Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The extracellular fluid potassium concentration normally is regulated at about 4.2 mEq/L, seldom rising or falling more than ±0.3 mEq/L. This precise control is necessary because many cell functions are sensitive to changes in extracellular fluid potassium concentration. For example, an increase in plasma potassium concentration of only 3 to 4 mEq/L can cause cardiac arrhythmias, and higher concentrations can lead to cardiac arrest or fibrillation.
A special difficulty in regulating extracellular potassium concentration is the fact that more than 98% of the total body potassium is contained in the cells, and only 2% is contained in the extracellular fluid ( Figure 30-1 ). For a 70-kg adult, who has about 28 liters of intracellular fluid (40% of body weight) and 14 liters of extracellular fluid (20% of body weight), about 3920 mEq of potassium are inside the cells, and only about 59 mEq are in the extracellular fluid. Also, the potassium contained in a single meal may be as high as 50 mEq, and the daily intake usually ranges between 50 and 200 mEq/day; therefore, failure to rapidly rid the extracellular fluid of the ingested potassium could cause life-threatening hyperkalemia (increased plasma potassium concentration). Likewise, a small loss of potassium from the extracellular fluid could cause severe hypokalemia (low plasma potassium concentration) in the absence of rapid and appropriate compensatory responses.
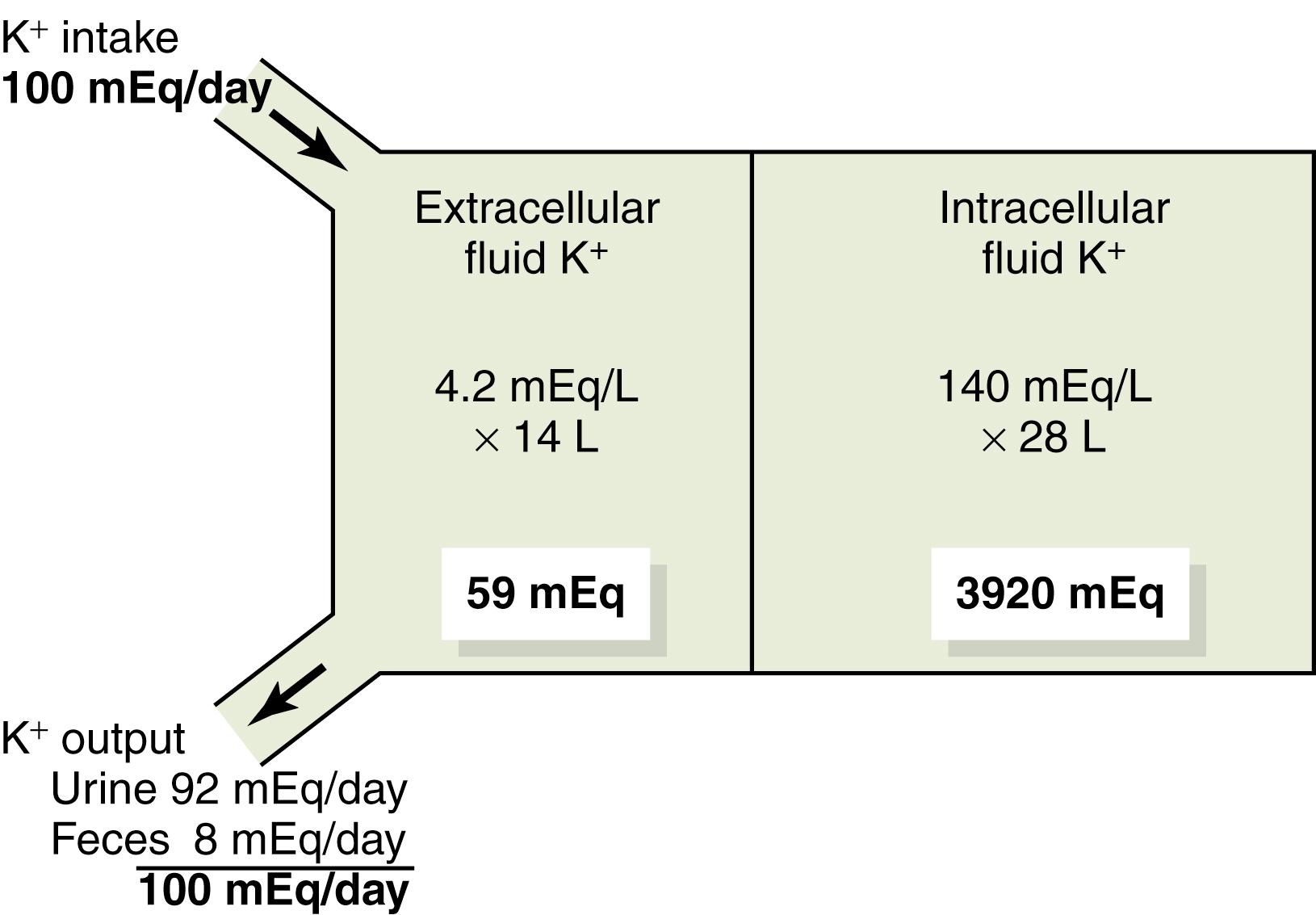
Maintenance of a balance between intake and output of potassium depends primarily on excretion by the kidneys because the amount excreted in the feces is only about 5% to 10% of the potassium intake. Thus, the maintenance of a normal potassium balance requires the kidneys to adjust their potassium excretion rapidly and precisely in response to wide variations in intake, as is also true for most other electrolytes.
Control of potassium distribution between the extracellular and intracellular compartments also plays an important role in potassium homeostasis. Because more than 98% of the total body potassium is contained in the cells, they can serve as an overflow site for excess extracellular fluid potassium during hyperkalemia or as a source of potassium during hypokalemia. Thus, redistribution of potassium between the intracellular and extracellular fluid compartments provides a first line of defense against changes in extracellular fluid potassium concentration.
After ingestion of a potassium-rich meal, extracellular fluid potassium concentration would rise to a dangerous level if the ingested potassium did not move into the cells rapidly. For example, absorption of 40 mEq of potassium (the amount contained in a meal rich in vegetables and fruit) into an extracellular fluid volume of 14 liters would raise plasma potassium concentration by about 2.9 mEq/L if all the potassium remained in the extracellular compartment. Fortunately, most of the ingested potassium rapidly moves into the cells until the kidneys can eliminate the excess. Between meals, plasma potassium concentration also remains nearly constant as potassium is released by the cells to balance the extracellular fluid potassium excreted by the kidneys. Table 30-1 summarizes some of the factors that can influence the distribution of potassium between the intracellular and extracellular compartments.
| Factors That Shift K + Into Cells (Decrease Extracellular [K + ]) | Factors That Shift K + Out of Cells (Increase Extracellular [K + ]) |
|---|---|
| Insulin | Insulin deficiency (diabetes mellitus) |
| Aldosterone | Aldosterone deficiency (Addison disease) |
| β-Adrenergic stimulation | β-Adrenergic blockade |
| Alkalosis | AcidosisCell lysisStrenuous exerciseIncreased extracellular fluid osmolarity |
Insulin stimulates sodium-potassium adenosine triphosphatase (ATPase) activity in many tissues, including skeletal muscle, which in turn transports potassium into the cells. Insulin is important for increasing cell potassium uptake after a meal. In people who have insulin-deficient diabetes mellitus, the rise in plasma potassium concentration after eating a meal is much greater than normal. Injections of insulin, however, can help correct the hyperkalemia.
Increased potassium intake also stimulates secretion of aldosterone, which increases cell potassium uptake. Excess aldosterone secretion ( Conn syndrome ) is almost invariably associated with hypokalemia, due in part to movement of extracellular potassium into the cells. Conversely, patients with deficient aldosterone production ( Addison disease ) often have clinically significant hyperkalemia due to accumulation of potassium in the extracellular space, as well as renal retention of potassium.
Increased secretion of catecholamines, especially epinephrine, can cause movement of potassium from the extracellular to the intracellular fluid, mainly by activation of β 2 -adrenergic receptors. Conversely, treatment of hypertension with β-adrenergic receptor blockers, such as propranolol, causes potassium to move out of the cells and creates a tendency toward hyperkalemia.
Metabolic acidosis increases extracellular potassium concentration, in part by causing loss of potassium from the cells, whereas metabolic alkalosis decreases extracellular fluid potassium concentration. Although the mechanisms responsible for the effect of hydrogen ion concentration on internal distribution of potassium are not completely understood, one effect of increased hydrogen ion concentration is to reduce activity of the Na + -K + ATPase pump. This reduction, in turn, decreases cellular uptake of potassium and raises extracellular potassium concentration. Alkalosis has the opposite effect, shifting potassium from the extracellular fluid into the cells and tending to cause hypokalemia.
As cells are destroyed, the large amounts of potassium contained in the cells are released into the extracellular fluid. This release of potassium can cause significant hyperkalemia if large amounts of tissue are destroyed, as occurs with severe muscle injury or with red blood cell lysis.
During prolonged exercise, potassium is released from skeletal muscle into the extracellular fluid. Usually the hyperkalemia is mild, but it may be clinically significant after heavy exercise, especially in patients treated with β-adrenergic blockers or in individuals with insulin deficiency. In rare cases, hyperkalemia after exercise may be severe enough to cause cardiac toxicity.
Increased extracellular fluid osmolarity causes osmotic flow of water out of the cells. The cellular dehydration increases intracellular potassium concentration, thereby promoting diffusion of potassium out of the cells and increasing extracellular fluid potassium concentration. Decreased extracellular fluid osmolarity has the opposite effect.
Renal potassium excretion is determined by the sum of three processes: (1) the rate of potassium filtration (glomerular filtration rate [GFR] multiplied by the plasma potassium concentration); (2) the rate of potassium reabsorption by the tubules; and (3) the rate of potassium secretion by the tubules. The normal rate of potassium filtration by the glomerular capillaries is about 756 mEq/day (GFR, 180 L/day, multiplied by plasma potassium concentration, 4.2 mEq/L). This rate of filtration is relatively constant in healthy persons because of the autoregulatory mechanisms for GFR discussed previously and the precision with which plasma potassium concentration is regulated. Severe decreases in GFR in certain renal diseases, however, can cause serious potassium accumulation and hyperkalemia.
Figure 30-2 summarizes the tubular handling of potassium under normal conditions. About 65% of the filtered potassium is reabsorbed in the proximal tubule. Another 25% to 30% of the filtered potassium is reabsorbed in the loop of Henle, especially in the thick ascending part, where potassium is actively co-transported along with sodium and chloride. In the proximal tubule and loop of Henle, a relatively constant fraction of the filtered potassium load is reabsorbed. Changes in potassium reabsorption in these segments can influence potassium excretion, but most of the daily variation of potassium excretion is not due to changes in reabsorption in the proximal tubule or loop of Henle. The collecting tubules and collecting ducts reabsorb potassium at variable rates, depending on the potassium intake.
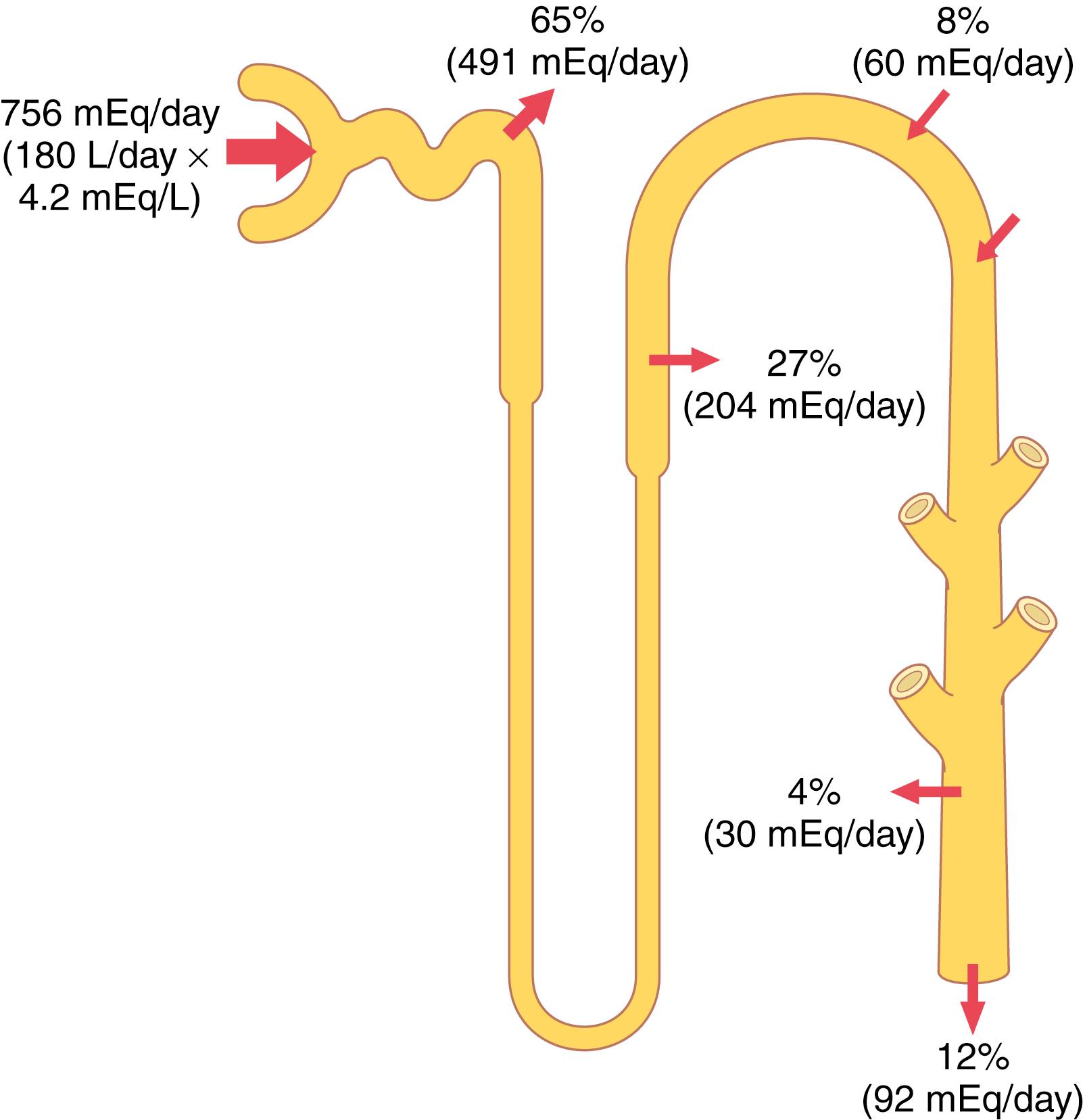
The most important sites for regulating potassium excretion are the principal cells of the late distal tubules and cortical collecting tubules. In these tubular segments, potassium can at times be reabsorbed or at other times can be secreted, depending on the needs of the body. With a normal potassium intake of 100 mEq/day, the kidneys must excrete about 92 mEq/day (the remaining 8 mEq are lost in the feces). About 60 mEq/day of potassium are secreted into the distal and collecting tubules, accounting for most of the excreted potassium.
With a high potassium intake, the required extra excretion of potassium is achieved almost entirely by increasing the secretion of potassium into the distal and collecting tubules. In fact, in those who consume extremely high-potassium diets, the rate of potassium excretion can exceed the amount of potassium in the glomerular filtrate, indicating a powerful mechanism for secreting potassium.
When potassium intake is low, secretion of potassium in the distal and collecting tubules decreases, causing a reduction in urinary potassium excretion. There is also increased reabsorption of potassium by the intercalated cells in the distal segments of the nephron, and potassium excretion can fall to less than 1% of the potassium in the glomerular filtrate (to <10 mEq/day). With potassium intakes below this level, severe hypokalemia can develop.
Thus, most of the daily regulation of potassium excretion occurs in the late distal and cortical collecting tubules, where potassium can be reabsorbed or secreted, depending on the needs of the body. In the next section, we consider the basic mechanisms of potassium secretion and the factors that regulate this process.
The cells in the late distal and cortical collecting tubules that secrete potassium are called principal cells and make up most of the epithelial cells in these regions. Figure 30-3 shows the basic cellular mechanisms of potassium secretion by the principal cells.
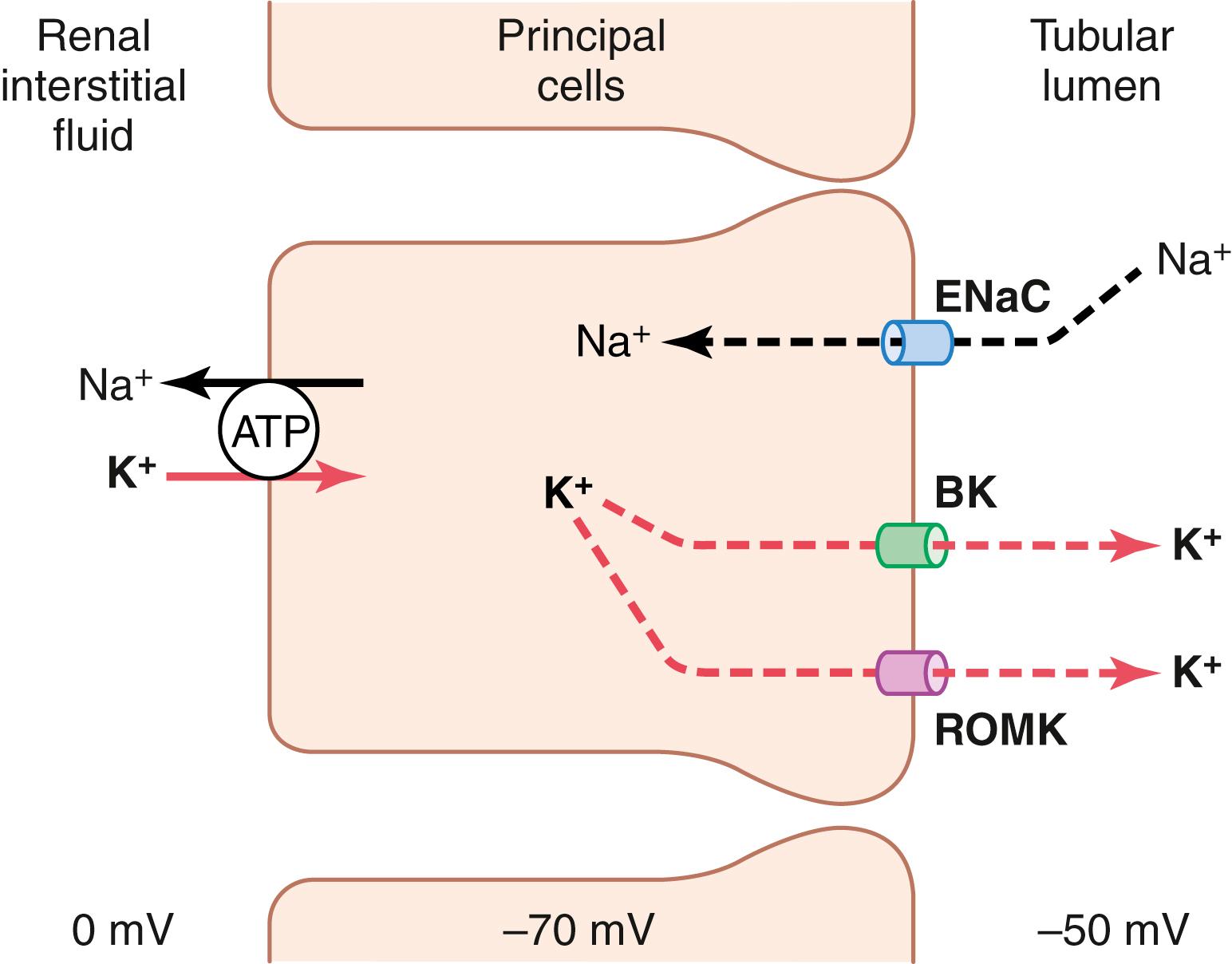
Secretion of potassium from the blood into the tubular lumen is a two-step process, beginning with uptake from the interstitium into the cell by the Na + -K + ATPase pump in the basolateral cell membrane. This pump moves sodium out of the cell into the interstitium and, at the same time, moves potassium to the interior of the cell.
The second step of the process is passive diffusion of potassium from the interior of the cell into the tubular fluid. The Na + -K + ATPase pump creates a high intracellular potassium concentration, which provides the driving force for passive diffusion of potassium from the cell into the tubular lumen. The luminal membrane of the principal cells is highly permeable to potassium because there are two types of special channels that allow potassium ions to diffuse across the membrane rapidly: (1) the renal outer medullary potassium (ROMK) channels , and (2) high-conductance, “big” potassium (BK) channels . Both types of potassium channels are required for efficient renal potassium excretion, and their abundance in the luminal membrane is increased during high potassium intake.
The primary factors that control potassium secretion by the principal cells of the late distal and cortical collecting tubules are the following: (1) the activity of the Na + -K + ATPase pump; (2) the electrochemical gradient for potassium secretion from the blood to the tubular lumen; and (3) the permeability of the luminal membrane for potassium. These three determinants of potassium secretion are, in turn, regulated by several factors, discussed later.
In circumstances associated with severe potassium depletion, there is a cessation of potassium secretion and a net reabsorption of potassium in the late distal and collecting tubules. This reabsorption occurs through the type A intercalated cells . Although this reabsorptive process is not completely understood, one mechanism believed to contribute is a hydrogen-potassium ATPase transport mechanism located in the luminal membrane (see Chapter 28 , Figure 28-13 ). This transporter reabsorbs potassium in exchange for hydrogen ions secreted into the tubular lumen, and the potassium then diffuses through basolateral membrane potassium channels into the interstitial fluid. This abundance of intercalated cell hydrogen-potassium ATPase transporters is enhanced with potassium depletion and hypokalemia, causing increased hydrogen ion secretion and alkalosis. Under normal conditions, however, potassium reabsorption by intercalated cells plays only a small role in controlling potassium excretion.
When there is excess potassium in the body fluids, type B intercalated cells in the late distal tubules and collecting tubules actively secrete potassium into the tubular lumen and have functions that are opposite to those of type A cells (see Chapter 28 , Figure 28-13 ). Potassium is pumped into the type B intercalated cell by a hydrogen-potassium ATPase transporter on the basolateral membrane, and it then diffuses into the tubular lumen through potassium channels.
Because the normal regulation of potassium excretion occurs mainly as a result of changes in potassium secretion by the principal cells of the late distal and collecting tubules, in this chapter we discuss the primary factors that influence secretion by these cells. The most important factors that stimulate potassium secretion by the principal cells include the following: (1) increased extracellular fluid potassium concentration; (2) increased aldosterone; and (3) increased tubular flow rate.
One factor that decreases potassium secretion is an increased hydrogen ion concentration (acidosis).
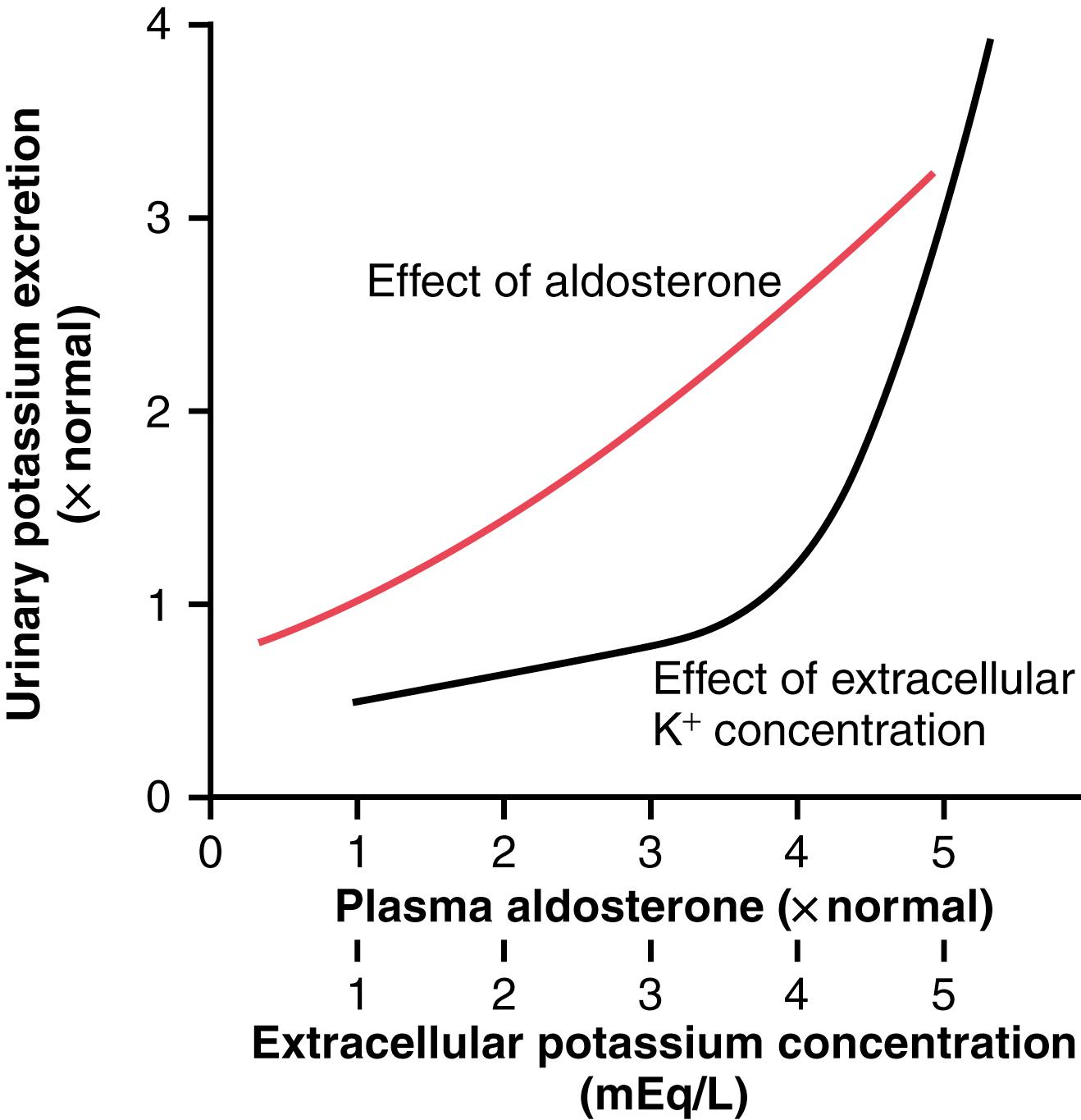
The rate of potassium secretion in the late distal and cortical collecting tubules is directly stimulated by increased extracellular fluid potassium concentration, leading to increases in potassium excretion, as shown in Figure 30-4 . This effect is especially pronounced when extracellular fluid potassium concentration rises above about 4.1 mEq/L, slightly less than the normal concentration. Increased plasma potassium concentration, therefore, serves as one of the most important mechanisms for increasing potassium secretion and regulating extracellular fluid potassium ion concentration.
Increased dietary potassium intake and increased extracellular fluid potassium concentration stimulate potassium secretion by at least four mechanisms:
Increased extracellular fluid potassium concentration stimulates the Na + -K + ATPase pump, thereby increasing potassium uptake across the basolateral membrane. This increased potassium uptake in turn increases intracellular potassium ion concentration, causing potassium to diffuse across the luminal membrane into the tubule.
Increased extracellular potassium concentration increases the potassium gradient from the renal interstitial fluid to the interior of the epithelial cell, which reduces backleakage of potassium ions from inside the cells through the basolateral membrane.
Increased potassium intake stimulates synthesis of potassium channels and their translocation from the cytosol to the luminal membrane, which, in turn, increases the ease of potassium diffusion through the membrane.
Increased potassium concentration stimulates aldosterone secretion by the adrenal cortex, which further stimulates potassium secretion, as discussed next.
Aldosterone stimulates active reabsorption of sodium ions by the principal cells of the late distal tubules and collecting ducts (see Chapter 28 ). This effect is mediated through a Na + -K + ATPase pump that transports sodium outward through the basolateral membrane of the cell and into the renal interstitial fluid at the same time that it pumps potassium into the cell. Thus, aldosterone also has a powerful effect on controlling the rate at which the principal cells secrete potassium and reabsorb sodium.
A second effect of aldosterone is to increase the number of potassium channels in the luminal membrane and therefore its permeability for potassium, further adding to the effectiveness of aldosterone in stimulating potassium secretion. Therefore, aldosterone has a powerful effect to increase potassium excretion, as shown in Figure 30-4 .
In negative feedback control systems, the factor that is controlled usually has a feedback effect on the controller. In the case of the aldosterone-potassium control system, the rate of aldosterone secretion by the adrenal gland is controlled strongly by extracellular fluid potassium ion concentration. Figure 30-5 shows that an increase in plasma potassium concentration of about 3 mEq/L can increase the plasma aldosterone concentration from nearly 0 to as high as 60 ng/100 ml, a concentration almost 10 times normal.
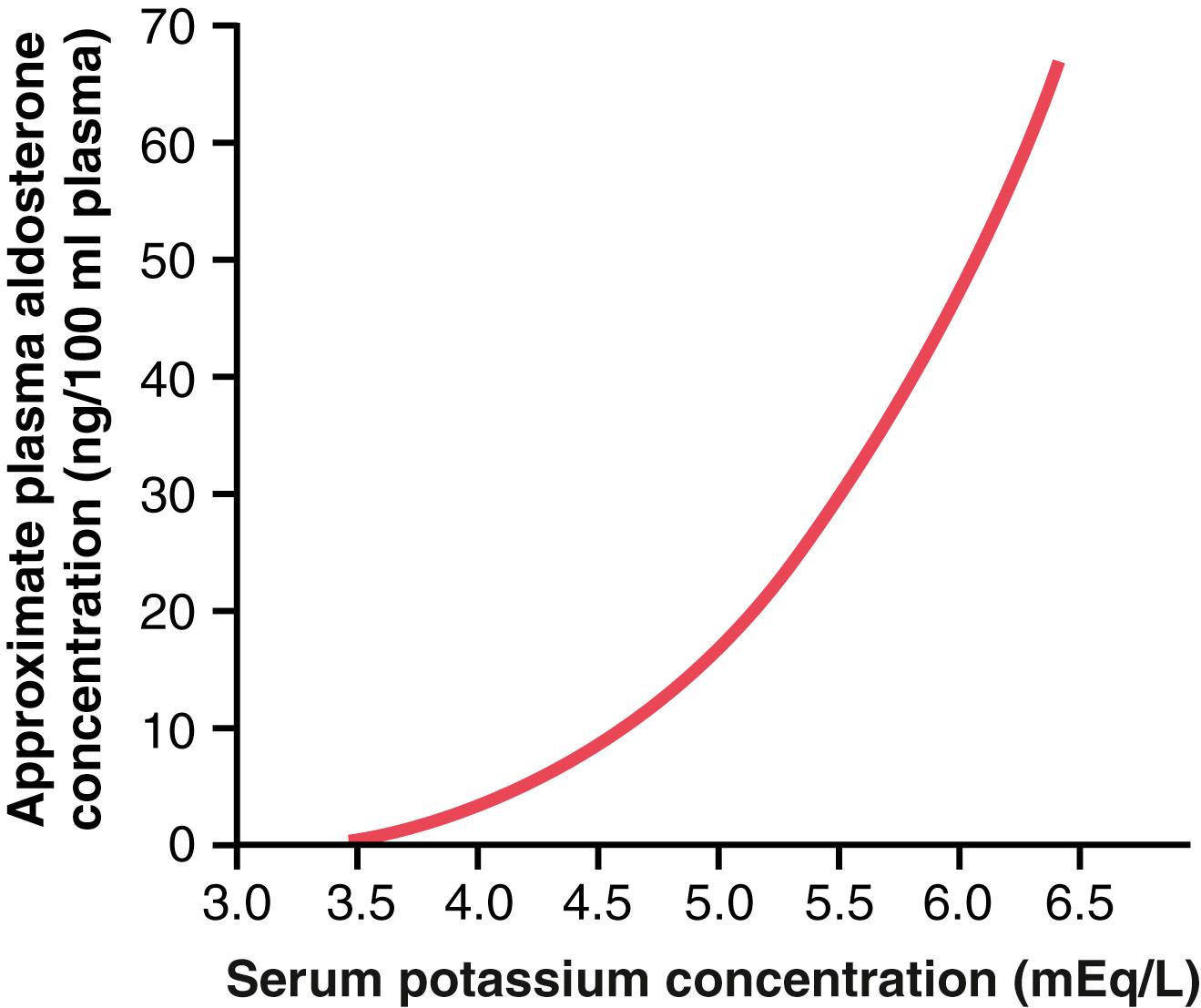
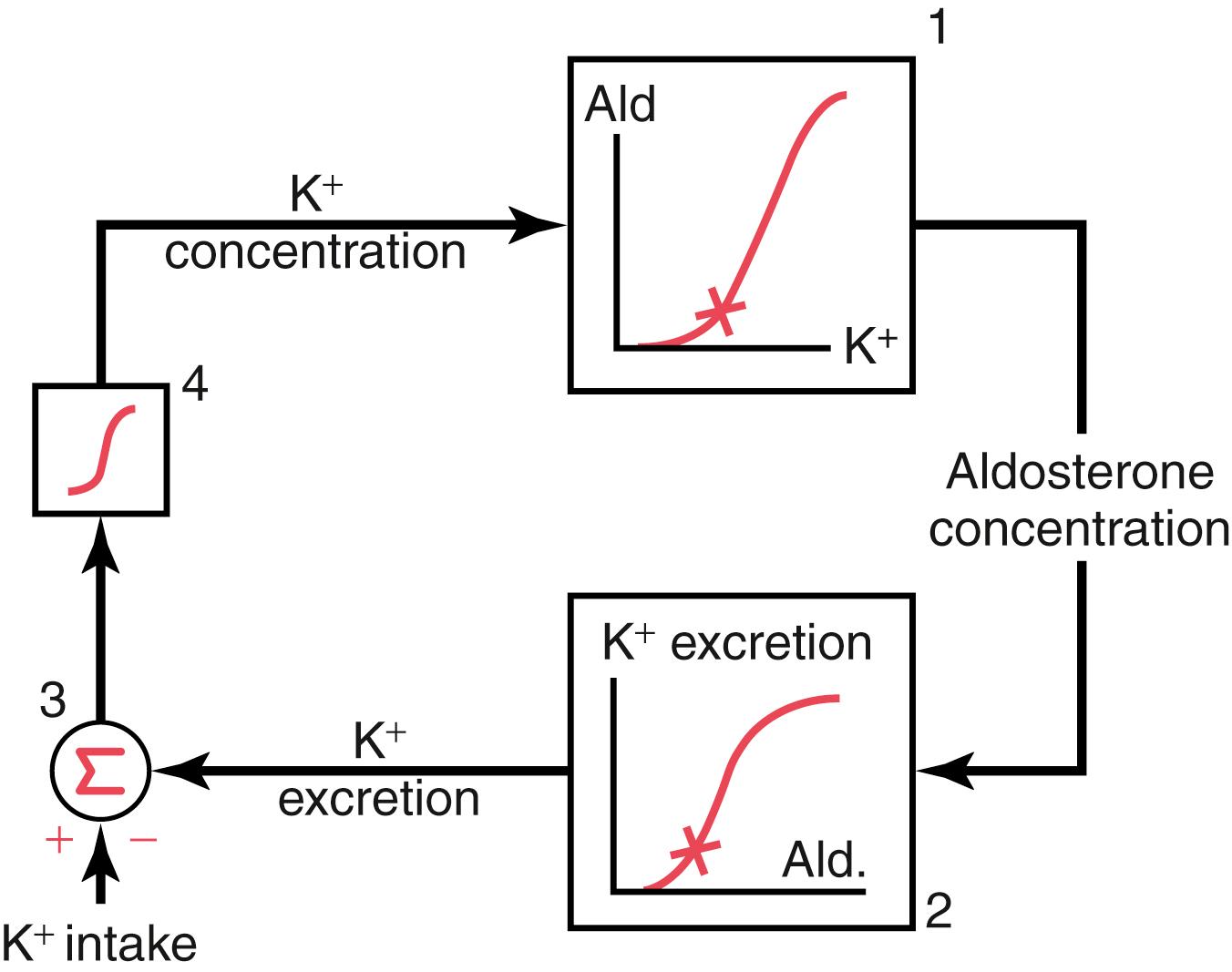
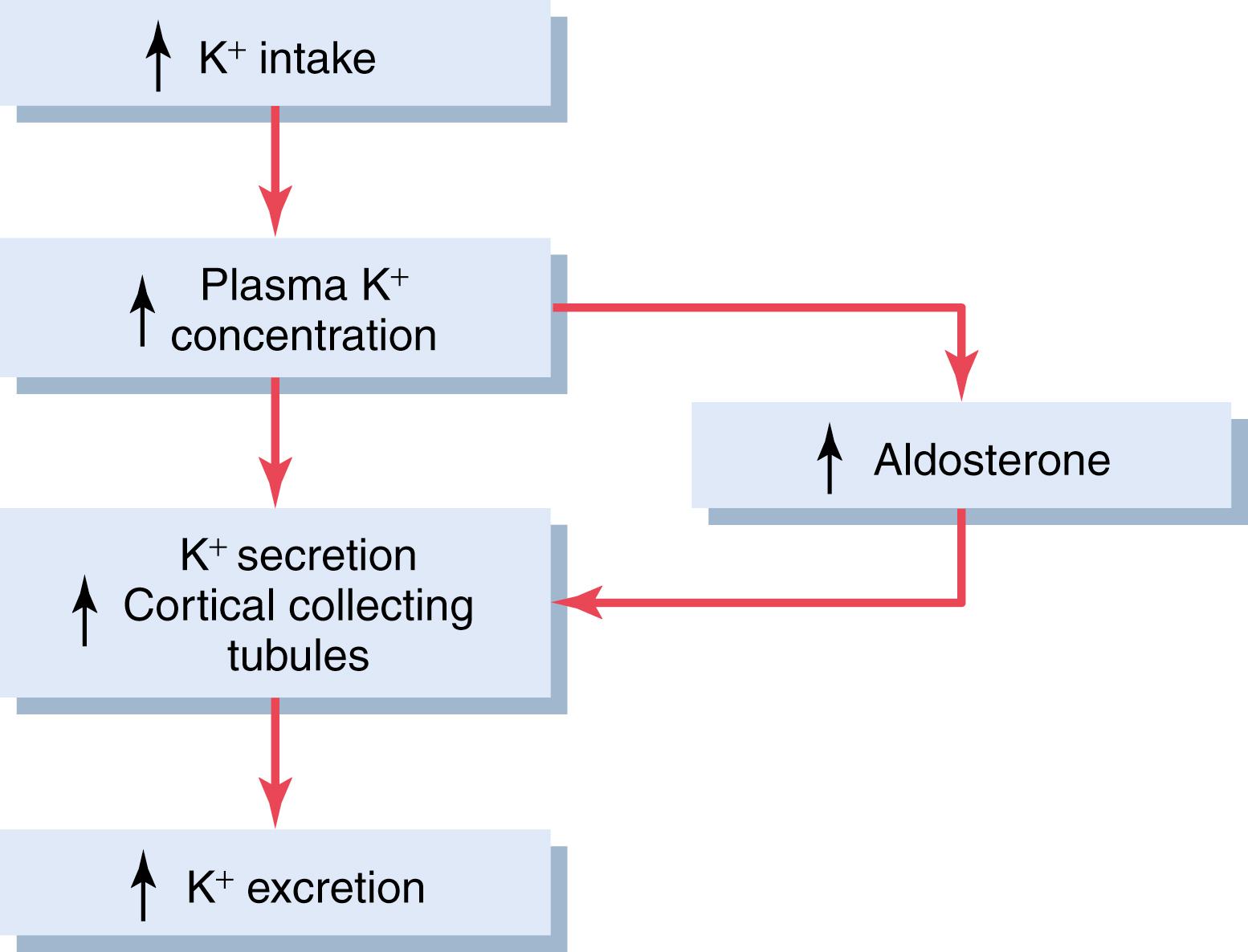
The effect of potassium ion concentration to stimulate aldosterone secretion is part of a powerful feedback system for regulating potassium excretion, as shown in Figure 30-6 . In this feedback system, an increase in plasma potassium concentration stimulates aldosterone secretion and, therefore, increases plasma aldosterone concentration (block 1). The increase in plasma aldosterone then causes a marked increase in potassium excretion by the kidneys (block 2). The increased potassium excretion then reduces the extracellular fluid potassium concentration back toward normal (circle 3 and block 4). Thus, this feedback mechanism acts synergistically, with the direct effect of increased extracellular potassium concentration to elevate potassium excretion when potassium intake is raised ( Figure 30-7 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here