determine the intensity of therapy. A detailed discussion of all the genetic anomalies associated with WT is beyond the scope of this chapter; however, a summary of the key genes and genetic anomalies is presented in Table 64.2 .
| Gene | Location | Type of Mutation | Frequency | Germline or Somatic? | Tumor Zygosity | Predicted Effect |
|---|---|---|---|---|---|---|
| WT1 | 11.13 | Whole or partial gene deletion Insert ion nonsense and missense |
∼20% | Both | Predominantly homozygous | Inactivation of protein |
| WT2 | 11pl5.5 | Genetic imprinting | ? | Both | Heterozygous | Abnormally high levels of IGF-2 mRNA |
| WTX | Xq11.1 | Whole or partial gene deletion and nonsense | ∼20% | Somatic only | Heterozygous and hemizygous | Inactivation of protein |
| CTNNbB1 | 3p21 | Inframe deletions and missense | ∼% | Somatic only | Heterozygous | Stabilization of protein |
| TP53 | Xqll.1 | Missense | ∼5% | Both | Heterozygous and homozygous | Inactivation of protein |
TP53 mutations in WT are found almost exclusively in tumors with anaplastic histology. Of anaplastic WTs, 75% have TP53 mutations. Interestingly, some tumors can contain both anaplastic and favorable histology. Studies in these tumors demonstrate that the TP53 mutation is found only in the areas of anaplasia and not in the area of favorable histology. This implies that TP53 mutations may be essential for anaplastic progression. A recent study looked in detail into the mechanistic action of TP53 in WT. NWTS samples were analyzed for TP53 mutations and copy loss. Integrative genomic analysis was performed on 39 selected diffuse anaplastic tumors. In this study, TP53 copy loss appeared to be important for both the development of anaplasia and volume (focal or diffuse) of anaplasia in the tumor. Other TP53 mutations may have an impact on the risk of relapse and death. If these observations are confirmed in future studies, TP53 mutation and copy loss could be helpful with therapeutic stratification.
CTNNB1 plays a central role in the Wnt signal transduction pathway. The Wnt signaling pathway describes a network of proteins known for their roles in embryogenesis and cancer. Deregulation of CTNNB1 has been linked to several malignancies and has been reported to occur in 15% of WT. The mutations resulted in areas of phosphorylation and degradation of beta catenin, which is highly correlated with WT1 mutation and the WTX gene.
The WTX gene (also known as AMER1 for adenomatous polyposis coli [APC] membrane recruitment 1) was found to be mutated in 15 of 51 (29%) WTs tested, making it the most common known gene mutation in WT. Localization of WTX on the X-chromosome results in complete inactivation by a single mutational event in males and in females if the active X-chromosome is affected. The exact clinical impact of WTX on WT development remains to be defined.
WT1 gene was the first gene to be linked with WT development. Evidence suggests that WT1 may act as a tumor suppressor gene or oncogene, and is located at chromosome 11p13. Wild-type WT1 is important for normal cell development and survival. WT1 is involved in cell growth, differentiation, and apoptosis. Patients heterozygous for WT1 germline mutations are predisposed to WT, and WT1 is inactivated in tumors. This implies the loss of WT1 function is associated with enhanced cell viability and/or proliferation. Ablation of WT1 at the initial stages of kidney development results in apoptosis and renal agenesis, indicating that it has a crucial role in maintaining cell viability. In some leukemias, the increased expression of WT1 compared with normal bone marrow cells, along with some reports of WT1 expression being a marker of poor prognosis, suggest that WT1 functions as an oncogene. In contrast, observations of WT1 inactivating mutations in leukemias suggest it functions as a tumor suppressor gene. It should be emphasized that children with congenital syndromes and WT constitute a very small proportion of the total WT population. However, LOH is seen in 30–40% of WT patients in the region of WT1 . Based on this observation, it would be expected that many patients with sporadic WT would have a mutation in the WT1 gene. Surprisingly, the incidence of mutations of WT1 associated with WT in the sporadic form of the disease is low (10–20%).
This second WT gene location was identified by linkage analysis in children with BWS. This site is not a single gene but contains several genes that may play a role in tumor development. In patients with BWS, it was found that the maternal allele of 11.15 was uniformly lost. The process that causes this is genomic imprinting, in which one allele is imprinted, in a parental-specific manner, to be functionally inactive.
LOH refers to loss of genetic material and allelic uniqueness. A major aim of NWTS-5 was to determine if tumor-specific LOH for chromosomes 11p, 1p, or 16q was associated with an adverse prognosis for children with favorable-histology WT, a finding suggested in earlier retrospective studies. The results of NWTS-5 demonstrated that outcomes for patients with LOH at 1p and 16q were at least 10% worse than those without LOH ( Fig. 64.1 ). Based on these results, increased therapy for stage I–IV favorable-histology WT with LOH at 1p and 16 q was utilized in COG studies ARENO532 (stage I and II) and 533 (stage III and IV). For stage I and II patients, increased therapy for children with LOH at 1 p and 16q resulted in an improved 4-year event-free survival (EFS) from 75% on NWTS-5 to 84%. For stage III and IV patients, increased therapy improved 4-year EFS from 66 to 96%.
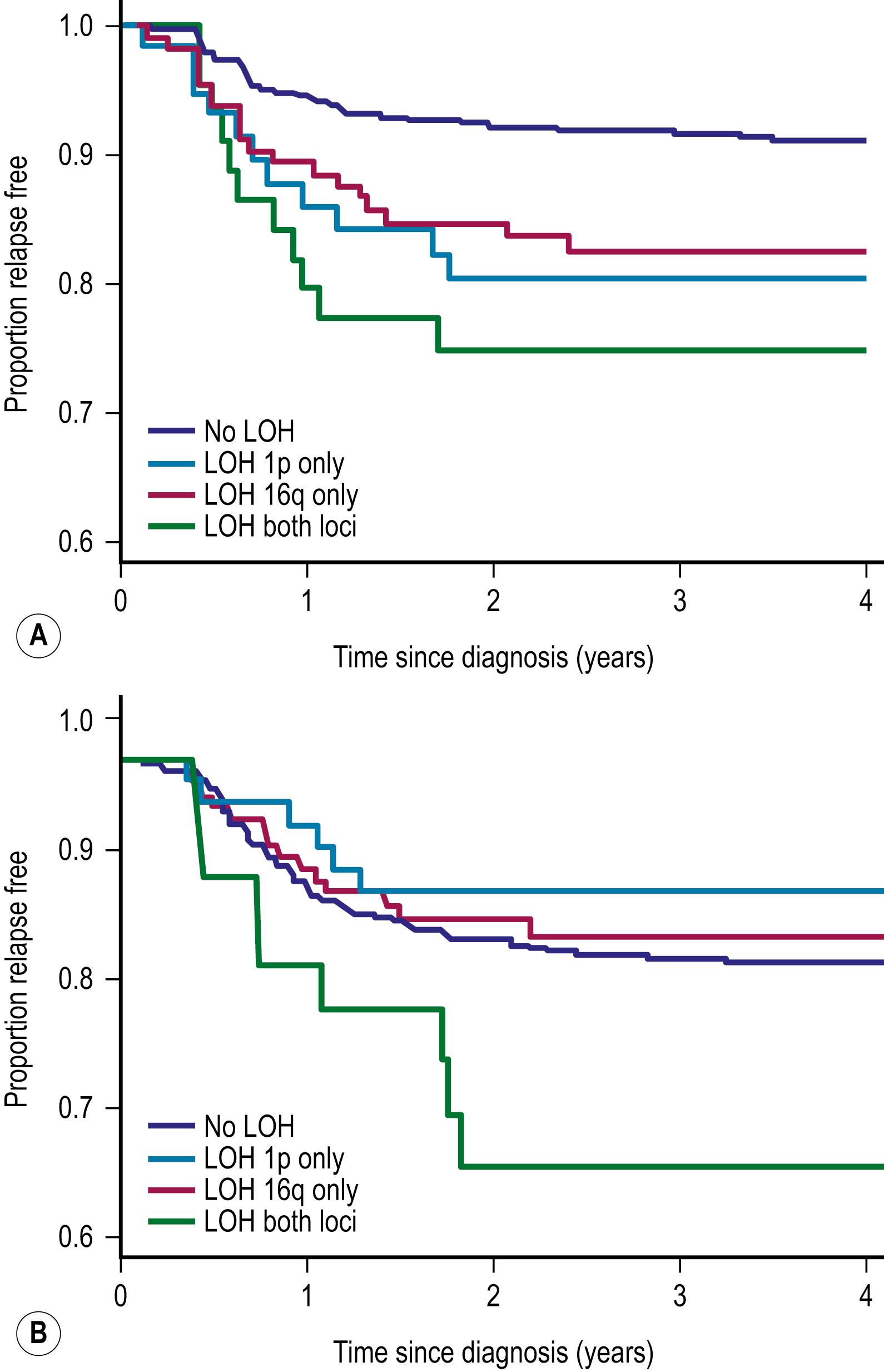
Although LOH is recognized as a negative prognostic finding, it is found in only 5% of patients. However, gain of chromosome 1q is one of the most common cytogenetic abnormalities in WT, observed in approximately 30% of tumors. Recent retrospective studies have shown that 1q gain is associated with inferior outcomes across all stages of favorable-histology WT. The relevant genes on 1q that confer adverse prognosis remain to be determined. However, in the upcoming COG studies, augmentation and reduction of therapy will be based on the presence or absence of 1q gain.
Nephrogenesis in the normal kidney is usually complete by 34–36 weeks of gestation. The presence of nephrogenic rests (NRs; persistent metanephric tissue in the kidney after the 36th week of gestation) has been associated with the occurrence of WT. NRs are considered precursor lesions to WT. However, only a small number develop clonal transformation resulting in the development of WT. The presence of multiple or diffuse NRs is termed nephroblastomatosis.
These NRs may occur in a perilobar (PLNRs) or intralobar (ILNRs) location and may be single or multiple ( Fig. 64.2 ). In children with aniridia or the Denys–Drash syndrome, the lesions are primarily ILNRs, whereas children with hemihypertrophy or BWS have predominantly PLNRs ( Table 64.3 ). NRs may be further classified by their growth phase, which has been separated into three phases: (1) incipient or dormant NRs, which show few well-formed tubular structures, but no evidence of proliferation and no mitoses; (2) hyperplastic NRs, which are composed of epithelial elements with nodular expansive growth; and (3) sclerosing rests, which consist of stromal and epithelial elements with few blastemal nephrogenic elements. Most NRs are dormant or in the sclerosing phase, and the majority will spontaneously resolve. Hyperplastic NRs can produce masses as large as a conventional WT and can present a diagnostic dilemma. NRs are rare in the general population. In an autopsy series of infants younger than 3 months, 9 of 1035 infants (0.87%) had PLNRs, whereas ILNRs were found in only 2 of 2000 cases (0.1%).
| +ILNR | –ILNR | +ILNR | –ILNR | |
|---|---|---|---|---|
| Clinical Phenotype | –PLNR (%) | +PLNR (%) | +PLNR (%) | –PLNR (%) |
| Denys–Drash | 60 | 0 | 4 | 37 |
| WAGR | 73 | 4 | 4 | 18 |
| Beckwith–Wiedemann | 18 | 3S | 27 | 20 |
| Male genitourinary anomalies | 43 | 0 | 5 | 44 |
The pathologic distinction between NR and WT can be very difficult. In fact, it may be impossible to distinguish a hyperplastic NR from a WT based on an incisional or needle biopsy specimen that does not include the margin between the NR and the remaining kidney. Most hyperplastic nodules lack a pseudocapsule at their periphery, whereas most WTs will have one. Thus, if the biopsy specimen does not contain the lesion and its margin, it will be difficult to differentiate between these two lesions. In these situations, the current recommendation is to use the term “nephrogenic process, consistent with a WT or a nephrogenic rest.” This is one reason that it is recommended to avoid percutaneous biopsies in children with renal masses.
The incidence of NRs is about 100 times greater than that of WT (1/10,000 infants) in perinatal autopsy series. In a review of cases of WT reported in the NWTS-4, 41% of the unilateral WTs were associated with NRs, whereas in children with synchronous bilateral WT, the incidence of NRs was 99%. These were primarily PLNRs. A child with a WT and NRs in the resected specimen is at increased risk of developing a metachronous tumor in the contralateral kidney. Other studies have looked at the incidence of rests in a large sample of WT with similar results. In a child under 1 year of age, this risk is very significant, and these children need to be followed very carefully with sequential ultrasound (US) examinations. It is very difficult to diagnose NRs on imaging. Although magnetic resonance imaging (MRI) may be helpful, there is no gold standard.
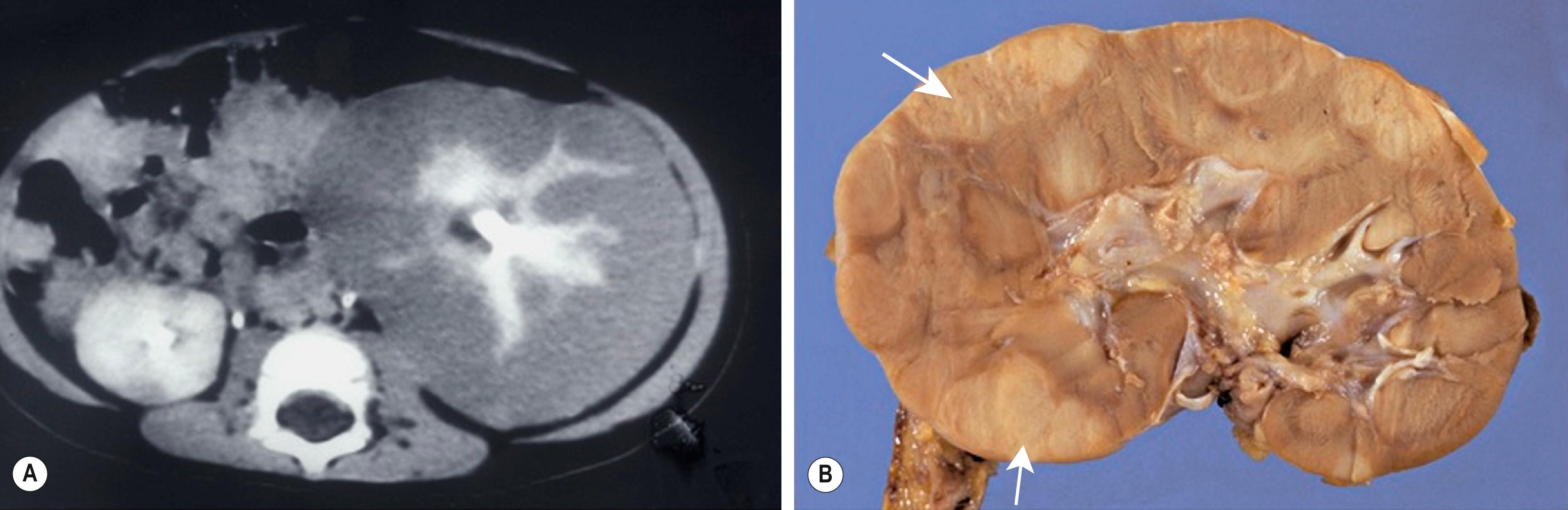
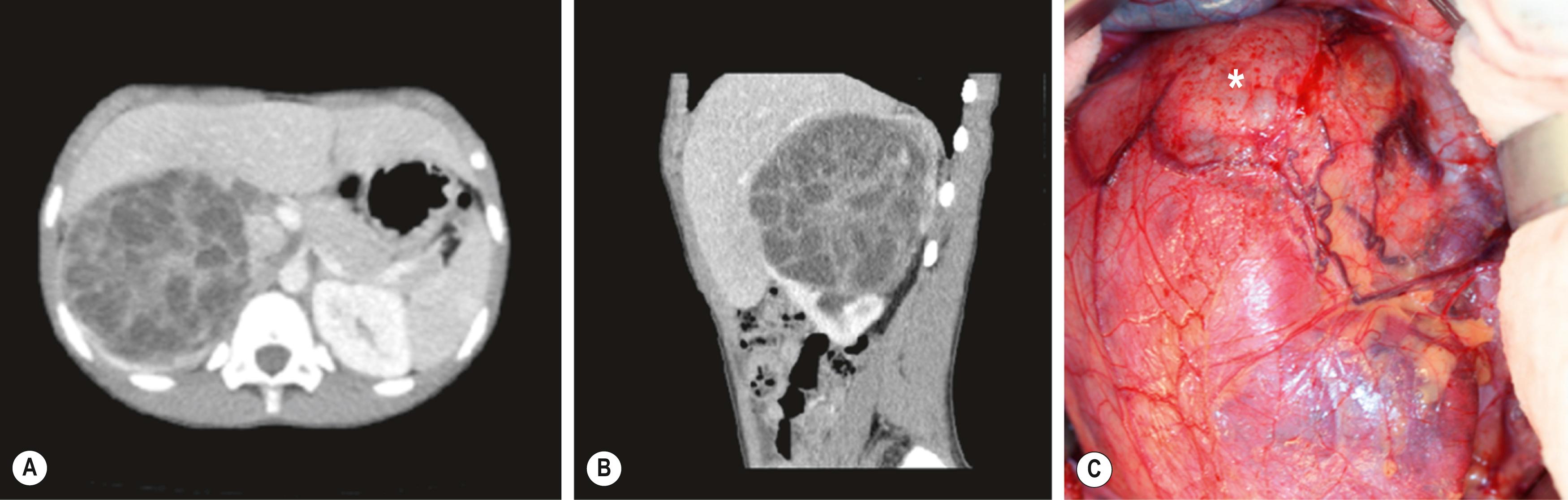
Diffuse hyperplastic perilobar nephroblastomatosis (DHPLN) is a unique category of nephroblastomatosis in which the rests form a thick rind around the periphery of the kidney. Infants with DHPLN may initially present with large unilateral or bilateral flank masses ( Fig. 64.3 ). A characteristic radiographic finding is massively enlarged kidneys that maintain their normal configuration and lack evidence of necrosis. As with the isolated NRs, proliferation of the thin rind of NRs on the periphery of the kidney will preserve the normal configuration of the kidney, but will result in marked enlargement. This is in contrast to WT, in which the normal renal configuration and collecting system are generally distorted and necrosis is present. The optimal diagnosis and management of a child with DHPLN is unclear. In a 2006 study of 52 children and 33 cases of DHPLN, histologic examination alone was unable to establish a diagnosis in 21 children who underwent biopsy at the time of initial presentation. In the same study, 24 children developed WT during long-term follow-up, with 8 being anaplastic; 13 had single tumors and 11 were multiple. Treatment was variable and included surgery, radiation and chemotherapy, or expectant observation. The 3 patients who received no therapy developed WT 10 months after diagnosis. Chemotherapy may decrease the proliferative element in NRs, but it is unclear whether it prevents the development of malignancy. In a retrospective review from 1989–2005, SIOP reported an interesting study in children with DHPLN. Thirteen patients had been pretreated for bilateral nephroblastomatosis. One patient was not treated, and 17 patients had upfront surgery. Preoperative treatment duration ranged from 1–12 weeks ( n = 103). Pretreatment for nephroblastomatosis was an independent risk factor (37 ± 14%/67 ± 13%; P < 0.001) for significantly worse outcome compared with those without treatment or less than three cycles of chemotherapy. A current COG study includes a treatment protocol to address some of the questions with respect to DHPLN and its optimal therapy.
An increased risk of WT arising in multicystic dysplastic kidneys has been suggested. This is primarily based on case reports, and all children have been under the age of 4 years. In contrast, a report describing 1041 infants and children with multicystic dysplastic kidneys found no case associated with a WT. The authors concluded that routine prophylactic nephrectomy was not needed in children with multicystic dysplastic kidneys. Furthermore, in a review of the NWTS pathology files, only three cases of dysplastic kidneys were identified in more than 7000 children with WT over a 26-year interval. Thus, prophylactic nephrectomy in children with multicystic dysplastic kidney for malignancy is not justified. Based on this report, a screening US in these children until age 4 years is suggested. A single institution retrospective study of 300 children over 30 years found no malignancies and suggested an algorithm in which children with specific characteristic (involution, no voiding cystourethrogram abnormality, or stable disease) do not need US after 1 year of age.
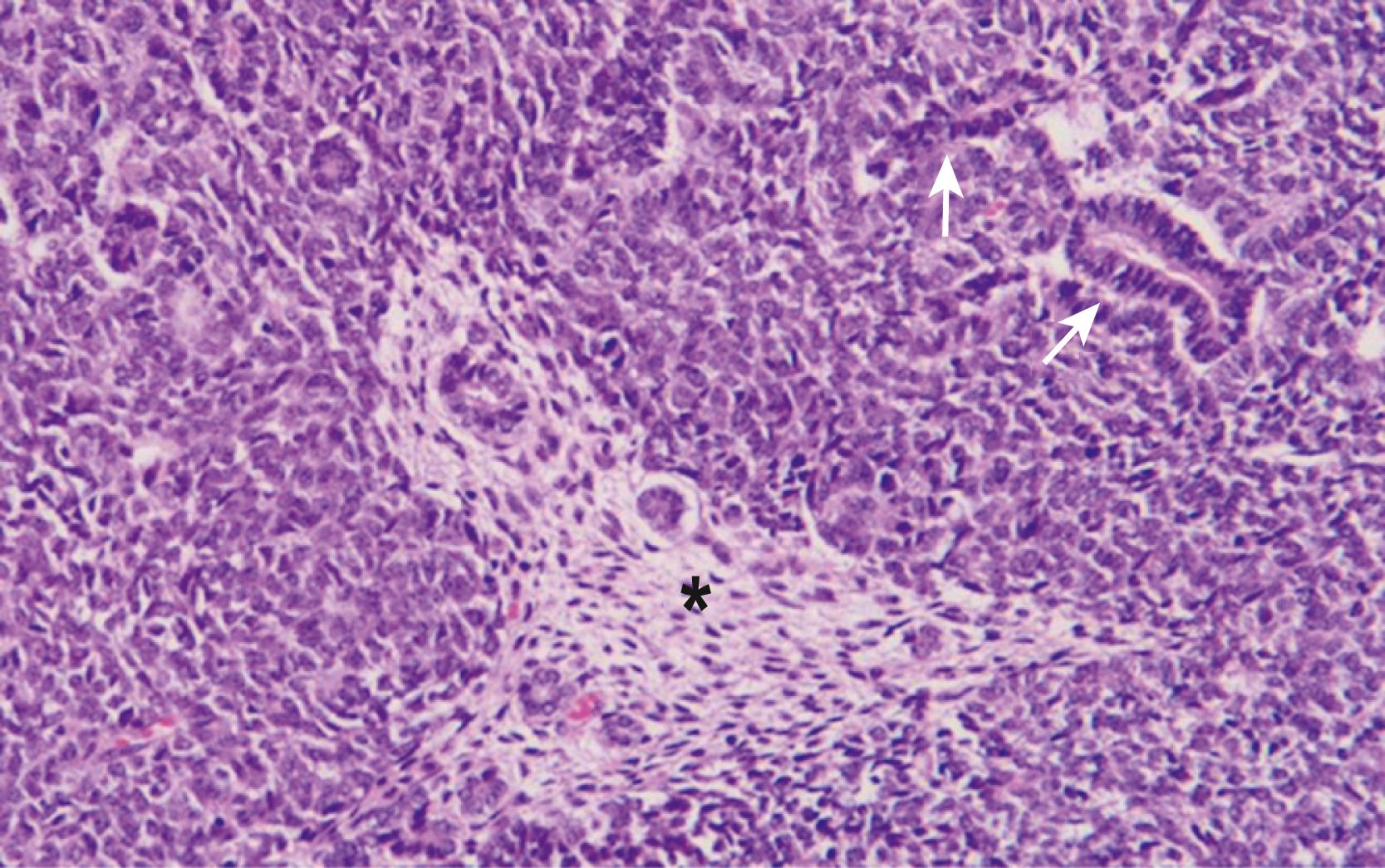
WTs are currently divided into those with favorable histology and those with unfavorable histology. Unfavorable histology proved to be the most important factor in patient outcome in NWTS-1, a finding that has remained consistent through the current trials. Fortunately, favorable histology accounts for 90% of the tumors. These tumors are the “classic WT” consisting of three elements: blastemal, stromal, and epithelial tubules ( Fig. 64.4 ). Tumors contain various proportions of each of these elements. Triphasic are the most characteristic, but biphasic and monophasic lesions also occur. The proportion of these three elements in WTs have been studied but have not been shown to predict outcomes. Under the current COG treatment protocols, they are not utilized to determine therapy. This is in contrast to SIOP, which uses a postchemotherapy staging system. Abnormal mucinous or squamous epithelium, skeletal muscle, cartilage, osteoid, and fat are less frequent elements found in WT.
Unfavorable tumors are those with focal or diffuse anaplasia. Anaplasia is defined by multipolar polyploid mitotic figures, marked nuclear enlargement (giant nuclei with diameters at least three times those of adjacent cells), and hyperchromasia ( Fig. 64.5 ). Determining whether a tumor has diffuse or focal anaplasia is important for prognosis and therapy. Focal anaplasia is defined as the presence of one or a few sharply localized regions of anaplasia within a primary tumor, the majority of which contains no nuclear atypia. Tumor designated as diffuse anaplasia must have at least one of the following four criteria: anaplastic cells outside of the kidney, presence of anaplasia in a random kidney biopsy, anaplasia in more than one region of the kidney, or anaplasia in one region with extreme nuclear pleomorphism in another site. Anaplasia occurs primarily in children older than 2 years. In NWTS-1, 66.7% of patients with anaplasia experienced relapse and 58.3% died of their tumor. Also, there was a higher frequency of relapse and death in the “diffuse” subgroup. Additionally, anaplasia in extrarenal tumor sites and a predominantly blastemal tumor pattern were both adverse prognostic factors. TP53 deletion on chromosome 17 has been associated with anaplasia. However, a recent study demonstrated recurrent anaplasia-specific genomic loss and underexpression in several additional regions, most strikingly 4q and 14q, suggesting anaplasia is not linked solely to deletions of chromosome 17, but is associated with loss at multiple loci.
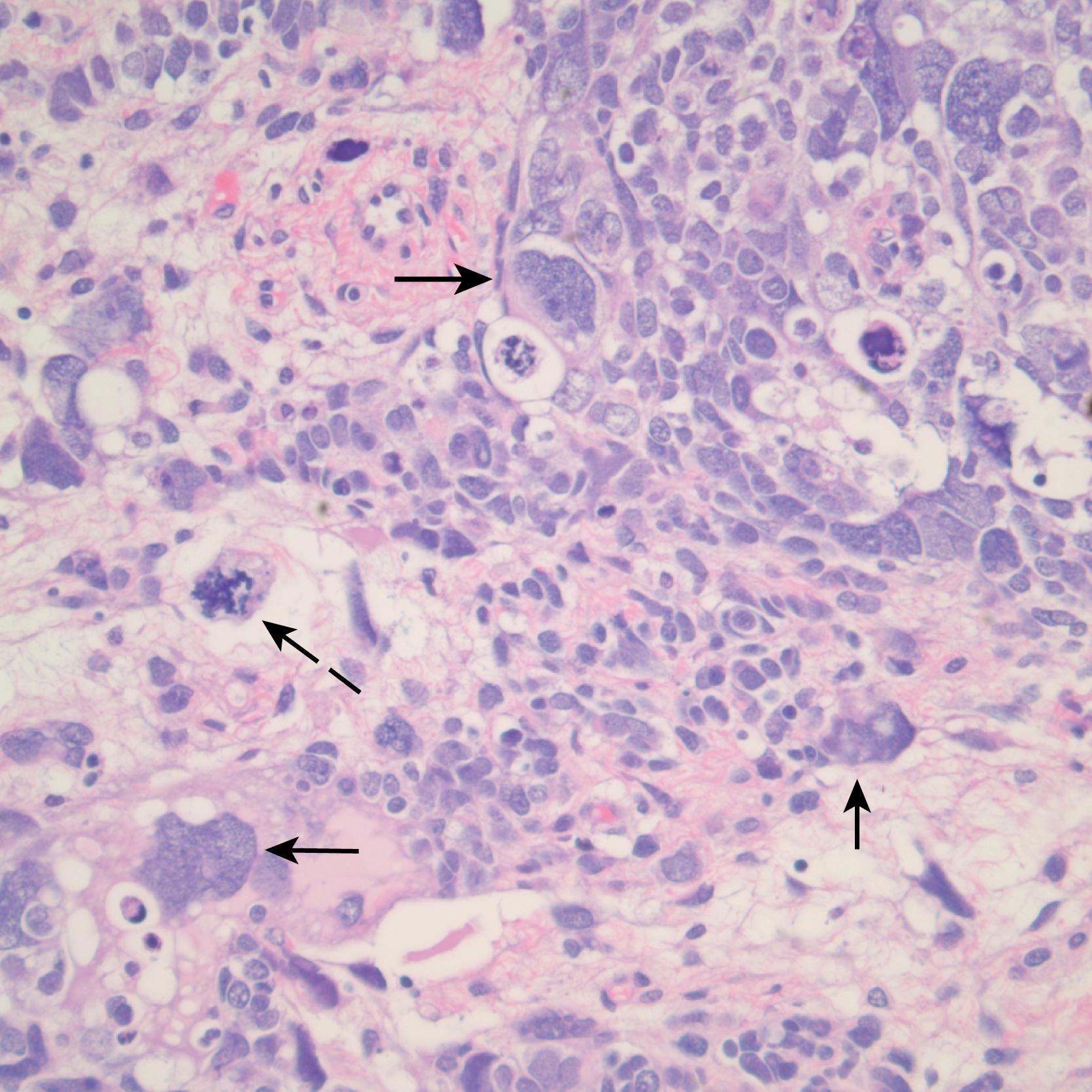
CCSK and malignant RTK were grouped in the initial NWTSG studies with the unfavorable-histology WT. They are now considered distinct entities from WT based on their pathologic appearance, biologic behavior, and response to different therapies. CCSK is a highly malignant tumor with an unusual proclivity for bony metastasis. Generally, it appears as a large unifocal and unilateral tumor with a homogeneous mucoid, tan, or gray/tan cut surface, often with foci of necrosis or prominent cysts ( Fig. 64.6 ). This tumor invades and surrounds the renal parenchyma rather than compressing the margin into a pseudocapsule as is seen in WT. Its classic appearance is that of a deceptively bland tumor with uniform oval nuclei with a delicate chromatin pattern, a prominent nuclear membrane, and sparse, poorly stained vacuolated “water-clear” cytoplasm with indistinct cell membranes. Although the cells generally appear in cords or nests divided by an arborizing network of vessels and supporting spindle cell septa, there are variations and nine major histologic patterns have been identified. Recently, a translocation t(10;17) and deletion (14q) also have been described in CCSK, suggesting that they may play a role in its pathogenesis. The cell of origin of this tumor is not known. In addition to osseous metastases, CCSKs also have a significant incidence of metastases to the brain. Long-term follow-up is important as one report found 30% of the relapses occur more than 2 years after diagnosis.
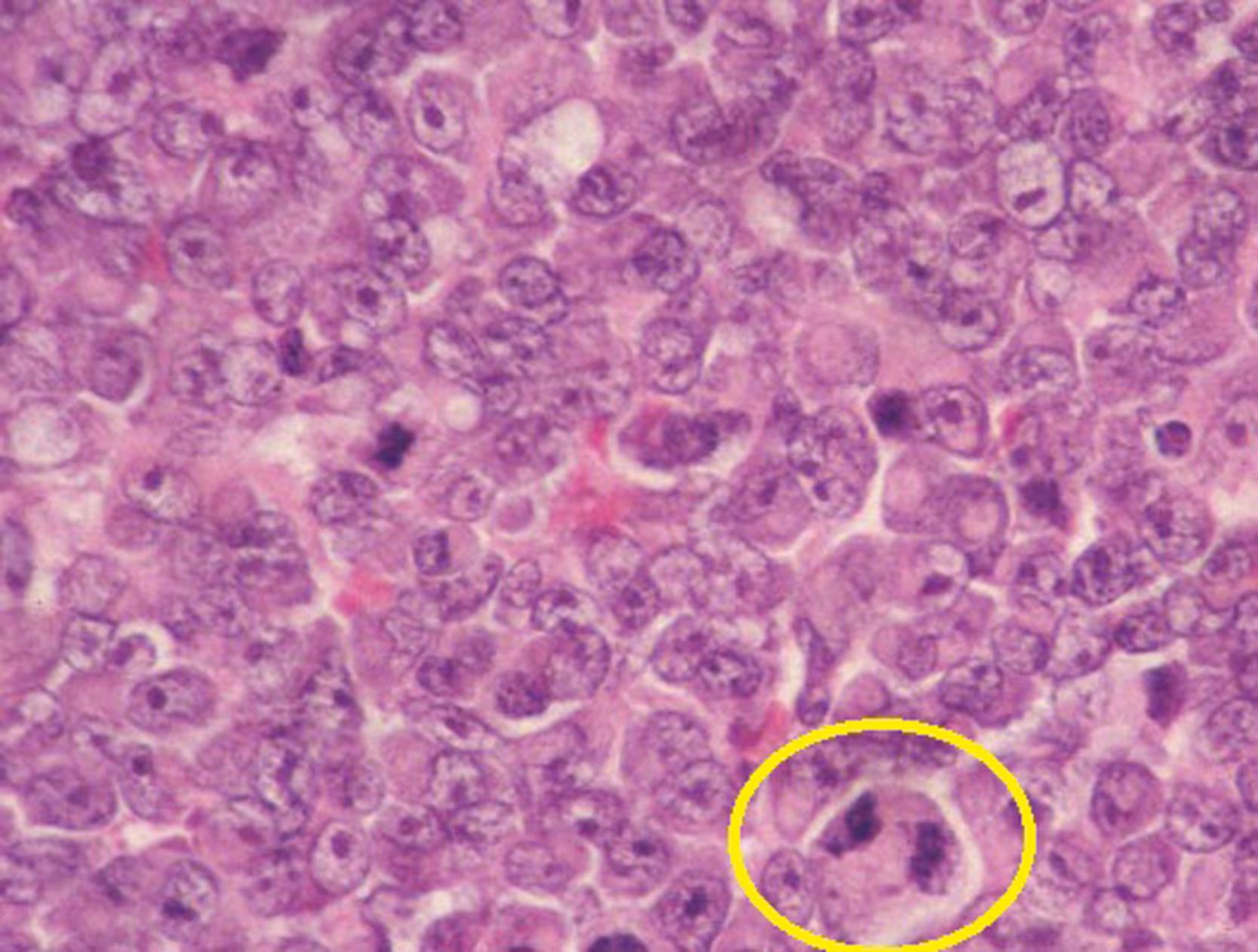
Malignant RTK occurs in young infants with a median age of 11 months. Most (85%) cases occur within the first 2 years of life. It is the most aggressive and lethal of all pediatric renal tumors, and fortunately only accounts for 2% of renal tumors. Grossly these tumors are unencapsulated and invasive with characteristic involvement of the perihilar renal parenchyma. Histologically, RTKs are characterized by monomorphous, discohesive, rounded to polygonal cells with acidophilic cytoplasm and eccentric nuclei containing prominent large “owl eye” nucleoli reminiscent of skeletal muscle, but lacking its cytoplasmic striations, ultrastructural features, and immunochemical markers ( Fig. 64.7 ). A large periodic acid–Schiff (PAS)-positive hyaline cytoplasmic inclusion occurs in a variable population of tumor cells and is a hallmark of this tumor. Ultrastructural examination reveals parallel cytoplasmic filamentous inclusions packed in concentric whorled arrays, a distinctive feature of this tumor, which suggests a neuroectodermal origin. The tumor tends to infiltrate the adjacent renal parenchyma rather than to compress it. These tumors are notable for the occurrence of a second primary tumor in the midline of the brain, resembling medulloblastoma. A consistent deletion (22q11-12) has been described in both renal and extrarenal rhabdoid tumors. These deletions delineate an area of overlap at the site of the hSNF5/INI1 gene, and tumors have biallelic alterations or deletions of this gene. For all renal tumors except RTK, immunohistochemical staining for the wild-type INI-1 protein shows nuclear positivity. In renal and extrarenal rhabdoid tumors, this is absent.
Two principal staging systems are used for children with WT ( Table 64.4 ). The COG system is based on pretreatment findings prior to administration of chemotherapy or radiotherapy. Patients are given a local stage and a disease stage. The local stage defines the extent of abdominal disease, and the disease stage considers both the local extent of disease and distant metastasis. Both factors determine therapy with the use of local RT to the tumor bed based on the local stage and the intensity of chemotherapy based on the disease stage.
| Stage | Criteria |
|---|---|
| COG Staging System | |
| I | The tumor is limited to the kidney and has been completely resected. |
| The tumor was not ruptured, nor was biopsy performed prior to removal. | |
| No penetration of the renal capsule or involvement of renal sinus vessels. | |
| II | The tumor extends beyond the capsule of the kidney but was completely resected with no evidence of tumor at or beyond the margins of resection. |
| There is penetration of the renal capsule or invasion of the renal sinus vessels. | |
| III | Gross or microscopic residual tumor remains postoperatively, including inoperable tumor, positive surgical margins, tumor spillage, regional lymph node metastases, positive peritoneal cytology, or transected tumor thrombus. |
| The tumor was ruptured or biopsied prior to removal. | |
| IV | Hematogenous metastases or lymph node metastases are present outside the abdomen (e.g., lung, liver, bone, brain). |
| V | Bilateral renal involvement is present at diagnosis, and each side may be considered to have a “local” stage. |
| SIOP Staging System | |
| I | The tumor is limited to the kidney or surrounded with a fibrous pseudocapsule if outside the normal contours of the kidney. |
| The renal capsule or pseudocapsule may be infiltrated with the tumor, but it does not reach the outer surface, and it is completely resected. | |
| The tumor may be protruding (bulging) into the renal pelvis and dipping into the ureter, but it is not infiltrating their walls. | |
| The vessels of the renal sinus are not involved. Intrarenal vessels may be involved. | |
| II | The tumor extends beyond the kidney or penetrates through the renal capsule and/or fibrous pseudocapsule into the perirenal fat but is completely resected. |
| The tumor infiltrates the renal sinus and/or invades blood and lymphatic vessels outside the renal parenchyma, but it is completely resected. | |
| The tumor infiltrates adjacent organs or vena cava but is completely resected. | |
| The tumor has been surgically biopsied (wedge biopsy) prior to preoperative chemotherapy or surgery. | |
| III | Incomplete excision of the tumor, which extends beyond resection margins (gross or microscopic tumor remains postoperatively). |
| Any abdominal lymph nodes are involved. Tumor rupture before or during surgery (irrespective of other criteria for staging). | |
| The tumor has penetrated the peritoneal surface. Tumor implants are found on the peritoneal surface. | |
| Tumor thrombi are present at resection margins of vessels, or ureter is transected or removed piecemeal by surgeon. | |
| IV | Hematogenous metastases (lung, liver, bone, brain, etc.) or lymph node metastases outside the abdominopelvic region. |
| V | Bilateral renal tumors at diagnosis. Each side has to be substaged according to above classifications. |
SIOP protocols generally recommend chemotherapy followed by nephrectomy, with surgicopathologic staging occurring at the time of nephrectomy. The SIOP classification was revised in 2010 based on a review of the histologic appearance of the tumors at resection (post neoadjuvant chemotherapy) and the corresponding outcomes. Tumors are now classified on SIOP protocols as completely necrotic (low-risk tumor), blastemal (high-risk tumor), and other histology (intermediate-risk tumors).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here