Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The etiology of neonatal renal failure encompasses both chronic conditions such as congenital defects of the kidney and urinary tract and acute insults such as hypoxia, ischemia, or drug toxicity leading to acute kidney injury (AKI) ( ). AKI in the neonate has been difficult to define, especially in the setting of prematurity, as standard definitions for both adult and pediatric patients rely on changes in serum creatinine and urine output ( ). During fetal development, the placenta provides renal-like clearance of plasma waste products. For the first day after birth, the newborn’s creatinine predominantly reflects maternal renal function. Glomerular filtration rate is relatively low in newborn infants and changes rapidly over the first few weeks of life. Nephrogenesis continues in the normal fetus until 34 to 35 weeks’ gestation, and thus a “normal” serum creatinine is difficult to define for infants born before 34 weeks’ gestation. In addition, neonatal AKI can present with severe oliguric or anuric renal failure that requires renal replacement therapy (RRT) but can also present with nonoliguric AKI, especially in the setting of injury from nephrotoxic medications. Thus given that there is no standard definition for neonatal AKI and because nonoliguric AKI may be unrecognized by clinicians, it is challenging to determine the true incidence of neonatal AKI. Whereas early studies of neonatal AKI often used a serum creatinine of 1.5 mg/dL or blood urea nitrogen (BUN) over 20 mg/dL as a cutoff indicating AKI for infants greater than 34 weeks’ gestation, more recent studies use a modified KDIGO (Kidney Disease: Improving Global Outcomes) definition for neonates that includes a rise in serum creatinine of 0.3 mg/dL or more from the lowest previous value, a 50% rise in serum creatinine from the lowest previous value, and/or urine output of less than 1 mL/kg/hour ( ; .)
A retrospective multinational multicenter study, Assessment of Worldwide Acute Kidney injury Epidemiology in Neonates (AWAKEN), has given new insights into the incidence of AKI and its complications ( ; , ; ). Overall, 30% of neonates developed AKI ( ). AKI risk varied by gestation, and there was a U-shape risk curve with youngest and oldest infants at highest risk. Not surprisingly, AKI affected the greatest percent of extremely premature (22–28 weeks’ gestation) infants, with almost one-half (48%) developing AKI. Infants with AKI were more likely to have hypoxic ischemic encephalopathy, congenital heart disease, and necrotizing enterocolitis ( ). Neonatal hypertension was more common in infants with AKI ( ) Early caffeine administration to preterm infants may be protective against AKI in the first 7 days of life ( ).
It has been increasingly recognized that neonatal AKI can lead to adverse long-term consequences. Neonatal AKI results in fourfold higher odds for mortality in the NICU and is associated with increased mortality following NICU discharge ( ). In addition, studies suggest that low birth weight, prematurity, and neonatal AKI may lead to increased risk for microalbuminuria, hypertension, and chronic end-stage kidney disease later in life ( ; ).
A baby boy is born to a 29-year-old gravida 3, para 2 mother at 35 2 ⁄ 7 weeks’ gestation. The pregnancy was complicated by oligohydramnios and a concern for right renal agenesis, left renal dysplasia, and preterm labor. Maternal testing: rapid plasma reagin (RPR) negative, hepatitis B surface antigen negative, group B Streptococcus (GBS) colonization negative, and human immunodeficiency virus (HIV) negative. Amniocentesis demonstrated a normal male karyotype. He was born via vaginal delivery (VD). His Apgar scores were 6/7/8, and his birth weight was 2510 g.
What is the initial diagnostic test that should be performed?
Kidney and bladder ultrasound
Measurement of serum creatinine
Voiding cystourethrogram
A. In the first 24 hours of life, a newborn’s serum creatinine will reflect maternal creatinine and thus is not useful for diagnosis of renal failure. This infant has a history of oligohydramnios and bilateral renal anomalies. Thus a kidney–bladder ultrasound is the best initial diagnostic step. The infant’s urine output should be monitored. No bladder abnormalities were described prenatally, but posterior urethral valves would be on the differential diagnosis of this infant’s anomalies. Posterior urethral valves involve obstruction at the level of the prostatic urethra. The initial management is relieving the obstruction by placement of a Foley catheter. Dilation of the prostatic urethra during voiding on a voiding cystourethrogram (VCUG) confirms the diagnosis of posterior urethral valves but would not be the initial diagnostic approach for the patient in this vignette ( Table 18.1 ).
| Prerenal | Intravascular volume depletion Reduced renal perfusion, such as with heart failure |
| Intrinsic Renal | Nephrotoxic medications Acute tubular necrosis secondary to hypoxia/ischemia/asphyxia Sepsis Hypotension Renal agenesis or hypodysplasia Cystic kidney disease Vascular: renal arterial or vein thrombosis |
| Postrenal | Posterior urethral valves |
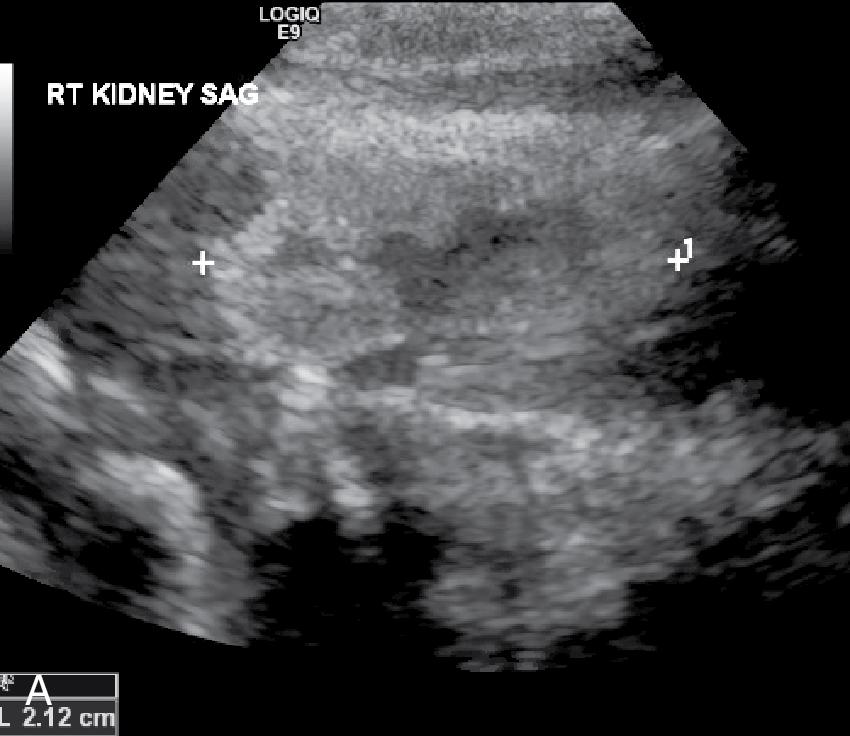
The patient was admitted to the NICU. A renal ultrasound showed a small, mildly echogenic right kidney (2.12 cm) without hydronephrosis and a slightly larger mildly echogenic left kidney (2.62 cm) with dilatation of the left renal pelvis without caliectasis. The bladder appeared normal. A VCUG revealed bilateral grade 3 vesicoureteral reflux and he was started on amoxicillin prophylaxis ( Fig. 18.1 ). The BUN is 13 mg/dL and the creatinine is 2.2 mg/dL.
Which of the following electrolyte abnormalities is most likely to occur in this infant?
Hypokalemia
Hyponatremia
Hypophosphatemia
B. There are three types of acute renal failure: prerenal, intrinsic renal, and postrenal failure. This infant likely has intrinsic renal failure due to bilateral renal hypodysplasia. Infants with abnormal kidney development and renal failure may exhibit polyuria or oliguria/anuria. Hyponatremia can be observed with both extremes of urine output. Many infants with polyuric renal failure and hyponatremia require water and sodium supplementation; studies have demonstrated improved linear growth with sodium supplementation. Despite the polyuria, hyperkalemia and hyperphosphatemia, rather than hypokalemia or hypophosphatemia, is likely to occur in infants with renal failure. The kidneys are the major sites for phosphorus excretion. In the setting of renal insufficiency, phosphorus excretion decreases and the elevations in serum phosphorus stimulate secretion of parathyroid hormone. Secondary hyperparathyroidism and reduced formation of the active form of vitamin D (1,25-[OH] 2 -vitamin D) due to decreased 1-hydroxylation that occurs in the kidney can lead to chronic kidney disease related mineral bone disease (CKD-MBD). In turn this leads to defective bone mineralization (renal rickets) and long-term cardiovascular complications due to deposition of calcium and phosphorus in the vasculature. Both breast milk and Similac PM 60/40 have lower potassium, calcium, and phosphorus content, making these better choices for neonates with renal insufficiency. High-calorie-containing formulas and breast milk fortifiers contain higher levels of potassium, calcium, and phosphorus and can lead to hyperkalemia and hyperphosphatemia in neonates with renal failure. In addition, following ablation of posterior urethral valves, many neonates will never recover function of the collecting ducts, which are the segments responsible for water reabsorption and will become polyuric. These infants require additional free water intake to compensate for water loss in their diluted urine; thus concentrated formulas (e.g., 22–24 kcal/oz versions) should be avoided in infants with persistently high urine volumes and low urine specific gravity.
Hyperkalemia (K >6.0 mEq/L) can be a life-threatening complication of neonatal AKI. Premature neonates have lower GFR and relative resistance to aldosterone, limiting their ability to excrete potassium. Therefore reducing potassium intake by lowering potassium from total parenteral nutrition (TPN) or intravenous fluid (IVF) and/or use of low potassium formula (Similac PM 60/40 or breast milk) is an important precaution in the management of neonatal AKI. Administration of sodium polystyrene resins can be used to achieve the exchange of potassium for sodium, but direct oral or rectal administration might carry risks in neonates as it has been reported to cause intestinal ischemia, obstruction, and perforation. Potassium intake can be further decreased by premixing sodium polystyrene with Similac PM 60/40 or breast milk allowing the potassium to precipitate out. After allowing the precipitate to settle, the supernatant is decanted for oral/gastric administration (so that there is no direct administration of the resin).
For acute management of hyperkalemia, an electrocardiogram (ECG) should be performed to evaluate the effects of hyperkalemia on the heart’s electrical activity. Calcium gluconate should be administered in the presence of ECG changes. As concomitant acidosis will drive potassium out of cells and elevate the serum potassium, sodium bicarbonate should be administered to treat acidosis but is not used when metabolic acidosis is not present. Administration of IV glucose and insulin can be used in the acute setting to stimulate potassium entry into cells and temporarily lower serum potassium. However, insulin and glucose will not enhance excretion of potassium. In the nonanuric patient, IV furosemide can be administered to increase potassium excretion but is not an evidence-based intervention. More recently, inhaled albuterol (salbutamol) has been used to treat infants with elevated serum potassium values. Small randomized clinical trials indicate the drug is effective, but it has not been evaluated in a large randomized trial. If medical management fails, dialysis is indicated for potassium removal.
On further questioning, it is discovered that the patient has an older sibling with a renal transplant for congenital kidney disease.
Which of the following is the next BEST diagnostic approach?
Echocardiogram to assess for associated heart malformation
Ophthalmic examination to assess for associated optic malformations
Genetic testing
C is the preferred answer.
Precision medicine using high throughput technologies has increasingly been integrated into CAKUT diagnosis and management. To date, about 40 monogenic causes have been reported. However, they account for less than 20% of the patients with CAKUT, suggesting that more genetic and perhaps environmental factors contributing to the diseases have yet to be identified ( ). Among genes mutated in CAKUT, some are transcriptional factors important for the development of kidney and urinary tract as well as other organs, which explains multiple organ malformations in a subset of CAKUT patients presenting with a syndrome. For mutations to be considered pathogenic, functional studies including experiments in animal models are required to establish the causality. The discovery of CAKUT-causing genes has been changing because of variable expressivity in individuals with the same mutations and incomplete penetrance that occurs in individuals with the mutation but who are otherwise well. It is expected that more pathogenic genetic defects will be identified and confirmed with advanced genomic and molecular technology. In addition, copy number variations (CNVs) that are generally defined as gain or loss of germline DNA ranging from 1 kilobases to several megabases have been shown to cause the CAKUT phenotype ( ). These gene-disrupting CNVs frequently involve more than one gene and are likely to be responsible for multiple organ defects. For example, CNV disorders detected in patients with renal hypodysplasia have also been associated with developmental delay or neuropsychiatric diseases ( ). Thus, discovering these CNVs provides a precise molecular diagnosis and guides multidisciplinary managements including early interventions for neurocognition.
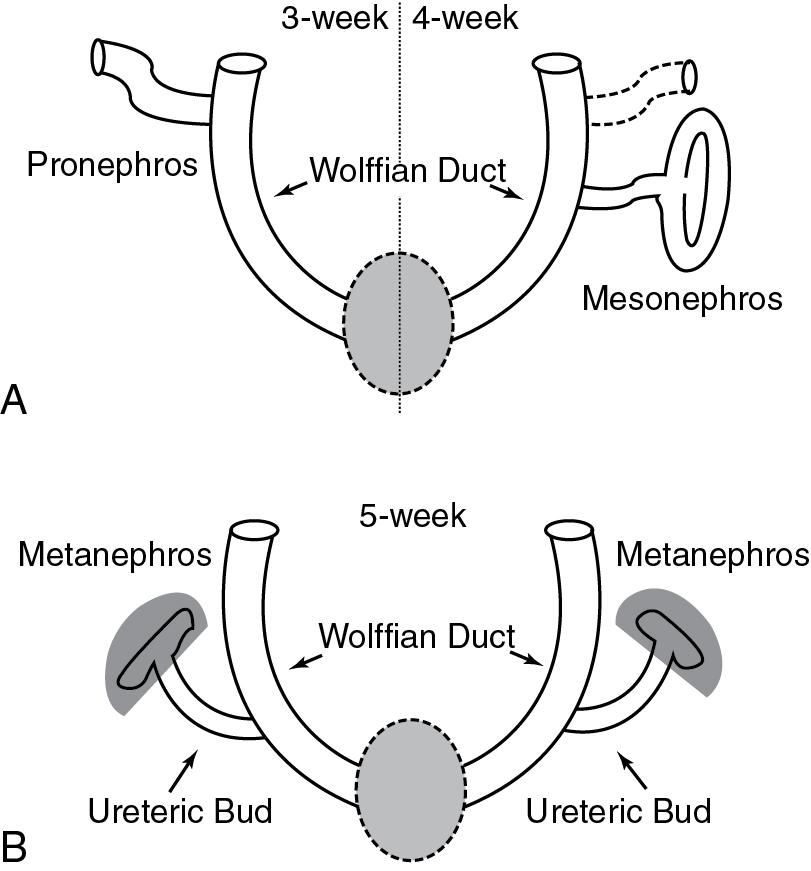
The kidneys are derived from the intermediate mesoderm. There are three sets of embryonic kidneys during embryogenesis: the pronephros (at 3 weeks’ gestation), mesonephros (at 4 weeks’ gestation), and metanephros (at 5 weeks’ gestation) ( Fig. 18.2 ). The pronephros and most of the mesonephros degenerate. The mesonephric duct (Wolffian duct) gives rise to epididymis, vas deferens, seminal vesicles, and ejaculatory ducts in males. The mature human kidneys develop from the metanephros. The ureteric bud, which is an outgrowth of the mesonephric duct in both males and females, invades the metanephric mesenchyme and undergoes sequential branching morphogenesis to form the collecting ducts. The tips of the ureteric bud induce nephron formation in the mesenchyme. Thus the number of ureteric bud branches will influence nephron number. Defects in the budding, migration, and branching of ureteric bud can lead to congenital abnormalities of the kidneys (e.g., renal aplasia, hypoplasia, dysplasia) and urinary tract deformities such as ureteral pelvic junction (UPJ) or ureteral vesical junction (UVJ) obstruction and vesicoureteral reflux.
The inductive signals originating from the ureteric bud stimulate mesenchymal to epithelial transition, and spheres of epithelial cells known as renal vesicles are the earliest form of the developing nephron. The developing nephrons go through a series of morphologic changes, forming a comma- and then S -shaped bodies. Segments of the S -shaped body will differentiate into the distal tubule, proximal tubule, and glomerular podocytes. Endothelial cells migrate into the cleft of the S -shaped body to form the glomerular endothelial cells of the capillaries. The molecular signals that drive nephrogenesis require reciprocal interactions between the ureteric bud and surrounding metanephric mesenchyme. Genetic defects in the signaling pathways can result in congenital anomalies of the kidney and urinary tract (CAKUT).
The first glomeruli form by 9 weeks, and nephrogenesis continues until 34 to 35 weeks’ gestation. The ureters and bladder form around the same time as the metanephric kidney. Glomerular filtration begins at the gestational age of 9 to 10 weeks. Fetal urine is the major source of amniotic fluid from approximately 16 weeks’ gestation onward. Thus obstruction of urinary outflow (as may occur in posterior urethral valves) or renal aplasia/severe dysplasia leads to low urine production and will present with oligohydramnios during the pregnancy. Oligohydramnios is associated with poor fetal lung development as the lack of sufficient amniotic fluid diminishes fetal breathing and thoracic movements that are required for proper lung maturation.
CAKUT are the leading causes of end-stage renal disease (ESRD) in children in North America. Obstructive uropathy from posterior urethral valves, followed by renal hypoplasia/aplasia/dysplasia, are the most common causes of children requiring dialysis in infancy.
Posterior urethral valves are membranes at the junction of the prostatic and bulbar urethra. Although the pathophysiology of this condition is not fully understood, these membranes are thought to form during normal development. However, failure to recanalize during urethral development leads to bladder outlet obstruction. In the face of high-pressure obstruction, bladder differentiation is impaired and a thick-walled bladder develops. The dilation of the prostatic urethra can lead to the classic keyhole sign on a fetal ultrasound. The exposure of the developing kidneys to obstruction results in varying degrees of renal dysplasia.
CAKUT may be associated with both syndromes (e.g., Trisomy 21) and monogenic defects ( ). Monogenic causes of CAKUT often have extrarenal manifestations. For example, PAX2 mutations are associated with renal colobomas. The renal coloboma syndrome is an autosomal dominant disorder associated with optic nerve colobomas or dysplasia and renal defects (including hypodysplasia with or without VUR). As discussed earlier, advanced genetic technologies can help discover genetic mutations that underlie these malformations. Identifying the underlying defect allows for appropriate studies and consultations to identify and manage extrarenal disease.
The clinical course over the next week was remarkable for fever with hemodynamic instability and a low urine output of 0.25 mL/kg/hour. He required multiple fluid boluses and was started on broad spectrum antibiotics. Table 18.2 lists other pertinent laboratory data and clinical information.
| Day | Na (mEq/L) | K (mEq/L) | Cl (mEq/L) | HCO 3 (mEq/L) | BUN (mg/dL) | Creatinine (mg/dL) | Calcium (mg/dL) | PO 4 (mg/dL) | Fluid intake (mL) | Fluid output (mL) | Other treatments |
| 0 | 132 | 4.4 | 106 | 21 | 8 | 1.6 | 7.7 | 186 | 12 | Hypotensive required fluid boluses and pressors | |
| 1 | 133 | 3.6 | 104 | 20 | 17 | 2 | 8.2 | 378 | 22 | ||
| 2 | 129 | 3.4 | 91 | 25 | 37 | 3.3 | 9.1 | 3.4 | 297 | 98 | |
| 3 | 130 | 3.6 | 94 | 24 | 45 | 3.7 | 9.2 | 3.7 | 250 | 135 | Furosemide started for fluid overload |
| 4 | 132 | 3.3 | 94 | 29 | 50 | 3.8 | 10 | 3.2 | 281 | 177 | |
| 5 | 138 | 5.7 | 96 | 32 | 55 | 4.2 | 10.3 | 2.8 | 251 | 156 | |
| 6 | 150 | 6.5 | 98 | 34 | 64 | 4.9 | 10.3 | 4.1 | 240 | 80 |
Length 52 cm, weight 3.75 kg, HC 33.5 cm
T 37.1°C, HR 149/minute, RR 60/minute, BP 81/58, O 2 sat 97%
General: Alert
HEENT: periorbital edema
Chest: bilateral decreased air entry, no retractions
Cardiovascular: normal S1, S2, and no murmur
Abdomen: soft, distended, nontender, liver 1 cm palpable at RCM
Genitourinary: Tanner 1 male, scrotal edema
Extremities: well perfused, edema bilaterally
Neurologic: normal
The most appropriate next step in care for this infant is:
Additional normal saline bolus to increase urine output
Palliative care
Placement of a peritoneal dialysis catheter
C. This infant has normal blood pressure and perfusion and does not appear to be volume depleted; instead, the infant is edematous and likely volume overloaded. A normal saline bolus should be avoided. Fluid overload is associated with increased mortality in children, and net positive fluid balance is associated with prolonged mechanical ventilation in neonates ( ; ; ; ; ; ; ; ). This infant has substantial fluid overload (weight gain of 1 kg) and thus dialysis is indicated.
Indications for dialysis include:
Fluid overload that results in congestive heart failure, pulmonary edema, and severe hypertension
Intractable acidosis
Hyperkalemia
Uremic complications (hemorrhage, encephalopathy, etc.)
Fluid removal to optimize nutrition and medication administration
The ethics of providing dialysis to neonates should always be a consideration, because this could indicate the potential need for lifelong renal replacement therapy (RRT) and ultimately renal transplantation. This infant was able to be extubated, suggesting that the degree of pulmonary hypoplasia was not life limiting. Furthermore, the infant appears to be neurologically intact without significant comorbidities. Thus the infant would be a candidate for dialysis. Neonatal survival at 1 year for those started on dialysis in infancy is close to 85%, although there is a slightly worse prognosis for anuric infants. The most common causes of death are from cardiopulmonary causes and infection in this age group ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here